Abstract
Very few unhealthy behaviors have healthy desirable alternatives. Sunless tanning is a relatively risk-free alternative to the unhealthy behavior of suntanning, but has not been well-studied in the context of skin cancer prevention.
Objective
The present study examined the impact of a skin cancer prevention intervention that promoted sunless tanning as a substitute for sunbathing.
Design
The study is a randomized controlled trial.
Setting
The intervention was conducted on public beaches in Massachusetts.
Participants
Women (N=250) were recruited to participate in the study during their visit to a public beach.
Intervention
The intervention included motivational messages to use sunless tanning as an alternative to UV tanning, instructions for proper use of sunless tanning products, attractive images of women with sunless tans, free trial of sunless tanning product, skin cancer education, and UV imaging. The control condition completed surveys.
Main Outcome Measures
The primary outcome was sunbathing 2 months and 1 year following the intervention. Secondary outcomes included sunburns, sun protection, and sunless tanning.
Results
At 2 months, intervention participants reduced their sunbathing significantly more than controls and reported significantly fewer sunburns and greater use of protective clothing. At 1 year, intervention participants reported significant decreases in sunbathing and increases in sunless tanning relative to control participants, but no differences on other outcomes.
Conclusion
The intervention which promoted sunless tanning as an alternative to UV tanning had a short-term impact on sunbathing, sunburns and protective clothing, and a longer term impact on sunbathing and sunless tanning.
Ultraviolet radiation (UVR) was recently upgraded to the highest cancer risk category, joining arsenic and mustard gas.1 UVR is linked to more cancers worldwide than any other carcinogen.2 Although skin cancer is preventable, 3, 4 rates of unprotected sun exposure remain high. 5 Whereas UVR exposure can be incidental, many people intentionally expose themselves to UVR for tanning. The desire to be tan to improve physical appearance is the strongest predictor of intentional UVR exposure.6–11 People who desire a tan are also the most resistant to sun safety recommendations.12–14 A novel approach to reaching tan seekers is to promote sunless tanning products (i.e., fake tanning). Products provide a tan without UVR exposure using a substance called dihydroxyacetone (DHA), a colorless vegetable-derived sugar that interacts with dead surface cells in the epidermis to stain the skin. 15 DHA was approved by the U.S. Food and Drug Administration (FDA) as a color additive for cosmetics in 1973.16 The data on whether sunless tanning is a helpful or harmful sun safety recommendation are scant.17–23 One promising randomized trial followed college students for one month after an intervention that included both sunless tanning and UV imaging, and found significant increases in self-efficacy and intentions to use sunscreen, and a nonsignificant trend toward less sunbathing and greater sun protection compared to a control group, suggesting no evidence of harm in promoting sunless tanning.23 The promotion of sunless tanning might have even greater impact in higher risk populations, such as sunbathers.
The present study extends this research by examining the impact of a beach-based intervention targeting female sunbathers and promoting sunless tanning as a safe alternative to sunbathing in the context of general sun safety recommendations. The primary goal of the study was to reduce sunbathing 2-months and 1-year following the intervention, compared to controls. The present study is of the first to explore whether sunless tanning can play a role in skin cancer prevention.
Methods
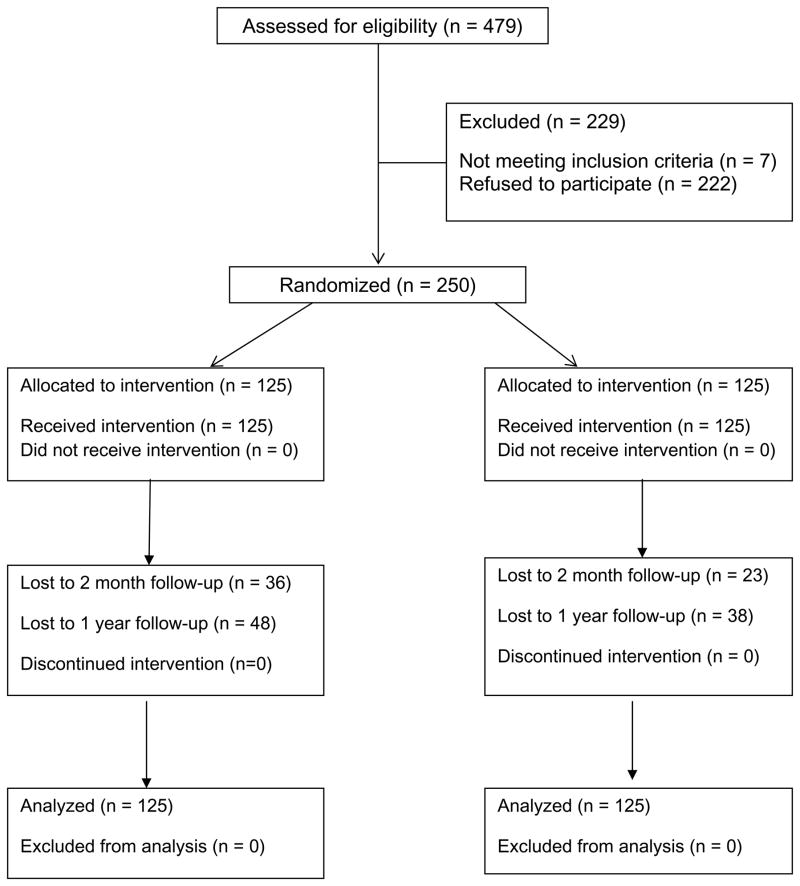
A detailed description of the study methodology is reported elsewhere.24 The protocol was approved by the institutional review board at the University of Massachusetts Medical School. Two public beaches in Massachusetts were randomly assigned to intervention or control conditions on 11 days in June and July 2006. Data collection occurred during peak UV hours (11 a.m. to 4 p.m.)25 on week and weekend days. Research assistants were given specific instructions to invite sunbathers to participate in a study on sunbathing requiring 20 minutes (intervention group) or 10 minutes (control group; see Figure 1). Only female beach visitors (N=250) were invited to participate because the acceptability of sunless tanners in men requires exploration given the vast majority of sunless tanning users are women.20 Eligibility criteria included 18 years of age or older, English speaking, able to read at the 6th grade level, and willingness to provide two or more types of contact information. 479 women were approached to participate, 257 agreed and 250 were eligible. Typical reasons for refusal included not wanting to be bothered during leisure time, plans to leave beach shortly, or attending to small children by oneself. Of the 7 participants ineligible at recruitment, 2 were excluded because they were not English speaking, 2 had to leave the beach before completing the surveys, 2 did not provide contact information, and 1 was under age 18. Intervention participants were told that participation would require completing questionnaires, taking a UV photo, and trying free product samples. Control participants were told that participation would require completing questionnaires. Eligible individuals were escorted to an unmarked study tent where they provided informed consent and completed questionnaires. Intervention participants were given the intervention described below, while control participants were given free cosmetic samples (unrelated to skin health), had their picture taken with an instant camera, and notified they would be contacted for follow-up.
Figure 1.
CONSORT diagram
Sunless Intervention
Research assistants who were trained in the protocol and use of the UV camera, educated about sunless tanning and users themselves, delivered the intervention. They first explained what sunless tanners are and gave written and verbal application instructions and an application demonstration. Participants applied the sunless tanner on their hand to observe the coloring effect on their skin. They were informed of the benefits of sunless tanning compared to sunbathing, of the safety and limitations (e.g., not a source of sun protection), and viewed sunless tans on female research staff. Participants were strongly encouraged to use sunless tanning instead of sunbathing. Participants also received a pamphlet about skin cancer and had their UV-filtered photo taken. UV-filtered camera photos reveal melanin deposits on the skin that are not visible to the naked eye. 26 UV imaging was included to heighten participant’s awareness of the sun damage on their skin and to serve as a cue to action to consider sunless tanning as a safe alternative. Participants were informed that the UV camera cannot be used to identify or diagnose a skin condition, including cancer. Participants were given copies of the photos. Finally, participants were given free samples of sunless tanning lotion and encouraged to use this for their tanning needs to prevent further sun damage to the skin and reduce their skin cancer risk. They also received free sunscreen. Ten months later, a UV photo was mailed to the participant with a reminder to avoid sunbathing in the upcoming summer.
Follow-up
Two-months and one-year following recruitment, participants were contacted by email, phone, or mail to complete follow-up surveys. Participants received a $10 gift card for completing questionnaires at 2-months and $20 at 1-year. In addition, participants were entered into a lottery to win a $500 gift card for completing each follow-up.
Outcome Measures
Sunbathing
The primary outcome was sunbathing. Participants were asked how much time they spent in the sun with the intention of getting a tan in the past two months using a 7-point scale ranging from 0 = never and 7 = everyday. Item wording was based on the recommended measurement of sunbathing in community and clinical research.27 The baseline measure reflects sunbathing in the 2 months previous to the study (May and June); the 2-month follow-up reflects sunbathing in the 2 months following recruitment (July and August); and one year follow-up reflects sunbathing in the first 2 months of summer (May and June) one year later.
Sunburns
Sunburn was assessed as the number of times participants reported a red or painful burn that lasted a day or more in the past 2 months using a 6-point scale from 0 = not at all to 5 = five times or more.27
Sunscreen and Other Sun Protection
Participants were asked to respond to a series of questions about how often they wear sunscreen, a shirt with sleeves, a hat, sunglasses, and how often they stay in the shade or under an umbrella in the past 2 months.27 For each item, responses were on a 5-point likert scale where 0 = never and 4 = always. Sunscreen and other protection items were examined separately. For the latter a mean sun protection score was calculated.
Sunless Tanning Behavior
Participants read a definition of sunless tanning and then indicated how many times they used sunless tanning products or spray-on tans in the last 2 months and in the past year.
Statistical Analyses
The trial was designed to have 80% power at a 5% significance level to test the hypothesized intervention effects at 2 months and 1 year on the primary outcome of sunbathing.
Preliminary analyses
Independent samples t-tests were conducted to examine whether the two groups were significantly different on age [t (247) = −3.13, p=.002] and skin type [t (248) = −0.17, p=.86]. The control group was significantly younger [mean=28.79; standard deviation (SD)=10.89] than the intervention group (mean=33.62; SD=13.29). Age was included as a covariate in all analyses. Chi-square tests revealed no differences in education [χ2(4) = 2.13, p=.71] or ethnicity [χ2(5) = 5.24, p=.39].
Analytic plan
Intention-to-treat analyses were used for all outcome variables. Mixed effects regression modeling, implemented via SAS PROC MIXED that incorporated a random intercept trend and the unstructured covariance as the covariance structure, was used to analyze the continuous outcomes. Generalized linear mixed models, implemented with SAS PROC GLIMMIX that incorporated a random intercept trend and a Poisson distribution were used to analyze sunless tanning. These analytic approaches include all participants that have data on at least one time point, which was true for all randomized cases. For sunbathing, sunburns, sunscreen, and protective clothing, all baseline and follow-up variables used a two month time frame which reflects summer months only. One model containing all 3 time points (baseline, 2 months, 1 year) was run for each dependent variable. Time was dummy coded into two variables (baseline and 2 months, baseline and 1 year). Fixed effects included the two time variables, the main effect of group, and each time x group interaction to examine whether the intervention condition resulted in greater change than the control group at the 2-month and 1-year follow-ups. Age and beach were included as covariates in the analyses.
Sunless tanning was assessed in two ways at baseline: sunless use in the past two months and in the past year. Thus, separate models were conducted for the baseline versus 2-month follow-up comparison and the baseline versus 1-year follow-up comparison for the sunless tanning outcome. Fixed effects included the time effect, the main effect of group, and the time x group interaction to examine whether the intervention group resulted in greater change than the control group at the 2-month and the 1-year follow-ups. Age and beach were included as covariates in the analyses.
Missing values were treated as missing in the analysis. At the 2-month follow-up, 71% of control participants had complete data for sunbathing and sunless tanning, while 82% and 81% of intervention participants had complete data for sunbathing and sunless tanning. For burns, sunscreen, and protective clothing, 61% of control and 77% of intervention participants had complete data. The sunbathing and sunless tanning variables had more complete data because participants who failed to complete follow-up surveys after 6 attempts were asked to complete at least these two items. All other missing data occurred due to participants skipping items or not returning surveys.
At the 1-year follow-up, 62% of control participants had complete data for sunbathing and 62% for sunless tanning while 69% of intervention participants had complete data for sunbathing and 70% for sunless tanning. For burns, sunscreen, and sun protection, 60–62% of control participants and 68–69% intervention participants had complete data. At 1-year follow-up, participants who failed to complete the follow-up surveys after 6 attempts were asked to complete the sunbathing and sunless variables.
Results
Participants had a mean age of 31.21 (SD=12.37). The majority of participants were Caucasian (84.4%), and many (44.8%) completed some college (Table 1).
Table 1.
Demographics among all participants and by group (N=250).
| All Participants | Intervention | Control | |
|---|---|---|---|
| Age (Mean (SD))* | 31.21 (12.36) | 33.62 (13.29) | 28.79 (10.89) |
| Ethnicity (n(%)) | |||
| Caucasian | 211 (88.7%) | 102 (86.4%) | 109 (90.8%) |
| Hispanic | 11 (4.6%) | 7 (5.9%) | 4 (3.3)% |
| African American | 4 (1.7%) | 2 (1.7%) | 2(1.7%) |
| American Indian | 1 (0.4%) | 0 (0%) | 1 (0.8%) |
| Multi-racial | 11 (4.6%) | 6 (5.1%) | 5 (4.2%) |
| Education (n(%)) | |||
| Less than a college degree | 158 (64.7%) | 77 (63.1%) | 81 (65.3)% |
| College degree | 56 (23.0%) | 27 (22.5%) | 29 (23.4%) |
| Graduate degree | 30 (12.3%) | 16 (13.3%) | 14 (11.3%) |
| Skin Type (n(%)) | |||
| I | 19 (7.6%) | 11 (8.8%) | 8 (6.4%) |
| II | 64 (25.6%) | 35 (28.0%) | 29 (23.2%) |
| III | 113 (45.2%) | 47 (37.6%) | 66 (52.8%) |
| IV | 54 (21.6%) | 32 (25.6%) | 22 (17.6)% |
| Family history of skin cancer (n(%)) | 75 (30.1%) | 39 (31.2%) | 36 (29.0%) |
| Personal history of skin cancer (n(%)) | 8 (3.2%) | 7 (5.6%) | 1 (0.8%) |
Note. Twelve participants (4.8%) were missing ethnicity data and six participants (2.4%) was missing education data.
p <.05
Sunbathing
Analyses revealed a significant time x group interaction on sunbathing at 2 months (t=−2.13, p=.03), such that participants in the intervention group reported a 33% decrease in sunbathing (t=−5.12; p<.001), compared to a 10% decrease in the control group (t=−2.28, p=.02; Cohen’s d=.32; Tables 2 and 3). At 1-year, the time x group interaction was also significant (t=−2.32; p=.02). Intervention participants reported a greater decrease in sunbathing (t=−5.07, p<.001), compared to control participants (t=−2.47, p=.01; Cohen’s d=0.32).
Table 2.
Unadjusted baseline, 2 months, and 1-year follow-up data for sunbathing, sunburns, protective clothing use, sunscreen use and sunless tanning.
| Baseline Mean (sd) | Two-months Mean (sd) | One Year Mean (sd) | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Sunbathinga | 4.12 (2.57) | 4.46 (2.13) | 2.77* (2.6) | 3.98 * (2.42) | 2.70* (2.61) | 3.81* (2.52) |
| Sunburnsb | 0.74 (1.06) | 0.71 (.80) | 0.20 *(.50) | 0.45* (0.72) | 0.43 (0.82) | 0.44 (0.66) |
| Protective clothingc | 1.77 (0.87) | 1.62 (0.78) | 2.34* (1.33) | 1.65* (0.85) | 1.97 (0.75) | 1.85 (0.68) |
| Sunscreenc | 2.41 (1.34) | 2.41 (1.34) | 1.94 (0.80) | 2.21 (1.37) | 2.74 (1.11) | 2.60 (1.27) |
| Sunless tanning | 7.50 (19.23) | 4.52 (10.34) | - | - | 15.90 (57.82) | 8.08 (25.38) |
Note.
p<.05,
0=never, 1=once, 2=twice, 3=once a week, 4=twice a week, 5=3–5 times a week, 6=everyday;
0=none, 1=once, 2=twice, 3=three, 4=four, 5=5 or more;
0=never, 1=rarely, 2=sometimes, 3=often, 4=always.
Table 3.
Results of the multivariate analyses for the significant time x condition interactions for the primary and secondary outcomes (N=250).
| Estimate | S.E. | t | p | |
|---|---|---|---|---|
| Sunbathing- 2 months | −0.22 | 0.09 | 2.38 | .02 |
| Sunbathing- 12 months | −0.68 | 0.34 | −2.00 | .04 |
| Sunburns- 2 months | −0.29 | 0.14 | −2.01 | .04 |
| Protective clothing- 2 months | 0.22 | 0.09 | 2.38 | .02 |
| Sunless tanning | .45 | .08 | 5.31 | <.0001 |
Sunburns
The time x group interaction was significant for sunburns at 2-months (t=−2.01; p=.04). Sunburn scores in the intervention group reduced by 73% over time (t=−5.51; p<.001), compared to 37% in the control group (t=−2.48; p=.01; Tables 2 and 3; Cohens’d=0.31). At 1-year, the interaction was not significant (t=−0.24; p=.81) but participants in both groups reported fewer burns at 1-year relative to baseline (t=−2.57, p<.01).
Protective clothing
The time x group interaction was significant for protective clothing at 2 months (t=2.15, p=.03), such that the intervention group reported a 32% increase in protective clothing (t=2.39, p=.02), relative to a 2% increase in the control group (t=−0.69, p=.49; See Tables 2 and 3; Cohen’s d=0.37). At 1-year, the interaction was not significant (t=−0.50; p=.61), but protective clothing increased over time for all participants (t=2.13=.03).
Sunscreen use
The time x group interaction did not significantly predict sunscreen use at 2 months (t=1.18; p=.24) or at 1 year (t=0.88; p=.38). However, sunscreen use decreased over time across groups at 2 months (t=−2. 32; p=.02), but did not change at 1 year (t=.94, p=.35).
Sunless Tanning
The time x group interaction was not significant for sunless tanning use at 2 months (t = −1.08, p=.28), but was significant at 1 year (t =5.31, p<.0001), such that participants in the intervention group significantly increased their total annual use of sunless tanning by an average of 8.40 uses (t=14.26, p<.0001), compared to the control group that increased their total annual use by 3.56 uses (t=2.92, p=.005).
Comment
Very few unhealthy behaviors have healthy desirable alternatives. Sunless tanning is a risk-free alternative to suntanning, but has not been well-studied in the context of skin cancer prevention. Results revealed that an intervention that promoted sunless tanning led to both short- and long-term behavior change. Sunbathers exposed to the intervention in the middle of summer reported declines in sunbathing and sunburns, as well as increased use of protective clothing during the rest of the summer, compared to their counterparts who only completed questionnaires. One-year following the intervention, effects were maintained for sunbathing.. Use of sunless tanning during the year following the intervention increased significantly more in the intervention group compared to the control group.
Nearly half of the intervention participants (48%) had used sunless tanning at least once prior to the study. Another 9% of intervention participants newly adopted sunless tanning at 2-months and about 6% at 1-year. Of the intervention participants who tried sunless tanning for the first time at 2-months and 1-year following the intervention, the majority (64% and 75%, respectively) used it more than once, suggesting that many who tried sunless tanning as a result of the study adopted it as a habit. In spite of the small increase in sunless tanning, intervention participants reduced their sunbathing. Further investigation is merited to determine what aspect or aspects of the intervention generated the observed effects. Sunless tanning was promoted within an intervention that provided skin cancer education and UV imaging. While education and UV imaging affect knowledge and intentions,28 neither has reduced sunbathing behavior (e.g.,29, 23, 30–32), which was the reason sunless tanning was added to this intervention. In a previous study of beach visitors, we tested an intervention that included all aspects of the present intervention (i.e., skin cancer education, free sunscreen, UV imaging) except the sunless tanning content. 29 Instead of sunless tanning, that study focused on sunscreen and other sun protection (e.g., clothing). Using the same measures, the previous study did not impact sunbathing, but did decrease sunscreen use. The focus of the present study on tanning alternatives may have better reinforced an abstinence message as opposed to a harm reduction message (i.e., using sun protection during exposure) as in the previous study. A message focused on sunscreen and other forms of sun protection might inadvertently reinforce the misconception that people can tan “safely.” Future research should examine which prevention messages reduce sunbathing, and which increase sun protection.
Results from the present study suggest that sun safety recommendations have the greatest impact during the season they are received. At 1-year, the intervention effect on sunbathing remained significant, but effects on secondary outcomes did not. Participants in all groups significantly reduced their sunburns and increased their use of protective clothing at 1-year, which could suggest either a social desirability bias in survey responses, an intervention effect of surveys, or that people in this region are exhibiting a trend toward healthier habits over time. Regardless, recurrent sun safety messages may be necessary to reinforce the impact on behavior. The present study also suggests that health messages received in the environments in which people sunbathe can deter future sunbathing.
Physicians might be reluctant to recommend sunless tanning due to concerns that it might inadvertently reinforce the patient’s desire to be tan. The literature is limited but does not seem to support this contention. Although one cross-sectional study found that people who use sunless tanning report more indoor tanning and sunburns than non-users, 21 this is probably because first adopters of sunless tanning are users of other forms of tanning. Several studies in the US and Australia have found that sunless users have higher rates of sunscreen use, which suggests that sunless tanning may cluster with other sun safety behaviors.17–20 The extent to which use of sunless tanning offsets a previously existing tanning habit has only been explored in one study. Almost three quarters (73%) of people receiving a sunless spray tan reported that they had decreased their indoor tanning since they began sunless tanning, while only 7% reported having increased their indoor tanning.22 These data suggest that sunless tanning might be associated with declining UVR tanning, which is a promising trend to capitalize on in skin cancer prevention efforts. The only other trial to test a sunless tanning intervention found promising effects on self-efficacy and intentions to use sunscreen, but no actual behavior change.23
As our study demonstrated, messages promoting sunless tanning might be of greater interest to people who are frequent tanners, such as beach visitors.
The present study had some limitations. The refusal rate was 46% which could have contributed to selection bias. Because sunless tanning was not specifically mentioned when participants were invited to participate, refusal would not have been related to attitudes about sunless tanning. Also possibly contributing to selection bias is that randomization occurred by beach instead of individual. Individual randomization is not feasible in this setting given multiple entrances and the transient nature of beach patrons. Because of the possibility of selection bias, we explored baseline differences on demographic variables. Age was significantly different between groups and was consequently entered as a covariate in all analyses. Another limitation is that all measures were self-report which may be subject to underreporting due to social desirability bias. Self-report measures are typically used in studies of sun-related behavior27 because very few practical, objective measures exist for large samples, and follow-up could not be done in person. Items used in the present study have recently been put forth as the standard in sun exposure and sun protection measurement.27
Effect sizes were fairly small. The intervention was a brief, one-shot, inexpensive approach to behavior change in the very setting in which high-risk behavior occurs. Results of the present study suggest that future studies that increase the intensity and length of the intervention are merited. Further investigation is merited to determine what aspect or aspects of the intervention generated the observed effects. Sunless tanning was promoted within an intervention that provided skin cancer education and UV imaging. While education and UV imaging affect knowledge and intentions,28 neither has reduced sunbathing behavior, (e.g.,29, 23, 30–32) which was our reason for adding sunless tanning to this standard intervention.
Loss to follow-up occurred and differed by group. The follow-up rate at 1-year was 66%, but is comparable to the 70% follow-up rate reported in the only other beach-based intervention study to follow participants for 1-year or longer.32 A systematic review revealed that two-thirds of skin cancer prevention studies follow participants for 6 weeks or less and over half follow participants for less than 3 months.28 The present study extends the literature by examining maintenance of intervention effects over the following summer. A marginally significant difference between study groups was apparent for missing data on the primary outcome at the 2 month follow-up [χ2(1) = 3.75, p=.05], such that more control participants had missing data (28.8%) than intervention participants (18.4%), but not at the 1 year follow-up [χ2(1) = 1.77, p=.18]. This transient difference in missing data between groups perhaps resulted from intervention participants receiving more personalized attention than control participants, which may have increased their sense of obligation to follow-up. Finally, even though DHA, the active ingredient in sunless tanning products has been FDA approved for cosmetic use since 1973 and has only received reports of rashes,33 studies of the safety of long-term use are lacking. One study in 2004 revealed that DHA lead to DNA damage in cultured keratinocytes, but it remains unknown if DHA induces the same effect in the human epidermis.34 Sunless tanning is the only safe means of tanning. Encouraging sunbathers to switch to sunless tanning could have an important health impact, but sunless tanning has been considered a cosmetic more so than a health care tool. Findings have implications for both public health and clinical efforts to prevent skin cancer. Promoting sunless tanning to sunbathers within the context of a skin cancer prevention public health message may be helpful in reducing sunbathing and sunburns, as well as promoting use of protective clothing. Future research should determine how to further convince tanners to switch to sunless tanning. Physicians should encourage patients who sunbathe to consider safe alternatives like sunless tanning. Finally, reinforcing sun safety messages every season is likely to be necessary to maximize the impact of the message.
Acknowledgments
Funding/Support: This project was supported by grant R21 CA 109670 awarded to Dr. Pagoto and funded by NIH/NCI.
Role of the Sponsor: The sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Financial Disclosures: The authors declare there are no conflicts of interest or financial disclosures to report.
Author Contributions: Dr. Pagoto had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pagoto; Analysis and interpretation of data: Schneider, Ma; Drafting of the manuscript: Pagoto, Oleski, Schneider, Ma; Critical revision of the manuscript for important intellectual content: Bodenlos, Pagoto, Schneider, Ma, Oleski; Statistical analysis: Schneider, Ma, Pagoto; Obtained funding: Pagoto; Administrative, technical or material support: Schneider, Oleski, Bodenlos.
References
- 1.El Ghissassi F, Baan R, Straif K, et al. A review of human carcinogens--part D: Radiation. Lancet Oncol. 2009;10(8):751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 2.Nyugen TH, Ho DQ. Nonmelanoma skin cancer. Curr Treat Opt Oncol. 2002;3:193–203. doi: 10.1007/s11864-002-0009-0. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer facts and figures, January 2002. Atlanta, GA: American Cancer Society; 2002. [Google Scholar]
- 4.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340(17):1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. National Center for Health Statistics. National Health Interview Survey. 2001. [Google Scholar]
- 6.Broadstock M, Borland R, Gason R. Effects of suntan on judgments of healthiness and attractiveness by adolescents. Journal of Applied Social Psychology. 1992;22:157–172. [Google Scholar]
- 7.Davis KJ, Cokkinides VE, Weinstock MA, O’Connell MC, Wingo PA. Summer sunburn and sun exposure among UAS youths ages 11 to 18: National prevalence and associated factors. Pediatrics. 2002;110(1 pt 1):27–35. doi: 10.1542/peds.110.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13(1):86–90. doi: 10.1037//0278-6133.13.1.86. [DOI] [PubMed] [Google Scholar]
- 9.Hillhouse JJ, Stair AW, 3rd, Adler CM. Predictors of sunbathing and sunscreen use in college undergraduates. J Behav Med. 1996;19(6):543–561. doi: 10.1007/BF01904903. [DOI] [PubMed] [Google Scholar]
- 10.Hoegh HJ, Davis BD, Manthe AF. Sun avoidance practices among non-Hispanic white Californians. Health Educ Behav. 1999;26(3):360–368. doi: 10.1177/109019819902600306. [DOI] [PubMed] [Google Scholar]
- 11.Robinson JK, Rigel DS, Amonette RA. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol. 1997;37(2 Pt 1):179–186. doi: 10.1016/s0190-9622(97)80122-3. [DOI] [PubMed] [Google Scholar]
- 12.Borland R, Hill D, Noy S. Being sunsmart: Changes in community awareness and reported behaviour following a primary prevention program for skin cancer control. Behav Change. 1990;7(3):126–135. [Google Scholar]
- 13.Borland RM, Hocking B, Godkin GA, Gibbs AF, Hill DJ. The impact of a skin cancer control education package for outdoor workers. Med J Aust. 1991;154(10):686–688. doi: 10.5694/j.1326-5377.1991.tb121261.x. [DOI] [PubMed] [Google Scholar]
- 14.Detweiler JB, Bedell BT, Salovey P, Pronin E, Rothman AJ. Message framing and sunscreen use: Gain-framed messages motivate beach-goers. Health Psychol. 1999;18(2):189–196. doi: 10.1037//0278-6133.18.2.189. [DOI] [PubMed] [Google Scholar]
- 15.Draelos ZD. Self-tanning lotions: Are they a healthy way to achieve a tan? Am J Clin Psychol. 2002;3(5):317–318. doi: 10.2165/00128071-200203050-00003. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services, Food and Drug Administration. Code of Federal Regulations, Title 21. 73. Vol. 1. 2002. p. 1150. [Google Scholar]
- 17.Beckmann KR, Kirke BA, McCaul KA, Roder DM. Use of fake tanning lotions in the South Australian population. Med J Aust. 2001;174(2):75–78. doi: 10.5694/j.1326-5377.2001.tb143158.x. [DOI] [PubMed] [Google Scholar]
- 18.Dixson H, Cappiello M, Borland R. Reaction to the 1994/1995 SunSmart campaign: Results from a representative household survey of Victorians. SunSmart Evaluation Studies 5. Melbourne: Anti-Cancer Council of Victoria; 1997. [Google Scholar]
- 19.Girgis A, Tzelepis F, Paul CL, Walsh RA, McElduff P, McKenzie J. Australians’ use of fake tanning lotions: Another piece of the puzzle. Aust N Z J Public Health. 2003;27(5):529–532. doi: 10.1111/j.1467-842x.2003.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 20.Stryker JE, Yaroch AL, Moser RP, Atienza A, Glanz K. Prevalence of sunless tanning product use and related behaviors among adults in the United States: Results from a national survey. J Am Acad Dermatol. 2007;56(3):387–390. doi: 10.1016/j.jaad.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 21.Brooks K, Brooks D, Dajani Z, et al. Use of artificial tanning products among young adults. J Am Acad Dermatol. 2006;54(6):1060–1066. doi: 10.1016/j.jaad.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DJ, Lesher JL., Jr The effect of sunless tanning on behavior in the sun: A pilot study. South Med J. 2005;98(12):1192–1195. doi: 10.1097/01.smj.0000189988.12031.ac. [DOI] [PubMed] [Google Scholar]
- 23.Mahler HI, Kulik JA, Harrell J, Correa A, Gibbons FX, Gerrard M. Effects of UV photographs, photoaging information, and use of sunless tanning lotion on sun protection behaviors. Arch Dermatol. 2005;141(3):373–380. doi: 10.1001/archderm.141.3.373. [DOI] [PubMed] [Google Scholar]
- 24.Pagoto SL, Schneider KL, Oleski J, Bodenlos JS, Merriam P, Ma Y. Design and methods for a cluster randomized trial of the Sunless Study: a skin cancer prevention intervention promoting sunless tanning among beach visitors. BMC Public Health. 2009;9:50. doi: 10.1186/1471-2458-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein L, Paluch R, Gordy C, Dorn J. Decreasing sedentary behaviors in treating pediatric obesity. Arch Pediatr Adolesc Med. 2000;154(3):220–226. doi: 10.1001/archpedi.154.3.220. [DOI] [PubMed] [Google Scholar]
- 26.Fulton JE., Jr Utilizing the ultraviolet (UV detect) camera to enhance the appearance of photodamage and other skin conditions. Dermatol Surg. 1997;23(3):163–169. doi: 10.1111/j.1524-4725.1997.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 27.Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008 Feb;144(2):217–222. doi: 10.1001/archdermatol.2007.46. [DOI] [PubMed] [Google Scholar]
- 28.Saraiya M, Glanz K, Briss PA, et al. Interventions to prevent skin cancer by reducing exposure to ultraviolet radiation: A systematic review. Am J Prev Med. 2004;27(5):422–466. doi: 10.1016/j.amepre.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Pagoto S, McChargue D, Fuqua RW. Effects of a multicomponent intervention on motivation and sun protection behaviors among midwestern beachgoers. Health Psychol. 2003;22(4):429–433. doi: 10.1037/0278-6133.22.4.429. [DOI] [PubMed] [Google Scholar]
- 30.Mahler HI, Kulik JA, Gibbons FX, Gerrard M, Harrell J. Effects of appearance-based interventions on sun protection intentions and self-reported behaviors. Health Psychol. 2003;22(2):199–209. doi: 10.1037//0278-6133.22.2.199. [DOI] [PubMed] [Google Scholar]
- 31.Kulik JA, Butler HA, Gerrard M, Gibbons FX, Mahler HI. Social norms information enhances the efficacy of an appearance-based sun protection intervention. Soc Sci Med. 2008;67(2):321–329. doi: 10.1016/j.socscimed.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstock MA, Rossi JS, Redding CA, Maddock JE. Randomized controlled community trial of the efficacy of a multicomponent stage-matched intervention to increase sun protection among beachgoers. Prev Med. 2002;35(6):584–592. doi: 10.1006/pmed.2002.1114. [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration. [Accessed December 17, 2009.]; Available at: http://www.fda.gov/Cosmetics/ProductandIngredientSafety/ProductInformation/ucm134064.htm.
- 34.Petersen A, Wulf HC, Gniadecki R, Gajkowska B. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutation Research. 2004;560:173–186. doi: 10.1016/j.mrgentox.2004.03.002. [DOI] [PubMed] [Google Scholar]