Abstract
The present study was the first to use the functional magnetic resonance imaging (fMRI) methodology to investigate the neural correlates of race categorization of own- and other-race faces. We found that Chinese participants categorized the race of Caucasian faces more accurately and faster than that of Chinese faces, replicating the robust effect of the other-race categorization advantage. Regions of interest (ROI) analyses revealed greater neural activations when participants were categorizing own-race faces than other-race faces in the bilateral ventral occipito-temporal cortex (VOT) such as the fusiform face areas (FFA) and the occipital face areas (OFA). Within the left FFA, there was also a significant negative correlation between the behavioral difference of own- and other-race face categorization accuracy and the activation difference between categorizing own- and other-race faces. Whole brain analyses showed that categorizing own-race faces induced greater activations in the right medial frontal cortex (MFC) and right inferior frontal gyrus (IFG) than categorizing other-race faces. Psychophysiological interaction (PPI) analyses revealed that the frontal cortical regions interacted more strongly with the posterior VOT during the categorization of own-race faces than that of other-race faces. Overall, our findings suggest that relative to the categorization of other-race faces, more cortical resources are engaged during the categorization of own-race faces with which we have a higher level of processing expertise. This increased involvement of cortical neural sources perhaps serves to provide more in-depth processing of own-race faces (such as individuation), which in turn paradoxically results in the behavioral other-race categorization advantage.
Keywords: face processing, other-race effect, face categorization, face recognition, fusiform face area, occipital face area, fMRI, cortical neural resources
1. Introduction
Behavioral research has well established that individuals process own- and other-race faces differently. One well-known effect is the other-race effect (ORE), or more precisely, the other-race recognition disadvantage. This effect refers to the fact that individuals recognize own-race faces more accurately and faster than other-race faces. Extensive research has been devoted to elucidate this phenomenon involving infants, children, and adults from various racial backgrounds (for reviews, see Hugenberg, Young, Bernstein, & Sacco, 2010; Meissner & Brigham, 2001; Lee, Anzures, Quinn, Pascalis, & Slater, 2011).
Overshadowed by the extensive research on the other-race recognition disadvantage is the work on the other-race categorization advantage. This effect refers to the paradoxical phenomenon whereby, contrary to the other-race recognition disadvantage, when individuals are asked to categorize faces according to their race, the processing time to categorize other-race faces is shorter than that to categorize own-race faces; in some cases, the categorizing accuracy of other-race faces is also higher than that of own-race faces (Caldara, Rossion, Bovet, & Hauert, 2004; Ge et al., 2009; Levin, 1996, 2000; Valentine & Endo, 1992; Zhao & Bentin, 2008).
Several social cognitive models have been proposed to explain either the other-race recognition disadvantage alone or the two other-race effects concurrently. For example, a contact hypothesis attempts to explain the other-race recognition disadvantage in terms of experience. It suggests that with increased exposure to own-race faces, individuals’ visual system becomes increasingly tuned to maximize differentiation among individual faces of one’s own race; however, that is not the case for individual other-race faces (Furl, Phillips, & O’Toole, 2002).
Built upon the contact hypothesis, Levin (1996, 2000) used a feature-selection hypothesis to explain the paradoxical phenomena of the two other-race effects. He attributed the effects to our automatic tendency to select race-specifying information in other-race faces, whereas to select individuating information in own-race faces. Sporer (2001) proposed an in-group/out-group model to add some social flavor to Levin’s hypothesis. He suggested that same-race faces are processed automatically in a configural manner due to our extensive expertise as well as their in-group status, whereas other-race faces are initially categorized for the detection of an out-group characterization cue. Hugenberg et al. (2010) further proposed a Categorization-Individuation Model (CIM). This model places the observers’ motivation to individuate in-group individuals as the main underlying socio-cognitive mechanism for the two other-race effects rather than the observers’ extensive experience with individuating in-group individuals. In addition, the neural mechanisms underlying the other-race effects have also be speculated. For example, a cognitive gating mechanism for racial information posited that same- and other-race faces are processed in different neural pathways depending on the outcome of the preceding racial categorization (MacLin & Malpass, 2001; MacLin, MacLin, Peterson, Chowdhry, & Joshi, 2009).
In contrast to the extensive behavioral studies and related theorization of the socio-cognitive-neural mechanisms underlying the two other-race effects, the actual neural mechanisms underlying these phenomena are largely unknown. Neuroimaging studies can help elucidate the neural mechanisms likely underlying such intriguing effects. For example, studies using electrophysiological methods (e.g., EEG and ERP) may reveal the temporal mechanisms underlying the processing of own- and other-race faces, and functional magnetic resonance imaging (fMRI) studies can localize these effects in a set of particular brain regions and associated networks. However, our survey of the literature on face processing showed surprisingly that very limited neuroimaging studies have examined the own- and other-race face processing in general, despite the fact that there has been an explosive increase in imaging work on face processing since the 1990s (for reviews, see Calder, Rhodes, Johnson, & Haxby, 2011; Ito & Bartholow, 2009).
Among the few existing neuroimaging studies, most have focused on racial prejudice and implicit socio-affective differences towards own- and other-race faces (Cunningham et al., 2004; Hart et al., 2000; Lieberman, Hariri, Jarcho, Eisenberger, & Bookheimer, 2005; Phelps et al., 2000; Platek & Krill, 2009; Ronquillo et al., 2007; Richeson et al., 2003; Richeson, Todd, Trawalter, & Baird, 2008). Several neuroimaging studies have also examined the other-race recognition disadvantage. A recent electrophysiological study investigated this effect using a paradigm of adaptation (Vizioli, Rousselet, & Caldara, 2010a). It revealed stronger neural repetition suppression (RS) to own-race faces of same identity on the N170 component, an early event-related potential (ERP) component originating from the occipito-temporal site and putatively associated specifically with face processing. They suggested that own-race faces were coded more efficiently. Several fMRI studies have also found the middle regions of the bilateral fusiform gyri to be activated significantly more when recognizing or discriminating own-race faces as opposed to recognizing other-race faces (Golby, Gabrieli, Chiao, & Eberhardt, 2001; Kim et al., 2006). The modulation of face race on activations in the fusiform gyrus may reflect the influence of experience on face discrimination and recognition, as some studies have attributed activations in the middle fusiform gyrus to visual expertise at face processing (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999; Gauthier, Skudlarski, Gore, & Anderson, 2000a; Tarr & Gauthier, 2000).
The other-race categorization advantage has also been investigated by some ERP studies. Using race-categorization tasks, Ito and Urland (2005) found that own-race faces elicited greater N170, whereas Caharel et al. (2011) showed an opposite result. Nevertheless, two studies (Caharel et al., 2011; Vizioli, Foreman, Rousselet, & Caldara, 2010b) found that the face inversion effect (FIE) was more evident for own-race faces than for other-race faces at the electrophysiological level, whereby own-race faces elicited a greater amplitude difference between inverted and upright faces. These results suggest that a more complicated neural tuning may exist for own-race faces at the early stage of face processing. Contrary to those ERP findings regarding the sensitivity of N170 to race, Caldara et al. (2004) found that the categorization advantage of other-race faces over own-race faces emerged at about 240 ms post stimulus onset, using a race-categorization task. They suggested that our weaker experience of other-race faces engenders fewer semantic representations of them, which in turn allow for the increased processing speed of them during categorization. However, the ERP methodology, though exquisite in temporal resolution, is inadequate in providing information about the specific brain regions and the neural networks among them that underlie the other-race categorization advantage. To address this major gap in the literature, we conducted the present study.
In the present study, unlike most of the existing studies on ORE that involved mainly either Caucasian participants or Asian or African participants living in a society with Caucasians as the majority, we recruited Chinese participants who live in China with Chinese as the majority and who had no direct contact with Caucasian individuals. During the experiment, participants were asked to categorize Chinese and Caucasian faces according to their race with the use of fMRI methodology (the race-categorization task). To ensure that any potential other-race effect could not be attributable merely to the differences in stimulus characteristics between faces from the two races, we used exactly the same Chinese and Caucasian faces from an existing behavioral study that obtained robust other-race categorization advantage from Chinese participants in China and Caucasian participants in the UK (Ge et al., 2009).
We first performed an analysis of behavioral data to ascertain the existence of the other-race categorization advantage. Then, we used a commonly used localizer task to individually identify the regions of interest (ROIs) in the ventral occipito-temporal cortex (VOT) that have been previously associated with face processing in general. Within the individually-defined ROIs, we used the fMRI data from the race-categorization task to determine the activation level differences between categorizing own- and other-race faces in the bilateral fusiform face areas (FFAs) and occipital face areas (OFAs) using the traditional percent signal change (PSC) analysis (Kanwisher, McDermott, & Chun, 1997; Gauthier et al., 2000b). We also performed the whole brain analyses to contrast activations induced by categorizing Chinese faces to that induced by categorizing Caucasian faces. To gain further insight into the neural networks involved in the categorization of own- and other-race faces, we used a psychophysiological interaction (PPI) analysis (Friston et al., 1997) to identify activated regions that showed an increased functional integration with the peak loci identified by the whole brain analyses.
Based on the extensive behavioral evidence (see Hugenberg et al., 2010; Levin, 1996, 2000; Sporer, 2001), we expected our behavioral results to replicate the robust other-race categorization advantage. More specifically, Chinese participants should categorize the other-race Caucasian faces faster and even more accurately than the own-race Chinese faces. Because except for several ERP studies (Caharel et al., 2011; Caldara et al., 2004; Ito & Urland, 2005), no fMRI studies have specifically examined the cross-race face categorization advantage, the extent to which the categorization task would activated various regions of the brain during the categorization of own-and other-race faces was unclear. On one hand, given the complete cross-over of the behavioral other-race categorization and recognition effects (Ge et al., 2009), one may expect that categorizing other-race faces would produce greater activations than categorizing own-race faces. On the other hand, due to our high level expertise with processing own-race faces, the existing fMRI studies have found greater activations for own-race faces than other-race faces when different tasks have been used (e.g., an identity-recognition task by Golby et al., 2001 and Kim et al., 2006, and a gender, age, or race perception/verbal-encoding task by Lieberman et al., 2005). It is thus possible that in the categorization task own-race faces would also generate greater activations than other-race faces in the VOT in general and in the bilateral fusiform gyri in particular. Also, the ERP study of Caldara et al. (2004) hinted that the categorization of the other-race faces might be more superficial and involve fewer semantic representations than that of own-race faces. Thus, we hypothesized that the whole brain analysis would reveal the categorization of other-race faces to engage fewer brain regions than that of own-race faces. Further, consistent with this prediction, the PPI analysis would show that the categorization of own- and other-race faces would induce differential levels of integrations of cortical regions, specifically with other-race faces engendering less of such inter-regional integration than own-race faces.
2. Methods
2.1. Participants
Thirty Han Chinese adults (11 females; Mage = 23 years, age range: 19–26 years) participated in our experiment. They all lived in Beijing, P.R. China where 99.99% of the population is Han Chinese. All participants were right-handed, with normal or corrected vision, with no known neurological or psychiatric disorders, and no metal implants. All participants were prescreened to ensure that they had no prior direct contact with any Caucasian individuals. A signed informed consent was obtained from each participant in accordance with protocols approved by the Human Research Protection Program of Tiantan Hospital, Beijing, China.
2.2. Stimuli and Experimental Design
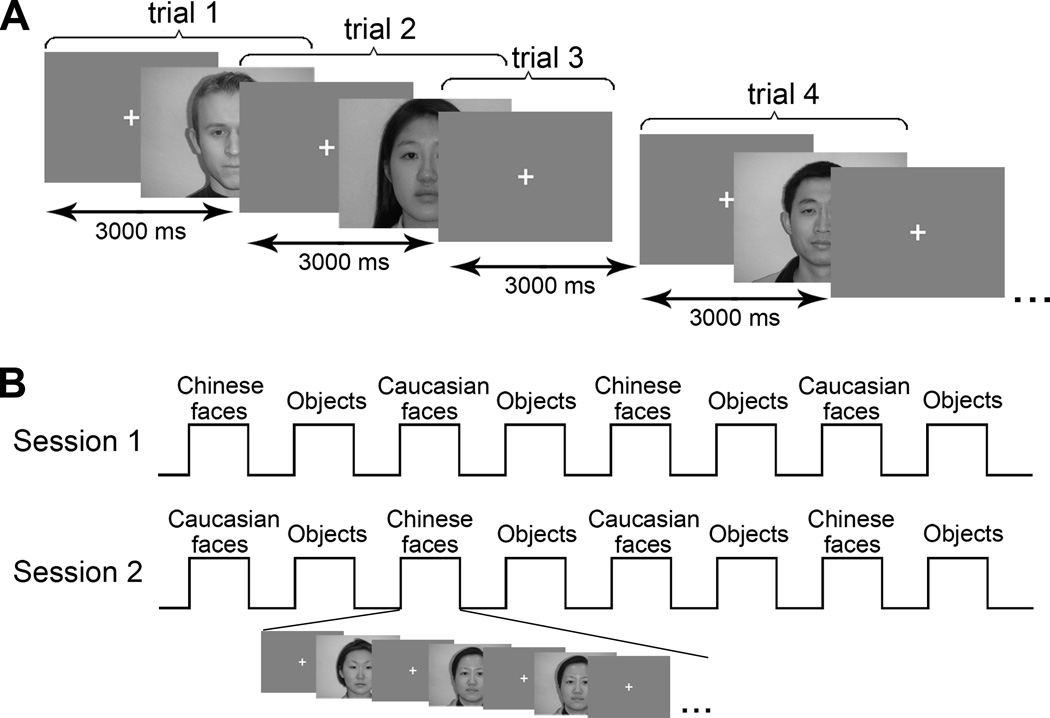
We used an event-related design in the race-categorization task and a traditional block design in the localizer task (see Figure 1). Sixty-four Caucasian and 64 Chinese young adults’ (half male and half female) upright frontal faces with neutral expressions were used in the race-categorizing task. The faces were chosen from a database that has been previously rated by Chinese and Caucasian adults in terms of attractiveness and distinctiveness. Thus, the faces from both races were matched on both dimensions. In addition, all the faces used in the current study have been used in an existing study (Ge et al., 2009) that produced a robust other-race categorization advantage for both Chinese participants in China and Caucasian participants in the UK: Chinese participants categorized Caucasian faces better than Chinese faces, whereas Caucasian participants categorized Chinese faces better than Caucasian faces. In other words, the robust other-race categorization advantage was not due to low-level stimulus characteristics. All stimuli were full-color photographic face images with a resolution of 640×480 pixels, taken under controlled illumination conditions. All of the faces were unfamiliar to the participants and participants never saw the same face twice during the entire race-categorization experiment. A cross hair located in the center of the screen served as a fixation point. Caucasian faces, Chinese faces, and fixations were shown on the screen in a pseudorandom manner with an inter-trial interval (ITI) of three seconds. The “fixation” trials served as the baseline in the subsequent statistical parametric mapping. Participants lay in the MRI machine and watched stimuli through a mirror, which was set up on the head coil. Participants were shown the faces individually and were instructed to categorize the race of each face they saw. They were also told to make their choices as quickly but as accurately as possible by pressing one of the assigned keys on the keypad. Half of the participants were required to push the left button on the keyboard for Caucasian faces and the right button for Chinese faces, while this assignment of response buttons was reversed for the other half of the subjects. No response was needed for the fixations.
Figure 1.
Experimental design. (A) The race-categorization task. In the task, 64 Caucasian faces, 64 Chinese faces, and 60 fixations were showed on the screen in a pseudorandom manner with an inter-trial interval (ITI) of three seconds. The faces used in the task were all different and shown only once. In the figure, trial 1 sets an example for the “Caucasian face” trials; trial 2 sets an example for the “Chinese face” trials; trial 3 demonstrates the “fixation” trials. (B) The localizer task. The localizer task was comprised of two sessions. Each session contained 8 blocks and the order of the eight blocks was counterbalanced in two sessions. The illustration on the bottom shows the structure of one block. There were 16 trials (faces or common objects) shown sequentially in each block and two pairs of them (only one pair was showed in the figure) were identical. Participants watched the stimuli and had to indicate whether two sequentially shown stimuli were identical.
The localizer task took place after the race-categorization task. The localizer task was composed of two sessions and each session included eight blocks, with each block containing 16 images from one of three categories (Caucasian faces, Chinese faces, and common objects), separated by blocks of fixation cross hairs. The order of the eight blocks was counterbalanced in two sessions. Participants were asked to simply view the images. However, like those used in the previous studies (e.g., Yovel & Kanwisher, 2004), we used occasional one-back trials whereby for each block, we presented two pairs of identical stimuli and asked participants to press a button to indicate the detection of such stimulus pairs. This procedure was to ensure that the participants were paying attention during the localizer task. The same Caucasian faces and Chinese faces used in the race-categorization task were used in the localizer task.
2.3. Data Acquisition
Participants were scanned using a 3.0 Tesla MRI scanner (Siemens Trio a Tim) at Tiantan Hospital. T1-weighted structural images with high-resolution of 1×1×1 mm3 were acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence with a field of view (FOV) of 256. The blood oxygen level-dependent (BOLD) responses were measured with a multislice echo planar imaging (EPI) sequence covering the whole brain with parameters as follows: TR = 2000 msec, TE = 30 msec, FOV = 256 mm, flip angle = 90°, matrix = 64×64, voxel size = 4×4×4 mm3, number of slices = 32. Each session contained 150 functional volumes for the race-categorization task and 161 functional volumes for the localizer task.
2.4. Statistical Parametric Mapping
We used SPM8 software (Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) to analyze the data. The first three volumes of each session were discarded due to T1 effects. The remaining volumes were realigned to the first volume of the first session, slice-time corrected, co-registered to the T1-weighted structural image, normalized to the MNI (Montreal Neurological Institute) template, resampled to 2×2×2 mm3 voxels, and spatially smoothed using an 8×8×8 mm3 FWHM Gaussian kernel.
Using the data from the race-categorization task, we constructed a general linear model (GLM) for each participant by which the effects of different experimental conditions, the session effects, and parameters of head movements served as regressors (Friston et al., 1995). Among these regressors, effects of different experimental conditions were produced by convolving stimulus functions with a canonical hemodynamic response function (HRF) to model the delayed and dispersed form of BOLD responses. In our GLM model, the different experimental conditions were “Caucasian faces” and “Chinese faces”. “Caucasian faces” and “Chinese faces” represented the effects of categorizing Caucasian faces and Chinese faces, respectively. We created a contrast of interest by subtracting the effect of “Caucasian faces” from the effect of “Chinese faces” for each participant. A second-level model was specified to carry out a one-sample t test on contrasts of interest for all participants. The mapping of the group analysis was obtained using a threshold of p < .0001 with an extent threshold of 30 voxels.
ROIs including the bilateral FFAs and OFAs were defined individually in the VOT according to the data collected in the localizer task. The FFA was located as the voxels within the lateral fusiform gyrus and/or adjacent areas that were significantly more active when viewing faces than objects. We defined FFAs using the contrast of “both Caucasian faces and Chinese faces > objects” to avoid biasing the result toward either own-race faces or other-race faces (Golby et al., 2001). Similarly, the OFA was defined as those voxels within the inferior/middle occipital gyrus which were significantly more active in the same condition. We first used a stringent but uncorrected threshold of p < .0001 (Kanwisher et al., 1997) to look for these ROIs. To include more of the participants’ data for analysis and thereby enhance the statistical power, we extended the threshold to p < .001, and once again to p < .005, t > 2.593 (Gauthier et al., 2000a). The ROIs selected using the three different p values constituted an ensemble of participants for our ROI analyses. Then, we drew ROIs according to those activated voxels and computed values of the PSCs for “Caucasian faces” and “Chinese faces” in each ROI using MarsBaR software (Brett, Anton, Valabregue, & Poline, 2002).
2.5. Psychophysiological interaction (PPI) analysis
Although traditional statistical parametric mapping and PSC analysis could reveal the neural correlates of the other-race face categorization advantage, network-level information was needed to ascertain the functional integrations of various cortical regions involved in categorizing own- and other-race faces. A psychophysiological interaction (PPI) analysis provided by SPM8 was thus used. The PPI analysis is a seed-region-based measure. It establishes predictive linkages of neural activity in one cortical area based on the activity in the seed region within the experimental or psychological context. PPI can thus reveal the interactive effect between the experimental condition and the predictive activity from the seed region (Friston et al., 1997). Although PPI analysis cannot provide detailed information about mutual modulatory facilitations among multiple cortical regions, it nevertheless provides data about how the activities in one seed region enhance those in other brain regions, which fittingly serves to test the specific hypotheses of the present study.
To perform PPI, we first treated each peak voxel determined by the “Chinese faces > Caucasian faces” contrast in the whole brain analysis as a reference seed region in the PPI analysis sequentially. We did not use the “Caucasian faces > Chinese faces” contrast because no supra-threshold clusters were found using this contrast (see below). To extract the time series from each seed region for each subject, we defined a volume of interest (VOI) as a 4-mm-radius sphere with the center located in a participant-specific local maximum closest to the group-level peak loci, using a contrast of “Chinese faces > Caucasian faces” and a threshold of p < .05, k ≥ 30 (uncorrected). This criterion was used so that a sufficient number of participants were available to ensure statistical power (Snijders, Petersson, & Hagoort, 2010). The distance between a participant-specific local maximum and a group-level peak voxel was set as less than 15 mm. We also ensured the anatomical labels of a group-level maximum and a homologous participant-specific local maximum to be consistent. Then, we computed PPI for each participant and for each VOI by multiplying the BOLD activity extracted from a previous-defined VOI with a psychological vector of interest (Chinese faces > Caucasian faces: 1 for categorizing Chinese faces, −1 for categorizing Caucasian faces). After that, we constructed a GLM with three fixed regressors, namely one regressor representing the BOLD activity from the seed region, one regressor reflecting the psychological variable of interest (Chinese face > Caucasian faces), and one regressor standing for the PPI, (i.e., the cross-product of the former two regressors). Finally, a statistical parametric mapping for each PPI was calculated. The resultant mappings were fed into a group-level random-effect analysis with a threshold of p <.0001 and k ≥ 30 (uncorrected).
3. Results
3.1. Behavioral Results
Paired sample t tests showed that participants spent more time categorizing own-race faces [Mean = 825.23 ms, SD = 106.72 ms; Median = 834.06 ms] than other-race faces [Mean = 723.37 ms, SD = 80.22 ms; Median = 705.01 ms] [t (29) = 6.558, p < .0001, two-tailed], and were more accurate with other-race faces [M = .98, SD = .03] than own-race faces [M = .95, SD = .05] [t (29) = 2.513, p < .02, two-tailed]. We computed the other-race latency advantage by subtracting each participant’s reaction time of correctly categorizing own-race faces from that of correctly categorizing other-race faces. We obtained the other-race accuracy advantage by subtracting each participant’s accuracy of categorizing own-race faces from that of categorizing other-race faces. Finally, a correlation analysis was performed and revealed a significantly negative correlation between the other-race latency advantage and the other-race accuracy advantage [r (30) = −.447, p < .02, two-tailed]: those participants who were more accurate at categorizing other-race faces also had a shorter latency period. Thus, a significant other-race advantage in face categorization in both latency and accuracy was found behaviorally.
3.2. fMRI ROI Analysis Results
With the use of the localizer task data, in total, we obtained ROIs for 25 of the 30 participants in the right FFA, and 17 of the 30 participants in the left FFA. Among the participants, we obtained the right FFA ROIs of 21 participants and the left FFA ROIs of 14 participants using a threshold of p < .0001. The right FFA ROIs of an additional two participants and the left FFA ROIs of an additional three participants were found using a threshold of p < .001. The remaining participants’ right FFA ROIs were found using a threshold of p < .005. The same strategy was used in the localization of the bilateral OFA ROIs. In total, we obtained the right OFA ROIs for 23 of the 30 participants and the left OFA ROIs for 16 of the 30 participants. Among them, the right OFAs of 14 participants and the left OFAs of 12 participants were found using a threshold of p < .0001. The right OFAs of an additional seven participants and the left OFAs of an additional four participants were found using a threshold of p <.001. The remaining participants’ right OFA ROIs were found using a threshold of p < .005. We used different levels of thresholds to obtain ROIs in order to enhance statistical powers for our subsequent ROI analyses.
The mean coordinates (mm) and standard deviations (mm) in the MNI template space were 42±4, −50±7, −19±5 for the right FFA; −40±2, −53±7, −21±6 for the left FFA; 39±7, −77±6, −13±5 for the right OFA; and −38±6, −78±7, −13±7 for the left OFA. These coordinates are consistent with what have been found in previous studies (Fairhall & Ishai, 2007; Ishai, Schmidt, & Boesiger, 2005; Kanwisher et al., 1997; Gauthier et al., 2000b; Li et al., 2010).
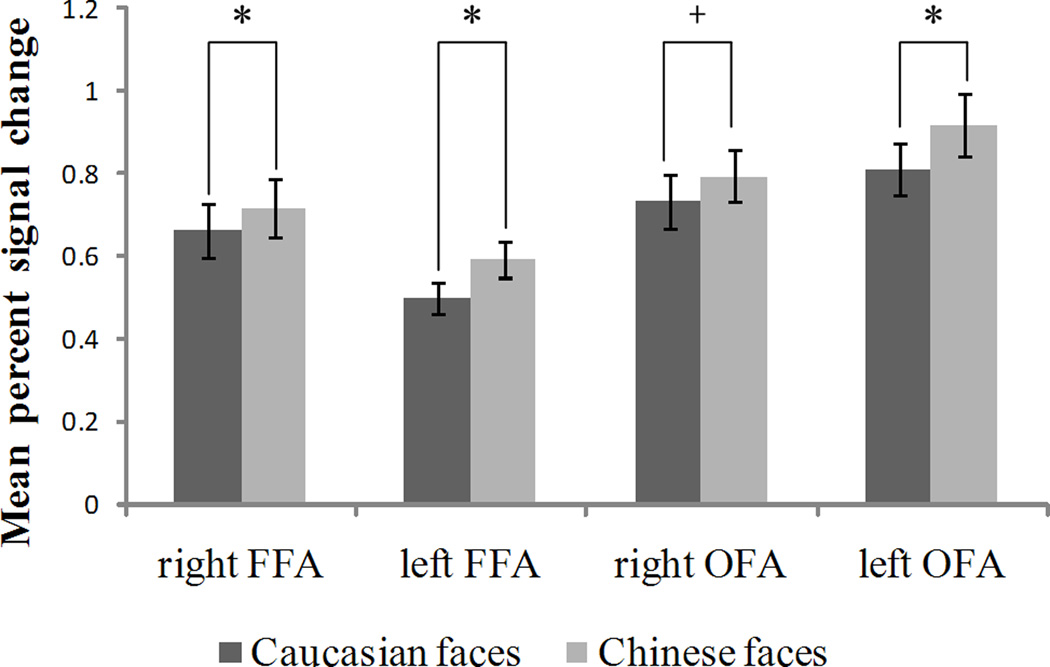
A traditional ROI analysis was performed to compare the intensities of activations in the ROIs across conditions. PSCs in the right FFA when categorizing own-race faces [M = .716, SD = .357] were significantly greater than those when categorizing other-race faces [M =.661, SD = .330] [t (24) = 3.486, p < .002, two-tailed]. PSCs in the left FFA when categorizing own-race faces [M = .593, SD = .176] were significantly greater than those when categorizing other-race faces [M = .498, SD = .153] [t (16) = 3.904, p < .002, two-tailed]. PSCs in the left OFA when categorizing own-race faces [M = .916, SD = .303] were significantly greater than those when categorizing other-race faces [M = .810, SD = .255] [t (15) = 4.562, p < .001, two-tailed]. A marginal significantly greater response to own-race faces [M = .795, SD = .296] than other-race faces [M = .733, SD = .316] was found in the right OFA [t (22) = 2.005, p = .057, two-tailed]. Those PSCs with SEMs for different conditions across all ROIs are shown in Figure 2.
Figure 2.
Results of the ROI analyses. Mean percent signal changes within the bilateral fusiform face areas (FFA) and the occipital face areas (OFA) when categorizing own-race (Chinese) and other-race (Caucasian) faces are shown with their SEMs. The symbol “*” indicates the difference between the two conditions is significant at the 0.05 level, while the symbol “+” indicates the difference between two conditions is marginally significant (p = .057). The MNI coordinates for right FFA, left FFA, right OFA, left OFA separately are (42±4, −50±7, −19±5), (−40±2, −53±7, −21±6), (39±7, −77±6, −13±5), (−38±6, −78±7, −13±7).
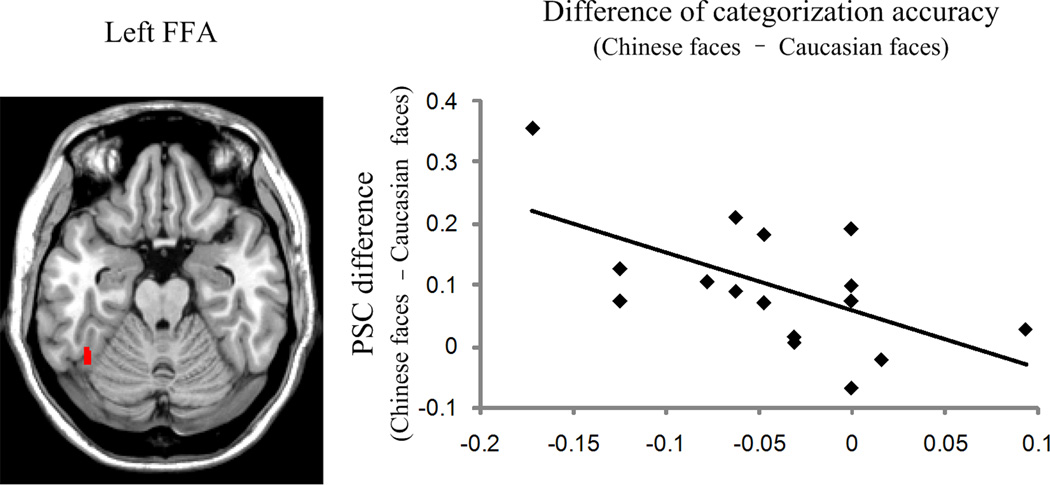
We investigated whether there was a correlation between behavioral other-race categorization advantages and PSC differences. The difference of categorization accuracy was calculated by subtracting each participant’s accuracy of categorizing other-race faces from that of categorizing own-race faces. The difference of categorization latency was computed by subtracting each participant’s reaction time of correctly categorizing other-race faces from that of correctly categorizing own-race faces. We subtracted PSCs of categorizing other-race faces from PSCs of categorizing own-race faces to obtain PSC differences. A significantly negative correlation [r (17) = −.594, p <.02, two-tailed] was found between the difference of categorization accuracy and the PSC difference within the left FFA: the larger the difference in activation between own- and other-race faces in the left FFA, the worse did the participant categorize the own-race face relative to the other-race face (Figure 3). None of the other brain-behavior correlations were significant.
Figure 3.
Correlation between behavioral difference of own- and other-race face categorization accuracy and the left FFA’s response to own-race faces versus other-race faces. The location of the left FFA from one participant is shown on an axial slice. The scatter plot demonstrates a negative correlation between PSC differences (PSC of own-race – PSC of other-race) and differences in the categorization accuracy (accuracy of own-race – accuracy of other-race) for 17 participants whose FFAs can be identified (r = −.594).
3.3. fMRI Whole Brain Analysis Results
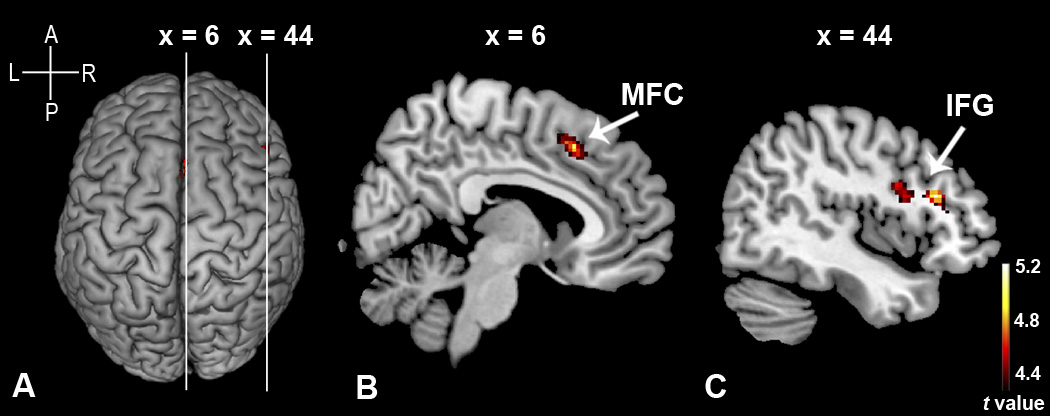
The group-level random-effect analyses were performed with either the “Chinese faces > Caucasian faces” contrast or the “Caucasian faces > Chinese faces” contrast, using the data from the race-categorization task. The “Chinese faces > Caucasian faces” contrast revealed that categorizing Chinese faces produced significantly greater activations than categorizing Caucasian faces in the right inferior frontal gyrus (IFG) and the right medial frontal cortex (MFC), using an uncorrected threshold of p < .0001 with an extent threshold of 30 voxels (Figure 4). The group peak loci (mm) in the MNI template space (Table 1) were 44, 26, 20 for the right IFG (pars triangularis); 42, 10, 18 for the right IFG (pars opercularis); and 6, 20, 46 for the right MFC. In contrast, no supra-threshold clusters were found in either cortical or subcortical regions with the “Caucasian faces > Chinese faces” contrast, using the same uncorrected threshold of p < .0001.
Figure 4.
Activation maps of the group random-effects analysis showed on a surface rendering (A) and sagittal views (B, C) for the contrast of “Chinese faces > Caucasian faces”. The white lines across the rendering are lines of cut to obtain cross-sectional views. (B) comes from the left line while (C) comes from the right line. The right medial frontal cortex (MFC) is illustrated in (B). The right inferior frontal gyrus (IFG) is illustrated in (C). In the two activated clusters shown in (C), the anterior one is IFG (pars triangularis) and the posterior one is IFG (pars opercularis). The figures on the top are the MNI coordinates of the two sagittal slices. This figure is generated using a stringent with an uncorrected threshold of p < .0001 and an extent threshold of 30 voxels. The cross on the top-left corner gives the directions for (A). A = anterior; P = posterior; L = left; R = right.
Table 1.
Clusters Activated More for Chinese Faces than Caucasian Faces as Revealed by the Group-Level Analyses
| Region | BA | MNI Coordinates (mm) |
Voxel | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R IFG (p. Triangularis) | 45 | 44 | 26 | 20 | 71 | 4.42 |
| R MFC | 32/6 | 6 | 20 | 46 | 115 | 4.29 |
| R IFG (p. Opercularis) | 44 | 42 | 10 | 18 | 61 | 4.21 |
| 44 | 44 | 6 | 26 | 3.99 | ||
Note. Threshold: p < .0001 uncorrected, k ≥ 30 voxels.
BA = Brodmann area; R = right; IFG = inferior frontal gyrus; MFC = medial frontal cortex.
Similar whole brain analyses were also carried out for the localizer data. We compared the effect of “Caucasian faces” with that of “Chinese faces” to ascertain whether there was a distinctive pattern of brain activities for own- and other-race faces during such a passive viewing task. Neither the “Chinese faces > Caucasian faces” contrast nor the “Caucasian faces > Chinese faces” contrast revealed any significant activation, using the uncorrected threshold of p < .0001.
3.4. PPI Results
The PPI analyses were performed to reveal brain networks enhanced by the right IFG (pars triangularis), right IFG (pars opercularis), and right MFC, separately, under the contrast of “Chinese faces > Caucasian faces”. Table 2 shows the resulting coordinates of VOIs in each individual participant and their means as well as standard deviations. The mean ± SD distance between each participant’s VOI and the group peak loci was 8.72 ± 3.52 mm for the right IFG (pars triangularis), 9.47 ± 2.94 mm for the right IFG (pars opercularis), and 7.39 ± 3.70 mm for the right MFC.
Table 2.
MNI Coordinates (mm) of the VOIs for Each Participant for the PPI analyses
| Subject | R IFG (Tri) |
R MFC |
R IFG (Oper) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | x | y | z | |
| 1 | — | — | 48 | 2 | 22 | ||||
| 2 | — | — | — | ||||||
| 3 | — | 10 | 16 | 42 | — | ||||
| 4 | 44 | 26 | 20 | 2 | 28 | 50 | 50 | 18 | 12 |
| 5 | — | 4 | 14 | 42 | — | ||||
| 6 | 52 | 38 | 18 | 4 | 26 | 48 | 42 | 14 | 28 |
| 7 | — | — | — | ||||||
| 8 | 52 | 24 | 18 | 6 | 18 | 44 | 48 | 4 | 26 |
| 9 | — | 6 | 16 | 46 | 46 | 8 | 8 | ||
| 10 | 46 | 16 | 28 | 0 | 30 | 40 | 40 | 4 | 18 |
| 11 | — | — | — | ||||||
| 12 | 40 | 30 | 24 | 8 | 32 | 42 | — | ||
| 13 | — | 4 | 16 | 40 | 32 | 6 | 12 | ||
| 14 | — | — | — | ||||||
| 15 | 40 | 20 | 24 | — | — | ||||
| 16 | 40 | 30 | 16 | 0 | 20 | 46 | 44 | 8 | 22 |
| 17 | 42 | 20 | 22 | 14 | 22 | 46 | 38 | 6 | 22 |
| 18 | 52 | 30 | 22 | — | — | ||||
| 19 | 54 | 26 | 24 | 6 | 24 | 44 | 48 | −2 | 12 |
| 20 | — | 6 | 18 | 46 | 46 | 4 | 22 | ||
| 21 | 56 | 30 | 22 | 8 | 24 | 52 | — | ||
| 22 | 52 | 28 | 16 | — | 46 | 4 | 20 | ||
| 23 | — | 4 | 30 | 50 | 38 | 12 | 30 | ||
| 24 | 44 | 26 | 24 | 6 | 22 | 46 | 40 | 8 | 22 |
| 25 | 52 | 26 | 20 | 2 | 32 | 54 | — | ||
| 26 | 50 | 22 | 16 | 8 | 20 | 48 | 48 | 10 | 14 |
| 27 | 44 | 40 | 16 | — | — | ||||
| 28 | 50 | 24 | 14 | 4 | 26 | 40 | 40 | 16 | 26 |
| 29 | 42 | 34 | 16 | 8 | 16 | 42 | 40 | 8 | 24 |
| 30 | 38 | 26 | 18 | 16 | 18 | 48 | 42 | 6 | 28 |
| Mean | 47 | 27 | 20 | 6 | 22 | 46 | 43 | 8 | 20 |
| SD | 6 | 6 | 4 | 4 | 6 | 4 | 5 | 5 | 6 |
Note. IFG = inferior frontal gyrus; MFC = medial frontal cortex; Tri = pars triangularis; Oper = pars opercularis; R = right.
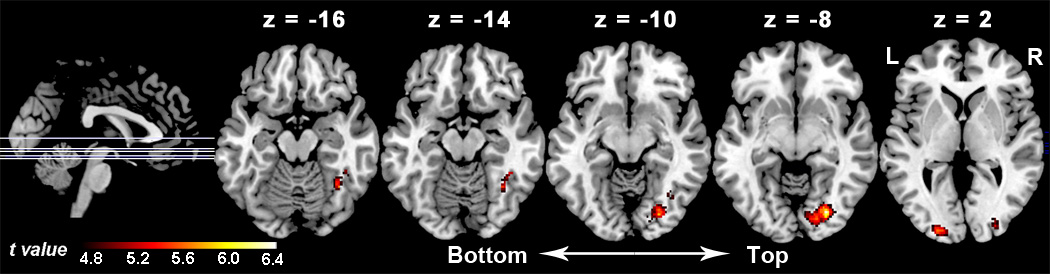
The results of the PPI analyses are shown in Table 3. At the threshold of p < .0001and k ≥ 30 voxels, we found that the right IFG (pars opercularis) enhanced activations in a wide swath of the visual cortex (the right visual cortex in particular) which spread roughly over the ventral visual pathway. The peak loci of the activated cortex are highly close to the coordinates of the FFA and OFA (Table 3). Brain regions enhanced by the right IFG (pars opercularis) are shown in Figure 5. The other two seed regions failed to produce supra-threshold enhanced integrations in any other cortical regions.
Table 3.
Brain Regions Enhanced by Three Seed Regions under the Modulation of the “Chinese Faces” > “Caucasian Faces” Contrast
| Seed region | Region | MNI Coordinates (mm) |
Voxel | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R IFG (Tri) | No supra-threshold clusters | |||||
| R MFC | No supra-threshold clusters | |||||
| R IFG (Oper) | R Occipital Lobe | 540 | ||||
| R Fusiform Gyrus | 26 | −78 | −8 | 4.52 | ||
| R Lingual Gyrus | 16 | −80 | −4 | 459 | 4.46 | |
| R MOG | 26 | −92 | 10 | 4.43 | ||
| R Fusiform Gyrus | 36 | −56 | −16 | 4.17 | ||
| R Fusiform Gyrus | 40 | −44 | −14 | 81 | 4.00 | |
| R IOG | 38 | −64 | −10 | 3.80 | ||
| L Occipital Lobe | 93 | |||||
| L MOG | −22 | −94 | 2 | 93 | 4.17 | |
Note. Threshold: p < .0001 uncorrected, k ≥ 30 voxels.
IFG = inferior frontal gyrus; IOG = inferior occipital gyrus; MOG = middle occipital gyrus; MFC = medial frontal cortex; Tri = pars triangularis; Oper = pars opercularis; R = right; L = left.
Figure 5.
Functional integration enhanced by the right inferior frontal gyrus (pars. opercularis) under the modulation of the “Chinese faces > Caucasian faces” contrast. The activation map is shown in five axial slices whose locations are illustrated in the sagittal view. The figures on the top are the MNI coordinates of these axial slices. This activation map is generated using a stringent with an uncorrected threshold of p < .0001 and an extent threshold of 30 voxels.
It should be noted that the PPI analyses were not performed for the contrast of “Caucasian faces > Chinese faces” because no significantly activated clusters were found in the whole brain analyses, which prevented us from selecting the appropriate seed VOIs for the PPI analyses.
4. Discussion
The present study was the first to investigate the neural correlates of the other-race face categorization advantage with the use of fMRI methodology. Our behavioral results once again confirmed the existence of the other-race advantage in face race categorization (e.g., Ge et al., 2009; Levin, 1996): Participants categorized the race of other-race Caucasian faces faster and more accurately than the race of own-race Chinese faces. This finding suggests the other-race categorization advantage to be a highly robust behavioral phenomenon.
4.1. FFA findings
The conventional ROI-based analyses revealed that in the individually-defined bilateral FFAs activations were greater when participants categorized own-race Chinese faces than when they categorized other-race Caucasian faces. Our findings were unlikely merely attributable to differences in the low-level stimulus characteristics between the Chinese and the Caucasian face sets per se. As mentioned above, the Chinese and Caucasian faces used in the present study were matched on two important perceptual dimensions (distinctiveness and attractiveness). More importantly, the two sets of faces also produced the same robust other-race categorization advantage behaviorally in Chinese and Caucasian participants in different ways: Chinese participants categorized Caucasian faces better than Chinese faces whereas Caucasian participants categorized Chinese faces better than Caucasian faces (Ge et al., 2009). At the same time, most relevant to the present study, the face race main effect was not significant for the categorization task. In other words, participants without considering their race overall categorized the Chinese and Caucasian faces at the same level of accuracy and speed (Ge et al., 2009). Furthermore, when we compared the activation differences between the own-race Chinese and other-race Caucasian faces in the localizer task which was a passive viewing task, we failed to find any significant difference between the Chinese and Caucasian faces, the same faces that were used in the categorization task. Nevertheless, our design is still less than ideal because we only recruited Chinese participants without direct contact with Caucasian individuals in the present study. An ideal design would require the recruitment of additional Caucasian participants who live in a western country without direct contact with Chinese individuals.
This shortcoming notwithstanding, our FFA results replicated findings of previous studies that also examined the processing of own- and other-race faces (Golby et al., 2001; Kim et al., 2006). Consistently, the previous and present studies showed the enhanced FFA responses to own-race faces compared to other-race faces, though Natu, Raboy, and O’Toole (2010) found this pattern only in the early stage of stimulus presentation when using a novel temporal analytic method. It should be noted, however, that the consistent findings were obtained in spite of the fact that the present and previous studies used face stimuli of different racial attributes and different active task demands. In the previous studies, because they recruited Caucasian participants from a society with Caucasians as the majority, the enhanced FFA activations were engendered by the own-race Caucasian faces relative to the other-race African or Asian faces (Golby et al., 2001; Lieberman et al., 2005; Natu et al., 2010). In contrast, in the present study, because we recruited Chinese participants from a society with Chinese as the majority, the enhanced FFA activations were engendered by the own-race Chinese faces relative to the other-race Caucasian faces. Furthermore, whereas participants in the present study performed the race-categorization task, participants in the previous studies performed a variety of active tasks: (1) an identity-recognition task that asked participants to recognize individual own- and other-race faces (Golby et al., 2001; Kim et al., 2006), (2) a same-different discrimination task that required participants to indicate whether a preceding faces was identical to the one currently seen (Natu et al., 2010), and (3) a perception /verbal-encoding task that required participants to encode own- and other-race faces according to their characteristics such as gender, age, or race (Lieberman et al., 2005).
These results from the present and previous studies taken together suggest that task demands and racial attributes of the faces per se may not be responsible for the enhanced responsiveness of the FFA to own-race faces relative to other-race faces. Rather, this enhanced activation was likely due to the fact that participants had extensive experience or expertise with processing own-race faces relative to other-race faces. Although it is still controversial as to whether the FFA is biologically dedicated to face processing (Kanwisher & Yovel, 2006; Gauthier et al., 2000a), it has been found that the FFAs are specifically responsive to visual objects with which we have extensive processing experience, or expertise (Gauthier et al., 1999). For example, it has been consistently found that the FFAs’ responses to own-race faces are greater than those to non-face objects such as cars, birds, and chairs. Further, when participants are experts at processing certain non-face objects (e.g., car or bird experts), the FFAs also become more responsive to such non-face objects (Gauthier et al., 2000a).Thus, Golby et al. (2001) and others (e.g., Kim et al., 2006) have attributed their FFA findings to the visual expertise and suggested that more extensive experience with own-race faces or greater attention to such faces induced by experience may be responsible for the differential own- vs. other-race activation in the FFA.
The present findings further suggest that the FFA’s enhanced responsiveness to the own-race faces may be so robust that may not be influenced by active task demands. No matter whether being asked to recognize the identity of a face or to categorize it by race, when seeing own-race faces, participants’ high-level processing expertise with these types of faces may automatically induce FFA to engage in an enhanced level of face processing. In contrast, the less experienced other-race faces may not have such a privileged processing status in the first instance (Rhodes, Locke, Ewing, & Evangelista, 2009), resulting in relatively lower level of activations in the bilateral FFAs.
4.2. OFA findings
Our ROI-based analyses also revealed greater activations in the individually-defined bilateral OFAs when participants categorized own-race faces than when they categorized other-race faces. A consistent result was reported by Natu et al. (2010) who used both Asian and Caucasian participants, and found that the neural activity patterns useful for dissociating own- and other-race faces span a relatively broad area in the ventral temporal cortex, including not only the fusiform gyri but also the ventral lateral occipital areas. Furthermore, the discriminability of own- and other-race faces depends on the neural activity pattern of the broader ventral temporal cortex rather than that of the FFA alone. According to the latest research on the functional division of labor between the FFA and OFA, the FFA is sensitive to perception of both face parts and configuration (Gilad, Meng, & Sinha, 2009), whereas the OFA is sensitive only to face parts (Liu, Harris, & Kanwisher, 2010). Also, it is believed that the OFA is recruited in the earlier stages of face processing than the FFA (Haxby, Hoffman, & Gobbini, 2000; Liu et al., 2010), and serves to send feed-forward information to be integrated in the FFAs (Fairhall & Ishai, 2007). Thus, the greater activations induced by own-race faces relative to other-race faces in the bilateral OFAs may again reflect participants’ expert-level ability to extract feature information of own-race faces, which will be further integrated in the FFAs.
4.3. Behavioral correlates of FFA activations
Our brain-behavior correlation analyses showed that the response difference for own-race faces compared to other-race faces in the left FFA negatively correlated with the categorization accuracy differences between own- and other-race faces. In other words, when the neural activity for own-race faces is greater than that for other-race faces, participants were less accurate in categorizing own-race faces relative to other-race faces (see Figure 3). This finding implies that a poorer performance at categorizing own-race faces than that at categorizing other-race faces might paradoxically be due to the fact that more neural resources were recruited to process own-race faces.
Why would more neural resources in processing own-race faces result in poorer behavioral performance in categorizing such faces? One possibility is that such additional neural resources might have been dedicated to individuation when categorizing own-race faces in contrast to when categorizing other-race faces. In line with the idea, Tanaka and Taylor (1991) showed behaviorally that increased processing experience with faces leads to a downward shift of the automatic default mode of processing from the basic level (i.e., is it a face?) to the individual level (i.e., is this Mary?). Without asking participants to pay attention to the identity information, an ERP study (Vizioli et al., 2010a) showed that the neural activity occurred as early as the perceptual stage, and it was sensitive to the identity of own-race faces, but not sensitive to the identity of other-race faces. Further support to this suggestion comes from the work by Golby et al. (2001) who asked participants to recognize own- and other-race faces. Contrary to our findings, they found that in the left FFA, the activation difference between own- and other-race faces was positively correlated with superior memory for own-race relative to other-race faces. In other words, when the neural activity for own-race faces was greater than that for other-race faces, participants were more accurate in recognizing own-race faces relative to other-race faces. Thus, whereas increased activations in the left FFA for own-race faces enhanced recognition of own-race faces behaviorally, the same increased activations had an opposite effect: It impaired the categorization performance of own-race faces relative to that of other-race faces. Thus, these behavior-brain correlational results taken together provide, at least partially, an explanation of the paradoxical behavioral effects of own-race recognition advantage and other-race categorization advantage.
Interestingly, the present and previous studies (Golby et al., 2001) both failed to find a significant brain-behavior correlation in the right FFA, although the greater activations for viewing own-race faces than for viewing other-race faces were found consistently in the region. In other words, individual differences in the right FFA’s response difference for own-race versus other-race faces did not predict either the individual differences in superior memory for own-race faces or superior categorization for other-race faces. There have been some suggestions that the right and left FFAs may play different roles in the face individuation with the right fusiform gyrus having an advantage in configural face processing (Maurer et al., 2007; Rossion et al., 2000) whereas the left fusiform gyrus may be more involved in feature-based processing (Rossion et al., 2000). If this functional division of labor is true, we speculate that the significant behavioral-brain correlations found in the left FFA in both the present and previous study may be due to the fact that participants were engaged in feature-based processing of faces.
4.4. Whole brain analysis results
Our whole brain analyses for the race-categorization tasks showed greater activations for categorizing own-race Chinese faces relative to categorizing other-race Caucasian faces in the right IFG (pars triangularis and pars opercularis) and right MFC, using the contrast “Chinese faces > Caucasian faces”. No significant activation was found at the same threshold using the contrast “Caucasian faces > Chinese faces”. This differential pattern of results suggests that the participants devoted more cortical resources to process the own-race faces than the other-race faces, particularly in the frontal regions such as the right IFG and right MFC. These regions are known as part of the extended neural systems for face processing which have been suggested to be involved in face processing at a higher level (Haxby et al., 2000). A number of studies on own-race face recognition or own-race facial configural encoding have also reported that the right IFG (pars triangularis), right IFG (pars opercularis), and medial frontal cortex were significantly activated. The loci of these activations (Courtney, Ungerleider, Keil, & Haxby, 1996; Haxby et al., 1996; Mason & Macrae, 2004; Maurer et al., 2007; Sergerie, Lepage, & Armony, 2005) are similar to those found in the present study that required participants to categorize faces by their race. The similarities in findings between the present and existing studies further suggest that when seeing own-race faces, participants’ high-level processing expertise may automatically engage an enhanced level of face processing not only in the core face processing network but also in the extended one. We speculate that these significantly-activated regions in the frontal cortex may serve as the source of top-down modulation to regulate face processing in the visual cortex. Consistent with this postulation, several previous studies indicated similar brain regions, whose coordinates were very close to those in the present study, were involved in top-down processing of visual stimuli, in particular, faces (Cardin, Friston, & Zeki, 2011; Esterman & Yantis, 2010; Ishai, Ungerleider, & Haxby, 2000; Li et al., 2010; Zhang et al., 2008).
Greater activations for other-race faces compared to own-race faces in the amygdala, anterior cingulate cortex, and dorsolateral prefrontal cortex have been reported in previous studies (Cunningham et al., 2004; Hart et al., 2000; Lieberman et al., 2005; Phelps et al., 2000; Platek & Krill, 2009; Ronquillo et al., 2007; Richeson et al., 2003; Richeson et al., 2008). In the present study, the “other-race Caucasian faces > own-race Chinese faces” contrast failed to reveal any significant activations in these cortical or subcortical regions. This discrepancy between the present and the previous findings might be due to a crucial difference. In all of these studies, the other-race faces were of African-American descent and were viewed by Caucasian participants who lived in a society where implicit and explicit racial prejudice and negative stereotypes against African-Americans still exist. In support of this idea, Lieberman et al. (2005) found that even African-American participants’ amygdalas were more responsive to African-American faces than Caucasian-American faces. In contrast, in the present study, the other-race faces were Caucasian faces viewed by Chinese university students who generally do not hold negative views against Caucasian individuals.
4.5. PPI findings
The results from the PPI analyses provided additional support to the neural resource hypothesis. Under the modulation of the “Chinese faces > Caucasian faces” contrast, the IFG (pars opercularis) enhanced the activations across a large area of the bilateral occipital cortexes, particularly in the right occipital cortex (see Table 3 for details). Interestingly, these areas overlap with those of the ventral visual pathway which is selectively tuned to processing object identities (Fang & He, 2005). Further, the peak loci of the activations in the ventral pathway are very similar to those of the FFA and OFA. Thus, it appears that relative to categorization of other-race faces, categorizing own-race faces engenders the frontal cortex to exert considerable feed-backward influence on the posterior visual cortex, such as the FFA and OFA, particularly in the right hemisphere. This finding is in line with some recent findings regarding top-down face processing. Recent studies have revealed that the right IFG (pars opercularis in particular) plays a significant top-down role in face processing. The IFG has been found to be activated not only when the face stimuli are highly visible, but also when they are ambiguous (Esterman & Yantis, 2010), non-existent (Li et al., 2010), and even imagined (Ishai et al., 2000). As already hinted by Caldara et al. (2004), the increased top-down influence from the IFG on the ventral visual cortex may serve to assist more in-depth processing of the own-race faces with which we have a higher level of processing expertise. Probably, as already alluded to earlier, the in-depth processing of the own-race faces includes the automatic processing of face information at the individual level. However, this more in-depth processing of the own-race faces assisted by the enhanced top-down influence may paradoxically increase the processing time and decrease in accuracy for the race categorization of the own-race faces relative to the other-face faces.
5. Summary
To the best of our knowledge, the present study is the first fMRI study on the other-race face categorization advantage. We not only replicated the effect behaviorally but also found significant differences in own- and other-race categorizations at the neural level. The ROI-based analysis revealed greater activations in the bilateral FFAs and OFAs for categorizing own-race faces than for categorizing other-race faces. In addition, the difference between the left FFA’s neural response to own-race faces vs. that to other-race faces had a significant negative correlation with the accuracy difference in categorizing own- vs. other-race faces. The whole brain analysis showed activations in the right IFG and right MFC when categorizing own-race faces compared to categorizing other-race faces. The follow-up PPI analysis suggested that the ventral visual cortex is significantly modulated by top-down effects originating from the right IFG (pars opercularis in particular) when categorizing own-race faces in contrast to categorizing other-race faces. These findings taken together indicate that more cortical resources are engaged during the categorization of own-race faces than that of other-race faces in the VOT and the frontal cortex as well as the interaction between the two.
The socio-cognitive-neural mechanisms for the neural activity differences observed in the present study are yet to be fully specified. The reason that more neural resources are devoted to categorizing own-race faces relative to other-race faces may be due to the observers’ extensive experience with processing own-race faces relative to other-race faces. The increased involvement of neural sources perhaps serves to provide more in-depth processing of own-race faces (such as individuation), which in turn paradoxically results in behaviorally slower and less accurate categorization of own-race faces than other-race faces. Such possibility needs to be further examined with neuroimaging studies in the future that not only recruit participants from different races (e.g., Chinese vs. Caucasians) and use faces of multiple races, but also call for different task demands (e.g., passive viewing vs. categorization vs. individuation). Such systematic investigation of the neural bases of own- vs. other-race face processing is highly important because evidence from such investigation will allow for the development of a comprehensive and multi-level account of not only own- and other-race face processing specifically but also the interaction between experience and face processing in general.
Highlights.
VOT is more active for categorizing own- than other-race faces in ROI analyses.
The other-race accuracy advantage correlates with PSC differences in the left FFA.
Group analyses reveal greater activations in IFG and MFC for own-race faces.
PPI analyses reveal that IFG modulates VOT more for own- than other-race faces.
Overall, more neural resources are used to categorize own- than other-race faces.
Acknowledgements
This work is supported by the National Natural Science Foundation of China under Grant No. 30970771, 60910006, 31028010, 81000640, the Knowledge Innovation Project of the Chinese Academy of Sciences under Grant No. KGCX2-YW-129, the National Basic Research Program of China (973 Program) under Grant No. 2011CB707700, and NIH (R01HD046526 & R01HD060595).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; 2002, June; Sendai, Japan. Available on CD-ROM in NeuroImage, Vol 16, No 2. [Google Scholar]
- Caharel S, Montalan B, Fromager E, Bernard C, Lalonde R, Mohamed R. Other-race and inversion effects during the structural encoding stage of face processing in a race categorization task: An event-related brain potential study. International Journal of Psychophysiology. 2011;79:266–271. doi: 10.1016/j.ijpsycho.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Caldara R, Rossion B, Bovet P, Hauert C. Event-related potentials and time course of the ‘other-race’ face classification advantage. Neuroreport. 2004;15:905–910. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Rhodes G, Johnson MH, Haxby JV. Handbook of face perception. Oxford: Oxford University Press; 2011. [Google Scholar]
- Cardin V, Friston KJ, Zeki S. Top-down modulations in the visual form pathway revealed with dynamic causal modeling. Cerebral Cortex. 2011;21:550–562. doi: 10.1093/cercor/bhq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychological Science. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Esterman M, Yantis S. Perceptual expectation evokes category-selective cortical activity. Cerebral Cortex. 2010;20:1245–1253. doi: 10.1093/cercor/bhp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nature Neuroscience. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Furl N, Phillips PJ, O’Toole AJ. Face recognition algorithms and the other-race effect: Computational mechanisms for a developmental contact hypothesis. Cognitive Science. 2002;26:797–815. [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000a;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience. 2000b;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Ge L, Zhang H, Wang Z, Quinn PC, Pascalis O, Kelly D, Lee K. Two faces of the other-race effect: Recognition and categorisation of Caucasian and Chinese faces. Perception. 2009;38:1199–1210. doi: 10.1068/p6136. [DOI] [PubMed] [Google Scholar]
- Gilad S, Meng M, Sinha P. Role of ordinal contrast relationships in face encoding. Proceedings of the National Academy of Sciences of the USA. 2009;106:5353–5358. doi: 10.1073/pnas.0812396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nature Neuroscience. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, Mclnerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proceedings of the National Academy of Sciences of the USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hugenberg K, Young SG, Bernstein MJ, Sacco DF. The categorization-individuation model: An integrative account of the other-race recognition deficit. Psychological Review. 2010;117:1168–1187. doi: 10.1037/a0020463. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Research Bulletin. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of proessing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Ito TA, Bartholow BD. The neural correlates of race. Trends in Cognitive Sciences. 2009;13:524–531. doi: 10.1016/j.tics.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: A cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yoon H, Kim B, Jeun S, Jung S, Choe B. Racial distinction of the unknown facial identity recognition mechanism by event-related fMRI. Neuroscience Letters. 2006;397:279–284. doi: 10.1016/j.neulet.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Lee K, Anzures G, Quinn PC, Pascalis O, Slater A. Development of face processing expertise. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. Handbook of face perception. Oxford: Oxford University Press; 2011. [Google Scholar]
- Levin DT. Classifying faces by race: The structure of face categories. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1996;22:1364–1382. [Google Scholar]
- Levin DT. Race as a visual feature: Using visual search and perceptual discrimination tasks to understand face categories and the cross-race recognition deficit. Journal of Experimental Psychology: General. 2000;129:559–574. doi: 10.1037//0096-3445.129.4.559. [DOI] [PubMed] [Google Scholar]
- Li J, Liu J, Liang J, Zhang H, Zhao J, Rieth CA, Lee K. Effective connectivities of cortical regions for top-down face processing: A dynamic causal modeling study. Brain Research. 2010;1340:40–51. doi: 10.1016/j.brainres.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Perception of face parts and face configurations: An fMRI study. Journal of Cognitive Neuroscience. 2010;22:203–211. doi: 10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLin OH, Malpass RS. Racial categorization of faces: The ambiguous race face effect. Psychology, Public Policy, and Law. 2001;7:98–118. [Google Scholar]
- MacLin OH, MacLin MK, Peterson D, Chowdhry O, Joshi P. Social psychophysics: Using psychophysics to answer “social” questions with PsychoPro. Behavior Research Methods. 2009;41:623–632. doi: 10.3758/BRM.41.3.623. [DOI] [PubMed] [Google Scholar]
- Mason MF, Macrae CN. Categorizing and individuating others: The neural substrates of person perception. Journal of Cognitive Neuroscience. 2004;16:1785–1795. doi: 10.1162/0898929042947801. [DOI] [PubMed] [Google Scholar]
- Maurer D, O’Craven KM, Grand RL, Mondloch CJ, Springer MV, Lewis TL, Grady CL. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45:1438–1451. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Meissner CA, Brigham JC. Thirty years of investigating the own-race bias in memory for faces: A meta-analytic review. Psychology, Public Policy, and Law. 2001;7:3–35. [Google Scholar]
- Natu V, Raboy D, O’Toole AJ. Neural correlates of own- and other-race face perception: Spatial and temporal response differences. NeuroImage. 2010;54:2547–2555. doi: 10.1016/j.neuroimage.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, Banaji MR. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Platek SM, Krill AL. Self-face resemblance attenuates other-race face effect in the amygdala. Brain Research. 2009;1284:156–160. doi: 10.1016/j.brainres.2009.05.076. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Locke V, Ewing L, Evangelista E. Race coding and the other-race effect in face recognition. Perception. 2009;38:232–241. doi: 10.1068/p6110. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, Shelton JN. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Todd AR, Trawalter S, Baird AA. Eye-gaze direction modulates race-related amygdala activity. Group Processes Intergroup Relations. 2008;11:233–246. [Google Scholar]
- Ronquillo J, Denson TF, Lickel B, Lu Z, Nandy A, Maddox KB. The effects of skin tone on race-related amygdala activity: An fMRI investigation. Social Cognitive and Affective Neuroscience. 2007;2:39–44. doi: 10.1093/scan/nsl043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Dricot L, Devolder A, Bodart J, Crommelinck M, Gelder B, Zoontjes R. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 2000;12:793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A face to remember: emotional expression modulates prefrontal activity during memory formation. NeuroImage. 2005;24:580–585. doi: 10.1016/j.neuroimage.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Snijders TM, Petersson KM, Hagoort P. Effective connectivity of cortical and subcortical regions during unification of sentence structure. NeuroImage. 2010;52:1633–1644. doi: 10.1016/j.neuroimage.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Sporer SL. Recognizing faces of other ethnic groups: An integration of theories. Psychology, Public Policy, and Law. 2001;7:36–97. [Google Scholar]
- Tanaka JW, Taylor M. Object categories and expertise: Is the basic level in the eye of the beholder? Cognitive Psychology. 1991;23:457–482. [Google Scholar]
- Tarr MJ, Gauthier I. FFA: A flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Valentine T, Endo M. Towards an exemplar model of face processing: The effects of race and distinctiveness. The Quarterly Journal of Experimental Psychology Section A. 1992;44:671–703. doi: 10.1080/14640749208401305. [DOI] [PubMed] [Google Scholar]
- Vizioli L, Rousselet GA, Caldara R. Neural repetition suppression to identity is abolished by other-race faces. Proceedings of the National Academy of Sciences of the USA. 2010a;107:20081–20086. doi: 10.1073/pnas.1005751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli L, Foreman K, Rousselet GA, Caldara R. Inverting faces elicits sensitivity to race on the N170 component: A cross-cultural study. Journal of Vision. 2010b;10:15. doi: 10.1167/10.1.15. [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. Face perception: Domain specific, not process specific. Neuron. 2004;44:889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Huber DE, Rieth CA, Tian J, Lee K. Detecting faces in pure noise images: A functional MRI study on top-down perception. Neuroreport. 2008;19:229–233. doi: 10.1097/WNR.0b013e3282f49083. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bentin S. Own- and other-race categorization of faces by race, gender, and age. Psychonomic Bulletin & Review. 2008;15:1093–1099. doi: 10.3758/PBR.15.6.1093. [DOI] [PubMed] [Google Scholar]