Introduction
Formal staging of cancer is fundamental in providing clinicians and patients with prognostic information, developing treatment strategies, and directing and analyzing clinical trials. Staging of cutaneous melanoma continues to evolve through identification and rigorous analysis of potential prognostic factors. The first multivariate analyses of prognostic factors for melanoma were published over three decades ago, and several well-designed reports have subsequently advanced our understanding of important prognostic indicators for this disease.1–3 Despite these important efforts, need for a unified melanoma staging system applicable to both clinical practice and research became evident.4–6 In 1998, the American Joint Committee on Cancer (AJCC) Melanoma Staging Committee, which included experts from North America, Europe, and Australia, developed the AJCC melanoma staging database, a first-in-kind international integrated compilation of prospectively accumulated melanoma outcome data from several centers and clinical trial cooperative groups.7 Analysis of this database resulted in major revisions to the Tumor-Node-Metastasis (TNM) staging system reflected in the 6th edition AJCC Cancer Staging Manual published in 2002. More recently, the committee's analysis of an updated melanoma staging database, including prospective data on over 50,000 patients, led to staging revisions adopted in the 7th edition AJCC Cancer Staging Manual published in 2009.8,9 This article highlights these revisions, reviews relevant prognostic factors and their impact on staging, and discusses emerging tools that will likely impact future staging systems and clinical practice.
AJCC 7th edition updates and highlighted changes from the 6th addition
Staging systems for melanoma continue to be refined as our understanding of the complex biology of this disease improves. In 2002, the 6th edition AJCC staging system included significant revisions to the prior system based on prognostic factor analysis of the original melanoma staging database.10,11 These included: new strata for primary tumor thickness, incorporation of primary tumor ulceration in the T and N classifications, the distinction of nodal tumor burden as a prognostic factor in patients with regional metastases, and new categories for stage IV disease. Analysis of an updated AJCC melanoma staging database was subsequently performed to provide further insight into the prognostic significance of several biologic factors and to refine the 6th edition. These updates are reflected in the 7th edition melanoma staging system of the AJCC Cancer Staging Manual published in 20098 (Tables 1 and 2). While this most recent staging schema remains largely intact compared to the prior version, several noteworthy revisions are briefly highlighted below and further detailed where appropriate throughout this article (Table 3).
Table 1.
TNM staging categories for cutaneous melanoma (7th edition).
| T classification | Thickness | Ulceration Status |
|---|---|---|
| Tis | NA | NA |
| T1 | ≥1.00 mm | a: w/o ulceration and mitosis ≥1/mm2 |
| b: with ulceration or mitoses ≥1/mm2 n/mm2 | ||
| T2 | 1.01 – 2.0 mm | a: w/o ulceration |
| b: with ulceration | ||
| T3 | 2.01 – 4.0 mm | a: w/o ulceration |
| b: with ulceration | ||
| T4 | >4.0 mm | a: w/o ulceration |
| b: with ulceration | ||
| N classification | # of Metastatic Nodes | Nodal Metastatic Burden |
|---|---|---|
| N0 | 0 | NA |
| N1 | 1 | a: micrometastasis* |
| b: macrometastasis** | ||
| N2 | 2–3 | a: micrometastasis* |
| b: macrometastasis** | ||
| c: in transit met(s)/satellite(s) without metastatic nodes | ||
| N3 | 4+ metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic nodes | |
| M classification | Site | Serum LDH |
|---|---|---|
| M0 | No distant metastases | NA |
| M1a | Distant skin, subcutaneous, or nodal metastases | normal |
| M1b | Lung metastases | normal |
| M1c | All other visceral metastases | normal |
| Any distant metastasis | elevated | |
Abbreviations: NA, not applicable; LDH, lactate dehydrogenase
Micrometastases are diagnosed after sentinel lymph node biopsy.
Macrometastases are defined as clinically detectable nodal metastases confirmed pathologically.
From Balch CM, Gershenwald JE, Soong S, et al, J Clin Oncol 27(36): 6199-206, 2009; with permission.
Table 2.
Anatomic stage groupings for cutaneous melanoma (7th edition).
| Clinical Staging* |
Pathologic Staging+ |
||||||
|---|---|---|---|---|---|---|---|
| T | N | M | T | N | M | ||
| 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 | IA | T1a | N0 | M0 |
| IB | T1b | N0 | M0 | IB | T1b | N0 | M0 |
| T2a | N0 | M0 | T2a | N0 | M0 | ||
| IIA | T2b | N0 | M0 | IIA | T2b | N0 | M0 |
| T3a | N0 | M0 | T3a | N0 | M0 | ||
| IIB | T3b | N0 | M0 | IIB | T3b | N0 | M0 |
| T4a | N0 | M0 | T4a | N0 | M0 | ||
| IIC | T4b | N0 | M0 | IIC | T4b | N0 | M0 |
| III | Any T | N > N0 | M0 | IIIA | Tl-4a | N1a | M0 |
| Tl-4a | N2a | M0 | |||||
| IIIB | Tl-4b | N1a | M0 | ||||
| Tl-4b | N2a | M0 | |||||
| Tl-4a | N1b | M0 | |||||
| Tl-4a | N2b | M0 | |||||
| Tl-4a | N2c | M0 | |||||
| IIIC | Tl-4b | N1b | M0 | ||||
| Tl-4b | N2b | M0 | |||||
| Tl-4b | N2c | M0 | |||||
| Any T | N3 | M0 | |||||
| IV | Any T | Any N | M1 | IV | Any T | Any N | M1 |
Clinical staging includes microstaging of the primary melanoma and clinical/radiologic evaluation for metastases. By convention, it should be used after complete excision of the primary melanoma with clinical assessment for regional and distant metastases.
Pathologic staging includes microstaging of the primary melanoma and pathologic information about the regional lymph nodes after partial (ie, sentinel lymph node biopsy) or complete lymphadenectomy. Pathologic Stage 0 or Stage IA patients are the exception; they do not require pathologic evaluation of their lymph nodes.
From Balch CM, Gershenwald JE, Soong S, et al, J Clin Oncol 27(36): 6199-206, 2009; with permission.
Table 3.
Differences between the previous (2002) and the current (2009) versions of the AJCC melanoma staging system
| Factor | 2002 Criteria | 2009 Criteria | Comments |
|---|---|---|---|
| Thickness | Primary determinant of T staging; thresholds of 1.0,2.0,4.0 mm | Same | Correlation of metastatic risk is a continuous variable |
| Level of invasion | Used only for defining T1 melanomas | No longer used | Clark levels IV or V may be used in rare instances as a criterion for defining T1b melanoma only if mitotic rate cannot be determined in a nonulcerated melanoma |
| Ulceration | Included as a second determinant of T and N staging | Same | Signifies a locally advanced lesion; dominant prognostic factor for grouping Stage I,II and III |
| Mitotic rate per mm2 | Not used | Used for categorizing T1 melanoma | Mitosis ≥ /mm2 used as a primary determinant for defining T1b melanoma |
| Satellite metastases | In N category | Same | Merged with in transit lesions |
| Immunochemical detection of nodal metastases | Not allowed | Allowed | Must include at least one melanoma-specific marker (e.g., HMB-45, Melan-A, MART 1) |
| 0.2 mm threshold of defined N-positive disease | Implied | No lower threshold of staging N-positive disease | |
| Number of Nodal metastases | Dominant determinant of N Staging | Same | Thresholds of 1 vs 2–3 vs. > 4 nodes |
| Metastatic "volume" | Included as a second determinant of N staging | Same | Clinically occult ("microscopic") vs. clinically apparent ("macroscopic") nodal volume |
| Lung metastases | Separate category as M1b | Same | Has a somewhat better prognosis than other visceral metastases |
| Elevated serum LDH | Included as a second determinant of M staging | Same | Recommend a second confirmatory LDH if elevated |
| Clinical vs. pathologic staging | Sentinel node results incorporated into definition of pathologic staging | Large variability in outcome between clinical and pathological staging; sentinel node staging encouraged for standard patient care and should be required prior to entry into clinical trials |
From Balch CM: Melanoma of the Skin. In Edge SB, Byrd DR, Compton CC, et al (eds): AJCC Cancer Staging Manual ed 7th. New York: Springer Verlag, 2009; with permission.
A fundamental change to the new staging system is the addition of mitotic rate as a criterion for defining T1b primary melanoma. Mitotic rate of the primary tumor, defined as mitoses/mm2, was included as a covariate in the staging analysis and was identified to have significant prognostic implications, further discussed below.
A second important change is the formal inclusion of immunohistochemical assessment, rather than just hematoxylin and eosin (H&E) staining, as acceptable in defining the presence of nodal metastases. Importantly, at least one melanoma-specific marker, such as HMB-45, Melan-A, or MART 1, should be used. Furthermore, unlike criteria used in breast cancer staging, there is no lower threshold of tumor burden used to define nodal micrometastases, reflecting the consensus that even small amounts of metastatic disease are potentially clinically relevant.
Historically, patients with melanoma with an unknown primary presenting with metastases arising in the skin, subcutaneous tissue, and/or lymph nodes, have variably been classified as having either stage III or stage IV disease, provided that a staging evaluation does not reveal other sites of disease. However, recent studies focused on patients with melanoma of unknown primary with metastases to lymph nodes have demonstrated a survival profile similar (if not more favorable) to patients with regional nodal disease and a known primary melanoma.12,13 In the updated staging system, metastatic melanoma to the skin, subcutaneous tissue, or lymph nodes with an unknown primary is classified as stage III disease. Accordingly, such patients should be offered surgical management and participation in adjuvant stage III trials.
Localized Melanoma (Stage I&II)
The prognosis for patients with localized melanoma is generally favorable. In the 6th edition AJCC melanoma staging system, tumor thickness and ulceration were identified as the dominant independent predictors of survival.10 However, based on emerging data from several single institution studies reporting tumor mitotic rate as an adverse prognostic factor,14–17 mitotic rate was included in the analysis of the updated AJCC melanoma staging database. Importantly, although some investigators predicted that ulceration would no longer maintain its prognostic significance for patients with localized disease, in fact, tumor thickness, mitotic rate, and the presence of ulceration were each found to be significant independent predictors of survival in this group of patients.8,9 Furthermore, in the 7th edition AJCC melanoma staging system, these three factors were used to define T categories (Tables 1 and 2).8,9
Primary tumor thickness was introduced as a prognostic factor by Alexander Breslow in 1970,18 and has subsequently been validated in multiple studies.1,19–21 Currently, the AJCC staging system uses tumor thickness cut points of 1.0 mm, 2.0 mm, and 4.0 mm to define T-category strata based on their statistical significance and importantly, their clinical utility in defining tumor thickness as thin (<1 mm), intermediate (1–4mm), and thick (>4mm) tumors.4,22 In analysis of over 27,000 patients from the AJCC melanoma staging database with Stage I or II disease, as primary tumor thickness increased, there was a significant decrease in survival (Figures 1A,B).9
Figure 1.
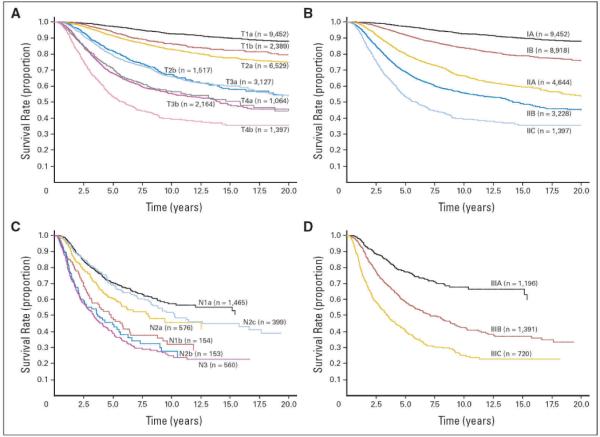
Survival curves from the 7th edition American Joint Committee on Cancer melanoma staging database comparing (A) the different T categories and (B) the stage groupings for stages I and II melanoma. Note that survival outcomes for patients with ulcerated tumors were remarkably similar to those of patients with nonulcerated tumors of the next highest T category. For patients with stage III disease, survival curves are shown comparing (C) the different N categories and (D) the stage groupings. Note in particular the marked heterogeneity in survival among these patients with stage III disease.
From Balch CM, Gershenwald JE, Soong S, et al, Journal of Clinical Oncology 27(36): 6199-206, 2009; with permission.
Ulceration is defined as the lack of an intact dermis overlying the primary tumor on histologic evaluation. Multiple studies demonstrate that the presence of ulceration represents a more aggressive tumor phenotype with a higher likelihood of metastasis and worse prognosis.23,24 For patients with ulcerated melanomas, survival is significantly lower than for patients with nonulcerated tumors of equivalent depth. Moreover, analysis of the original AJCC melanoma staging database (published in the 2002 AJCC Cancer Staging Manual) demonstrated that survival outcomes for patients with ulcerated tumors were remarkably similar to those of patients with nonulcerated tumors of the next highest T category.10 This finding was validated in the 2008 database analysis and is reflected in the 2009 AJCC melanoma staging system (Figure 1A and 1B).8,9
Primary tumor mitotic rate deserves special mention, as it represents a fundamental change in the revised melanoma staging system. This change is based on a body of data showing a significant correlation between increasing mitotic rate and decreased survival. Salman and Rodgers first suggested the prognostic importance of the mitotic index of the primary tumor, identifying that it was associated with a higher rate of metastasis in patients with thin lesions.25 Several other investigators have subsequently confirmed tumor mitotic rate as an independent prognostic factor.14–17 Multivariate analysis of 10,233 patients from the updated AJCC melanoma staging database with localized melanoma (stages I & II) revealed mitotic rate as the second most important predictor of survival, after tumor thickness, and was particularly pronounced among patients with T1 melanoma.9 Accordingly, in a multivariate analysis of 4,861 patients with T1 melanoma, tumor thickness, mitotic rate, and ulceration were all powerful predictors of survival; level of invasion was no longer statistically significant when mitotic rate and ulceration were included in the analysis.9 The 10-year survival rate was 95% for nonulcerated T1 melanomas with a mitotic rate of < 1/mm2, and dropped to 88% if the mitotic rate was ≥1/mm2 (P<.0001).9 Although ulcerated T1 melanomas were associated with a mitotic rate ≥ 1/mm2 in 78% of patients, the 10-year survival rate was the same regardless of whether the mitotic rate was < 1 or ≥1/mm2 (85% v 87%; P=.41). Based on these data, mitotic rate (operationally defined in the 7th edition melanoma staging system as a dichotomous variable) replaced Clark level of invasion as a primary criterion for defining T1b melanoma.8,9
The mitotic rate of the primary melanoma should be assessed following biopsy. The suggested approach is detailed in the 7th edition of the AJCC Cancer Staging Manual.8 Briefly, the recommended technique is to first find the area within the dermis containing the most mitotic figures, the so-called “hot spot”. After counting mitoses in the hot spot, the count is extended to adjacent fields until an area of 1 mm2 is assessed. The count is then expressed as mitoses/mm2. If no hot spot can be identified and mitoses are randomly scattered throughout the lesion, then a representative mitosis is chosen, and beginning with that field, the count is then extended until an area corresponding to 1 mm2 is assessed. Individual microscopes should be calibrated for accurate recording. If the invasive area of a tumor is less than 1 mm2, then the number of mitoses present in 1 mm2 of dermal tissue that includes the tumor should be determined and recorded as mitoses/mm2. Alternatively, in these tumors, the simple presence or absence of a mitosis can be designated as at least 1/mm2 (i.e., “mitogenic”) or 0/mm2 (i.e., “nonmitogenic”), respectively.
Determining mitotic rate is important not only in providing prognostic information, but also in discussing and planning extent of surgery. In the 6th edition of the AJCC Staging Manual, sentinel lymph node (SLN) biopsy was recommended for patients with T1b tumors, based on an approximately 10% incidence of identifying occult nodal metastasis in patients with thin melanomas that were either ulcerated or had Clark level IV invasion.10 While the updated AJCC melanoma staging database does not permit a precise estimation of predicting nodal micrometastasis in this cohort, others have demonstrated increased mitotic activity in the primary tumor to be a predictor of SLN positivity.14–17,26 In a preliminary report based on a multivariate analysis of patients with T1 melanoma who underwent sentinel node biopsy, Caudle and colleagues found that a mitotic rate ≥1/mm2 was an independent predictor of sentinel lymph node histologic status.27 Although this clinical question is not yet fully resolved, available data suggests that in addition to using other potential prognostic factors, consideration should be given to offering sentinel lymph node (SLN) biopsy in patients with thin (≤1mm) melanoma if the primary tumor mitotic rate is ≥1/mm2.
For years, Clark level of invasion has been known to have prognostic significance, and has served as a criterion in several melanoma staging systems.28 Nonetheless, several investigators have demonstrated that level of invasion is less reproducible among pathologists, and is less accurate in providing prognostic information compared to tumor thickness.1,29–31 In the 6th edition AJCC melanoma staging system, Clark level of invasion of at least IV (or ulceration) was used to define T1b tumors. However, in the T1 category-specific AJCC multivariate analysis, level of invasion was no longer an independent predictor of survival relative to mitotic rate and ulceration.9 In the 7th edition AJCC melanoma staging system, level of invasion is only to be used to define T1b tumors in the rare occurrence that mitotic rate cannot be accurately determined.
Stage III Melanoma
Patients with regional metastasis (ie, regional lymph node, satellite, and/or in-transit metastasis) represent a heterogeneous group with regard to staging and prognosis. It is well established that regional lymph nodes are the most common first site of metastasis in melanoma patients.32 The 6th edition AJCC melanoma staging system identified the number of regional lymph nodes harboring metastatic disease, regional node tumor burden (empirically classified as microscopic versus macroscopic), and ulceration of the primary tumor as independent predictors of survival in this cohort.10,11 Recent analysis of patients from the AJCC melanoma staging database used for the 7th edition melanoma staging system confirm these criteria as important prognostic factors, and includes patients with long-term follow-up of patients staged in the era of sentinel lymph node biopsy.
Regional lymph nodes
In previous staging systems, the size of metastasis-containing regional lymph nodes was the primary criterion used in stratifying stage III patients.5 However, more recent analyses have demonstrated that in patients with regional metastasis, the number of nodes harboring metastatic disease is the most important predictor of survival.4,10,11,33–36 The current AJCC N-category stratifies patients according to number of nodes involved based on best statistical grouping: 1 (N1) versus 2–3 (N2) versus 4 or more (N3) nodes.8
Regional node tumor burden, empirically defined in the AJCC melanoma staging system as microscopic or macroscopic metastasis, was the second most important prognostic factor in patients with stage III disease in the AJCC database analysis. Microscopic disease refers to metastatic deposits detected on histologic analysis following elective lymph node dissection, or more commonly, SLN biopsy. Macroscopic disease refers to nodal metastases that are clinically or radiographically apparent and pathologically confirmed. Importantly, these definitions are based on method of detection, not the size or “visibility” of the nodal metastasis. This criterion is used to sub-categorize the N classification in the current staging system. For example, N1a–N3a refers to patients with micrometastasis and N1b-N3b to patients with macrometastasis. Analysis of patients in the AJCC melanoma staging database demonstrated significant differences in survival when accounting for nodal tumor burden (Figure 1C,D and Table 4).8,9
Table 4.
Five-year survival rates for stage III (nodal metastases) patients stratified by number of metastatic nodes, primary tumor ulceration, and nodal tumor burden (microscopic or macroscopic) (n=2313).
| Primary tumor ulceration | No. of Nodal Micrometastases % ±S.E. (n=1,872) | No. of Nodal Macrometastases % ± S.E. (n=441) | ||||
|---|---|---|---|---|---|---|
| 1 | 2–3 | ≥4 | 1 | 2–3 | ≥4 | |
| Absent | 81.5 ± 1.9 (777) | 73.2 ± 3.7 (246) | 38.0 ± 8.5 (46) | 51.6 ± 7.2 (75) | 46.6 ± 7.9 (67) | 45.4 ± 9.1 (50) |
| Present | 56.6 ± 2.9 (531) | 53.9 ± 4.2 (223) | 34.0 ± 8.3 (49) | 49.4 ± 6.2 (88) | 37.7 ± 6.2 (93) | 29.2 ± 6.7 (68) |
From Balch CM, Gershenwald JE, Soong SJ, et al: Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 28:2452, 2010; with permission.
Lymphatic mapping and sentinel node biopsy is now widely used as the standard method of staging patients deemed to have significant risk of clinically occult regional nodal metastasis. Certainly, most contemporary series reveal that the majority of patients with stage III disease present with micrometastasis, usually detected on SLN biopsy.9,37–40 Recently, analysis of patients from the AJCC melanoma staging database with stage III disease was performed to identify and compare independent predictors of survival between those with micrometastases and macrometastases.37 This investigation confirmed significant survival differences based on nodal tumor burden, and remarkable heterogeneity in survival among substages of patients with stage III disease. Multivariate analysis demonstrated differences in independent predictors of survival when stage III patients were stratified by nodal tumor burden (Table 5).37 In both groups, number of positive lymph nodes remained the most significant predictor of survival. In addition, older age was found to be an independent adverse prognostic factor, regardless of nodal tumor burden. However, in patients with nodal micrometastases, features of the primary tumor including thickness, mitotic rate, ulceration, and anatomic location were found to significantly impact survival. In contrast, these primary tumor features were not independent predictors in patients with nodal macrometastases (Table 5). These results reveal important differences regarding prognosis based on nodal tumor burden (Figures 1C,D and Table 4), and provide the groundwork for further refinement in prognostic assessment of stage III patients, particularly, but not exclusively, among the dominant cohort with nodal micrometastases.
Table 5.
Multivariate Cox regression analyses for nodal micrometastases and for nodal macrometastases with mitotic rate data available (N=1338)
| Nodal Micrometastases (n=1070) | Nodal Macrometastases (n=268) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | d.f. | Chi-Square(χ2) | P-value | Hazard Ratio | Chi-Square(χ2) | P-value | Hazard Ratio |
| # of Positive Nodes | 2 | 43.0 | >0.001 | 6.1 | 0.0480 | ||
| 1** | — | — | — | 1.00 | — | — | 1.00 |
| 2–3 | 1 | 2.2 | 0.1343 | 1.25 | 0.2 | 0.6800 | 1.11 |
| ≥4 | 1 | 43.0 | >0.001 | 3.59 | 5.7 | 0.0172 | 1.83 |
|
| |||||||
| Thickness (mm) | 3 | 21.4 | >0.001 | 5.7 | 0.1267 | ||
| 0–2.00** | — | — | — | 1.00 | — | — | 1.00 |
| 2.01–4.00 | 1 | 7.0 | 0.0083 | 1.62 | 0.8 | 0.3842 | 0.74 |
| 4.01–6.00 | 4.3 | 0.0380 | 1.58 | 0.3 | 0.5811 | 1.22 | |
| >6.00 | 1 | 21.2 | >0.001 | 3.00 | 0.6 | 0.4210 | 1.34 |
|
| |||||||
| Ulceration | 1 | 11.1 | 0.001 | 2.8 | 0.0959 | ||
| Absent** | — | — | — | 1.00 | — | — | 1.00 |
| Present | 1 | 11.1 | 0.001 | 1.59 | 2.7 | 0.0959 | 1.45 |
|
| |||||||
| Clark Level | 1 | 0.003 | 0.9597 | 0.4 | 0.5140 | ||
| II/III** | — | — | — | 1.00 | — | — | 1.00 |
| IV/V | 1 | 0.003 | 0.9597 | 1.01 | 0.4 | 0.5140 | 1.27 |
|
| |||||||
| Mitotic Rate | 2 | 23.4 | >0.001 | 2.71 | 0.2582 | ||
| <1** | — | — | — | 1.00 | — | — | 1.00 |
| 1–19.99 | 1 | 0.02 | 0.8993 | 0.96 | 2.6 | 0.1085 | 0.45 |
| ≥20 | 1 | 6.1 | 0.0134 | 2.69 | 2.4 | 0.1239 | 0.41 |
|
| |||||||
| Age (year) | 2 | 12.7 | 0.0018 | 13.8 | 0.0010 | ||
| <50** | — | — | — | 1.00 | — | — | 1.00 |
| 50 — 69 | 1 | 2.5 | 0.1160 | 1.26 | 1.6 | 0.2121 | 0.75 |
| ≥70 | 1 | 12.6 | 0.0004 | 1.83 | 6.3 | 0.0118 | 1.92 |
|
| |||||||
| Site | 1 | 5.0 | 0.0251 | 0.4 | 0.5413 | ||
| Extremity** | — | — | — | 1.00 | — | — | 1.00 |
| Axial | 1 | 5.0 | 0.0251 | 1.36 | 0.4 | 0.5413 | 1.13 |
|
| |||||||
| Gender | 1 | 1.1 | 0.2959 | 0.9 | 0.3435 | ||
| Male** | — | — | — | 1.00 | — | — | 1.00 |
| Female | 1 | 1.1 | 0.2959 | 0.86 | 0.9 | 0.3435 | 0.79 |
From Balch CM, Gershenwald JE, Soong SJ, et al: Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 28:2452, 2010; with permission.
Primary tumor ulceration was first included as a stratification criterion for stage III melanoma in the 6th edition AJCC melanoma staging system based on data demonstrating its significance as an independent adverse predictor of survival in this cohort.4,10,11,41 This criterion was upheld in the recently published 7th edition AJCC staging system (Tables 4 and 5).8,9 Similar to its impact on survival estimates for stage I and II melanoma, primary tumor ulceration is associated with decreased survival in stage III, essentially upstaging such a patient whose primary tumor is ulcerated to that of a patient with a nonulcerated primary who has a higher nodal tumor burden category (Figure 1C,D and Table 4). This criterion is therefore again used to define N-category substages (Table 2).8,9 For example, 5-year survival is 53.9% and 46.6%, respectively, in patients with 2–3 microscopically involved regional nodes and an ulcerated primary melanoma versus 2–3 macroscopically involved lymph nodes in patients whose primary tumor is not ulcerated (Table 4).37
The criteria discussed above revealed marked heterogeneity in the prognosis for patients with stage III disease, and are used to define stage III substages into IIIA, IIIB, and IIIC (Figure 1C,D and Tables 2 and 4).9 Future studies involving staging and management of patients with regional metastases, particularly those with microscopic nodal tumor burden, are likely to be better refined by incorporating other features of the primary tumor, including mitotic rate.
In-transit and satellite disease
The final criterion for defining stage III disease is the presence of intralymphatic metastases in the form of either in-transit disease or satellite lesions. In both the previous and most recent editions of the AJCC melanoma staging system, a designation of N2c is given to patients with in-transit or satellite disease in the absence of nodal metastases, while patients with concomitant nodal metastases and in-transit and satellite lesions are classified as having N3 disease.8–11 Based on current analyses of the AJCC melanoma staging database, patients with N2c disease have 5- and 10-year survival rates of 69% and 52%, respectively. These survival rates are actually higher than that of patients with N2a and N2b disease, and more favorable than in prior reports.4,42–45 Nonetheless, the survival for patients with intralymphatic metastases (in the absence of nodal metastases) still fits best into the stage IIIB category (Tables 1 and 2).
Stage IV Melanoma
The prognosis for patients with distant metastases is generally poor, with historical 5-year survival rates of less than 10%.46–48 Several factors have been examined in attempt to better predict survival in this group.3,48–50 Beginning with the 6th edition AJCC melanoma staging system, patients with stage IV melanoma were categorized as having M1a (metastasis to distant skin, subcutaneous tissues, and/or lymph nodes), M1b (metastasis to the lungs), and M1c (metastasis to any non-pulmonary visceral site) disease. In addition, patients with an elevated serum LDH were assigned to the M1c category, regardless of site of distant metastasis. Analysis of the updated database, including over 8000 patients, validated these criteria as significant independent prognostic factors in patients with stage IV disease.8,9
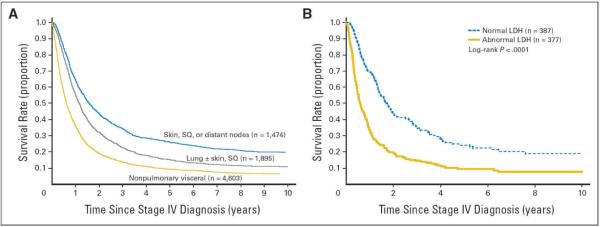
Based on this most recent analysis, patients with metastasis to distant skin, subcutaneous tissues, and/or lymph node basins (M1a), have the highest one-year survival rate (62%) among patients with stage IV disease. Patients with pulmonary metastasis (M1b) have an intermediate prognosis (one-year survival rate, 53%). Finally, patients with non-pulmonary visceral metastases and/or an elevated serum LDH (M1c) have the worst one-year survival among stage IV patients (33%) (Table 2 and Figure 2,A,B).8,9
Figure 2.
Survival curves of 7,635 patients with metastatic melanoma at distant sites (stage IV) subgrouped by (A) the site of metastatic disease and (B) serum lactate dehydrogenase (LDH) levels. LDH values are not used to stratify patients. Curves in (A) are based only on site of metastasis. The number of patients is shown in parentheses. SQ, subcutaneous.
From Balch CM, Gershenwald JE, Soong S, et al, Journal of Clinical Oncology 27(36): 6199-206, 2009, with permission.
Serum markers are uncommonly used in staging solid tumors. However, multiple reports have consistently demonstrated an elevated serum LDH to represent a highly significant, independent adverse prognostic factor in patients with stage IV melanoma.51–53 These findings were recapitulated in the 7th edition AJCC melanoma database analysis, and revealed that 1- and 2-year survival rates for stage IV patients with a normal LDH were 65% and 40%, respectively, compared to 32% and 18%, respectively, in those patients with an elevated LDH level (Figure 2A,B).9 Although the exact pattern of elevated LDH isoforms is nonspecific in melanoma patients, and the mechanism for LDH elevation is not fully understood, an overwhelming amount of clinical data supports its use as a prognostic factor in patients with stage IV disease. Accordingly, it is recommended that serum LDH levels are measured in all melanoma patients when diagnosed with distant metastasis (Table 2 and Figure 2A,B).
Several studies have reported the number of distant metastases to be a relevant prognostic factor in patients with metastatic melanoma.46,54,55 Although analysis of stage IV patients in the updated staging database confirms this finding,8,9 the challenge of standardizing the diagnostic modalities used to identify and quantify distant disease makes it difficult to incorporate this as a formal criterion in the current staging system.
Emerging Themes for Staging and Prognosis for Cutaneous Melanoma: Conditional Survival Estimates, Electronic Prognostic Models, and Molecular Profiling
Conditional Survival Estimates
For staging purposes, survival estimates for melanoma patients are determined from the time of melanoma diagnosis and are typically reported using the methods of Kaplan and Meier. Although well-characterized, stage-specific 5-year and 10-year survival estimates based on analysis of large patient populations at time of initial melanoma diagnosis are available, such traditional survival estimates become less relevant for patients surviving several years beyond diagnosis and treatment, as a patient's cancer-specific risk profile changes over time, particularly for patients with advanced disease at initial presentation. Over the past decade, the concept of conditional survival – ie, having survived “x” years since initial diagnosis, what is “my” predicted survival from that point forward? - has emerged as an important technique to estimate survival for cancer survivors. Conditional survival estimates have been published for a variety of malignancies.56–63 Recently, analyses of patients with cutaneous melanoma have demonstrated that conditional survival estimates increase over time in patients with advanced disease. 64–66 For instance, in an analysis of melanoma patients using the SEER database, 5-year conditional survival estimates in patients with stage II, III, and IV melanoma improved from 72% to 86%, 51% to 87%, and 19% to 84%, respectively, in which that latter estimate in each range above corresponded to the subset of patients that survived 5 years following initial diagnosis.66 Furthermore, among stage III patients treated at the University of Texas MD Anderson Cancer Center, 5-year conditional disease-specific survival estimates for patients with stage IIIA, IIIB, and IIIC improved from 78% to 90%, 54% to 79%, and 39% to 78%, respectively.65
Understanding that survival estimates are not static, but rather improve for melanoma survivors who initially present with advanced disease, provides an opportunity for more accurate prognostic assessment for patients and clinicians alike. Conditional survival estimates provide quantitative information that educates clinicians, may reduce patient anxiety about risk of cancer recurrence and death, and potentially serve as motivation for clinicians to continue to pursue aggressive treatment strategies in patients with advanced disease.
Beyond TNM-Based Staging
Despite the strong evidenced-based predictive capacity of the current AJCC melanoma staging system, it is de facto constrained by the rigorous structure inherent in its TNM-based design. AJCC database analyses demonstrate several important predictors of survival not included in the current staging system.9 Variables such as age, gender, primary tumor site, extent of microscopic tumor burden, and number of sites of distant metastases have been shown to have prognostic relevance. Tools that allow clinicians to incorporate these demographic and clinicopathologic data for a specific patient can ultimately yield personalized and ever more accurate estimates of recurrence and survival. As our understanding of the biology of melanoma, as well as stage-specific prognostic factors continues to expand, greater emphasis on development and implementation of individualized patient prognostic models is essential to continue to improve patient care. An ideal system would incorporate state-of-the-art prognostic factor analyses, permit healthcare providers to remotely enter relevant data, and provide real-time feedback.
Recently, the first electronic predictive tool for patients with localized melanoma based on the large AJCC melanoma staging database was published.67 Based on this model, an individual patient's 1-, 2-, 5-, and 10-year survival with associated 95% confidence intervals are available. Refined risk stratification schema to allow for treatment and surveillance planning, selection of patients appropriate for clinical trials, and comparison of effectiveness of therapies for well-defined patient subgroups within trials is possible. An initial version of this model is available on the internet (http://www.melanomaprognosis.org). Following on-screen prompts, data for patients with localized melanoma, as well as those with regional metastases, are entered using drop-down menus, and survival estimates are immediately displayed. This model serves as a template for providing patients and clinicians with prognostic information and a foundation on which to plan future individualized treatment studies.
Molecular-based Profiling and Melanoma Biomarkers
In the future, it is likely that molecular profiling endeavors will provide additional information pertinent to staging and prognosis for cutaneous melanoma. Certainly, recent developments in targeted therapies for patients with metastatic melanoma are based on an improved understanding of disease biology at the molecular level.68–73 Furthermore, studies attempting to provide “genetic signatures” for individual patients are underway and are beginning to shed light on the potential use of these techniques in prognostic models and treatment planning.74–76 Of note, the nascent phase II effort of the NIH Cancer Genome Atlas Project (TCGA) specifically includes melanoma, and will hopefully provide invaluable insight into the molecular biology of melanoma in years to come. Further information regarding this exciting initiative can be found at http://cancergenome.nih.gov.
Biomarkers for melanoma identification, prediction of disease progression, prognosis, and treatment planning are lacking. Although serum LDH is part of the current staging system, it is non-specific and cannot readily be used to evaluate response to therapy. While a discussion of emerging melanoma biomarkers is beyond the scope of this article, identification of relevant biomarkers will likely contribute to enhanced prognostic assessment and potentially hasten clinical trial development and evaluation. It is hopeful that this area of intense investigation will yield meaningful surrogates for selecting future therapies, monitoring treatment response, and add to individualized prognostic modeling.
Summary
The AJCC melanoma staging database forms the foundation for the current melanoma staging system; future analyses based on this robust platform will likely continue to serve as a foundation for future improvements in melanoma staging. As our understanding of the biology of this complex tumor system continues to evolve, both clinical and molecular factors that may have significant prognostic implications will undoubtedly be unveiled. Notable updates to melanoma staging published in the 7th edition AJCC melanoma staging system include: incorporation of mitotic rate into T1 criteria, inclusion of immunohistochemical detection of nodal micrometastases, and categorization of patients with melanoma of an unknown primary (ie, metastatic melanoma arising in the skin, subcutaneous tissue, or regional lymph nodes in a patient whose staging evaluation does not reveal other sites of disease) as stage III, rather than stage IV.
Based on the results of the AJCC melanoma staging database analysis, future prognostic factor studies should evaluate the formal impact of mitotic rate across all stages of disease, further assess the influence of microscopic nodal tumor burden in patients with stage III disease in this era of SLN biopsy, and continue to refine staging and prognosis for patients with stage IV melanoma. Moreover, continued development and application of conditional survival estimates in melanoma patients, increased use of prognostic tools which incorporate relevant criteria beyond the scope of TNM-based staging, molecular profiling endeavors (including, for example, lessons learned from the nascent and ongoing NIH-sponsored Cancer Genome Atlas Project [TCGA] which specifically includes melanoma), and identification of melanoma-specific biomarkers, will hopefully provide opportunities for more accurate staging and individualized prognosis for melanoma patients in the future.
Acknowledgments
This work was supported in part by The University of Texas MD Anderson Cancer Center Melanoma SPORE (P50 CA93459) and the Grossman Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose
References
- 1.Balch CM, Murad TM, Soong SJ, et al. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Annals of Surgery. 1978;188:732. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eldh J, Boeryd B, Peterson LE. Prognostic factors in cutaneous malignant melanoma in stage I. A clinical, morphological and multivariate analysis. Scand J Plast Reconstr Surg. 1978;12:243. doi: 10.3109/02844317809013000. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Esch EP, Cascinelli N, Preda F, et al. Stage I melanoma of the skin: evaluation of prognosis according to histologic characteristics. Cancer. 1981;48:1668. doi: 10.1002/1097-0142(19811001)48:7<1668::aid-cncr2820480732>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol. 1997;15:1039. doi: 10.1200/JCO.1997.15.3.1039. [DOI] [PubMed] [Google Scholar]
- 5.Gershenwald JE, Buzaid AC, Ross MI. Classification and staging of melanoma. Hematol Oncol Clin North Am. 1998;12:737. doi: 10.1016/s0889-8588(05)70021-6. [DOI] [PubMed] [Google Scholar]
- 6.Ross M. Modifying the criteria of the American Joint Commission on Cancer staging system in melanoma. Curr Opin Oncol. 1998;10:153. doi: 10.1097/00001622-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Thompson JF, Shaw HM, Hersey P, et al. The history and future of melanoma staging. J Surg Oncol. 2004;86:224. doi: 10.1002/jso.20082. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM. Melanoma of the Skin. In: Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. ed 7th Springer Verlag; New York: 2009. [Google Scholar]
- 9.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the american joint committee on cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 11.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the american joint committee on cancer melanoma staging system. J Clin Oncol. 2001;19:3622. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 12.Cormier JN, Xing Y, Feng L, et al. Metastatic melanoma to lymph nodes in patients with unknown primary sites. Cancer. 2006;106:2012. doi: 10.1002/cncr.21835. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, Faries MB, Wanek LA, et al. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26:535. doi: 10.1200/JCO.2007.14.0285. [DOI] [PubMed] [Google Scholar]
- 14.Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97:1488. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 15.Barnhill RL, Katzen J, Spatz A, et al. The importance of mitotic rate as a prognostic factor for localized cutaneous melanoma. J Cutan Pathol. 2005;32:268. doi: 10.1111/j.0303-6987.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 16.Busam KJ. The prognostic importance of tumor mitotic rate for patients with primary cutaneous melanoma. Ann Surg Oncol. 2004;11:360. doi: 10.1245/ASO.2004.02.910. [DOI] [PubMed] [Google Scholar]
- 17.Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25:1129. doi: 10.1200/JCO.2006.08.1463. [DOI] [PubMed] [Google Scholar]
- 18.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 20.Breslow A, Cascinelli N, van der Esch EP, et al. Stage I melanoma of the limbs: assessment of prognosis by levels of invasion and maximum thickness. Tumori. 1978;64:273. doi: 10.1177/030089167806400305. [DOI] [PubMed] [Google Scholar]
- 21.Shaw HM, Balch CM, Soong SJ, et al. Prognostic histopathological factors in malignant melanoma. Pathology. 1985;17:271. doi: 10.3109/00313028509063766. [DOI] [PubMed] [Google Scholar]
- 22.Buttner P, Garbe C, Bertz J, et al. Primary cutaneous melanoma. Optimized cutoff points of tumor thickness and importance of Clark's level for prognostic classification. Cancer. 1995;75:2499. doi: 10.1002/1097-0142(19950515)75:10<2499::aid-cncr2820751016>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Wilkerson JA, Murad TM, et al. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45:3012. doi: 10.1002/1097-0142(19800615)45:12<3012::aid-cncr2820451223>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.McGovern VJ, Shaw HM, Milton GW, et al. Ulceration and prognosis in cutaneous malignant melanoma. Histopathology. 1982;6:399. doi: 10.1111/j.1365-2559.1982.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 25.Salman SM, Rogers GS. Prognostic factors in thin cutaneous malignant melanoma. J Dermatol Surg Oncol. 1990;16:413. doi: 10.1111/j.1524-4725.1990.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 26.Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity:lessons learned forom the generation of a probabalistic model. Ann Surg Oncol. 2004;11:247. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Caudle AS, Ross MI, Prieto VG, et al. Mitotic rate predicts sentinel lymph node involvement in melanoma: Impact of the 7th edition AJCC Melanoma Staging System. Society of Surgical Oncology 63rd Annual Cancer Symposium; St. Louis. 2010. p. S8. [Google Scholar]
- 28.Clark WH, Jr., From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705. [PubMed] [Google Scholar]
- 29.Breslow A. Tumor thickness in evaluating prognosis of cutaneous melanoma. Ann Surg. 1978;187:440. doi: 10.1097/00000658-197804000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morton DL, Davtyan DG, Wanek LA, et al. Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer. 1993;71:3737. doi: 10.1002/1097-0142(19930601)71:11<3737::aid-cncr2820711143>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Vollmer RT. Malignant melanoma. A multivariate analysis of prognostic factors. Pathol Annu. 1989;24(Pt 1):383. [PubMed] [Google Scholar]
- 32.Gershenwald JE, Hwu P. Melanoma. In: Hong WK, Bast RC, Halit WN, et al., editors. Cancer Medicine. ed 8 People's Medical Publishing House-USA; Shelton: 2010. [Google Scholar]
- 33.Buzaid AC, Anderson CM. The changing prognosis of melanoma. Curr Oncol Rep. 2000;2:322. doi: 10.1007/s11912-000-0025-9. [DOI] [PubMed] [Google Scholar]
- 34.Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16:2253. doi: 10.1200/JCO.1998.16.6.2253. [DOI] [PubMed] [Google Scholar]
- 35.Gershenwald JE, Fischer D, Buzaid AC. Clinical classification and staging. Clin Plast Surg. 2000;27:361. [PubMed] [Google Scholar]
- 36.White RR, Stanley WE, Johnson JL, et al. Long-term survival in 2,505 patients with melanoma with regional lymph node metastasis. Ann Surg. 2002;235:879. doi: 10.1097/00000658-200206000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMasters KM, Noyes RD, Reintgen DS, et al. Lessons learned from the Sunbelt Melanoma Trial. J Surg Oncol. 2004;86:212. doi: 10.1002/jso.20084. [DOI] [PubMed] [Google Scholar]
- 39.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 40.Scolyer RA, Murali R, Satzger I, et al. The detection and significance of melanoma micrometastases in sentinel nodes. Surg Oncol. 2008;17:165. doi: 10.1016/j.suronc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Balch C, Soong S, Murad T, et al. A multifactorial analysis of melanoma: prognostic factors in melanoma patients with lymph node metastases (stage II) Ann Surg. 1981;193:377. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascinelli N, Bufalino R, Marolda R, et al. Regional non-nodal metastases of cutaneous melanoma. Eur J Surg Oncol. 1986;12:175. [PubMed] [Google Scholar]
- 43.Day CL, Jr., Harrist TJ, Gorstein F, et al. Malignant melanoma. Prognostic significance of “microscopic satellites” in the reticular dermis and subcutaneous fat. Ann Surg. 1981;194:108. doi: 10.1097/00000658-198107000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leon P, Daly JM, Synnestvedt M, et al. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991;126:1461. doi: 10.1001/archsurg.1991.01410360031006. [DOI] [PubMed] [Google Scholar]
- 45.Pawlik TM, Ross MI, Thompson JF, et al. The risk of in-transit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol. 2005;23:4588. doi: 10.1200/JCO.2005.12.245. [DOI] [PubMed] [Google Scholar]
- 46.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases [see comments] Journal of the American College of Surgeons. 1995;181:193. [PubMed] [Google Scholar]
- 47.Manola J, Atkins M, Ibrahim J, et al. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 48.Unger JM, Flaherty LE, Liu PY, et al. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer. 2001;91:1148. doi: 10.1002/1097-0142(20010315)91:6<1148::aid-cncr1111>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Eton O, Legha SS, Moon TE, et al. Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol. 1998;16:1103. doi: 10.1200/JCO.1998.16.3.1103. [DOI] [PubMed] [Google Scholar]
- 50.Ryan L, Kramar A, Borden E. Prognostic factors in metastatic melanoma. Cancer. 1993;71:2995. doi: 10.1002/1097-0142(19930515)71:10<2995::aid-cncr2820711018>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 51.Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26:624. doi: 10.1080/07357900802027073. [DOI] [PubMed] [Google Scholar]
- 52.Keilholz U, Martus P, Punt CJ, et al. Prognostic factors for survival and factors associated with long-term remission in patients with advanced melanoma receiving cytokine-based treatments: second analysis of a randomised EORTC Melanoma Group trial comparing interferon-alpha2a (IFNalpha) and interleukin 2 (IL-2) with or without cisplatin. Eur J Cancer. 2002;38:1501. doi: 10.1016/s0959-8049(02)00123-5. [DOI] [PubMed] [Google Scholar]
- 53.Sirott MN, Bajorin DF, Wong GY, et al. Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer. 1993;72:3091. doi: 10.1002/1097-0142(19931115)72:10<3091::aid-cncr2820721034>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 54.Balch CM. Cutaneous melanoma: prognosis and treatment results worldwide. Semin Surg Oncol. 1992;8:400. doi: 10.1002/ssu.2980080611. [DOI] [PubMed] [Google Scholar]
- 55.Staudt M, Lasithiotakis K, Leiter U, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102:1213. doi: 10.1038/sj.bjc.6605622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi M, Fuller CD, Thomas CR, Jr., et al. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109:203. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 57.Fuller CD, Wang SJ, Thomas CR, Jr., et al. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109:1331. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 58.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer. 1995;76:237. doi: 10.1002/1097-0142(19950715)76:2<237::aid-cncr2820760213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 59.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. 2001;92:2211. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 60.Merrill RM, Bird JS. Effect of young age on prostate cancer survival: a population-based assessment (United States) Cancer Causes Control. 2002;13:435. doi: 10.1023/a:1015764507609. [DOI] [PubMed] [Google Scholar]
- 61.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41:1097. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 62.Wang SJ, Emery R, Fuller CD, et al. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer. 2007;10:153. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 63.Wang SJ, Fuller CD, Emery R, et al. Conditional Survival in Rectal Cancer: A SEER Database Analysis. Gastrointest Cancer Res. 2007;1:84. [PMC free article] [PubMed] [Google Scholar]
- 64.Bowles TL, Xing Y, Hu CY, et al. Conditional survival estimates improve over 5 years for melanoma survivors with node-positive disease. Ann Surg Oncol. 2010;17:2015. doi: 10.1245/s10434-010-1051-y. [DOI] [PubMed] [Google Scholar]
- 65.Rueth NM, Groth SS, Tuttle TM, et al. Conditional survival after surgical treatment of melanoma: an analysis of the surveillance, epidemiology, and end results database. Ann Surg Oncol. 2010;17:1662. doi: 10.1245/s10434-010-0965-8. [DOI] [PubMed] [Google Scholar]
- 66.Xing Y, Chang GJ, Hu CY, et al. Conditional survival estimates improve over time for patients with advanced melanoma: results from a population-based analysis. Cancer. 2010;116:2234. doi: 10.1002/cncr.24966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soong SJ, Ding S, Coit D, et al. Predicting survival outcome of localized melanoma: an electronic prediction tool based on the AJCC Melanoma Database. Ann Surg Oncol. 2010;17:2006. doi: 10.1245/s10434-010-1050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hingorani SR, Jacobetz MA, Robertson GP, et al. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198. [PubMed] [Google Scholar]
- 69.Jiang X, Zhou J, Yuen NK, et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin Cancer Res. 2008;14:7726. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 70.Sumimoto H, Miyagishi M, Miyoshi H, et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene. 2004;23:6031. doi: 10.1038/sj.onc.1207812. [DOI] [PubMed] [Google Scholar]
- 71.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010 doi: 10.1038/nature09454. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 74.Jonsson G, Busch C, Knappskog S, et al. Gene expression profiling-based identification of molecular subtypes in stage IV melanomas with different clinical outcome. Clin Cancer Res. 2010;16:3356. doi: 10.1158/1078-0432.CCR-09-2509. [DOI] [PubMed] [Google Scholar]
- 75.Philippidou D, Schmitt M, Moser D, et al. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res. 2010;70:4163. doi: 10.1158/0008-5472.CAN-09-4512. [DOI] [PubMed] [Google Scholar]
- 76.Rother J, Jones D. Molecular markers of tumor progression in melanoma. Curr Genomics. 2009;10:231. doi: 10.2174/138920209788488526. [DOI] [PMC free article] [PubMed] [Google Scholar]