Abstract
Three key reactions, an efficient Ugi four-component coupling, a regiospecific, base-mediated elimination reaction, and an intramolecular nitrone/alkene [3+2] cycloaddition, were used to achieve an effective synthesis of the tricyclic molecular framework of the immunosuppressant FR901483. The outcome of a control experiment supports the idea that an internal deprotonation by an alkoxide ion is the origin of the site selectivity observed in the base-induced elimination of hydroxy mesylate 17.
Keywords: Ugi four-component coupling, elimination reaction, [3+2] cycloaddition
Scientists from the Fujisawa Pharmaceutical Company isolated the natural product FR901483 (1) from the fermentation broth of the fungus Cladobotryum sp. No. 11231 in the course of a search for new immunosuppressive agents with mechanisms of action different from cyclosporine A and FK-506.2 The unique and rigid molecular architecture of 1 (Figure 1), which seems to conceal most of the atoms of two molecules of tyrosine, was revealed by an X-ray crystallographic analysis. The phosphate ester is another interesting element of this substance that is essential to its potent immunosuppressive activity in vitro. FR901483 significantly extends graft survival time in the rat skin allograft model and is believed to exert its immunosuppressive function by inhibiting purine nucleotide biosynthesis.
Figure 1.

The molecular structure of FR901483 (1)
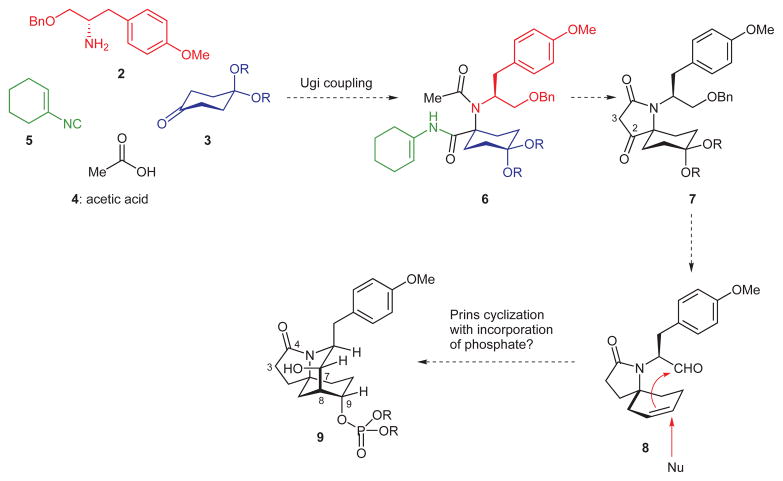
The status of 1 as a potential immunosuppressant and its attractive structure stimulated much creative research in the field of chemical synthesis. Five laboratories, including ours, published syntheses of the full structure of this natural product.3–7 In addition to these achievements, several laboratories described either formal syntheses of FR901483 or novel concepts for constructing its rigid, azatricyclic core structure.8 In 2000, our laboratory described an enantiospecific, nature-inspired synthesis of this natural product from two simple tyrosine derivatives featuring the following key transformations: a C–N bond-forming oxidative dearomatization, an aldol cyclization, and a late-stage Mitsunobu substitution that simultaneously produced the phosphate ester of 1 and the desired stereochemistry at C-9.4 While the brevity of this design for synthesis was attractive, we were not entirely satisfied with the yields of these three transformations or with the level of selectivity (both regio- and diastereoselectivity) for the aldol cyclization step. This circumstance engendered a new way of thinking about the problem of gaining a rapid access to the azaspirotricyclic core structure of FR901483. The general features of this alternative synthesis are outlined below in Scheme 1.
Scheme 1.
A strategy for constructing the azatricyclic architecture of FR901483 from four simple starting materials
The idea that compounds 2–5 would join spontaneously by the Ugi coupling process9 to give compound 6 was a key aspect of this design. If it succeeded, this union would produce a polyfunctional compound having the crowded C–N bond and all of the carbon atoms found in the tricyclic core structure of FR901483 from four simple compounds. Indeed, amine 2 and Armstrong’s cyclohexenyl isocyanide 510 can be readily prepared, while the monoketal of 1,4-cyclohexandione 3 and acetic acid (4) are commercially available. In the wake of converting the enamide moiety in 6 to an ester (or a thioester), we would then be in a position to generate the C2–C3 bond in compound 7 via a Dieckmann condensation.11 A few seemingly straightforward functional group manipulations and an alkene synthesis, which would have to be selective, could then afford alkenyl aldehyde 8. While the alkene in 8 is disubstituted and not especially electron-rich, the π-electrons of this group would enable the desired ring formation if a Prins cyclization12 could be achieved. In essence, this would involve a trans addition of an activated aldehyde carbonyl carbon and an external nucleophile to the disubstituted alkene in 8. By far the most attractive manifestation of this idea would be a direct conversion of 8 into 9 by a Prins cyclization in which the trapping nucleophile is a simple phosphate ion. To the best of our knowledge, Prins reactions with incorporation of phosphate are not found in the literature. Nevertheless, we were drawn to the potential of 8 as a useful intermediate in our synthesis and confident that we would identify a reaction suitable for forming the needed ring.
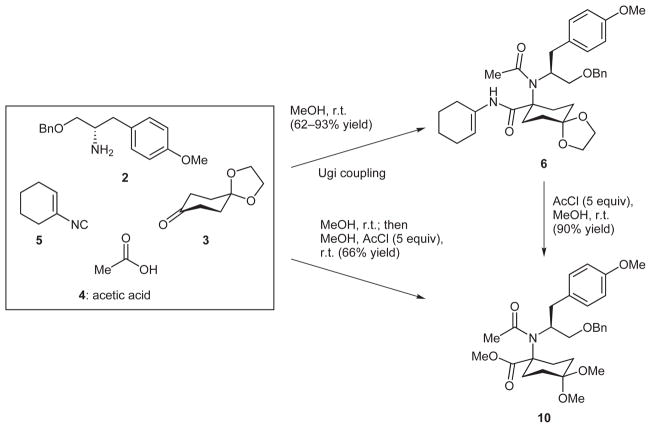
Within the family of multicomponent coupling reactions, the Ugi reaction is unique in its ability to rapidly produce complex molecular structures from four simple inputs and broad applicability to problems in chemical synthesis.13 To our delight, a simple mixing of Armstrong’s isocyanide 510 with compounds 2, 3, and 4 in methanol at room temperature resulted in the formation of 6, a compound replete with useful functionality, in yields ranging from 62– 93% (Scheme 2). Exposure of enamide 6 to anhydrous HCl in methanol, generated by methanolysis of acetyl chloride, gave rise to compound 10 via methyl ester formation and concomitant 1,3-dioxolane–dimethyl ketal exchange. In fact, it was possible to directly form 10 in one pot simply by adding methanol and acetyl chloride to the Ugi product generated by the union of compounds 2–5 in methanol. This single laboratory operation effectively assembles a structure with a proper placement of carbon atoms and the needed nitrogen atom for a synthesis of the tricyclic core framework of FR901483. Moreover, this process generates a chirotopic, nonstereogenic center14 and thus is not complicated by the production of diastereoisomers.
Scheme 2.
The Ugi reaction enables direct syntheses of compounds 6 and 10
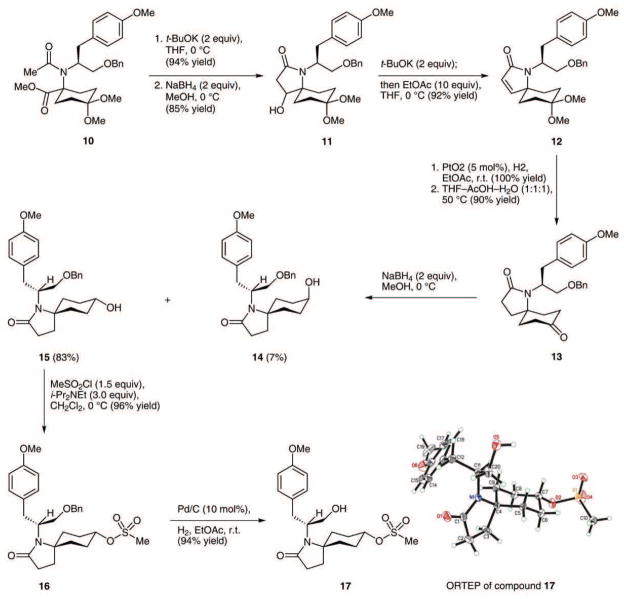
We were pleased with our direct synthesis of compound 10 and could, from this vantage point, make rapid progress toward the larger objective. To this end, deprotonation of the acetamide in 10 with potassium tert-butoxide in THF at 0 °C triggered an efficient Dieckmann cyclization11 to a β-keto amide (i.e. compound 7, R = Me), which was subsequently reduced with sodium borohydride to the epimeric mixture of secondary alcohols shown as 11. To remove the unneeded hydroxyl group, ethyl acetate was added to the potassium alkoxide salts formed by deprotonating the epimeric mixture of alcohols by potassium tert-butoxide; this action resulted in a rapid and efficient formation of α,β-unsaturated lactam 12. This simple procedure, which presumably involves an in situ formation and subsequent elimination of acetate esters, was developed by Funk and co-workers.6 After hydrogenation of the newly formed alkene over Adams’s catalyst, a straightforward, acid-catalyzed hydrolysis of the ketal protecting group gave rise to keto lactam 13. The five-step sequence starting from the Ugi product 10 did not require any chromatographic purifications and produced keto lactam 13 in 73% overall yield with an average yield of 94% for each step.
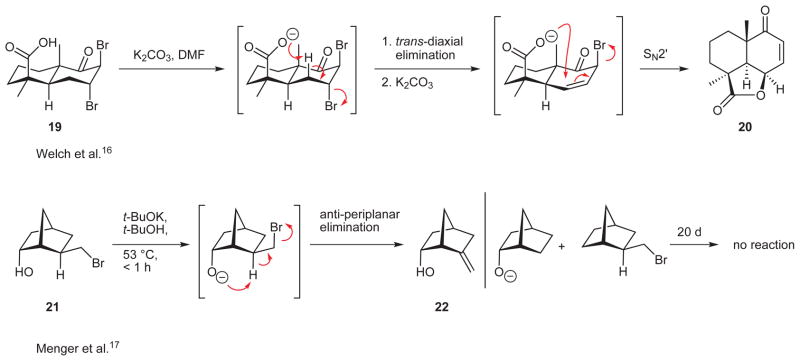
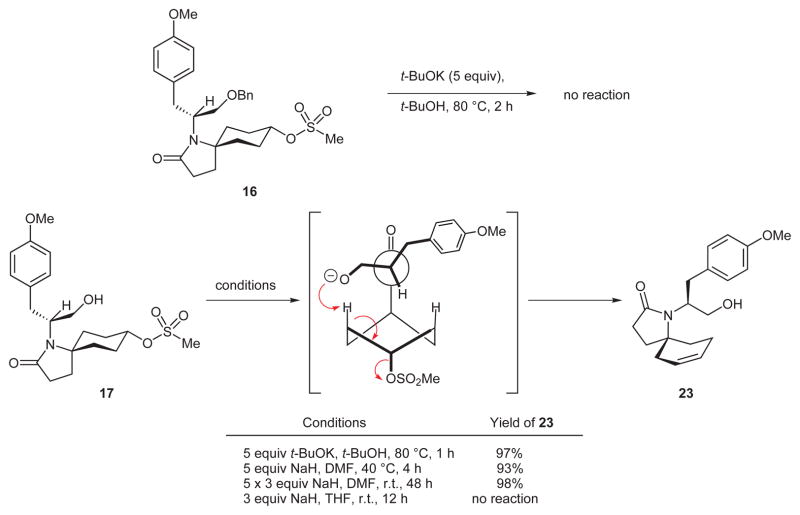
From the outset, we were aware that the advantage gained by using the simple, symmetrical, and commercially available ketone 3 in the Ugi construction of 10 would evaporate if we could not achieve a selective placement of the alkene into the six-membered ring on the path to key intermediate 8 (Scheme 1). Of course, there are some popular methods to transform ketones into alkenes, but it was unclear how any of these would enable us to generate the desired alkene in a selective fashion. This nontrivial selectivity problem stimulated the intriguing idea that methanesulfonate ester 17 might, under basic conditions, undergo the desired elimination with acceptable margins of selectivity. This idea followed from two assumptions (Figure 2). First, we surmised that a minimization of both steric repulsion between the cyclohexane ring and the side chains on C6 and destabilizing allylic strain might hinder rotation about the N–C6 bond. If this were so, then the hydroxyl function of 17 would be hovering over only one of the axial C–H bonds with an antiperiplanar relationship15 to the mesylate leaving group (see Newman projection B); the assumption here was that Newman projection B might be a close approximation of the reactive conformation through which the elimination reaction would take place. Second, we assumed that a suitably strong base would deprotonate the free hydroxyl group in 17 and that the resulting alkoxide ion would be well-positioned to attack the axial Ca–H bond, thus triggering the formation of the desired alkene via an intramolecular, anti elimination of methanesulfonate (see arrows in 18).
Figure 2.
A concept for achieving a selective alkene synthesis
The carboxylate ion- and alkoxide ion-initiated anti eliminations of HBr published by the laboratories of Welch16 and Menger,17 respectively (19 → 20, 21 → 22, Scheme 4), are instructive examples where advantage was taken of an ‘internal base’ to bring about efficient elimination behavior.18 If it succeeded in the context of alcohol mesylate 17, this type of proximity-dependent tactic would provide a simple solution to the challenging problem of generating the desired cyclohexene.
Scheme 4.
Two examples of eliminations by internal bases
To set the stage for the pivotal elimination reaction, ketone 13 was reduced with sodium borohydride to a 12:1 mixture of epimeric alcohols 14 and 15 favoring the desired equatorial alcohol diastereoisomer 15 (Scheme 3). Treatment of this compound with methanesulfonyl chloride and Hünig’s base gave rise to methane sulfonate ester 16 in 96% yield. A high-yielding hydrogenolysis of the benzyl ether in 16 afforded the crystalline hydroxy mesylate 17. In the solid state, compound 17 is a close, chairflipped conformational relative of the Newman projections shown in Figure 2. Interestingly, while benzyl ether mesylate 16 was recovered unchanged after exposure to five equivalents of potassium tert-butoxide in tert-butyl alcohol at 80 °C for two hours, exposure of hydroxy mesylate 17 to the same conditions for one hour resulted in the formation of a single cyclic alkene 23 in 97% yield (Scheme 5). Excellent yields of alkene 23 were also obtained by exposure of hydroxy mesylate 17 to sodium hydride in DMF, although THF was unsuitable as a reaction solvent. The observation that benzyl ether mesylate 16 is impervious to the action of the same bases that cause a smooth and regiospecific alkene synthesis from alcohol 17 is consistent with our prediction that a C7 alkoxide ion is serving the role of internal base in an anti elimination of the secondary mesylate. Analysis of the elimination experiments conducted with compound 17 by 400 MHz NMR before purification clearly indicated that only one alkene product was formed. However, owing to the symmetrical nature of the newly formed cyclohexene, we were unable to make a confident structural assignment by NMR and tentatively assigned the structure of the alkene product as 23 based on our supposition that alcohol mesylate 17 would eliminate in a selective fashion. The outcome of a transformation described below ultimately proved that compound 23 does indeed have the structure shown.
Scheme 3.
Synthesis of key intermediate 17
Scheme 5.
A regiospecific, base-mediated elimination of hydroxy mesylate 17
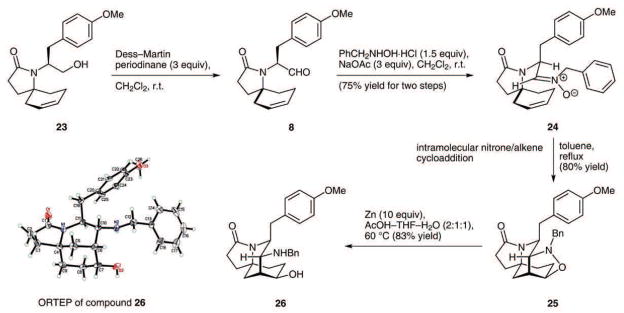
With an effective solution to the problem of generating the desired cyclohexene, attention was turned to the testing of the tandem vicinal difunctionalization strategy outlined in Scheme 1. An oxidation of alcohol 23 with the Dess–Martin periodinane did accomplish the formation of the desired alkenyl aldehyde 8 (Scheme 6). However, all attempts to achieve Prins cyclizations with incorporation of heteroatom nucleophiles at C9 were unsuccessful, presumably due to the unreactive nature of the disubstituted ring alkene; these experiments led only to a recovery of unreacted alkenyl aldehyde 8.19 Efforts to achieve an intramolecular Paterno–Büchi photocycloaddition and a samarium diiodide mediated, reductive aldehyde/alkene cyclization with compound 8 also did not produce the desired products.
Scheme 6.
Completion of tricyclic carbon skeleton of FR901483
In the wake of these experiments, the decision was made to explore the feasibility of intramolecular [3+2] dipolar cycloaddition reactions. To this end, the aldehyde function of 8 was efficiently converted to the corresponding oxime; however, efforts to oxidize the oxime to a nitrile oxide with either sodium hypochlorite or chloramine T gave only unreacted starting material and no evidence of a productive nitrile oxide/alkene cycloaddition. By contrast, nitrone 24, produced by the condensation of N-benzylhydroxyl amine with aldehyde 8, demonstrated useful reactivity. When a solution of this compound in toluene was heated to 130 °C for 18 hours, the desired nitrone/alkene [3+2] dipolar cycloaddition took place and afforded isoxazolidine 25 in 80% yield. The structure of compound 25 was supported by extensive NMR analysis, including H–H COSY, C–H HMQC, and NOESY techniques. It was also possible to reduce the N–O bond of 25 by the action of zinc powder in a warm (60 °C) solvent mixture of AcOH–THF–H2O to reveal 26, a compound embodying the tricyclic core structure of FR901483. This substance gave a crystal suitable for X-ray crystal structural analysis, which unambiguously confirmed its structure and, incidentally, the regiochemical course of the pivotal elimination step shown in Scheme 5.
The primary aim of this project, namely the development of a rapid synthesis of the type of topology found in the potent immunosuppressive natural product FR901483 (1), was achieved. An Ugi coupling of four simple compounds, two of which are commercially available, gave direct access to the fully substituted, nitrogen-bearing carbon and a structure having all of the required carbons. This process, the intramolecular elimination that generated the desired alkene in a regiospecific fashion, and a [3+2] dipolar cycloaddition reaction were the main transformations. Our most advanced FR-like tricycle, compound 26, was not converted to the natural product that inspired this effort. However, it is quite likely that the chemistry described here could be adapted to concise syntheses of even closer structural relatives of FR901483.
Acknowledgments
This work was supported by the Skaggs Institute for Chemical Biology, The Scripps Research Institute (TSRI), the National Institute of General Medical Sciences (GM065483), the 2001 AstraZeneca Award for Excellence in Organic Chemistry (EJS), and the Merck Research Laboratories. A predoctoral fellowship from the Heiwa Nakajima Foundation to H.S. is also gratefully acknowledged. We sincerely thank Professor K. C. Nicolaou for generously providing laboratory space and facilities to H.S. during the period of September, 2003 to January, 2004. We also thank Dr. Raj Chadha (TSRI) for the X-ray crystal structural determinations, Dr. L. B. Pasternack and Dr. D. H. Huang (TSRI) for NMR spectroscopic assistance, and Dr. G. Siuzdak (TSRI) for mass spectrometric assistance.
Footnotes
This paper is dedicated to the pioneering achievements of Professor Yoshito Kishi in the field of organic chemistry on the occasion of his 70th birthday.
References and Notes
- 1.This manuscript is based on Chapter 2 of the Ph.D. thesis of Hirofumi Seike, The Scripps Research Institute, 2003. Experimental procedures and characterization data for the new compounds described in this manuscript are available from the corresponding author upon request.
- 2.Sakamoto K, Tsujii E, Abe F, Nakanishi T, Yamashita M, Shigematsu N, Izumi S, Okuhara M. J Antibiot. 1996;49:37. doi: 10.7164/antibiotics.49.37. [DOI] [PubMed] [Google Scholar]
- 3.This synthesis established the absolute stereochemistry of FR901483: Snider BB, Lin H. J Am Chem Soc. 1999;121:7778.For an earlier model study, see: Snider BB, Lin H, Foxman BM. J Org Chem. 1998;63:6442.
- 4.Scheffler G, Seike H, Sorensen EJ. Angew Chem Int Ed. 2000;39:4593. doi: 10.1002/1521-3773(20001215)39:24<4593::aid-anie4593>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ousmer M, Braun NA, Ciufolini MA. Org Lett. 2001;3:765. doi: 10.1021/ol015526i. [DOI] [PubMed] [Google Scholar]; (b) Ousmer M, Braun NA, Bavoux C, Perrin M, Ciufolini MA. J Am Chem Soc. 2001;123:7534. doi: 10.1021/ja016030z. [DOI] [PubMed] [Google Scholar]
- 6.Maeng JH, Funk R. Org Lett. 2001;3:1125. doi: 10.1021/ol015506g. [DOI] [PubMed] [Google Scholar]
- 7.Kan T, Fujimoto T, Ieda S, Asoh Y, Kitaoka H, Fukuyama T. Org Lett. 2004;6:2729. doi: 10.1021/ol049074w. [DOI] [PubMed] [Google Scholar]
- 8.(a) Yamazaki N, Suzuki H, Kibayashi C. J Org Chem. 1997;62:8280. doi: 10.1021/jo9715579. [DOI] [PubMed] [Google Scholar]; (b) Bonjoch J, Diaba F, Puigbó G, Solé D, Segarra V, Santamaría L, Beleta J, Ryder H, Palacios JM. Bioorg Med Chem. 1999;7:2891. doi: 10.1016/s0968-0896(99)00250-3. [DOI] [PubMed] [Google Scholar]; (c) Suzuki H, Yamazaki N, Kibayashi C. Tetrahedron Lett. 2001;42:3031. [Google Scholar]; (d) Brummond KM, Lu J. Org Lett. 2001;3:1347. doi: 10.1021/ol010029n. [DOI] [PubMed] [Google Scholar]; (e) Wardrop DJ, Zhang W. Org Lett. 2001;3:2353. doi: 10.1021/ol0161514. [DOI] [PubMed] [Google Scholar]; (f) Puigbó G, Diaba F, Bonjoch J. Tertahedron Lett. 2003;59:2657. [Google Scholar]; (g) Bonjoch J, Diaba F, Puigbó G, Peidró E, Solé D. Tetrahedron Lett. 2003;44:8387. [Google Scholar]; (h) Panchaud P, Ollivier C, Renaud P, Zigmantas S. J Org Chem. 2004;69:2755. doi: 10.1021/jo035843y. [DOI] [PubMed] [Google Scholar]; (i) Brummond KM, Hong SP. J Org Chem. 2005;70:907. doi: 10.1021/jo0483567. [DOI] [PubMed] [Google Scholar]; (j) Kropf JE, Meigh IC, Bebbington MWP, Weinreb SM. J Org Chem. 2006;71:2046. doi: 10.1021/jo052466b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Simila STM, Reichelt A, Martin SF. Tetrahedron Lett. 2006;47:2933. [Google Scholar]; (l) Diaba F, Ricou E, Bonjoch J. Tetrahedron: Asymmetry. 2006;17:1437. [Google Scholar]; (m) Kaden S, Reissig HU. Org Lett. 2006;8:4763. doi: 10.1021/ol061538y. [DOI] [PubMed] [Google Scholar]; (n) Gotchev DB, Comins DL. J Org Chem. 2006;71:9393. doi: 10.1021/jo061677t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Asari A, Angelov P, Auty JM, Hayes CJ. Tetrahedron Lett. 2007;48:2631. [Google Scholar]
- 9.(a) Ugi I, Steinbrüchner C. Angew Chem. 1960;72:267. [Google Scholar]; (b) Gokel G, Luedke G, Ugi I. In: Isonitrile Chemistry. Ugi I, editor. Academic Press; New York: 1971. p. 145. [Google Scholar]
- 10.(a) Keating TA, Armstrong RW. J Am Chem Soc. 1995;117:7842. [Google Scholar]; (b) Keating TA, Armstrong RW. J Am Chem Soc. 1996;118:2574. [Google Scholar]; (c) Armstrong RW, Combs AP, Tempest PA, Brown SD, Keating TA. Acc Chem Res. 1996;29:123. [Google Scholar]; (d) Hassner A, Lorber ME, Heathcock C. J Org Chem. 1967;32:540. doi: 10.1021/jo01278a006. [DOI] [PubMed] [Google Scholar]; (e) Baldwin JE, Bottaro JC, Riordan PD, Derome AE. J Chem Soc, Chem Commun. 1982:942. [Google Scholar]; (f) Baldwin JE, Yamaguchi Y. Tetrahedron Lett. 1989;30:3335. [Google Scholar]
- 11.For a review of the Dieckmann condensation and related processes, see: Davis BR, Garratt PJ. In: Comprehensive Organic Synthesis: Additions to C–X p-Bonds. Part 2. Trost BM, Fleming I, editors. Vol. 2. Pergamon; New York: 1991. pp. 795–863. Chap. 3.6.De Risi C, Spalluto G, Zanirato V. Chem Rev. 1995;95:1065.Kürti L, Czakó B. Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Academic Press; Massachusetts: 2005. pp. 138–139.
- 12.For reviews of the carbonyl ene and Prins reactions, see: Adams DR, Bhatnagar SP. Synthesis. 1977:661.Snider BB. Acc Chem Res. 1980;13:426.Snider BB. In: Comprehensive Organic Syntheses: Additions to C– X p-Bonds. Part 2. Trost BM, Fleming I, editors. Vol. 2. Pergamon; Oxford: 1991. pp. 527–561. Chap. 2.1.Whitesell JK. Stereoselective Synthesis (Houben- Weyl) Vol. 5. Thieme; New York: 1996. pp. 3271–3297.Kürti L, Czakó B. Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Academic Press; Massachusetts: 2005. pp. 364–365.For selected mechanistic studies, see: Snider BB, Ron E. J Am Chem Soc. 1985;107:8160.Song Z, Beak P. J Am Chem Soc. 1990;112:8126.Marshall JA, Andersen MW. J Org Chem. 1992;57:5851.
- 13.Dömling A, Ugi I. Angew Chem Int Ed. 2000;39:3168. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u.For a recent example of the Ugi coupling reaction in a total synthesis of a complex natural product, see: Endo A, Yanagisawa A, Abe M, Tohma S, Kan T, Fukuyama T. J Am Chem Soc. 2002;124:6552. doi: 10.1021/ja026216d.
- 14.Mislow K, Siegel J. J Am Chem Soc. 1984;106:3319. [Google Scholar]
- 15.For discussions of the mechanisms of β-elimination reactions, see: McLennan DJ. Tetrahedron. 1975;31:2999.Saunders WH., Jr Acc Chem Res. 1976;9:19.Jones M., Jr . Organic Chemistry. 3. W. W. Norton & Co; New York: 2005. pp. 315–327.
- 16.Welch SC, Hagan CP, White DH, Fleming WP, Trotter JW. J Am Chem Soc. 1977;99:549. doi: 10.1021/ja00444a039. [DOI] [PubMed] [Google Scholar]
- 17.Menger FM, Chow JF, Kaiserman H, Vasquez PC. J Am Chem Soc. 1983;105:4996. [Google Scholar]
- 18.For discussions of intramolecular deprotonations and effective molarity, see: Menger FM. Acc Chem Res. 1985;18:128.For a recent, impressive example of a cyclopropanation initiated by an internal deprotonation event, see: Li WDZ, Yang YR. Org Lett. 2005;7:3107. doi: 10.1021/ol051141e.
- 19.An effort to initiate a Prins cyclization of compound 8 with phosphoric acids was also unsuccessful.