Abstract
The preparation and characterization of two π-cation radical derivatives of copper β-oxo porphyrins is described. [3,3,8,8,13,13,17,18-Octaethyl-(3H,8H,13H)-porphine-2,7,12-trionato (2-)] copper π-cation radical, [Cu(2,7,12-trioxoOEHP.)]+, and [3,3,8,8,12,13,17,18-octaethyl-(3H,8H)-porphine-2,7-dionato(2-)] copper π-cation radical, [Cu(2,7-dioxoOEiBC.)]+, have been prepared and characterized by single-crystal X-ray determinations, UV/vis/NIR, and IR spectroscopies. Both molecules have modest distortion from the planarity and show monomeric units in the solid state. [Cu(2,7-dioxoOEiBC.)]+ shows a concentration dependent near-IR band at 1410 nm. Crystal data for [Cu(2,7,12-trioxoOEHP.)][SbCl6]: tetragonal, space group P42/n, a = 31.085 (14) Å, c = 9.410 (4) Å, V = 9093 Å3, Z = 8, T = 127 K. Crystal data for [Cu(2,7-dioxoOEiBC.)][SbCl6]: monoclinic, space group P21/n, a = 9.655 (4) Å, b = 20.592 (8) Å, c = 43.347 (17) Åβ = 89.97(1)°, V = 8618. Å3, Z = 8, T = 100 K.
Introduction
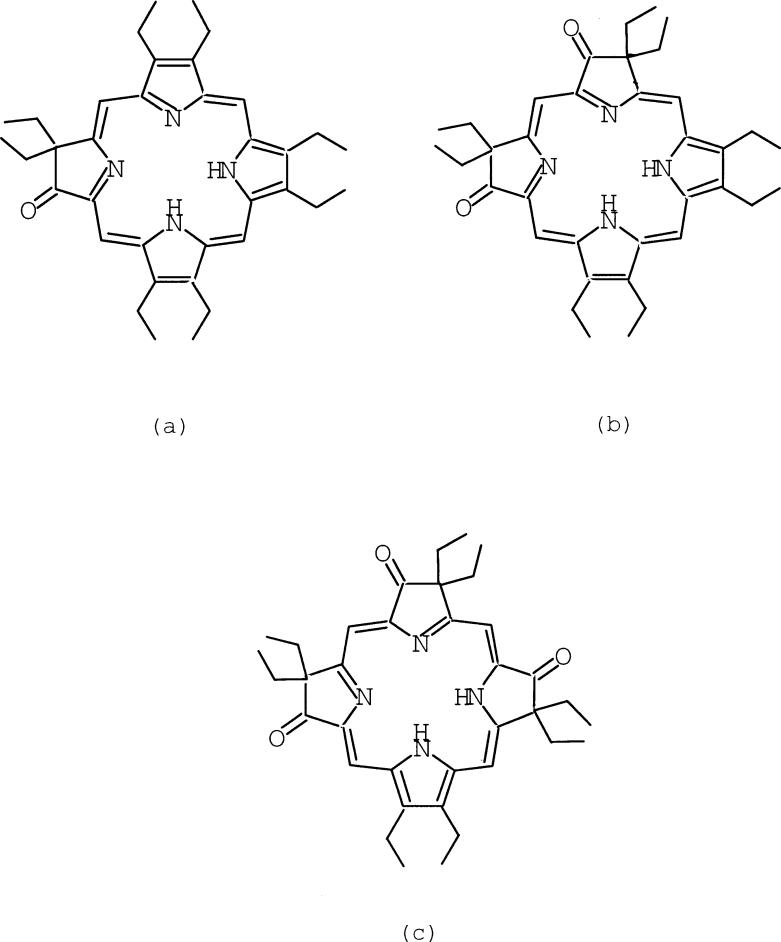
There is an increasing interest in the conformational change of the porphyrin core in metalloporphyrin systems1–3 stimulated, inter alia, by an important review by Shelnutt.3 Core deformations of porphyrin derivatives are increasingly evident in the crystal structures of reactions centers and heme proteins.4–6 The nonplanarity of the core has potential effects on the redox and excited state properties of porphyrin derivatives.7 This raises the question of whether there are intrinsic effects on the porphyrin core conformation upon ring oxidation. It has been observed in some severely distorted π-cation radical systems that the magnitude of core distortions increases upon oxidation. For example, the core of the neutral species [Cu(OETPP)]8 is extremely nonplanar and displays a strongly S4-saddled distortion. The magnitude of the core distortions in the π-cation radical, [Cu(OETPP.)]+, are larger than that in the neutral homologue.9, 10 However, in contrast to these observations, the magnitude of the core distortions of metallo-β-oxochlorin derivatives, [Cu(oxoOEC)] and [Fe(oxoOEC)Cl], decreases upon oxidation to the corresponding π-cation radical.11, 12 In these derivatives, an inter-ring interaction has potential effects on the core conformation. We now report the structural distortion of the more substituted metallo-β-oxoporphyrin π-cation radical derivatives, [Cu(2,7,12-trioxoOEHP.)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6], where no inter-ring interactions are found in the solid state structures. Scheme 1 shows chemical structures of (a) H2(oxoOEC), (b) H2(2,7-dioxoOEiBC), and (c) H2(2,7,12-trioxoOEHP).
Scheme 1.
Structures of oxoOEC (a), 2,7-dioxoOEiBC (b), and 2,7,12-trioxoOEHP (c).
Experimental Section
General Information
H2OEP was purchased from Midcentury Chemicals. Dichloromethane and hexane were distilled under argon from CaH2 or sodium/benzophenone, respectively. UV/vis/NIR electronic absorption spectra were obtained on a Perkin-Elmer UV/vis/NIR Lambda 19 spectrophotometer. IR spectra were recorded on a Perkin-Elmer 883 spectrometer.
Synthesis of 2,7-dioxoOEiBC and 2,7,12-trioxoOEHP
The procedure was based on the literature13, 14 with modification. H2OEP (1.00 g, 1.87 mmol) was dissolved in 100 mL of concentrated sulfuric acid, cooled in an ice bath, and stirred with a magnetic bar. To this solution was added dropwise 5.0 mL of 30% H2O2 such that the temperature was kept below 10 °C. After the addition was completed, the green solution was stirred for an additional 20 minutes at room temperature. The reaction was quenched by pouring the solution into a large beaker containing solid sodium acetate (~100 g) and crushed ice (~300 g). The next day the reaction mixture was filtered and the precipitate was washed with water and dried. The crude product was separated on a silica gel column (60–200 mesh) using CH2Cl2/hexane (70:30). The fifth fraction, containing 2,7-dioxoOEiBC and 2,7,12-trioxoOEHP, was collected. Yield: 11% (120 mg). Insertion of copper into H2(2,7-dioxoOEiBC) and H2(2,7,12-trioxoOEHP) was accomplished by the reaction of the free base mixture and copper(II) acetate in DMF.15, 16 The reaction product was chromatographed on a silica gel column (60–200 mesh) using CH2Cl2/hexane (50:50) since it contains the two different copper β-oxoporphyrin complexes. Green [Cu(2,7-dioxoOEiBC)] was eluted first and then dark green [Cu(2,7,12-trioxoOEHP)]. [Cu(2,7-dioxoOEiBC)]: UV-vis(CH2Cl2 solution): λmax 392(Soret), 433, 586, 620 nm. IR(KBr): νC=O 1720 cm−1. [Cu(2,7,12-trioxoOEHP)]: UV-vis(CH2Cl2 solution): λmax 431(Soret), 632, 693, 755 nm. IR(KBr): νC=O 1710 cm−1.
Oxidation of [Cu(2,7-dioxoOEiBC)] and [Cu(2,7,12-trioxoOEHP)]
[Cu(2,7-dioxo-OEiBC.)][SbCl6] was prepared by chemical oxidation of [Cu(2,7-dioxoOEiBC)] with tris(4-bromophenyl)aminium hexachloroantimonate. [Cu(2,7-dioxoOEiBC)](25 mg, 0.040 mmol) and tris(4-bromophenyl)aminium hexachloroantimonate(34 mg, 0.042 mmol) were placed in a 100-mL Schlenk flask and dried for 30 minutes. After drying, dichloromethane was added to the Schlenk flask and the solution immediately turned brown. After stirring for 30 minutes, hexane was added to the solution. The mixture was filtered, and the brown solid was dried in vacuo; the yield was quantitative. Suitable crystals of [Cu(2,7-dioxoOEiBC.)][SbCl6] were obtained by the slow diffusion of hexane into a chloroform solution of [Cu(2,7-dioxoOEiBC.)][SbCl6]. UV-vis-NIR(CH2Cl2 solution): λmax 380(Soret), 415, 514, 670, 1410 nm. IR(KBr): νC=O 1734 cm−1. The synthesis of [Cu(2,7,12-trioxoOEHP.)][SbCl6] followed the previous procedure. Suitable crystals of [Cu(2,7,12-trioxoOEHP.)][SbCl6] were obtained by the slow diffusion of hexane into a dichloromethane solution of [Cu(2,7,12-trioxoOEHP.)][SbCl6]. UV-vis(CH2Cl2 solution): λmax 383, 424, 481 nm. IR(KBr): νC=O 1727 cm−1.
X-ray Structure Determinations
A brief summary of crystal data, intensity collection parameters and refinement parameters is listed in Table 1 and more completely in the Supporting Information (Table S1). All data collected were used including negative intensities. Individual details are given below.
Table 1.
Brief Crystallographic Data and Data Collection Parameters for [Cu(2,7,12-trioxoOEHP)][SbCl6] and [Cu(2,7-dioxoOEiBC)][SbCl6].
| [Cu(2,7,12-trioxoOEHP)]-[SbCl6]·1.25(CH2Cl2) | [Cu(2,7-dioxoOEiBC)]-[SbCl6]·0.5CHCl3 | |
|---|---|---|
| formula | CuN4C37.25O3H46.5SbCl8.5 | CuN4C36.5O2H44.5SbCl7.5 |
| FW, amu | 1077.34 | 1022.43 |
| a, Å | 31.085 (14) | 9.655(4) |
| b, Å | - | 20.592(8 |
| c, Å | 9.410 (4) | 43.347(17) |
| β, deg | - | 89.97(1) |
| V, Å3 | 9093 (7) | 8618(8) |
| space group | P42/n | P21/n |
| Z | 8 | 8 |
| Dc, g/cm3 | 1.574 | 1.576 |
| F(000) | 4335 | 4120 |
| μ, mm–1 | 1.601 | 1.622 |
| absorption correction | SADABS | DIFABS |
| 0.71073 Å | 0.71073 Å | |
| temperature, K | 127(2) | 100(2) |
| total data collected | 28222 | 63639 |
| unique data | 8981 (Rint = 0.0544) | 15176 (Rint = 0.035) |
| unique observed data [I > 2σ(I)] | 7338 | 11785 |
| refinement method | Full-matrix least-squares on F2 | |
| final R indices [I > 2σ(I)] | R1 = 0.0397, wR2 = 0.1000 | R1 = 0.0525, wR2 = 0.0948 |
| final R indices (all data) | R1 = 0.0553, wR2 = 0.1171 | R1 = 0.0753, wR2 = 0.1145 |
[Cu(2,7,12-trioxoOEHP.)][SbCl6]
Single crystals of [Cu(2,7,12-trioxoOEHP.)][SbCl6] were examined on a Nonius FAST area-detector diffractometer at 127 K with a graphite-monochromated Mo rotating anode source (λ = 0.71073 Å).17 Detailed methods and procedures for small molecule X-ray data collection with the FAST system have been described elsewhere.18 Intensity data were corrected for Lorentz and polarization effects. The correction of the absorption effects was made by using the DIFABS procedure.19 The structures of [Cu(2,7,12-trioxoOEHP.)][SbCl6] was solved by the direct methods program SHELXS20 and the remaining nonhydrogen atoms were located by difference Fourier syntheses. Structures were refined on F2 using the program SHELXL. The structure of the target is well-defined. However, two well-separated but disordered solvent molecules are also present in the asymmetric unit of structure. One disordered dichloromethane molecule had two orientations in which one chlorine atom occupied two sites and the carbon and the other chlorine atom occupied positions that were common to both sites. The second dichloromethane was apparently grotesquely disordered; the several chlorine atom positions of this solvent molecule were assigned occupancy factors from the difference Fourier peak heights and then adjusted so that the combined occupancy factors/thermal parameters had reasonable values. All hydrogen atoms, except those attached to the disordered carbon atoms, were idealized with the standard idealization methods and were included in subsequent least-square refinement cycles. The final least-squares refinement with anisotropic displacement parameters for all the non-hydrogen atoms led to discrepancy indices of R1 = 0.040, wR2 = 0.100 (for 7338 observed data based on I ≥ 2.0σ(I)), and R1 = 0.055, wR2 = 0.117 (for the 8981 unique data).21 The largest electron density peak from the final difference Fourier syntheses was 1.16 e/Å3 at a distance of 0.86 Å A from the antimony atom.
[Cu(2,7-dioxoOEiBC.)][SbCl6]
A crystalline sample of [Cu(2,7-dioxoOEiBC.)][SbCl6] was placed in inert oil, mounted on a glass pin, and transferred to the cold gas stream of the diffractometer. Crystal data were collected and integrated using a Bruker Apex system, with graphite monochromated Mo-Kα(λ = 0.71073 Å) radiation at 100K. The structure was solved by heavy atom methods using SHELXS-97 and refined using SHELXL-97. Non-hydrogen atoms were found by successive full matrix least squares refinement on F2 and refined with anisotropic displacement parameters. All hydrogen atoms were idealized with the standard idealization methods and were included in subsequent least-square refinement cycles. Although the unit cell refined as nearly orthorhombic, intensities of equivalent reflections were not consistent with orthorhombic space groups. Therefore, the space group was determined to be P21/n with pseudomerohedral twinning (matrix applied: 100 0–10 00–1), resulting in two ion pairs and a chloroform solvent molecule per asymmetric unit. The two components of the twin were determined to be present in a 51% to 49% ratio. The chloroform molecule was found to be two-fold disordered with occupancies of 56% and 44% for the two components.
The refinement converged to a final value of R1 = 0.052 and wR2 = 0.095 for 11785 observed unique reflections based on I ≥ 2.0σ(I)), and R1 = 0.075 and wR2 = 0.114 for the 15176 unique data including also negative intensities. The weighted R-factors, wR, are based on F2 and conventional R-factors, R, on F, with F set to zero for negative intensities. The I ≥ 2.0σ(I) criterion was used only for calculating R1. R-indices based on F2 are statistically about twice as large as those based on F. The maximum and minimum electron density on the final difference Fourier map was 0.96 e/Å3 and -1.24 e/Å3, respectively, close to the Sb atom.
Results
The oxidation of [Cu(2,7-dioxoOEiBC)] and [Cu(2,7,12-trioxoOEHP)] with tris(4-bromophenyl)-aminium hexachloroantimonate results in the formation of the π-cation radicals, [Cu(2,7-dioxo-OEiBC.)][SbCl6] and [Cu(2,7,12-trioxoOEHP.)][SbCl6], respectively. The electronic spectra of the oxidized complexes have a blue-shifted and broadened Soret band and the bands in the visible region have decreased in intensity. [Cu(2,7-dioxoOEiBC.)][SbCl6] shows one new broad, concentration-dependent, near-IR band at 1410 nm, but [Cu(2,7,12-trioxoOEHP.)][SbCl6] does not show any new near-IR bands at the highest concentration we were able to use.
The IR carbonyl band for the neutral compounds shifts upfield by about 15 cm−1 upon formation of π-cation radicals; the values are given in the Experimental. [Cu(2,7-dioxoOEiBC.)][SbCl6] and [Cu(2,7,12-trioxoOEHP.)][SbCl6] show a π-cation radical IR marker band at 1550 cm−1 and 1570 cm−1, respectively.
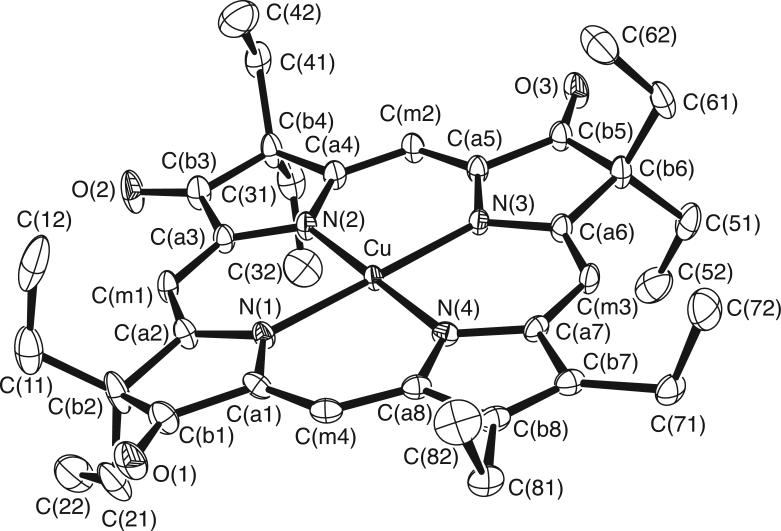
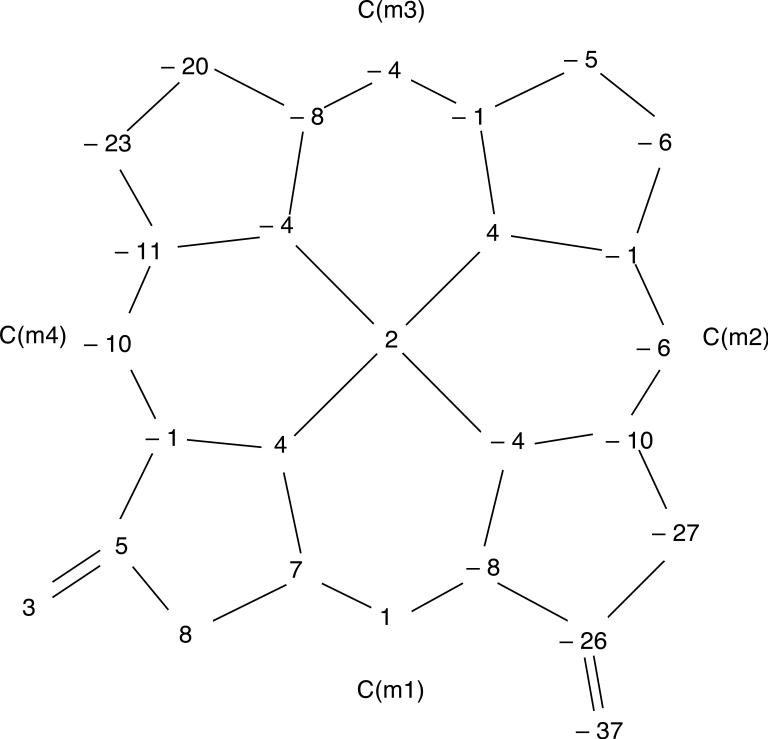
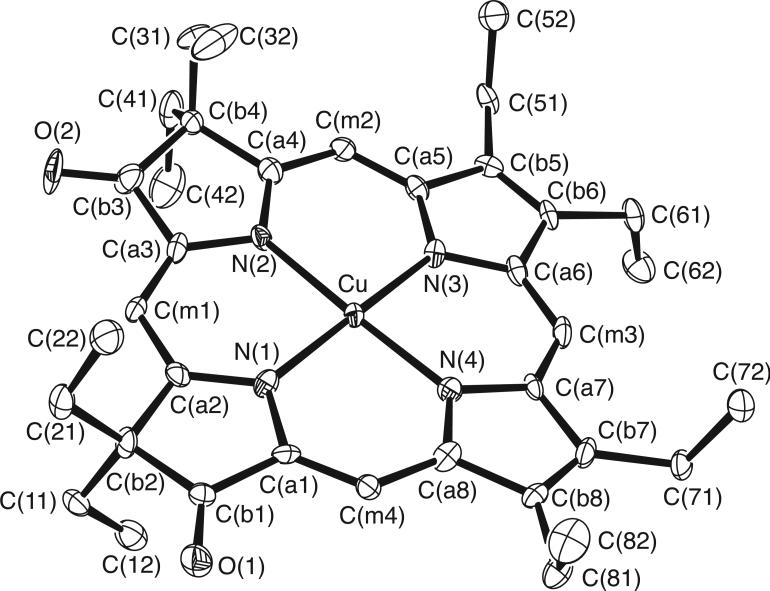
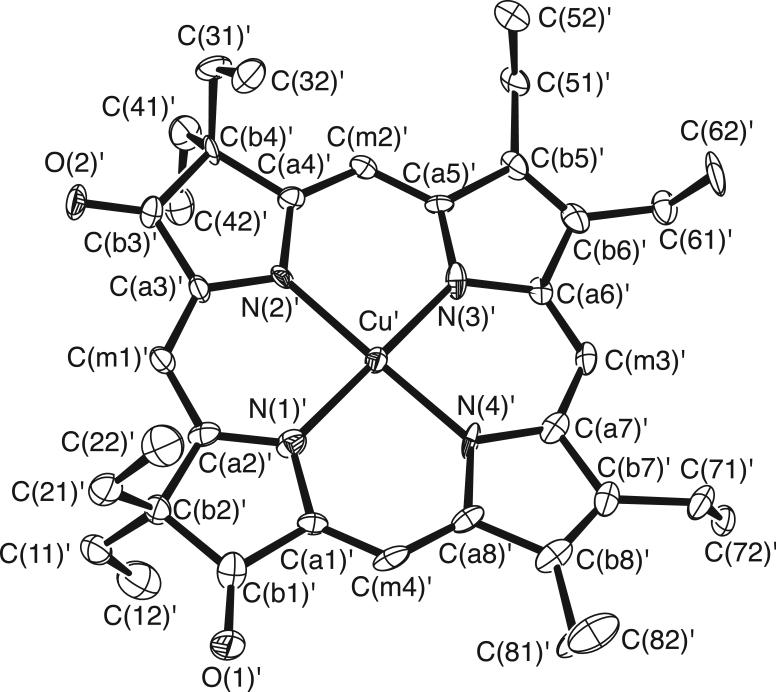
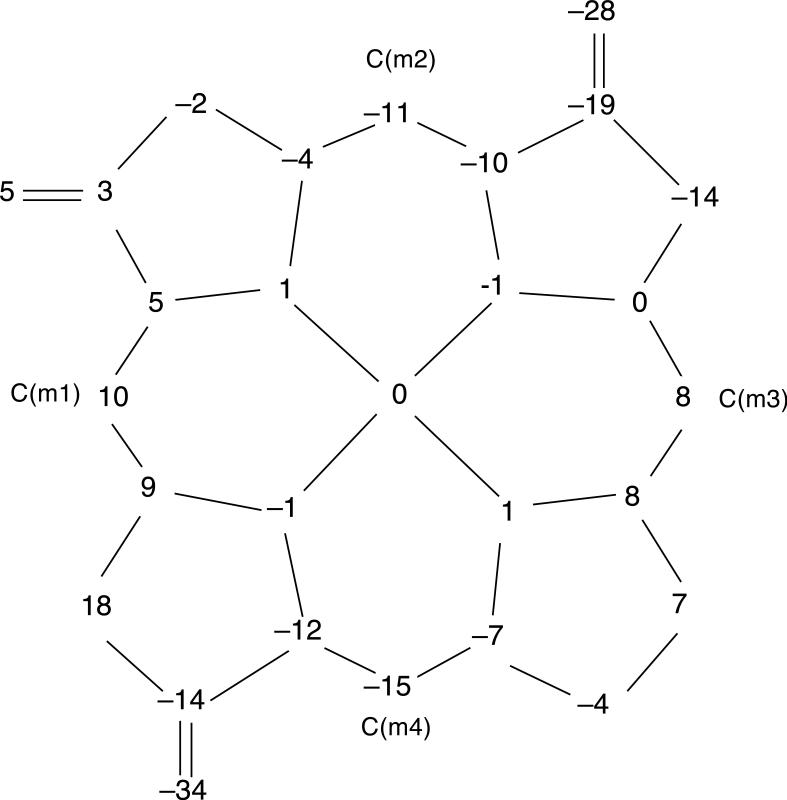
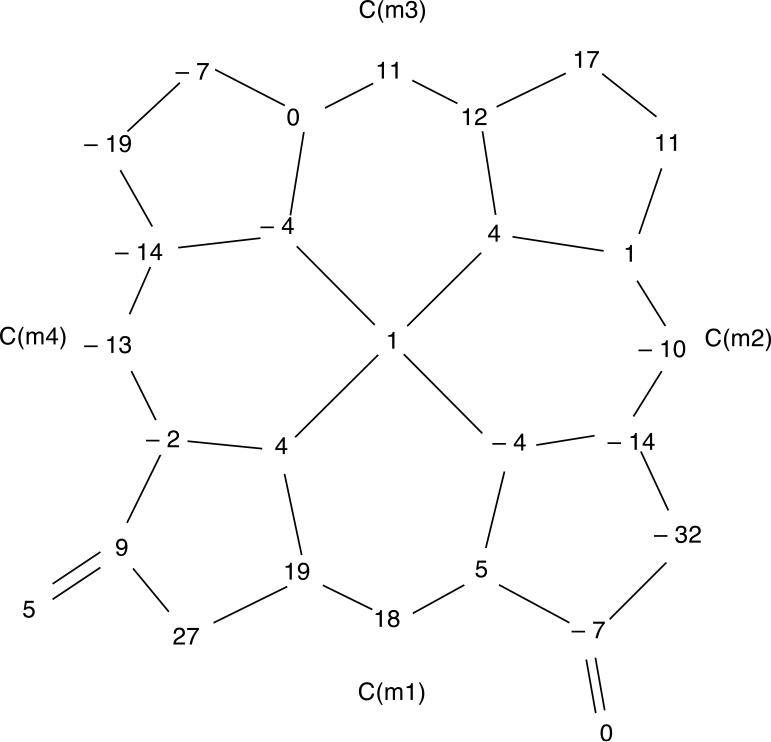
The molecular structures of [Cu(2,7,12-trioxoOEHP).)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6] have been determined by X-ray crystallography. Figures 1 to 6 show labelled ORTEP diagrams and formal diagrams giving the perpendicular displacements of each atom from the four nitrogen mean plane for [Cu(2,7,12-trioxoOEHP).)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6], respectively. The largest absolute displacements of the core atoms from the four nitrogen mean plane are C(b5) of [Cu(2,7,12-trioxoOEHP.)][SbCl6] at 0.19 Å and C(b4) of [Cu(2,7-dioxoOEiBC.)][SbCl6] at 0.32 Å. Table 2 contains selected bond distances and angles for [Cu(2,7,12-trioxoOEHP.)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6].
Figure 1.
ORTEP diagram of the [Cu(2,7,12-trioxoOEHP.)]+ cation. Anisotropic displacement ellipsoids are drawn at the 50% probability level. Porphyrin hydrogen atoms are omitted for clarity.
Figure 6.
Formal diagram of the porphinato core of molecule B of [Cu(2,7-dioxoOEiBC.)][SbCl6], displaying the perpendicular displacements, in units of 0.01 Å, of each atom from the four nitrogen mean plane.
Table 2.
Selected Bond Lengths and Angles for [Cu(2,7,12-trioxoOEHP)][SbCl6] and [Cu(2,7-dioxoOEiBC)][SbCl6].
| bond lengths (Å) and angles (deg) | ||
|---|---|---|
| [Cu(2,7,12-trioxoOEHP)] [SbCl6] | [Cu(2,7-dioxoOEiBC)][SbCl6] | |
| Cu-N(pyrrolinone) | ||
| Cu-N(1) | 2.041(3) | 2.035(3) |
| Cu-N(2) | 2.035(3) | 2.027(8) |
| Cu-N(3) | 2.029(3) | |
| Cu-N(pyrrole) | ||
| Cu-N(3) | 1.998(1) | |
| Cu-N(4) | 1.997(3) | 2.008(5) |
| Cα-Cβ (carbonyl) | ||
| C(a1)-C(b1) | 1.486(5) | 1.490(6) |
| C(a3)-C(b3) | 1.500(5) | 1.494(10) |
| C(a5)-C(b5) | 1.493(5) | |
| Cα-Cβ (gem-diethyl) | ||
| C(a2)-C(b2) | 1.525(5) | 1.499(10) |
| C(a4)-C(b4) | 1.521(5) | 1.517(4) |
| C(a6)-C(b6) | 1.525(5) | |
| Cβ-Cβ (pyrrolinone) | ||
| C(b1)-C(b2) | 1.503(5) | 1.510(1) |
| C(b3)-C(b4) | 1.515(5) | 1.512(1) |
| C(b5)-C(b6) | 1.509(5) | |
| Cα-Cβ((pyrrole) | ||
| C(b5)-C(b6) | 1.337(4) | |
| C(b7)-C(b8) | 1.356(5) | 1.334(11) |
| Cα-N-Cα (pyrrolinone) | ||
| C(a1)-N(1)-C(a2) | 108.5(3) | 108.1(1) |
| C(a3)-N(2)-C(a4) | 108.6(3) | 108.8(1) |
| C(a5)-N(3)-C(a6) | 108.8(3) | |
| Cα-N-Cα (pyrrole) | ||
| C(a5)-N(3)-C(a6) | 105.9(4) | |
| C(a7)-N(4)-C(a8) | 105.4(3) | 105.6(4) |
Discussion
The crystal structures of [Cu(2,7,12-trioxoOEHP.)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6] have been determined. Two types of Cu–N distances are observed in each porphyrin ring: “short” Cu–N distances (1.997(3) Å) to the pyrroles and “long” Cu–N distances (2.029(3)–2.041(3) Å) to the pyrrolinone rings of [Cu(2,7,12-trioxoOEHP.)][SbCl6], and “short” Cu–N distances (1.998(1)–2.008(5) Å) to the pyrroles and “long” Cu–N distances (2.027(8)–2.035(3) Å) to the pyrrolinone rings of [Cu(2,7-dioxoOEiBC.)][SbCl6]. The short distances are typical of Cu–N bond lengths found in planar copper porphyrinates,22–28 while the long distances are typical of Cu–N distances found in the reduced rings of [Cu(oxoOEC)], [Cu(2,7-dioxoOEiBC)], [Cu(oxoOEC.)][SbCl6],11, 29 and in copper hydroporphyrinates.30–32 This bond distance difference is also found in other metal complexes including [Ni(oxoOEC)], [Ni(dioxoOEiBC)], [Ni(trioxoOEHP)],33 [Fe(oxoOEC)(Cl)], [Fe(oxoOEC.)Cl][SbCl6],12 and [Fe(dioxoOEiBC))Cl)].34
In addition to the Cu–N distances, a number of other geometric parameters also vary for reduced rings. The pyrrolinone rings have lengthened Cβ–Cβ bonds and widened Cα–N–Cα angles expected for reduced rings; Cβ–Cβ(pyrrolinone) = 1.509(5) versus Cβ–Cβ(pyrrole) = 1.356(5) Å and Cα–N–Cα(pyrrolinone) = 108.6(8) versus Cα–N–Cα(pyrrole) = 105.4(3)° for [Cu(2,7,12-trioxoOEHP.)][SbCl6], Cβ–Cβ(pyrrolinone) = 1.511(1) versus Cβ–Cβ(pyrrole) = 1.336(2) Å and Cα–N–Cα(pyrrolinone) = 108.5(5) versus Cα–N–Cα(pyrrole) = 105.8(2)° for [Cu(2,7-dioxoOEC.)][SbCl6]. The average Cα–Cβ bond containing the carbonyl group is shorter than the average Cα–Cβ containing the gem-diethyl group; the difference suggests that the keto group could be conjugated with the π system of the macrocycle; Cα–Cβ(carbonyl) = 1.475 versus Cα–Cβ(gem-diethyl) = 1.510 Å for [Cu(2,7,12-trioxoOEHP.)][SbCl6]; Cα–Cβ(carbonyl) = 1.492 versus Cα–Cβ(gem-diethyl) = 1.508 Å for [Cu(2,7-dioxoOEC.)][SbCl6]. These phenomena are also found in all other crystallographically characterized metallo-β-oxochlorin, metallo-β-dioxoisobacteriochlorin, and metallo-β-trioxohexahydroporphyrin complexes.11,12,33,34
Various core conformations have been found in copper-β-oxo species. For example, [Cu(2,7-dioxoOEiBC)] is planar, [Cu(oxoOEC)](general position) and [Cu(oxoOEC.)][SbCl6] is saddled, and [Cu(oxoOEC)](special position) has a ruffed conformation. [Cu(2,7,12-trioxoOEHP.)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6] show ruffed conformations and have no ring-ring interactions. As shown in Table 3, the ruffed conformation is observed in the copper-β-oxo species with longer Cu· · ·Cu separations.
Table 3.
Comparison of Geometry of Copper-β-oxo Species
It has been noted in some severely distorted π-cation radical systems that the magnitude of the core distortion increases upon oxidation. For example, neutral [Cu(OETPP)] adopts a saddled conformation in which the β carbons are displaced by ±1.1–1.2 Å from the 24-atom mean plane. After oxidation, the saddling of the [Cu(OETPP.)]+ π-cation radical increases with the β-carbon displacements now at ±1.36 Å. In contrast to these observations, the saddle distortions decreases after oxidation of [Cu(oxoOEC)] to [Cu(oxoOEC.)][SbCl6],11 and [Fe(oxoOEC)(Cl)] to [Fe(oxoOEC.)(Cl)][SbCl6].12 We have further examined the core conformation change of the more substituted metallo-β-oxoporphyrin π-cation radical complexes [Cu(2,7-dioxoOEiBC.)][SbCl6] and [Cu(2,7,12-trioxoOEHP.)][SbCl6]. The neutral [Cu(2,7-dioxoOEiBC)] complex has a planar conformation with the β-carbon displacements ranging from ± 0.01–0.12 Å and an absolute value average of 0.09 Å from the plane of the four nitrogens. [Cu(2,7-dioxoOEiBC)] molecules form dimers in which pyrrole rings overlap with a mean plane separation of 3.77 Å and a closest Cu· · ·Cu distance of 6.12 Å.29 After oxidation to the π-cation radical, Molecule A of [Cu(2,7-dioxoOEiBC.)][SbCl6] has a ruffed as well as saddled conformation with the β-carbon displacements of 0.07–0.32 Å and an absolute value average of 0.16 Å from the plane of the four nitrogens. Molecule B of [Cu(2,7-dioxoOEiBC.)][SbCl6] has a more distorted structure than molecule A with the β-carbon displacements of 0.05–0.27 Å and an absolute value average of 0.15 Å from the plane of the four nitrogens.Thus the magnitude of the core distortion increases upon oxidation to [Cu(2,7-dioxoOEiBC.)][SbCl6]; this core deformation is consistent with no ring-ring interaction. [Cu(2,7-dioxoOEiBC.)][SbCl6] forms a monomeric species with the closest Cu· · ·Cu separations longer than 9.0 Å. The closest Cu· · ·Cu′ separations i about 11.5 Å. [Cu(2,7,12-trioxoOEHP.)][SbCl6] shows a ruffled structure with β-carbon displacements of 0.02–0–.19 Å and an absolute value average of 0.10 Å from the plane of the four nitrogens and has no ring-ring interaction. We tried to obtain crystals of neutral [Cu(2,7,12-trioxoOEHP)] to compare structural properties, but we failed to harvest crystals. The structurally characterized [Ni(2,7,18-trioxoOEHP)] derivative shows no ring-ring interaction. In the π-cation radical species of [Cu(2,7-dioxoOEiBC)] and [Cu(OETPP)], the magnitude of the core distortions in the radical derivatives is larger than that in the neutral homologue. However, in the π-cation radical derivatives of [Cu(oxoOEC)] and [Fe(oxoOEC)(Cl)], which have ring-ring interactions from dimer formation, the magnitude of the core distortion in the radical derivatives is smaller than that in the neutral homolog. These observations suggest that the degree of the core distortion in radicals is strongly related to the presence of ring-ring interactions.
Although the complete overlap of two rings in metallo-β-oxo species is prevented by the gem-diethyl groups, some degree of ring-ring overlap is possible. [Cu(oxoOEC)](general position), [Cu(oxoOEC)](special position), [Cu(2,7-dioxoOEiBC)], [Cu(oxoOEC.)][SbCl6], [Fe(oxoOEC)(Cl)], [Fe(oxoOEC.)][SbCl6], and [Fe(2,7-dioxoOEiBC)Cl] complexes have a ring-ring interaction even though the degree of the ring-ring interaction is different.
Optical and IR spectral data indicate that the oxidation of [Cu(2,7-dioxoOEiBC)] and [Cu(2,7,12-trioxoOEHP)] results in the formation of π-cation radical derivatives. In the electronic spectrum, the Soret band blue shifts and broadens upon oxidation and the bands in the visible region decrease in intensity. In the case of [Cu(2,7-dioxoOEiBC.)][SbCl6], a very broad, near-IR band is observed at 1410 nm; the neutral [Cu(2,7-dioxoOEiBC)] complex does not absorb in this region. This band was studied over the concentration range 0.96 × 10–3 to 0.29 × 10–3 M. A plot of the absorbance at 1410 nm against concentration for the [Cu(2,7-dioxoOEiBC.)][SbCl6] system reveals deviations from linearity at high concentration; the absorbance was larger at higher concentrations. This observation of concentration-dependent near-IR band suggests the presence of a dimeric species in solution while a monomeric species is present in the solid state. Such near-IR bands have been observed in metallooctaethylporphyrin π-cation radical derivatives, where they all result from the formation of dimeric π-cation radical species, [M(OEP.)]22+. These near-IR “dimer bands” are modestly metal and porphyrin dependent and can be counterion dependent.35–37 Absorption maxima are found in the region of 900–960 nm for nickel, copper, palladium, and zinc octaethylporphyrin π-cation radicals; the vanadyl complex has a red-shifted near-IR band at 1375 nm.38 Similarly, the π-cation radical complex of the copper oxooctaethylchlorin, [Cu(oxoOEC.)]22+, has two overlapped near-IR absorption bands at 1285 and 1548 nm that are significantly broader than those seen for the OEP species. All of these near-IR absorption bands are concentration dependent. Upon oxidation of [Cu(2,7,12-trioxoOEHP)], no near-IR band is observed in the region 900–3000 nm at the highest concentration we were able to use. These observations suggest that the [Cu(2,7,12-trioxoOEHP.)] radical is monomeric both in solution and the solid state because the increased number of peripheral substituents prevents aggregation of the molecule.
[Cu(2,7-dioxoOEiBC.)][SbCl6] and [Cu(2,7,12-trioxoOEHP.)][SbCl6] show a π-cation radical IR marker band at 1550 and 1570 cm–1, respectively. This band is similar to the π-cation radical IR marker bands found in octaethylporphyrinate and β-alkyl-substituted porphyrinate π-cation radical derivatives (~1550 cm–1); meso-tetraaryl substituted porphyrin π-cation radicals exhibit diagnostic π-cation radical IR marker bands at ~1280 cm–1.39, 40 Upon oxidation of [Cu(2,7-dioxoOEiBC)], the carbonyl band in the infrared shifts from 1720 to 1734 cm–1, that of [Cu(2,7,12-trioxoOEHP.)] shifts from 1710 to 1727 cm–1. These large oxidation-induced shifts of the carbonyl band indicate that the oxidation of [Cu(2,7-dioxoOEiBC)] and [Cu(2,7,12-trioxoOEHP)] accompanies a significant change of the electronic structure of the macrocycle. More specifically, the increase in energy of the carbonyl band upon oxidation is consistent with the removal of electron density from the porphyrin ring, forming the π-cation radical.
Summary
We have reported the characterization of two copper-β-oxo π-cation radicals, [Cu(2,7,12-trioxoOEHP.)][SbCl6] and [Cu(2,7-dioxoOEiBC.)][SbCl6]. The [Cu(2,7,12-trioxoOEHP.)]+ radical is monomeric both in solution and solid state. The neutral [Cu(2,7-dioxoOEiBC)] complex has a planar conformation and forms dimers in which pyrrole rings overlap with a mean plane separation of 3.77 Å. After oxidation to the π-cation radical, the magnitude of the core distortion increases and [Cu(2,7-dioxoOEiBC.)][SbCl6] forms monomeric species in the solid state. However, in the π-cation radical derivatives of [Cu(oxoOEC)] and [Fe(oxoOEC)(Cl)], which have ring-ring interactions from dimer formation, the magnitude of the core distortion in the radical derivatives is smaller than that in the neutral homologue. It appears that the degree of the core distortion in radicals is strongly related to the presence of ring-ring interaction.
Supplementary Material
Synopsis.
The preparation and characterization of two π-cation radical derivatives of copper β-oxo porphyrins, [Cu(2,7,12-trioxoOEHP.)]+ and [Cu(2,7-dioxoOEiBC.)]+, is reported. Both molecules have modest distortion from the planarity and show monomeric units in the solid state. [Cu(2,7-dioxoOEiBC.)]+ shows a concentration dependent near-IR band at 1410 nm.
Figure 2.
ORTEP diagram of ion A of the [Cu(2,7-dioxoOEiBC.)]+ cation. Anisotropic displacement ellipsoids are drawn at the 50% probability level. Porphyrin hydrogen atoms are omitted for clarity..
Figure 3.
ORTEP diagram of ion B of the [Cu(2,7-dioxoOEiBC.)]+ cation. Anisotropic displacement ellipsoids are drawn at the 50% probability level. Porphyrin hydrogen atoms are omitted for clarity.
Figure 4.
Formal diagram of the porphinato core of [Cu(2,7,12-trioxoOEHP.)][SbCl6], displaying the perpendicular displacements, in units of 0.01 Å, of each atom from the four nitrogen mean plane.
Figure 5.
Formal diagram of the porphinato core of molecule A of [Cu(2,7-dioxoOEiBC.)][SbCl6], displaying the perpendicular displacements, in units of 0.01 Å, of each atom from the four nitrogen mean plane.
Acknowledgments
We thank the National Institutes of Health for support of this research under Grant GM-38401 to WRS. Funds for the purchase of the FAST area detector diffractometer was provided through NIH Grant RR-06709 to the University of Notre Dame.
Footnotes
Supporting Information
The CIF files for the crystal structures were deposited at the Cambridge Crystallographic Data Centre (CCDC). The CCDC deposition numbers of [Cu(2,7-dioxoOEiBC.)][SbCl6], and [Cu(2,7,12-trioxoOEHP.)][SbCl6] are 809817, and 809816, respectively. Copies can be obtained on request, free of charge, via www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223-336-033 or deposit@ccdc.cam.ac.uk).
Tables S1 to S10 giving complete crystallographic details, fractional coordinates for the nonhydrogen atoms, complete bond lengths and angles, anisotropic displacement parameters and fixed hydrogen atom coordinates of both complexes. Figures S1 to S5, unit cell packing diagram and 24-atom mean plane of both complexes.
References and Notes
- 1.Scheidt WR. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 3. Academic; New York: 2000. [Google Scholar]
- 2.Scheidt WR, Lee YJ. Struct. Bonding (Berlin) 1987;64:1. [Google Scholar]
- 3.Shelnutt JA, Song XZ, Ma JG, Jia SL, Jentzen W, Medforth CJ. J. Chem. Soc. Rev. 1998;27:31. [Google Scholar]
- 4.Crane BR, Siegel LM, Getzoff ED. Science. 1995;270:59. doi: 10.1126/science.270.5233.59. [DOI] [PubMed] [Google Scholar]
- 5.Ravichandran KG, Boddupalli SS, Hasemann CA, Peterson JA, Diesenhofer J. Science. 1993;261:731. doi: 10.1126/science.8342039. [DOI] [PubMed] [Google Scholar]
- 6.Barkigia KM, Chantranupong L, Smith KM, Fajer J. J. Am. Chem. Soc. 1988;110:7566. [Google Scholar]
- 7.Parusel ABJ, Wondimagegn T, Ghosh A. J. Am. Chem. Soc. 2000;122:6371. [Google Scholar]
- 8.Abbreviations used in this paper: H OEP, octaethylporphyrin; (OEP..), the π-cation radical of OEP; H2oxoOEC, oxooctaethylchlorin; (oxoOEC..), the π-cation radical of oxoOEC; H2OETPP, octaethyltetraphenylporphyrin; (2,7-dioxoOEiBC), 3,3,8,8,12,13,17,18-octaethyl-(3H,8H)-porphine-2,7-dionato(2−); (2,7,12-trioxoOEHP), 3,3,8,8,13,13,17,18-octaethyl-(3H,8H,13H)-porphine-2,7,12-trionato(2−); (2,7-dioxoOEiBC.), the π-cation radical of 2,7-dioxoOEiBC; (2,7,12-trioxoOEHP.), the π-cation radical of 2,7,12-trioxoOEHP; DMF, N,N-dimethylformamide.
- 9.Renner MW, Barkigia KM, Zhang Y, Medforth CJ, Smith KM, Fajer J. J. Am. Chem. Soc. 1994;116:8562. [Google Scholar]
- 10.Renner MW, Barkigia KM, Fajer J. Inorg. Chim. Acta. 1997;263:181. [Google Scholar]
- 11.Neal TJ, Kang SJ, Schulz CE, Scheidt WR. Inorg. Chem. 1999;38:4294. doi: 10.1021/ic991052w. [DOI] [PubMed] [Google Scholar]
- 12.Neal TJ, Kang SJ, Turowska-Tyrk I, Schulz CE, Scheidt WR. Inorg. Chem. 2000;39:872. doi: 10.1021/ic991052w. [DOI] [PubMed] [Google Scholar]
- 13.Inhoffen HH, Nolte W. Justus Liebigs Ann. Chem. 1969;725:167. doi: 10.1002/jlac.19697250121. [DOI] [PubMed] [Google Scholar]
- 14.Chang CK. Biochemistry. 1980;19:1971. doi: 10.1021/bi00550a037. [DOI] [PubMed] [Google Scholar]
- 15.Stolzenberg AM, Glazer PA, Foxman BM. Inorg. Chem. 1986;25:983. [Google Scholar]
- 16.Adler AD, Longo FR, Kampas F, Kim J. J. Inorg. Nucl. Chem. 1970;32:2443. [Google Scholar]
- 17.Programs used in this study include: FAST data collection used MADNES routines (J. W. Pflugrath, Cold Spring Harbor, and A. Messerschmidt, Max-Plank-Institute für Biochemie, Martinsried, unpublished) and data reduction the program ABSURD (I. Tickle, Birbeck College and P. Evans, MRC, unpublished)
- 18.Scheidt WR, Turowska-Tyrk I. Inorg. Chem. 1994;33:1314. [Google Scholar]
- 19.The process is based on an adaptation of the difabs logic Walker NP, Stuart D. Acta Crystallogr., Sect. A. 1983;A39:158. to area detector geometry by Karaulov: Karaulov AI School of Chemistry and Applied Chemistry, University of Wales, College of Cardiff, Cardiff CF1 3TB, UK, personal communication.
- 20.Analysis programs used in this study included shelxs and shelxl Sheldrick GM. Acta Cryst. A64. 2008. p. 112. and local modifications of Johnson's ortep2. Wilson, editor. Scattering factors were taken from International Tables for Crystallography. C. AJC Kluwer Academic Publishers; Dordrecht: 1992.
- 21.R1 = Σ||Fo| – |Fc||/Σ|Fo| and wR2 = {Σ[w(Fo2 – Fc2)2]/Σ[wFo4]}1/2.
- 22.Pak R, Scheidt WR. Acta Crystallogr., Sect. C. 1991;C47:431. doi: 10.1107/s0108270190008010. [DOI] [PubMed] [Google Scholar]
- 23.Moustakali I, Tulinsky A. J. Am. Chem. Soc. 1973;95:6811. doi: 10.1021/ja00801a047. [DOI] [PubMed] [Google Scholar]
- 24.Scheidt WR, Lee YJ. Struct. Bonding (Berlin) 1987;64:1. [Google Scholar]
- 25.Senge MO, Medforth CJ, Sparks LD, Shelnutt JA, Smith KA. Inorg. Chem. 1993;32:1716. [Google Scholar]
- 26.Byrn MP, Curtis CJ, Hsiou Y, Khan SI, Sawin PA, Tendick SK, Terzis A, Strouse CE. J. Am. Chem. Soc. 1993;115:9480. [Google Scholar]
- 27.Byrn MP, Curtis CJ, Goldberg I, Hsiou Y, Khan SI, Sawin PA, Tendick SK, Strouse CE. J. Am. Chem. Soc. 1991;113:6549. [Google Scholar]
- 28.Kumar RK, Balasubramanian S, Goldberg I. Inorg. Chem. 1998;37:541. doi: 10.1021/ic971259u. [DOI] [PubMed] [Google Scholar]
- 29.Chang CK, Barkigia KM, Hanson LK, Fajer J, Strouse CE. J. Am. Chem. Soc. 1986;108:1352. [Google Scholar]
- 30.Senge MO, Ruhlandt-Senge K, Lee SJ, Smith KM. Z. Naturforsch., Teil B. 1995;50:969. [Google Scholar]
- 31.Senge MO, Kallisch WW, Ruhlandt-Senge K. J. Chem. Soc., Chem. Commun. 1996:2149. [Google Scholar]
- 32.Jaquinod L, Khoury RG, Smith KM. J. Chem. Soc., Chem. Commun. 1996:2581. [Google Scholar]
- 33.Connick PA, Haller KJ, Macor KA. Inorg. Chem. 1993;32:3256. [Google Scholar]
- 34.Barkigia KM, Chang CK, Fajer J, Renner MW. J. Am. Chem. Soc. 1992;114:1701. [Google Scholar]
- 35.Brancato-Buentello KE, Kang SJ, Scheidt WR. J. Am. Chem. Soc. 1997;119:2839. [Google Scholar]
- 36.Fuhrhop JH, Wasser P, Riesner D, Mauzerall D. J. Am. Chem. Soc. 1972;94:7996. doi: 10.1021/ja00778a011. [DOI] [PubMed] [Google Scholar]
- 37.Fajer J, Borg DC, Forman A, Dolphin D, Felton RH. J. Am. Chem. Soc. 1970;92:3451. doi: 10.1021/ja00714a038. [DOI] [PubMed] [Google Scholar]
- 38.Schulz CE, Song H, Lee YJ, Mondal JU, Mohanrao K, Reed CA, Walker FA, Scheidt WR. J. Am. Chem. Soc. 1994;116:7196. [Google Scholar]
- 39.Hu S, Spiro TG. J. Am. Chem. Soc. 1993;115:12029. [Google Scholar]
- 40.Shimomura ET, Phillippi MA, Goff HM, Scholz WF, Reed CA. J. Am. Chem. Soc. 1981;103:6778. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.