Abstract
Dishevelled/Dsh proteins (Dvl in mammals) are core components of both Wnt/Wg-signaling pathways: canonical β-catenin signaling and Frizzled (Fz)-planar cell polarity (PCP) signaling. Although Dsh is a key cytoplasmic component of both Wnt/Fz-pathways, regulation of its signaling specificity is not well understood. Dsh is phosphorylated, but the functional significance of its phosphorylation remains unclear. We have systematically investigated the phosphorylation of Dsh by combining mass-spectrometry analyses, biochemical studies, and in vivo genetic methods in Drosophila. Our approaches identified multiple phospho-residues of Dsh in vivo. Our data define three novel and unexpected conclusions: (1) Strikingly and in contrast to common assumptions, all conserved serines/threonines are non-essential for Dsh function in either pathway; (2) phosphorylation of conserved Tyrosine473 in the DEP domain is critical for PCP-signaling - DshY473F behaves like a PCP-specific allele; and (3) defects associated with the PCP specific dsh1 allele, DshK417M, located within a putative Protein Kinase C consensus site, are likely due to a post-translational modification requirement of Lys417, rather than phosphorylation nearby. In summary, our combined data indicate that while many Ser/Thr and Tyr residues are indeed phosphorylated in vivo, strikingly most of these phosphorylation events are not critical for Dsh function with the exception of DshY473.
Keywords: Phospho-protein, Dishevelled, Wingless, Wnt-signaling, Signaling specificity, PCP
Introduction
Frizzled (Fz) family receptors act as transducers of the Wnt/Wg growth factor family, commonly signaling through the canonical Wnt (Wg)-Fz/β-catenin pathway (Logan and Nusse, 2004; Polakis, 2000). In addition, a distinct Wnt-Fz pathway regulates cellular polarity within the plane of an epithelium, referred to as non-canonical Wnt or Fz/planar cell polarity (PCP) signaling (Adler, 2002; Keller, 2002; Lawrence et al., 2007; Seifert and Mlodzik, 2007; Wang and Nathans, 2007; Wu and Mlodzik, 2009). Fz/PCP signaling diverges from the β-catenin pathway downstream of the cytoplasmic component Dishevelled (Dsh in Drosophila, Dvl 1–3 in mammals). Since both pathways require Fz and Dsh to transduce signaling information, a key question is how Fz and potential associated factors can differentially activate Dsh. In many tissues, both pathways act in the same cells and thus a tight regulation of Fz-Dsh signaling specificity is essential. In deregulated, mutant scenarios, the selection of the wrong Fz-Dsh pathway can lead to disease (i.e. polycystic kidney disease or cancer (Logan and Nusse, 2004; Polakis, 2000; Simons and Mlodzik, 2008)). Although a molecular framework of the two pathways has been established (Klein and Mlodzik, 2005; Logan and Nusse, 2004; Mlodzik, 2002; Strutt, 2003; Veeman et al., 2003), little is known about the molecular aspects regulating Fz-Dsh pathway selection and signaling specificity. Here we investigate this with focus on Dsh phosphorylation.
Drosophila is a well-suited model system to address Wnt-signaling specificity mechanisms. For example, in the fly eye, activation of the Fz/β-catenin pathway leads to the restriction of the eye field and later the removal of excess photoreceptor cells through apoptosis (Lin et al., 2004), whereas Fz/PCP signaling is required for correct induction of specific photoreceptor fates and their patterning (Mlodzik, 1999; Strutt and Strutt, 1999). Similarly, during wing development the two pathways have distinct non-overlapping functions: canonical Wg-signaling is required for correct wing blade/margin formation, while Fz/PCP signaling orients wing cells in the proximal-distal axis. Moreover, as Fz/PCP aspects are universal to metazoans (Adler, 2002; Keller, 2002; Lawrence et al., 2007; Seifert and Mlodzik, 2007; Wang and Nathans, 2007; Wu and Mlodzik, 2009), Drosophila is well suited to study Dsh function, because of lack of Dsh redundancy (a single dsh gene) and its function being testable in rescue assays of null mutants.
In Wnt-Fz/β-catenin signaling, the combined action of Fz, LRP5/6-Arrow (a Fz co-receptor), and Dsh is to antagonize the APC/GSK3/Axin complex that causes β-catenin (Arm in Drosophila) phosphorylation and degradation. Upon Wnt binding, the two transmembrane proteins associate, leading to a physical interaction of Dsh and Axin (via an interaction through their DIX domains) at the membrane receptor complex (Bilic et al., 2007; Zeng et al., 2008). This leads to the stabilization of β-catenin/Arm and allows its accumulation in the nucleus, where it interacts with TCF transcription factors on Wnt-signaling target sites (Logan and Nusse, 2004; Polakis, 2000). In contrast, during Fz/PCP signaling, Fz “activates” Dsh through an unknown mechanism in an LRP5/6-Arrow independent manner, with Dsh actng on a distinct set of effectors. The components of Wnt-Fz/β-catenin that act downstream of Dsh are not required in the Fz/PCP pathway, and vice versa (e.g. (Axelrod et al., 1998; Boutros et al., 1998)). The Fz-Dsh pair thus activates distinct pathways, a feature conserved between flies and vertebrates (Mlodzik, 2002; Sahai et al., 1998; Strutt, 2003; Veeman et al., 2003).
The regulation of signaling specificity remains largely obscure. Some insights have come from studies in Drosophila, with focus on the cytoplasmic (C-) tails of Fz and Fz2 identified as critical in regulating the specificity through their effects on subcellular localization (Boutros et al., 2000; Wu et al., 2004). Other domains of Fz and/or Fz2 (e.g. the Wnt interacting extracellular CRD) are also important in determining signaling outcome (Boutros et al., 2000; Rulifson et al., 2000; Strapps and Tomlinson, 2001; Wu and Mlodzik, 2008). All Dsh-family members (ranging from nematodes to humans) share three conserved domains: a DIX domain, a central PDZ domain and a C-terminal DEP domain, which have been implicated in protein-protein interactions, and thus Dsh likely serves as an adapter molecule (rev. in Boutros and Mlodzik, 1999; Wallingford and Habas, 2005). Dsh is recruited by Fz to the membrane (Boutros et al., 2000; Wu et al., 2004). Although a Fz-Dsh interaction surface has been identified (Wong et al., 2003), additional mechanisms help to stabilize the membrane complex (Simons et al., 2009). In cell culture, when Dsh is not in a membrane-associated state, it tends to be in cytoplasmic aggregates with multimerization mediated by DIX domains (Schwarz-Romond et al., 2005). Regulation of Dsh activity and localization is therefore likely instrumental for signaling outcomes.
PCP specific Dsh recruitment appears more stable than its general basolateral membrane recruitment for β-catenin signaling (Wu et al., 2004), suggesting that Dsh receives different “local” input (for example through post-translational modification) for its membrane association (Metcalfe et al., 2010). Dsh/Dvl proteins also use different domains for downstream pathway functions: DIX and PDZ domains are essential for β-catenin signaling, while the PDZ and DEP-C terminal domains are critical for PCP signaling (rev. in Boutros and Mlodzik, 1999; Wallingford and Habas, 2005)(also Fig. 1A). Upon “activation”, Dsh is subject to two major changes: (1) translocation from the cytosol to membrane and (2) hyper-phosphorylation, usually assessed by changes in gel mobility. Both aspects are often used as a measure of “Dsh activation”, although what “activation” entails remains unclear. At least for phosphorylation, it is only a correlated phenomenon without a defined contribution to signaling function (Axelrod, 2001; Cong et al., 2004; Klein et al., 2006; Ossipova et al., 2005; Strutt et al., 2006). In Drosophila, both the dsh1 and fz- mutants (with PCP specific defects) show markedly reduced Dsh phosphorylation as evident by band-shift (Axelrod, 2001). In vertebrate cell culture models, both canonical and non-canonical (PCP) Wnts, in particular Wnt3A and Wnt5A respectively, lead to induction of Dsh phosphorylation as detected with Dsh/Dvl band-shift assays. Multiple kinases have been suggested and shown to phosphorylate Dsh in vitro, with potential regulatory requirement(s) in overexpression experiments (Cong et al., 2004; Klein et al., 2006; Ossipova et al., 2005; Strutt et al., 2006).
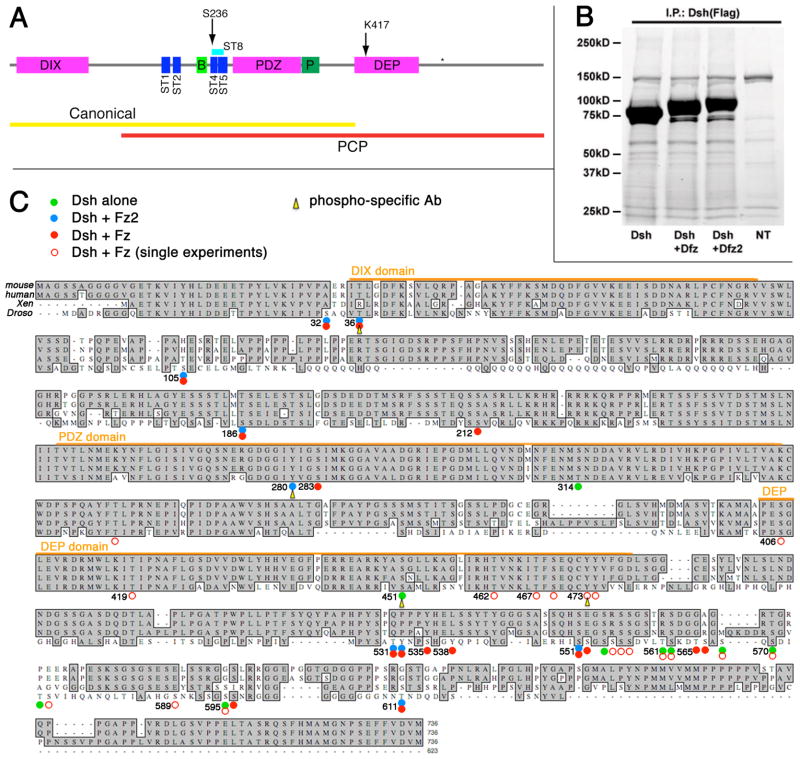
Figure 1. Identification of phosphorylated residues of Dsh.
(A) Schematic of Dsh with the DIX, PDZ, and DEP domains indicated in purple. Light and dark green domain correspond to the Basic and Proline rich regions, respectively. Dark blue boxes represent the Serine/Threonine clusters that were mutated in Penton et al. (2002). ST8 (light blue box) corresponds to the 8 S/T residues that were mutated in Strutt et al. 2006 and have been identified as potential Par1 sites (Ossipova et al 2005). S236 corresponds to the CK1ε site in Klein et al. 2006. K417 and (*) depict the position of the dsh1 mutation and the BlpI site used to engineer the C-terminal mutations, respectively. Note that re-sequencing of the ST5 region of the mutant constructs from Penton et al. (2002) showed that T252 is not mutated in the ST5 containing constructs. The defined functional requirements for the two Wnt-signaling pathways (Boutros et al. 1998; 1999; Wallingford and Habas, 2005) are indicated as yellow (canonical pathway) and red (PCP signaling) bars below the scheme.
(B) Purification of transfected Dsh protein from HEK293T cells. Protein staining (Simply Blue, Invitrogen) of SDS-PAGE gel is shown. 3xFlag-tagged Drosophila Dsh was transfected alone or co-transfected with either Dfz or Dfz2 as indicated. Cell lysates were immunoprecipitated with anti-Flag antibodies. The respective products were analyzed in 4–15% gradient SDS-PAGE gel (Biorad; BSA was used to estimate protein concentration). NT lane: untransfected control. The upper bands/region (hyperphosphorylated Dsh) were cut out, eluted and subjected to mass-spectrometry (MS) studies (see Methods).
(C) Phosphorylated residues of Drosophila Dsh as identified by mass-spectrometry, Drosophila Dsh sequence is aligned with mouse Dvl2, human Dvl2, and Xenopus Dsh. Marked residues were detected as phosphorylated in independent transfections and MS experiments. The results shown reflect three independent experimental analyses; phosphorylation events detected only in one of the three analyses are shown with open circles instead of dots. Green dots: Dsh transfected alone (naïve state); blue dots: Dsh co-tranfected with Fz2 (“canonical” signaling state); red dots: Dsh co-transfected with Fz (“PCP” state); the respective signaling states were assigned based on observations of Dsh membrane recruitment (T.J. Klein and MM, unpublished) and activation of the Top-flash Wnt-signaling reporter assay. Orange color bars indicate the extent of the three conserved domains: DIX (residue 35–83), PDZ (252–338) and DEP (404–478). Yellow arrowheads indicate residues that were chosen for anti-phospho-residue antiserum generation. All four phospho-residues were confirmed by in vivo analyses.
A comprehensive analysis of Dsh phosphorylation has been especially challenging since for example over 15% of Dsh residues are serines and theronines. Here we have explored mass-spectroscopic, biochemical, and genetic approaches to identify phosphorylated residues on Dsh and systematically define their functional significance in in vivo in rescue assays. Besides many Ser/Thr residues identified biochemically, our approach also identified tyrosine phosphorylation. Strikingly, in contrast to common assumptions and the fact that a phosporylation dependent band-shift of Dsh/Dvl-proteins is often used as pathway activation read-out (Axelrod, 2001; Cong et al., 2004; Klein et al., 2006; Ossipova et al., 2005; Strutt et al., 2006), all of the Ser/Thr residues tested (although conserved) are dispensable for Dsh function in vivo. We also show that the Dsh gel-shift can be uncoupled from Dsh activity. However, Tyr473 located within the DEP domain is essential for Dsh function for PCP signaling in vivo. Furthermore, dissection of the dsh1 allele (DshK417M) suggests that K417 could undergo direct post-translational modification rather than affect a phosphorylation event in its flanking PKC site consensus. Our data indicate that while many Ser, Thr, and Tyr-residues are phosphorylated, most of these phosphorylation events are, unexpectedly, not critical for Dsh function.
Material and methods
DNA constructs, fly genetics, and Rescue Assay
dsh>DshGFP was kindly provided by Jeff Axelrod. Point mutagenesis of the Dsh ORF was performed by excising it with either KpnI (from 278nt of ORF to the end of polyA tail) or EcoRI (from about 135nt ahead of start codon to the end of polyA tail), subcloned into pBlueScript, and mutated by standard protocols. For in vivo purification, dsh>Dsh-3XFlag was made by replacing C-terminal GFP sequence from the original construct with 3XFlag sequence (Sigma). To analyze the potential requirements of S/T residues in the C-terminal part of Dsh, we have mutated them to Ala in three blocks (separated by introduced silent restriction sites) indicated by purple, blue and red (Fig. 2). 2 cluster combinations, referred to as C-term#1-2, C-term#1-3 or C-term#2-3 in Table 1 were made by swapping combining individual clusters. The respective mutant clusters in the context of full-length Dsh were then tested in gel-shift assays after transfection into 293T cell with or without Fz. Finally, we also generated a Dsh that lacked all sequences downstream of the Blp1 site, referred to as ΔC-term (Table 1; note that the mutant constructs lack all but one Ser, while ΔC-term contains no Ser/Thr C-terminal to the DEP domain). A detailed cloning strategy is available upon request.
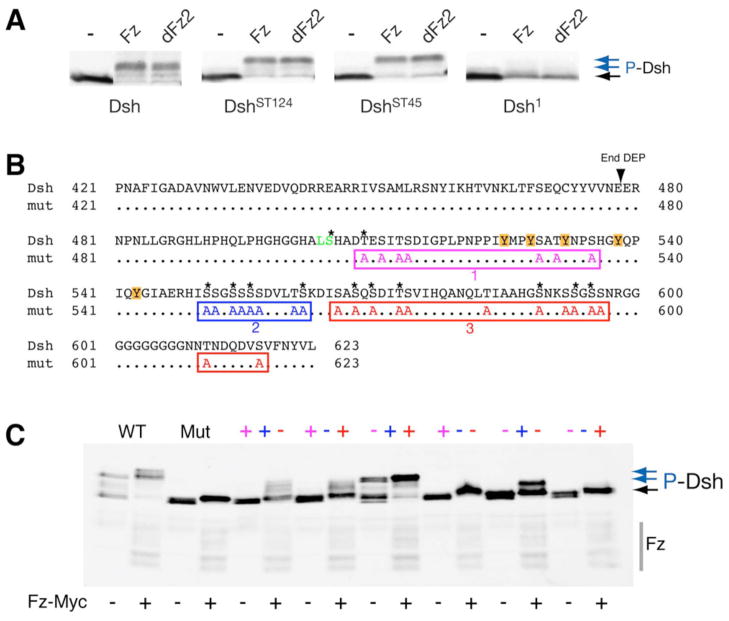
Figure 2. Dsh mobility shift assays.
(A) Gel-shift assays show that DshST124 and DshST45 phosphorylation (blue arrows on right) can be induced by co-transfections of Fz or dFz2 in S2 cells. As previously shown in vivo (Axelrod 2001), dsh1 barely shows a gel-shift under these conditions. (C, D) The C-terminal S/T residues affect the Dsh gel-shift. (B) Sequence of the C-terminus of Dsh with the end of the DEP domain indicated. To assess the contribution of the 29 S/T residues of the C-tail to the phosphorylation induced gel-shift, we mutated 28 of them in three clusters (#1: purple, #2: blue, and #3: red in color code; note that the first serine is part of the BlpI site (green) and was not mutated for cloning reasons). Asterisks indicate conserved S/Ts. The region around cluster #1 also contained 5 Tyrosines (marked by orange boxes). (C) Myc-Fz induced Dsh gel-shift assay in 293T cells. In contrast to wild-type Dsh, a Dsh with a fully mutant C-terminus is not shifted (Mut; blue arrows indicate phosphorylated forms of Dsh; note the strong shift due to endogenous Dsh activation of WT-Dsh in the 293T cells used). Analysis of mutations of individual clusters shows that the second and third clusters have bigger effects on the gel-shift than the first cluster. Combinations of the clusters, however, indicate that the first cluster also contributes to Dsh phosphorylation as assessed by gel-shifts. Grey bar on the right shows migration of Myc-Fz.
Table 1.
Rescue analysis of dshV26 by respective Dsh phosphorylation mutants.
| Protein mutant (all in dshV26 null background) | viability | PCP aspect | Domain location |
|---|---|---|---|
| dshV26 | -- | defective (in clones) | |
| dsh-DshGFP | +++ | wild type | |
| dsh-Dsh3xFlag | +++ | wild type | |
| dsh1(K417M) | +* | defective | DEP |
| Y24F | +++ | wild type | DIX |
| T36A | +++ | wild type | DIX |
| T252A | +++ | wild type | PDZ |
| S254A | +++ | wild type | PDZ |
| S266A | +++ | wild type | PDZ |
| S271A | +++ | wild type | PDZ |
| Y280F | +++ | wild type | PDZ |
| S283A | +++ | wild type | PDZ |
| T314A | +++ | wild type | PDZ |
| T349A | +++ | wild type | proline rich |
| S406A | +++ | wild type | DEP |
| T419A | +++ | wild type | DEP |
| S451A | +++ | wild type | DEP |
| T462A | +++ | wild type | DEP |
| T467A | +++ | wild type | DEP |
| S469A | +++ | wild type | DEP |
| Y473F | +* | defective | DEP |
| Y473F in abl−/+ | +* | defective | DEP |
| Y474F | +++ | wild type | DEP |
| S406E | +++ | wild type | DEP |
| T419E | +++ | wild type | DEP |
| S451E | +++ | wild type | DEP |
| DEP6A | +++ | wild type | DEP |
| K417R | +* | defective | DEP |
| C-term#2 | +++ | wild type | C-terminus |
| C-term#1-2 | +++ | wild type | C-terminus |
| C-term#1-3 | +++ | wild type | C-terminus |
| C-term#2-3 | +++ | wild type | C-terminus |
| C-term5Y-F | +++ | wild type | C-terminus |
| DshΔC | +++ | wild type | C-terminus |
The table indicates the location of the mutation (domain that is affected), capability to rescue flies to viability, and PCP features. All data are based on rescue of the following genotype: dshV26, f-/Y; dsh-DshGFP**/+, multiple lines were analyzed for all constructs.
PCP aspects were recorded based on wing, thorax, and eye phenotypes (see also Figure 4).
note that the dsh1 allele and the other two DEP domain mutants that did rescue to viability with PCP defects showed reduced viability.
To estimate effects of mutants, dsh>Dsh(mutant)GFP strains were balanced these on second or third chromosomes and crossed to the dshv26 null mutants. Note that the dshv26 chromosome used was also marked with forked (f) to control for non-disjunction events. Rescue index was calculated as: rescue index (R.I.)=rescued/control. For each group of rescue assay, dsh>DshwtGFP served as baseline control. R.I. of wild-type Dsh is in theory 1. Practical R.I. (wild-type) was used to calibrate R.I. of mutants.
y, w, f36a, dshV26/FM6 was obtained from the Bloomington Stock center.
Peptides and antibodies
The phospho-peptide sequences used as antigens were:
pS36: CSAQV(pT)LRDFK
pY280: RGGDGGI(pY)VGSI
pS451: CRREARRIV(pS)AMLR
pY473: GGEQC(pY)YVVNE
Peptides were synthesized by ProSci and Tufts University Mass-Spectrometry Core Facility, and antisera were generated by ProSci (California). Anti-Fmi antiserum was from Developmental Studies Hybridoma Bank
Collection of fly samples and protein purification
There is no documented setup to collect Drosophila larvae and pupae at large scale. We modified a regular trash-can to a “larval farm”. 15–20 bottles of fly cultures (larval stages) were transferred to the “farm”. Small amount of water was added to make it moist and a thin layer of yeast pellets was spread on top, the surface was increased with plastic foil/film to allow larvae to crawl up and covered with a glass panel and wet towel. The setup was kept at room temperature and inspected every 12 hours to adjust for moisture. 48 hours after set-up, plastic films were taken out to collect larvae/pupae (about a 1:1 ration of pupae and larvae were the preferred stage). A larval “farm” produced about 20g sample each time.
To detect phosphorylation of Dsh protein in vivo, Dsh protein needed to be purified from >500 larvae/pupae. The sample was homogenized in PBS with 1% SDS, 1% Triton X-100, 1mM DTT, 1:100 dilution of protease inhibitor cocktail, phosphatase inhibitor cocktail I and II (from Sigma). Lysate was spun at 4°C 14,000g for 10min, supernatant filtered with Whatman paper, followed by (NH4)2SO4 precipitation (Dsh-3Xflag was enriched in 5–10% saturated fraction). 50–100ng of pure Dsh-3XFlag was obtained from 50g larva/pupae.
Transfected Dsh-3Xflag in HEK293T cells was immunoprecipitated with M2-agarose gel (Sigma). Cell lysis buffer (same for immunoprecipitation) contained 50mM Tris-HCl(pH7.4), 140mM NaCl, 1% Triton X-100, 1:100 dilution of protease inhibitor cocktail (Sigma), and phosphatase inhibitor cocktails I and II (both Sigma). Protein was eluted with 0.1M glycine (pH2.8) and precipitated with methanol/chloroform.
Mass spectrometry
Mass spectrometry was performed at the Tufts University Protein Core Facility. Excised bands were subjected to in-gel reduction, alkylation, and enzymatic digestion (Roche Applied Science, Indianapolis) in a HEPA-filtered hood to reduce keratin background. LC/MS/MS analysis was performed on the in-gel digest extracts using Agilent (Santa Clara, CA) 1100 binary pump directly coupled to a mass spectrometer. 2–8ul of sample was injected on column using a LC Packings (Sunnyvale, CA) FAMOS autosampler. Nanopore electrospray columns were constructed from 360mm o.d., 75 mm i.d. fused silica capillary with the column tip tapered to a 15 mm opening (New Objective, Woburn, MA). The columns were packed with 200 Å 5 um C18 beads (Michrom BioResources. Auburn, CA.), a reverse-phase packing material, to a length of 10 cm. The flow through the column was split precolumn to achieve a flow rate of 300 nL/min. The mobile phase used for gradient elution consisted of (A) 0.3% acetic acid 99.7 % water and (B) 0.3% acetic acid 99.7 % Acetonitrile. Tandem mass spectra (LC/MS/MS) were acquired on a Thermo LTQ ion trap mass spectrometer (Thermo Corp., San Jose, CA). Needle voltage was set to 3 kV, isolation width was 3 Da, relative collision energy was 30%, and dynamic exclusion was used to excluded recurring ions. Ion signals above a predetermined threshold automatically triggered the instrument to switch from MS to MS/MS mode for generating fragmentation spectra.
Histology and confocal microscopy
Wings of adult flies were collected and soaked in PBS with 0.1% Triton X-100 for 4 hours. The wings were carefully mounted in 80% glycerol PBS solution. Eye sections were performed by standard protocols (Gaengel and Mlodzik, 2008).
Pupae were dissected at 30APF, and fixed with 4% formaldehyde for 1 hour. Dissected wing disc samples were treated with 5% FBS for 30min followed by antibody staining (1:10 anti-Fmi; 1:500 anti-GFP) overnight at 4°C. Following several washes, pupal wings were mounted in Vectashield (Vector Labs), and imaged with a Zeiss LSM5 confocal microscope. Single optical sections were analyzed with the Zeiss Image Browser and Adobe Photoshop software.
Results
Identification of Dsh phosphorylation sites by mass-spectrometry
Dsh phosphorylation is detected in many systems, ranging from in vivo assays to cell transfection experiments and is readily observed on gels by an associated change in mobility (e.g Axelrod, 2001; Cong et al., 2004; Klein et al., 2006). Although some phosphorylated residues have been identified in vitro (Cong et al., 2004; Klein et al., 2006; Ossipova et al., 2005; Strutt et al., 2006; also Fig. 1A), Dsh phosphorylation has not been addressed comprehensively. Moreover, although the phosphorylation-associated slower migration (band-shift) of Dsh/Dvl proteins is often used as read-out for pathway activation, its functional significance has never been demonstrated.
To systematically address Dsh phosphoryation, we first decided to identify phospho-residues through mass-spectroscopic analyses of purified Dsh from different cell culture conditions. HEK293T cells appeared well suited as they could be transfected efficiently (in contrast to Drosophila S2). After transfection into HEK293T cells alone or with Fz or dFz2, tagged Dsh was purified by immunoprecipitation (Fig. 1B; see Methods). The slower migrating hyper-phosphorylated bands were cut out and eluted (see Methods). The phospho-Dsh proteins from the three states tested: Dsh alone (representing a “naïve” Dsh), Dsh plus Fz (representing a Fz/PCP associated state), and Dsh plus Fz2 (likely representing non PCP membrane association) were analyzed by a tandem mass-spectrometry/HPLC approach of tryptic fragments. This identified multiple phosphorylation events on Ser/Thr as well as Tyrosine residues (Fig. 1C), suggesting wide-spread phosphorylation of Dsh.
The phospho-residues thus identified were distributed throughout the protein, including the conserved DIX, PDZ, and DEP domains. In addition, the less conserved C-terminal Dsh region was found to harbor many phosphorylated residues. There was a noticeable and reproducible difference among the samples from the three distinct Dsh transfections/“states”, suggesting that several phosphorylation events were due to distinct “signaling”-response(s). Despite the potential overlap between Fz and dFz2 in canonical signaling in vivo (canonical Wg-signaling is controlled redundantly by both Fz and dFz2; Bhanot et al., 1999; Bhat, 1998; Chen and Struhl, 1999; Mueller et al., 1999), dFz2 appears to be the dedicated receptor for canonical signaling (Boutros et al., 2000). Accordingly, distinct phosphorylation patterns induced by the two Fz-receptors suggested distinct function(s) in this cell culture system, further confirmed by the fact that the mechanism of membrane recruitment of Dsh mediated by Fz and Fz2 is distinct in cell culture (Simons et al., 2009; T.J. Klein and MM unpublished).
Among three independent rounds of mass-spectrometry analysis, consistent data was obtained with respect to the phosphorylated residues throughout Dsh (Fig. 1C). Nevertheless, due to the pattern of tryptic fragments, the S/T-rich area between the basic-region and PDZ domain (known to be phosphorylated in vitro) (Cong et al., 2004; Klein et al., 2006; Ossipova et al., 2005; Strutt et al., 2006) and part of the DEP domain could not be covered well by this approach. However, as it already has been documented that the S/T-rich region just upstream of the PDZ domain does not require major phosphorylation for function in vivo (Strutt et al., 2006; also Fig. 1A), we did not analyze this region in vivo in further detail. Moreover, our biochemical data demonstrate that Ala-substitutions within this region (Dsh-ST124 and Dsh-ST45 in Fig 1A and 2A; Penton et al., 2002 for original description) did not affect Dsh phosphorylation as detected by Fz or dFz2 induced band-shifts (Fig. 2A).
Although the mass-spectrometry approach provided a useful framework for subsequent in vivo studies (see below), it was clear that it could not substitute for equivalent physiological analyses. We therefore complemented and confirmed the mass-spectrometry data by functional studies and specific antibodies raised against individual phosphorylated residues (see below).
Residue specific phosphorylation of Dsh in vivo
The phospho-residue mapping via mass-spectrometry was performed on cell culture samples for technical reasons, making it possible that some candidate phosphorylation sites were due to overexpression.
We thus next employed in vivo assays to further dissect the role of potential phosphorylation sites in Dsh. First, we wished to confirm that the sites in question were indeed phosphorylated in vivo at the relevant stages of development during PCP signaling. To address this, phospho-residue specific antibodies to Thr36, Tyr280, Ser451 and Tyr473 were generated (Fig. 1C). Peptides containing the phospho-residues were used to immunize rabbits (see Methods). The respective anti-sera were first characterized with dot-blots by comparing sensitivity between phospho-peptides and their non-phosphorylated counterparts (see Suppl. Fig. S1 for example). Batches of responsive sera were affinity purified using phospho-peptide conjugated columns. Purified antibodies were further tested on cell culture samples: most of the phospho-peptide antibodies detected specific bands on Dsh co-transfected with Fz (Suppl. Fig. S2 shows an example of anti-pS451). In vitro kinase assays were used as positive controls for Tyrosine specific antibodies (the Src kinase could phosphorylate the PDZ domain in vitro; Supp Fig. S3A). The sole (and phosphorylated) Tyrosine residue within the Dsh PDZ domain were Tyr280 and anti-pTyr280 (anti-pY280) antibodies detected specific bands on Src-treated Dsh protein (Suppl. Fig. S3), indicating its specificity.
Next, endogenous Dsh protein samples were analyzed for phosphorylation of these residues using dsh-Dsh3XFlag, dshV26 rescued animals (Table 1 and below). As expected from dot blot assays (Suppl. Fig S1), a direct Western blot approach was not sensitive enough, and thus Dsh protein was enriched from late larval/pupal stages by immunoprecipitation (the 3XFlag tag allows efficient affinity purification; see Supp. Data/Methods for details). Distinct Dsh protein banding patterns were reproducibly detected with phospho-specific antisera on Western blots (probing immunoprecipitated Dsh from dsh-Dsh3XFlag, dshV26 animals; Fig. 3), which ran as several major bands in a Fz-dependent manner (Fig. 3, lane 1), consistent with previous work (e.g. Cong et al., 2004; Klein et al., 2006). Strikingly, anti-pY280, anti-pS451, and anti-pY473 recognized the top band, while anti-pS36 stained all three bands (Fig. 3, lanes 2, 3, 4 and 5; as anti-pY473 and anti-pS451 stained specifically only one of the Dsh bands, the other Dsh bands served as controls demonstrating the specificity of the antibodies). These data confirm that several residues, S36, Y280, T451, and Y473, were phosphorylated in vivo. Although the antisera work on Western-blots (Fig. 3) a large amount of Dsh protein needs to be enriched for these studies, and thus the sensitivity of these sera is not high enough to detect protein in situ.
Figure 3. Phosphorylation of Dsh protein in vivo.

Immunoprecipitated Dsh protein from late larval and pupal stages was analyzed with phospho-residue specific antibodies (see Methods for details).
Purified Dsh protein from dsh-Dsh3xFlag, dshV26 flies were probed with the following antibodies (all control lanes are BSA): 1: anti-Dsh; 2: anti-phosphoThr36; 3: anti-phosphoTyr280; 4: anti-phosphoSer451; 5–7: anti-phosphoTyr473 with phosphorylated antigen peptide as competitor (lane 6) and non-phosphorylated antigen peptide as competitor (lane 7).
The detection of phosphorylation states of individual residues in vivo is physiologically relevant, and comparing it with our mass-spectrometry data, these data confirm many consistent phosphorylation events between the two assays. This approach also helped to confirm phosphorylated residues that were detected by mass-spectrometry only in some experiments, such as Y473 (Fig. 1C). Furthermore, these data also confirmed our analysis of Abl-mediated DshY473 phosphorylation and function (Singh et al., 2010, see also below).
A quantitative Dsh rescue assay
To address the functional relevance of phosphorylation of individual residues or phosphorylated clusters, we established an in vivo rescue assay of a dsh null allele. Although cell culture based reporter assays (such as Top-Flash activation) or in vivo over-expression assays are fast and gather initial insights into functional questions, altered expression levels of Dsh can cause increased and/or aberrant signaling. Thus, to compare Dsh-activity levels accurately, a functional rescue assay that faithfully represented the endogenous physiological situation was necessary. A similar transgenic approach using endogenous dsh control sequences (dsh-DshGFP) has been used to illustrate asymmetric membrane localization of Dsh (Axelrod, 2001).
First confirmed that dsh-DshGFP was indeed expressed at endogenous levels (Suppl. Fig. S4) and that the dsh-DshGFP transgene was capable to fully rescue dsh- null animals. dshV26 (a.k.a. dsh3) animals were fully rescued by all dsh-DshGFP transgenic lines (Table 1) and indistinguishable from wild type demonstrating that canonical Wg/Wnt signaling required for patterning is fully functional. They displayed a wild-type PCP pattern in all tissues analyzed (not shown and below). Similarly, the equivalent transgene with a different tag, dsh-Dsh3XFlag (which provides an advantage for immunoprecipitation and biochemical studies, see above) also fully rescued the dshV26 null (Table 1 and not shown).
Analysis of Serine/Threonine phosphorylation mutants of Dsh in vivo
THis in vivo dsh− rescue assay was then used to stringently test whether the respective phospho-residues play a physiological role in Dsh signaling. We focused on phospho-residues that were either highly conserved or reproducibly detected in the mass-spectrometry analyses or both (Fig. 1C; due to the large number of candidate S/T residues, 110 in total in Dsh, the mass-spectrometry pre-selection was essential). We first targeted 14 conserved S/T residues in the DIX, PDZ, DEP and proline rich domains as individual mutations and the conserved tyrosine residues located in the PDZ (Y280) and DEP domains (Y473, Y474). Strikingly, in the dshV26, dsh-DshGFP* rescue assay (*indicating the individual mutations), none of the S/T residues, when mutated to Ala (A), had a detectable effect on either viability and patterning (canonical Wg-signaling) or PCP (Table 1; all mutants have been analyzed in multiple independent lines and expression levels were confirmed with the GFP-tag; see below and not shown). The extent of rescue by the individual transgenes/mutants was quantified (Table 1 and not shown). It is worth noting that, even though the PCP specific dsh1 allele is thought not to affect canonical Wnt-signaling (and does not in cell culture studies; see below), the dsh-Dsh1 transgene rescued dshV26 null background to viability with only a reduced recovery of adult flies (Table 1 and not shown; the rescue level denoted “+” in Table 1 allowed keeping of a homozygous stock). This observation is consistent with the behavior of the dsh1 allele that also displays reduced viability (Boutros et al., 1998).
As none of the individual S/T mutations tested affected phenotypic aspects of Dsh function, we mutated groups of related S/T-residues. We focused on the DEP domain and the C-terminal region of Dsh, since the DEP domain/C-terminal regions have been linked to PCP signaling and are required for Fz-dependent Dsh membrane recruitment (e.g. Boutros et al., 1998; Klein et al., 2006; Rothbächer et al., 1998). Within the DEP domain, several PKC phosphorylation consensus sites are present and a PKC member has been implicated in Dsh membrane association in Xenopus (Kinoshita et al., 2003). We thus mutated several S/T-residues within potential PKC substrate sites (Table 1; also Fig. 1C), including a mutant with all S/T sites in the DEP domain mutated (DshDEP6A; Table 1). Strikingly, the DshDEP6A mutant fully rescued all aspects of dsh function to wild type (Table 1 and not shown).
Besides the three conserved domains, Dsh/Dvl proteins also have a C-terminal region (C-terminal to DEP domain) with many conserved S/T residues (Fig. 1C and 2C). This region has been implicated in Dsh function in PCP signaling (Park et al., 2008). Moreover, we have detected several of these S/T-residues to be indeed phosphorylated in the mass-spectrometry studies (Fig. 1C). We thus targeted S/T-clusters in the C-terminal region by first establishing three mutant clusters individually and then combining them in different combinations (Fig. 2B, C). Strikingly, mutating these clusters to Ala affected the band-shift of Dsh as induced by Fz co-transfections (Fig. 2B, C). When all three of these clusters were mutated the Dsh shift was almost completely lost (Fig. 2C, lanes labeled “Mut”). Similarly, single mutant clusters also affected the level of the band shift (Fig. 2C, a notable exception being the most proximal cluster labeled in pink in Fig. 2B; see Fig. 2C legend for details). Despite these strong effects in biochemical assays, none of the three clusters affected Dsh function in either viability or PCP signaling, when tested in pairs in the dsh-DshGFP* rescue assays (Table 1 and not shown). Strikingly, even when this entire region was deleted (dsh-DshΔC; deletion from residue 505 onward, generating a truncated protein that lacks all of the 29 C-terminal S/T-residues) the mutant isoform fully rescued both canonical Wg-signaling requirements and all PCP aspects (Table 1; Figure 4E, J; and not shown). These data demonstrated that phosphorylation of the C-tail or the C-tail of Dsh per se is not essential for its function in vivo (see also Discussion).
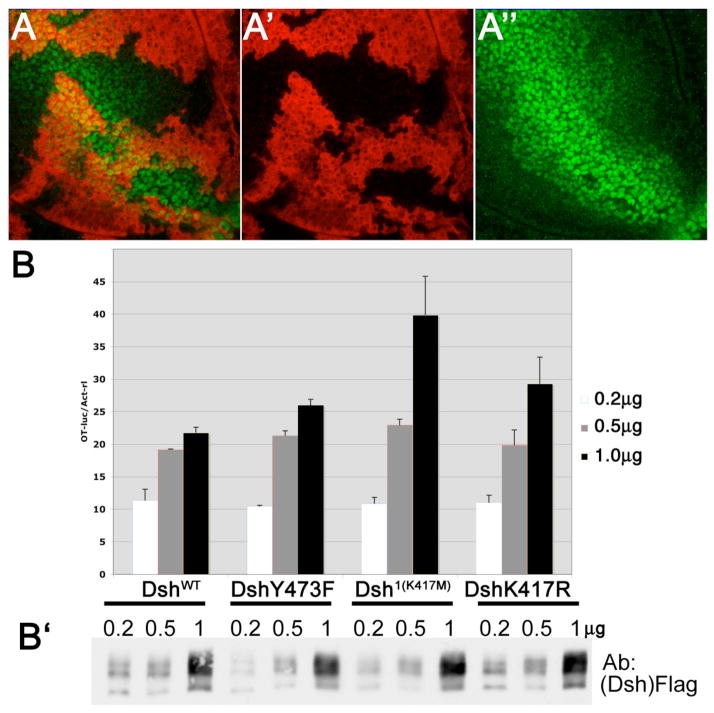
Figure 4. Canonical Wg-signaling activity of Dsh1, DshY473F, and DshK417R mutants.
(A) dsh-DshY473F (in a dsh- background) fully rescues the expression of Wg-signaling target genes during wing disc development. Example shown is Distalless (Dll). Senseless/Sens was also tested and rescued (not shown).
3rd instar larval wing disc stained for the clonal marker lacZ (red, marks wild-type regions) and Distalless (Dll; green), expressed along the dorso-ventral margin flanking the Wg expression stripe and weaker throught the wing blade anlage. Dll is a direct target of Wg. dshV26 clones loose Dll expression (not shown). The presence of DshY473F fully rescued the mutant dshV26 effect. Dll expression showed no difference between mutant and wild-type tissue. (A′) and (A″) show single channels for lacZ and Dll, respectively.
(B-B′) Dsh mutants do not affect expression levels of Top-flash reporter. Dsh mutant expression constructs were transfected with Top-flash luciferase reporter (and SV40-renilla luciferase reporter as control) in HEK293T cells. The ratios of Top-flash vs. renilla were normalized against empty vector control at three doses of transfection as indicated. Note that DshY473 showed no significant reduction as compared to wild-type Dsh (DshWT); the same was the case with Dsh1(K417M) and DshK417R mutants. (B″): Western-blot against Dsh to visualize similar protein levels.
In summary, all S/T mutants tested, including the S/T cluster mutants in the DEP domain and C-terminal region, were fully functional (Table 1). Detailed analyses of such animals for potential visible defects, PCP or other, also revealed no aberrant phenotypes. We thus conclude that all S/T-residues tested were non-essential for either canonical Wg or PCP signaling. Previous work analyzing the S/T clusters between the basic region and the PDZ domain also revealed that these sites, although phosphorylated, were not essential for Wg-signaling (Penton et al., 2002; Strutt et al., 2006) and only a minor PCP requirement was seen when at least 8 of these were mutated (Strutt et al., 2006). Thus to date, including our data here for the C-tail, no S/T phosphorylation on Dsh has been shown to be essential for canonical Wnt/Wg-signaling.
Dsh Tyrosine473 is required for PCP signaling
Although previous work has suggested that Dsh is mainly phosphorylated on S/T residues and not tyrosines (Willert et al., 1997a), we have detected Tyr(Y)-phosphorylation in our mass-spectrometry studies and, importantly, confirmed Y280 and Y473 to be phosphorylated in vivo (Figs. 1 and 3). We thus tested for a potential physiological role of the highly conserved Y280 (PDZ domain) and Y473, Y474 residues (DEP domain). DshY280 to phenylalanine (DshY280F) substitution had no effects on either viability or PCP (Table 1). In contrast, DshY473F displayed the strongest effect in the viability rescue in the same range as the Dsh1 mutant (Table 1), although a rescued (homozygous) stock was viable, consistent with our previous data in the Abl context (Singh et al., 2010). We did not, however, detect defects in Wg-signaling target gene expression or signaling strength during development, and also a quantitative cell culture assay did not reveal a significant difference of DshY473F as compared to DshWT (Fig. 4). We thus conclude that the Y473 is not required for canonical signaling and the reduced viability might have to do with other Dsh functions not yet described in Drosophila.
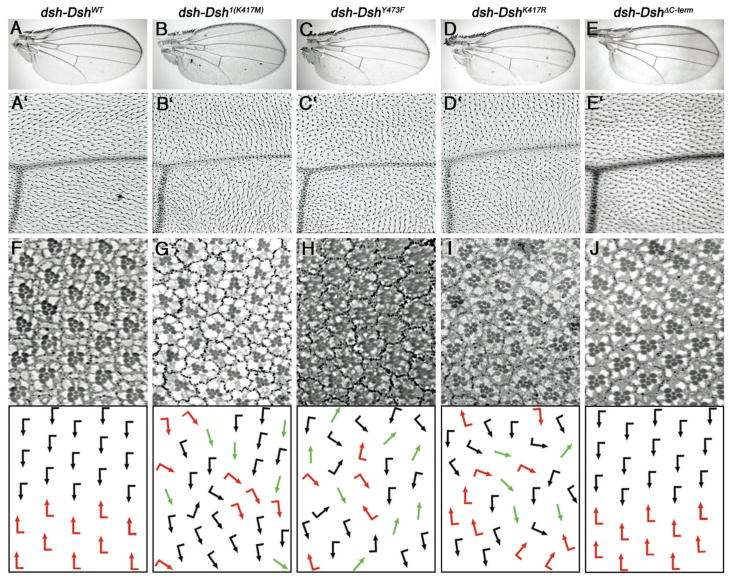
Strikingly, the dshY473F flies displayed strong PCP defects in all tissues analyzed (Table 1 and Fig. 5C, H). The neighboring Y474 had no effect and the double mutant (DshY473, 474F) behaved like DshY473F alone (Table 1 and not shown). As these PCP defects were strikingly similar to the dsh1 allele, we compared their phenotypic PCP defects in more detail. In both cases, dsh1 and dshY473F, the rescued individuals displayed classical PCP defects in the thorax and wings (Figure 5A–C′ and not shown), and had rough eyes, with randomized chirality, symmetrical clusters, and misrotations again resembling dsh1 and classical PCP mutants in general (Figure 5F–H). Moreover, dsh-DshY473F did not rescue the PCP defects of the dsh1 allele (not shown). Taken together, these data indicate that DshY473F behaves like a strong PCP specific dsh allele and thus Y473 appears to be required for PCP regulation.
Figure 5. Functional requirements of specific Dsh residues in vivo.
All rescue constructs were expressed under the control of the endogenous dsh promoter in a dshV26 (null), f− background; the only Dsh protein present is provided by the transgenic lines (note that all genotypes are forked [f] to control for dshV26, giving the cellular hairs a wavy appearance). For all constructs, several independent lines behaved identically and displayed very similar expression levels, as expected (see Suppl. Fig. S5).
(A–E′) Wings of rescued flies of indicated genotype are shown; low magnification (A–E) highlight normal Wg-signaling: development with of normal sized wing and normal wing margin development demonstrating correct canonical Wnt signaling. High magnification (panels A′-E′) show PCP arrangements of the respective wings. The high magnification pictures show a region near the posterior cross vein and L4. Note PCP defects in the genotypes dsh-Dsh1, dsh-DshY473F, dsh-DshK417R. (F–J) Adult eye sections of the respective genotypes with schematic presentation in lower panels; black and red arrows represent ommatidia with chiral arrangements, which are normally arranged in a mirror-image across the dorso-ventral midline (see panel F for wild-type); green arrows represent symmetric ommatidia. Note that in G–I, the mirror-image arrangement is lost and a random distribution of the two chiral forms and symmetrical ommatidia is detected, reflecting typical PCP defects.
These data are consistent with our observation that Y473 can be phosphorylated by the Abl kinase, which is also required for PCP signaling but not canonical Wnt-signaling (Singh et al., 2010). The neighboring and downstream Tyr residues can also be phosphorylated by Abl in vitro (Singh et al., 2010), and thus we asked whether any of these could be functionally important in the absence Y473 in a redundant manner. We thus tested for potential phenotypic consequences of dsh-, dsh-DshY473F flies in an abl−/+ background. Neither viability nor the PCP defects were different in abl−/+ as compared to abl+ backgrounds (Table 1, Figure 5 and not shown), suggesting that none of the other Tyr residues in the DEP/C-term region of Dsh is (partially) redundant with Y473.
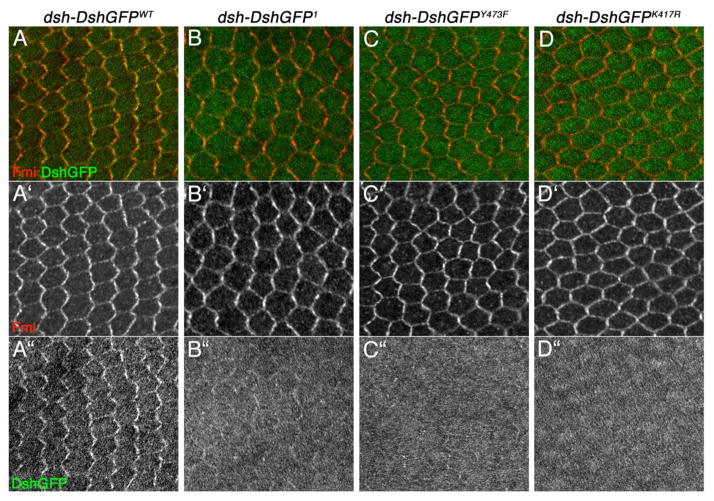
Stable membrane recruitment of Dsh is a hallmark of its role in PCP signaling (Axelrod, 2001; Boutros et al., 2000; Rothbächer et al., 1998) and, for example, reduced in dsh1 mutants or lost altogether in fz− mutant animals (Axelrod, 2001). We thus analyzed the subcellular localization of DshY473F in pupal wings around 30–34 hrs APF. Strikingly, and in contrast to DshWT (Fig. 6A), DshY473F (in the dsh null background) displayed hardly any membrane association in pupal wings (or elsewhere), very similar to the Dsh1 mutant isoform (Fig. 6B–C″). This defect in membrane association is in contrast with any of the S/T-residue cluster mutants that all localized normally to the membrane (data not shown), further indicating a specific requirement of Y473 in PCP signaling.
Figure 6. Localization of mutated Dsh isoforms in pupal wings during PCP signaling. Confocal images of DshGFP (green) and Fmi (red) staining in pupal wings at the 30 to 34hrs APF stage are shown. The respective genotypes are indicated above.
A′-D′ display anti-Fmi monochrome images (highlighting membranes and the PCP complexes localized there), and A″-D″ show monochromes of DshGFP (A–D are the merged channels). Note that in B/B″, C/C″, and D/D″ the membrane levels of DshGFP are reduced or even absent, although the overall signal and protein levels are not affected (see also Suppl. Figure S5).
Similar to the S/T site mutations, expression levels of DshY280F or DshY473F (as detected on Western blots) were very similar to wild-type DshGFP or Dsh1, indicating that protein stability in imaginal discs was unaffected by the DshY473F mutation (Fig. 7; also Fig. S5). However, an obvious difference between wild-type DshGFP and the DshY473F mutant isoform was their protein migration pattern, as detected on Western blots with imaginal disc samples (Fig. 7): DshY473F lacked the slower migrating hyperphosphorylated bands, very similar to Dsh1 (Fig. 7; Axelrod, 2001; Klein et al., 2006). This was in contrast to DshY280F or DshY474F, which displayed a normal Dsh banding pattern (Fig. 7 and not shown).
Figure 7.
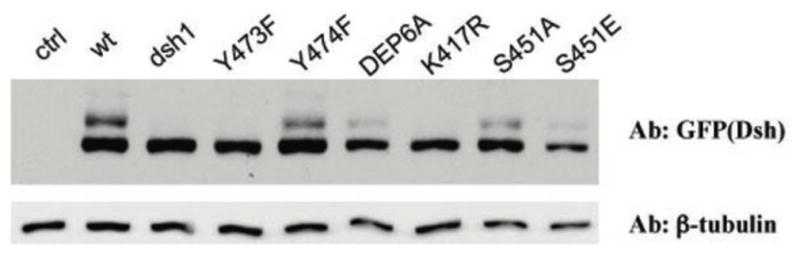
Migration behavior and phophorylation status of mutant DshGFP isoforms as detected by Western blots of late 3rd instar/early pupal tissues. The respective Dsh isoforms are indicated above the gel and were detected with an anti-GFP antibody (“ctrl” lane has no DshGFP protein present as control). All lines are expressed from the endogenous dsh-expression cassette and exhibit very similar protein levels (also Suppl. Figure S5). Note that Dsh1, DshY473F and DshK417R show markedly reduced gel shift, indicative of reduced Dsh phosphoprylation and correlating with their defective behavior in PCP signaling.
DshK417R disrupts PCP signaling very similarly to Dsh1 (DshK417M)
The original PCP specific and first allele of dsh, dsh1, a K to M transition at residue 417, lies within a Protein Kinase C (PKC) consensus site. This observation led to the hypothesis that it could affect Dsh phosphorylation as it also reduces Dsh phosphorylation in cell culture (Klein et al., 2006). However, as mentioned above, the DEP domain PKC site S/T-mutations, including the respective DshT419A or even the DshDEP6A mutant isoform, affecting all potential DEP-domain PKC sites, had no detectable effect on Dsh function. Thus, we reasoned that other types of post-translational modification such as acetylation or ubiquitination that occur on lysines may be involved. K417 is one of five lysines/arginines (K/R) within the DEP domain. A sequence alignment of Dsh of various species revealed that K417 and K465 are exclusively lysines, whereas the other three lysines and arginines appear interchangeable (Fig. 1B). Therefore, we tested whether a replacement of K with R would phenocopy the dsh1 allele (DshK417M) or be fully functional, the latter indicating the importance of the positive charge. DshK417R restores viability/canonical Wg-signaling of dshV26 comparable to dsh1(Table 1 and Fig. 5B) Intriguingly, DshK417R failed to rescue PCP and revealed PCP defects very similar to dsh1 in all tissues analyzed (Fig. 5D, I and not shown). Importantly, in analogy to Dsh1 (DshK417M), subcellular localization/membrane association of DshK417R was affected as well (Fig. 6D-D″). In 34h APF pupal wings, DshK417R was barely found at membranes, very similar to the Dsh1 protein. Further analysis suggested that DshK417R also lacked the slow migrating Dsh bands on Westerns, suggesting reduced activation (Fig. 7). Therefore, we conclude that Lysine417 in the Dsh DEP domain is essential, not merely due to a requirement of electric charges, but possibly due to covalent modification(s) required for PCP function (also Discussion).
Discussion
In vivo, Dsh becomes hyperphosphorylated upon Wnt-pathway activation and/or membrane recruitment (PCP signaling) and due to this correlation, phosphorylation has often been used as a read-out for pathway activation. Previous work has identified a region N-terminal to the PDZ domain, which is rich in Ser/Thr residues and can be phosphorylated in vitro, but it remained questionable whether phosphorylation in this region is functionally significant (Cong et al., 2004; Klein et al., 2006; Ossipova et al., 2005; Strutt et al., 2006). The significance of other predicted phosphorylation sites and events has not been addressed. Our data indicate that, surprisingly, all Ser/Thr residues tested, although highly conserved and phosphorylated, are dispensable for Dsh function in vivo. In particular, it is a striking observation that the lack of all Ser/Thr residues C-terminal to the DEP domain or within the DEP domain has no effect on Dsh function in either signaling pathway. This, taken together with previous observations that the Ser/Thr-rich region N-terminal to the PDZ domain is not essential for Dsh function (Strutt et al., 2006), strongly suggests that Ser/Thr phosphorylation of Dsh/Dvl proteins is generally (almost) non-essential, which is on its own a very surprising and unexpected finding.
In contrast, our data suggest that tyrosine phosphorylation, which previous attempts failed to identify (Willert et al., 1997b), is critical for Dsh function. In particular, Y473 within the DEP domain is critical in PCP signaling and likely phosphorylated by Abl type kinases (Singh et al., 2010). Our data further suggest that the original mutant dsh1 allele (a K417M transition) does not affect phosphorylation, but is likely to cause defects via affecting post-translational modification of Dsh on K417.
Dsh phosphorylation in vivo
Our mass-spectrometry approach identified several phosphorylation sites, including Tyr phosphorylation of Dsh, which has not been previously observed (Willert et al., 1997a). Importantly, several of the individual sites were confirmed to be indeed phosphorylated in vivo through tissue samples and phospho-specific antibodies. Nevertheless, the respective Ser/Thr residues are functionally non-essential (or possibly redundant) for Dsh function in either pathway. Due to the large number of Ser/Thr residues in Dsh/Dvl proteins (>110 in approx. 650 total residues) it is almost impossible to address phosphorylation redundancy in the whole protein and thus, we have focused mostly on the C-terminal region including the DEP domain. All mutations analyzed that contained multiple mutant residues, including all Ser/Thr within the DEP domain or lacked all C-terminal Ser/Thr residues, were fully functional in rescue assays (Wg-signaling and PCP) of a dsh- null mutant background. Taken together with our individual mutations in conserved residues in other regions of Dsh/Dvl and the earlier data on the S/T cluster between the basic region and PDZ domain (Penton et al., 2002; Strutt et al., 2006), our data suggest that Ser/Thr phosphorylation of Dsh is far less important than generally assumed.
In contrast, Tyr-phosphorylation of DshY473 within the DEP domain is essential for Dsh function in PCP signaling (this study and also Singh et al., 2010). The respective DshY473F mutation behaves like the original PCP specific dsh1 alelle: (1) dsh null flies can be rescued to viability, (2) the mutant protein is not recruited stably to the membrane during PCP signaling in vivo, and (3) the mutant protein shows an overall reduced phosphorylation as assessed by gel-shift (Fig. 7; compare also to Dsh1 analysis, e.g. Axelrod, 2001; Klein et al., 2006). Strikingly, Y473 is the only Tyr residue that is functionally required, although Dsh/Dvl proteins contain several tyrosines that are absolutely conserved across all species. For example, Y280 within the PDZ domain, which, although phosphorylated in vivo and conserved in all Dsh/Dvls, appears dispensable in functional rescue assays.
Overall, our data suggest that most phosphorylation target residues, although phosphorylated in vivo, are not essential for Dsh function with the notable exception is Y473, which behaves like a PCP specific dsh allele when mutated.
The Dsh C-terminal region
A striking observation is the lack of a requirement of the C-terminal region of Dsh (C-terminal to the DEP domain). Not only are all potential phosphorylation sites, S/T-sites and tyrosines, dispensable (Table 1), but even a complete deletion of the region C-terminal to residue 504 has no apparent functional consequences: The respective DshΔCterm isoform fully rescued all aspects of Dsh function. This is surprising as the equivalent C-terminal region of Xenopus Dsh has been suggested to be sufficient to localize GFP to the basal body of cilia and to interfere with their polarity when overexpressed (Park et al., 2008). Although patches of Ser/Thr residues are conserved within this C-terminal region (Fig. 1C and 2B), the sequence alignment of vertebrate and Drosophila Dsh proteins show overall less conservation in this protein region. The vertebrate Dvl proteins are highly conserved among themselves in this region, however. Therefore, it is likely that vertebrate Dsh/Dvls share functional requirements within these sequences that are not conserved between Drosophila and vertebrates and might lie outside the canonical Wnt-pathway and PCP signaling.
The nature of the dsh1 allele
The original allele of Dsh, dsh1, a Lys-to-Met transition at position 417 within the DEP domain, is a PCP specific allele (Axelrod et al., 1998; Boutros et al., 1998). As this Lysine is located within a potential PKC consensus site, it has been speculated that it could affect phosphorylation of the nearby serine (Boutros and Mlodzik, 1999; Wallingford and Habas, 2005). We demonstrate that this is not the case and that potential PKC phosphorylation within the DEP domain is not essential for Dsh function. However, our data indicate that it is the Lysine itself that is essential and a mutation to the related Arginine residue (which is similarly charged and often can substitute for Lys) also causes the same defects as the original DshK417M mutation. These data suggest that it is a post-translational modification such as ubiquitination or acetylation of Lys417, as it does not appear to be of structural importance (Wong et al., 2000). This notion is consistent with recent work in mammalian cell culture (Ganner et al., 2009), although we cannot exclude a direct involvement of Lys417.
In summary, our analyses have revealed that many phosphorylation events on Dsh are functionally dispensable in vivo, even when they occur on highly conserved residues. This is in stark contrast to many previous assumptions and suggestions and thus an important observation. In addition, we have identified that Dsh is phosphorylated on Tyr residues, which has not been observed earlier. Consistent with recent data on an Abl requirement in PCP (Singh et al., 2010), Tyr-phosphoryation events on at least one site, DshY473, are critical for Dsh function in PCP signaling.
Supplementary Material
Research Highlights.
Although Dsh is a key cytoplasmic component of both Wnt/Fz-pathways, regulation of its signaling specificity is not well understood.
Dsh is phosphorylated, but the functional significance of its phosphorylation remains unclear.
We have systematically investigated the phosphorylation of Dsh by combining mass-spectrometry analyses, biochemical studies, and in vivo genetic methods in Drosophila.
Our approaches identified multiple phospho-residues of Dsh in vivo. Our data define three novel and unexpected conclusions:
(1) Strikingly and in contrast to common assumptions, all conserved serines/threonines are non-essential for Dsh function in either pathway;
(2) phosphorylation of conserved Tyrosine473 in the DEP domain is critical for PCP-signaling - DshY473F behaves like a PCP-specific allele;
(3) defects associated with the PCP specific dsh1 allele, DshK417M, located within a putative Protein Kinase C consensus site, are likely due to a post-translational modification requirement of Lys417, rather than phosphorylation nearby.
our data indicate that while many Ser/Thr and Tyr residues are indeed phosphorylated in vivo, strikingly most of these phosphorylation events are not critical for Dsh function with the exception of DshY473.
Acknowledgments
We thank J. Axelrod, S. Cohen, R. Nusse, and the Bloomington Drosophila Stock Center for fly strains and reagents. We are grateful to Sophy Okello and Gustavo Garcia for technical help, Melissa Mariani and Katya Serysheva for careful editing of the manuscript, and all Mlodzik and Jenny lab members for helpful comments and suggestions throughout these analyses. This work was supported by NIH grants EY13256 to MM and GM088202 to AJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential requirement of Dishevelled provides signaling specificity in the Wingless and planar cell polarity signaling pathways. Genes & Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and DFrizzled-2 function as redundant receptors for wingless during drosophila embryonic development [In Process Citation] Development. 1999;126:4175–86. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Bhat KM. Frizzled and Frizzled2 play a partially redundant role in Wingless signaling and have similar requirements to Wingless in neurogenesis. Cell. 1998;95:1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–22. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mihaly J, Bouwmeester T, Mlodzik M. Signaling specificity by Frizzled receptors in Drosophila. Science. 2000;288:1825–1828. doi: 10.1126/science.288.5472.1825. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Chen C-N, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24:2000–11. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollar GL, Weber U, Mlodzik M, Sokol SY. Regulation of Lethal giant larvae by Dishevelled. Nature. 2005;437:1376–80. doi: 10.1038/nature04116. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Mlodzik M. Microscopic analysis of the adult Drosophila retina using semithin plastic sections. Methods Mol Biol. 2008;420:277–87. doi: 10.1007/978-1-59745-583-1_17. [DOI] [PubMed] [Google Scholar]
- Ganner A, Lienkamp S, Schafer T, Romaker D, Wegierski T, Park TJ, Spreitzer S, Simons M, Gloy J, Kim E, Wallingford JB, Walz G. Regulation of ciliary polarity by the APC/C. Proc Natl Acad Sci U S A. 2009;106:17799–804. doi: 10.1073/pnas.0909465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–76. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Curr Biol. 2006;16:1337–43. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–63. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Rogulja A, Cadigan KM. Wingless eliminates ommatidia from the edge of the developing eye through activation of apoptosis. Development. 2004;131:2409–18. doi: 10.1242/dev.01104. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Mendoza-Topaz C, Mieszczanek J, Bienz M. Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization. J Cell Sci. 2010;123:1588–99. doi: 10.1242/jcs.067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Mueller H, Samanta R, Wieschaus E. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development. 1999;126:577–586. doi: 10.1242/dev.126.3.577. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell. 2005;8:829–41. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. 2008 doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Nat Genet. 2008 Jul;40(7):871–9. doi: 10.1038/ng.104. Epub 2008 Jun 15. Nat Genet, 40, 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton A, Wodarz A, Nusse R. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics. 2002;161:747–62. doi: 10.1093/genetics/161.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes & Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Rothbächer U, Laurent MN, Cho KWY, Fraser SE. A dual role for Dishevelled protein and its involvement in early dorsal-ventral patterning of Xenopus. 1998 submitted. [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–26. [PubMed] [Google Scholar]
- Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–77. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–94. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Yanfeng WA, Grumolato L, Aaronson SA, Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 2010;24:2157–68. doi: 10.1101/gad.1961010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapps WR, Tomlinson A. Transducing properties of Drosophila Frizzled proteins. Development. 2001;128:4829–35. doi: 10.1242/dev.128.23.4829. [DOI] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–13. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006;16:1329–36. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Polarity determination in the Drosophila eye. Curr Opin Genet Dev. 1999;9:442–446. doi: 10.1016/S0959-437X(99)80067-7. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–36. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–58. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. Embo J. 1997a;16:3089–96. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. Embo J. 1997b;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H-C, Bourdelas A, Krauss A, Lee H-J, Shao Y, Wu D, Mlodzik M, Shi D-L, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Mao J, Nguyen JT, Srinivas S, Zhang W, Liu B, Li L, Wu D, Zheng J. Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat Struct Biol. 2000;7:1178–84. doi: 10.1038/82047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Klein TJ, Mlodzik M. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:1004–1014. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–9. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–75. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.