Abstract
The dorsal motor nucleus of the vagus (DMV) contains preganglionic neurons that control gastric motility and secretion. Stimulation of different parts of the DMV results in a decrease or an increase in gastric motor activities, suggesting a spatial organization of vagal preganglionic neurons in the DMV. Little is known about how these preganglionic neurons in the DMV synapse with different groups of intragastric motor neurons to mediate contraction or relaxation of the stomach. We used pharmacological and immunohistochemical methods to characterize intragastric neural pathways involved in mediating gastric contraction and relaxation in rats. Microinjections of l-glutamate (l-Glu) into the rostral or caudal DMV produced gastric contraction and relaxation, respectively, in a dose-related manner. Intravenous infusion of hexamethonium blocked these actions, suggesting mediation via preganglionic cholinergic pathways. Atropine inhibited gastric contraction by 85.5± 4.5%. Gastric relaxation was reduced by intravenous administration of l-NAME (52.5±11.9%) or VIP antagonist (56.3±14.9%). Combined administration of l-NAME and VIP antagonist inhibited gastric relaxation evoked by l-Glu (87.8±4.3 %). Immunohistochemical studies demonstrated choline acetyltransferase immunoreactivity in response to l-Glu microinjection into the rostral DMV in 88% of c-Fos–positive intragastric myenteric neurons. Microinjection of l-Glu into the caudal DMV evoked expression of nitric oxide (NO) synthase and VIP immunoreactivity in 81% and 39%, respectively, of all c-Fos–positive intragastric myenteric neurons. These data indicate spatial organization of the DMV. Depending on the location, microinjection of l-Glu into the DMV may stimulate intragastric myenteric cholinergic neurons or NO/VIP neurons to mediate gastric contraction and relaxation.
Keywords: brain stem, autonomic, vagal control, gastric motility, parasympathetic motor neurons
Introduction
The dorsal motor nucleus of the vagus (DMV) contains the cell bodies of the vagal preganglionic neurons that control gastric motility and secretion (7, 19, 23, 24,). Using retrograde (16) and anterograde (3) neural tracers, it has been estimated that greater than 80% of neurons in the DMV have projections to the stomach. Most of these neurons projecting to the stomach are cholinergic in nature, as gastric contractions or relaxations mediated by the vago-vagal reflex usually are blocked by hexamethonium (11, 35). Cholinergic preganglionic neurons in the DMV may synapse with intragastric cholinergic neurons mediating gastric contraction, whereas others may synapse with intragastric nitric oxide (NO) or vasoactive intestinal polypeptide (VIP) neurons regulating gastric relaxation (5, 24, 25, 35, 38). In this manner, the vagal pathways to the stomach may mediate both gastric contraction and gastric relaxation.
Neurons in the feline DMV, retrogradely labeled with horseradish peroxidase injected into the region of the lower esophageal sphincter, can be divided into two groups: one rostral and the other caudal to the obex (25). Stimulation of different parts of the DMV results in either a decrease or increase in lower esophageal sphincter pressure and gastric motor activity and both responses are abolished by vagotomy (25). This suggests spatial organization of the vagal preganglionic neurons in the DMV. Stimulation of distinct populations of vagal preganglionic neurons in the DMV may result in activation of the vagal nerve mediating excitatory and inhibitory pathways to the stomach. In keeping with the aforementioned study in the cat, subsequent studies from ours and other laboratories have demonstrated that microinjections of l-glutamate (l-Glu) into different regions of the DMV cause a different pattern of gastric responses in rodents. Microinjection of glutamate into the rostral part of the DMV evokes gastric contraction, whereas injection to the caudal part causes gastric relaxation in rats (8, 13, 20, 43) and mice (20). Although these observations support the existence of vagal excitatory and inhibitory pathways, the specific nature of the neurons in the gastric myenteric plexus to mediate these actions remains to be characterized.
In vitro preparations have demonstrated a frequency-dependent release of various neurotransmitters in response to vagal stimulation. Low-frequency (2–5 Hz) stimulation of the myenteric nerve in the guinea pig myenteric plexus selectively evokes acetylcholine (ACh) release, whereas high-frequency (10–50 Hz) stimulation mainly stimulates VIP release (1). Yokotani et al. (40) demonstrated that maximum release of ACh in response to vagal stimulation was observed at 5 Hz in the rat stomach. Relaxation of rat fundic strips evoked by transmural stimulation at lower frequencies was completely abolished by NG-nitro-l-arginine (l-NNA), a NO synthesis inhibitor (17). On the other hand, responses to stimulation at higher frequencies were reduced by trypsin. This suggested that relaxation of the rat gastric fundus in response to low-frequency stimulation was mediated by NO whereas peptidergic neurotransmitter(s) were released at higher frequencies (4). Takahashi and Owyang (36) confirmed these observations by demonstrating that vagal stimulated release of NO and VIP from the gastric myenteric neurons are frequency dependent. Vagal stimulation provoked a significant increase of NO in the stomach at 2.5 Hz; VIP release occurred mainly with vagal stimulation at a frequency greater than 10 Hz. In the same study, Takahashi and Owyang (36) showed that a NO synthesis inhibitor, l-NAME markedly antagonized the rapid relaxation but had no effect on the delayed relaxation, whereas the VIP antagonist significantly reduced the delayed relaxation without affecting the rapid relaxation. Gastric contraction in response to vagal stimulation (5 Hz) was abolished by atropine (36,40). Hence, these studies demonstrate that activation of the vagus nerve evokes ACh and NO/VIP release from the intragastric myenteric neurons via nicotinic synapse, which causes contraction and relaxation of the stomach, respectively. However, the central mechanisms controlling specific neurotransmitter release are not clear.
It has been demonstrated that microinjection of substance P into the nucleus raphe obscurus evokes vagal mediated gastric relaxation, a reaction that is partially blocked by the NO synthase (NOS) inhibitor and VIP antagonist (14). This indicates involvement of NO and VIP release to mediate gastric relaxation in whole animal. On the other hand, gastric relaxation may also be achieved by dysfacilitation of cholinergic input to the stomach. The central control of these two vagal pathways to mediate gastric relaxation is unknown.
Glutamate is a major excitatory neurotransmitter within the mammalian CNS. Several lines of evidence indicate that glutamate is involved in controlling the activities of DMV neurons. First, glutamate immunoreactivity is found in both axon terminals and cell bodies within the nucleus of the solitary tract and the DMV (27, 34). Second, in vitro experiments have shown glutamate-induced excitatory synaptic currents (37). Finally, several studies have demonstrated changes in gastric motor activity in response to microinjection of glutamate into the DMV of the rat (6, 8, 13, 15, 22, 30). In the present study we used pharmacological and immunohistochemical methods to characterize the vagus pathways and the intragastric myenteric neurons mediating gastric contraction and relaxation. We hypothesized that depending on the location, microinjection of L-Glu into the DMV may stimulate intragastric cholinergic neurons or NO/VIP neurons to mediate gastric contraction and relaxation respectively. Three makers, choline acetyltransferase (ChAT), the enzyme which synthesizes acetylcholine, neuronal nitric oxide synthase (nNOS), the enzyme which synthesizes nitric oxide, and VIP, an inhibitory non-adrenergic, non-cholinergic transmitter in the gastro-intestinal tract were used in this study. Acetylcholine was considered to be a main excitatory neurotransmitter whereas NO and VIP were considered to be principal inhibitory transmitters in the GI tract (5, 38).
Materials and Methods
Animal preparation
Experiments were performed on adult male Sprague-Dawley rats (250–350 g), obtained from Charles River Laboratories (Wilmington, MA, USA), in accordance with NIH guidelines and as approved by the University of Michigan Health Center Institutional Animal Care and Use Committee. Before each experiment, food was withheld overnight but water was provided. The rats were anesthetized with an intraperitoneal (i.p.) injection of urethane (1.0–1.25 g/kg). Catheters were inserted into the femoral artery and vein to monitor arterial blood pressure and drug injection, respectively. The depth of anesthesia was assessed by changes in blood pressure or by the reflex response to toe pinches. Both cervical vagus nerves were carefully isolated and the area was moistened with saline. A homeothermic blanket was used to maintain the body temperature at 37 ± 1°C. If necessary, a tracheal cannula was used to facilitate artificial ventilation with room air using a small animal ventilator (Harvard Apparatus, Holliston, MA).
The surface of the medulla was exposed via a dorsal approach. Muscle covering the occipital part of the skull was carefully removed, and part of the occipital plate was then removed with a small rongeur. The dura was cut under the dissection microscope using a custom-made needle. The cerebellum was retracted slightly and the subarachnoid covering was carefully removed. The calamus scriptorius (CS), which was defined as the caudal most pole of the area postrema (AP), was used as a landmark to locate the DMV (8).
Intragastric pressure measurement
Intragastric pressure (IGP) was measured by a method previously described by Lu and Owyang (18). Briefly, following laparotomy, an intragastric balloon made from the little finger of a small latex glove was tied around a section of polyethylene tubing (PE 160) and inserted into the body of the stomach via a small incision made in the duodenum. The balloon was secured with a suture to prevent movement. The tubing was connected to a pressure transducer, which was connected to an amplifier (TBM4M, WPI). The stomach was inflated by introducing warm saline (1.0–2.0 mL) into the balloon to achieve a baseline pressure of 5–10 cm H2O. The animal was then positioned in a stereotaxic apparatus. Intragastric pressure was displayed on-line using a Cambridge Electronic Design Micro1401 data acquisition system (Cambridge, UK).
Microinjection studies
Five-barreled glass micropipettes were used for microinjection of l-Glu (Sigma-Aldrich, St. Louis, MO). The multibarreled micropipettes (tip diameter, 15–30 µm) filled with l-Glu (0.1, 0.25, 0.5, and 1.0 pmol/nL, respectively), saline, and Fluoresbrite microbeads (0.50 µm, Polysciences, Warrington, PA) in separate barrels were connected to a nanopump (PV830, WPI). The glass micropipettes were guided by a micromanipulator.
The DMV was divided into rostral and caudal regions based on its anatomical relationship with the AP. The portion of the DMV extending from the CS to the anterior border of the AP was defined as the ‘rostral DMV’, and the portion of the nucleus extending caudal from the CS was defined as the ‘caudal DMV’. Injections were made at the level of the rostral most region of the AP (i.e. the rostral DMV) and at the level caudal to the CS (i.e. the caudal DMV). All microinjections were delivered either on the right or left DMV. Injections were given in volumes of 20 nl over a period of 10–15 s. Stereotaxic coordinates were originally chosen based on histological materials presented in Paxinos & Watson (21). Final coordinates for placements of the tip of the multibarrel glass micropipette within the rostral DMV (coordinates: 0.2–0.6 mm rostral to the CS, 0.3–0.6 lateral from the midline, and 0.5–0.7 mm ventral from the dorsal surface of the medulla) were selected based on our previous studies (8) wherein microinjection of l-Glutamate produced transient elevations in IGP. Conversely, coordinates for placements within the caudal DMV (coordinates: 0.0 mm to 0.3 mm caudal from the CS, 0.3–0.5 mm lateral from the midline, and 0.6–0.8 mm ventral from the dorsal surface of the medulla) were determined from sites where injections of l-Glu evoked gastric relaxation.
The injection site was marked by fluorescein-labeled fluorescent microbeads for postmortem identification at the end of the experiment. At the end of each experiment, rats were sacrificed by intravenous injection (i.v.) of an overdose of urethane and the brain was quickly removed and fixed with 4% buffered paraformaldehyde over 72 h. The medulla was sectioned (50-µm cross sections) on a cryostat. The sections were initially examined with a fluorescence microscope to determine the location of the injection sites. Fluoresbrite microspheres (excitation, 360 nm) florescence was visualized using a UV filter set 01 (excitation 365±12 nm, emission LP 397 nm). Computer images of the sections were digitized, and then sections were stained with neutral red for identification. The location of the center of each injection (determined as the center of the visible fluorescence produced by the beads) was placed on the cross sections plot. The section with the largest cross-sectional area of the beads was used to determine the center of the injection using the atlas of Paxinos and Watson (21).
Experimental protocol for l-Glu–induced gastric responses
IGP responses after microinjection of l-Glu into the DMV were studied. To characterize the neurotransmitters of the vagal efferent pathways, the following chemicals were injected intravenously: hexamethonium bromide (10 mg/kg), atropine (0.5 mg/kg), l-NAME (10 mg/kg), and a VIP antagonist (10−6 M) (Bachem, Torrance, CA). The doses of antagonists used were based on previous studies demonstrating effective blocking of gastric contraction or relaxation evoked by vagal stimulation (36). To determine whether the glutamate-induced changes in IGP were due to activation of preganglionic motor neurons in the DMV, cervical vagotomy was performed to test whether drug-induced changes in intragastric pressure were vagally mediated. In these experiments, IGP studies were only performed in those rats whose blood pressure and breathing had returned to baseline and remained stable for at least 15 min (8).
Analysis of microinjection data
Data from IGP measurements were analyzed using the Spike2 program designed for the CED data acquisition system. Prior to initiation of microinjections, values were calculated over a 5 min control period during which the baseline IGP trace remained stable. The lowest points of the IGP trace obtained over the 5 min segment were averaged, and the results were used as an index of gastric tone. After microinjections of drug into the DMV, the maximum or minimum value in the IGP trace (also derived from a 5 min segment) was taken to represent the largest change in gastric tone. The peak increases and decreases in IGP were compared with the pre-injection baseline levels.
Tissue preparation for immunofluorescent labeling
Rats were anesthetized with urethane (1.0–1.25 g/kg, i.p.). Stimulation of the DMV with l-Glu was performed in two groups of rats. In one group (n = 6), l-Glu was microinjected into the left rostral part of the DMV and in the other group (n = 5), microinjection was performed in the left caudal part of the DMV. Detection of l-Glu–stimulated c-Fos expression in the intragastric myenteric neurons was performed. l-Glu (10 nL, 1 pmol/nL) was injected over a period of 10–15 s each time with a 5-min interval, for 30 min. Saline injections were performed in a similar fashion in another group of rats (n = 4), which served as the controls. At the end of the experiment, the microinjection electrode was removed and the rats were left for 60 min before tissue preparation. Sixty min was allowed because it has been shown that maximal expression of c-Fos occurs at 60 min after termination of stimulation (41). Our preliminary studies confirmed that there was no significant difference between 60 and 90 minutes and this period appeared to be optimal for cFos expression studies. Rats were euthanized with an overdose of urethane. For preparation of tissue sections, rats were perfused transcardially with 100 mL of PBS (0.1 M), followed by 300–500 mL of ice-cold 4% paraformaldehyde in PBS (0.1 M, pH 7.4). The brain stem was removed for histological identification of the microinjection site. The stomach was removed and divided along the curvatures into ventral (ipsilateral to the microinjection site in the brain stem) and dorsal (contralateral to the microinjection site in the brain stem) parts. Both parts were postfixed overnight in the same fixative in PBS and placed in 20% sucrose in PBS (0.1 M) for 2 days. Subsequently, the tissues were rinsed in PBS, cut into 5×5 mm blocks, cryoprotected in sucrose, and frozen in a mixture of 20% sucrose and Tissue-Tek (Bayer, Elkhart, IN) OCT embedding compound. Coronal sections (20-µm thick) were cut using a cryostat and mounted on SuperFrost glass slides. The slides with brain stem slices under coverslips were placed in a humid immunohistochemistry chamber for immunofluorescent labeling. To obtain whole-mount gastric preparations, the stomach was removed and opened along the greater and lesser curvature. The gastric fundus and corpus were pined flat in a sylgard coated Petri dish filled with ice-cold Krebs’ solution. The mucosa was removed and the tissue was fixed for 2 hours in Zamboni’s solution containing 2% paraformaldehyde. The tissue was rinsed in PBS (0.1M) and the whole-mount preparations were prepared by removal of the circular muscle layer.
Immunofluorescent labeling
Immunohistochemical detection of c-Fos protein in activated neurons and neurochemical coding of these neurons were performed using immunofluorescent labeling procedures. Sections or whole-mount preparations that contained the myenteric plexus were washed (3 times for 5 min each time) in PBS and preincubated in a blocking solution containing 5% normal donkey serum and 0.3% Triton X-100 in PBS. The preparations were then incubated with the primary antibodies for 12–22 h at room temperature. The following antiserum and antibodies were used: rabbit anti Fos (Ab-5, 1:10,000, Oncogene), goat anti-choline acetyltransferase (ChAT, 1:250, Chemicom), mouse anti-neuronal NOS (1:500, BD Biosciences, San Jose, CA), and sheep anti-VIP (1:1,000, Chemicon). After incubation with the primary antibodies, the preparations were washed 3 times in PBS (5 min each time) and then incubated for 60 min at room temperature with species-specific secondary antibodies conjugated to fluorescein isothiocyanate (FITC, 1:100, Jackson ImmunoResearch) or Alexa Fluor 488 (1:250, Molecular Probes) and cyanine dye Cy3 (1:250, Jackson ImmunoResearch). Tissues were washed 3 times (5 min each time) in PBS. Subsequently, whole-mount preparations were mounted on gelatin-coated glass slides. The sections and whole-mount preparations and were coverslipped using anti-fading aqueous mounting medium (Gel/Mount, Biomeda, Foster City, CA). Tissues were examined and photographed using a Zeiss Axioplan2 fluorescent microscope. The FITC, Alexa Fluor 488 or rhodamine labeled elements were visualized using appropriate filters. Filter set 10 (excitation 450–490 nm and emission 515–565 nm), was used for the visualization of FITC and Alexa Fluor 488; and the filter cube used for Cy3 visualization was filter set 15 (excitation 546±12 nm and emission LP 590 nm). Images were recorded using AxioCam camera (Zeiss) and AxioVision software (Zeiss). Specificity of immunohistochemistry labeling was tested by a variety of control experiments. Omission of the primary antibody resulted in no staining. Preparations were also examined under a LSM 510 Laser Scanning Confocal Microscope mounted on a Zeiss Axiovert 100M inverted microscope. Digital camera images of 1012×1012 pixels were processed using image analysis software (Zeiss LSM Image browser V3.5).
Quantitative analysis of c-Fos expression in intragastric myenteric neurons that displayed nNOS, ChAT, or VIP immunoreactivity
l-Glu was microinjected into the left part of the DMV. Stomach tissues from either the ventral part (ipsilateral to the site of stimulation of the DMV) or the dorsal part (contralateral to the site of stimulation) of the corpus were examined by double immunostaining of c-Fos with ChAT, nNOS, or VIP antibody. Using a fluorescent microscope (Axioplan2, Zeiss) with 20× or 40× objectives, ChAT-, nNOS- and VIP- immunoreactive neurons in myenteric ganglia were counted in each ganglion. Only neurons within the border of intact myenteric ganglia were counted. Similar numbers of either ChAT-, nNOS- or VIP-immunoreactive neurons in the dorsal part of the stomach were examined for c-Fos expression as an experimental control to ensure that c-Fos expression in the intragastric myenteric ganglia was the result of activation of the vagal pathway by l-Glu stimulation of the brain stem and not due to nonspecific stimulation-induced effects such as gastric contraction or relaxation and others. Four rats injected with saline were used and a similar number of gastric myenteric neurons in the stomach were also examined for experimental control.
Data analysis
For both the immunochemistry studies and intragastric pressure studies, values were expressed as means ± SE. Statistical analysis was performed using either one-way ANOVA followed by student Newman Keuls multiple-comparisons test or the student t test. Significance was accepted at the level of P < 0.05.
Results
l-Glu–induced changes in intragastric pressure
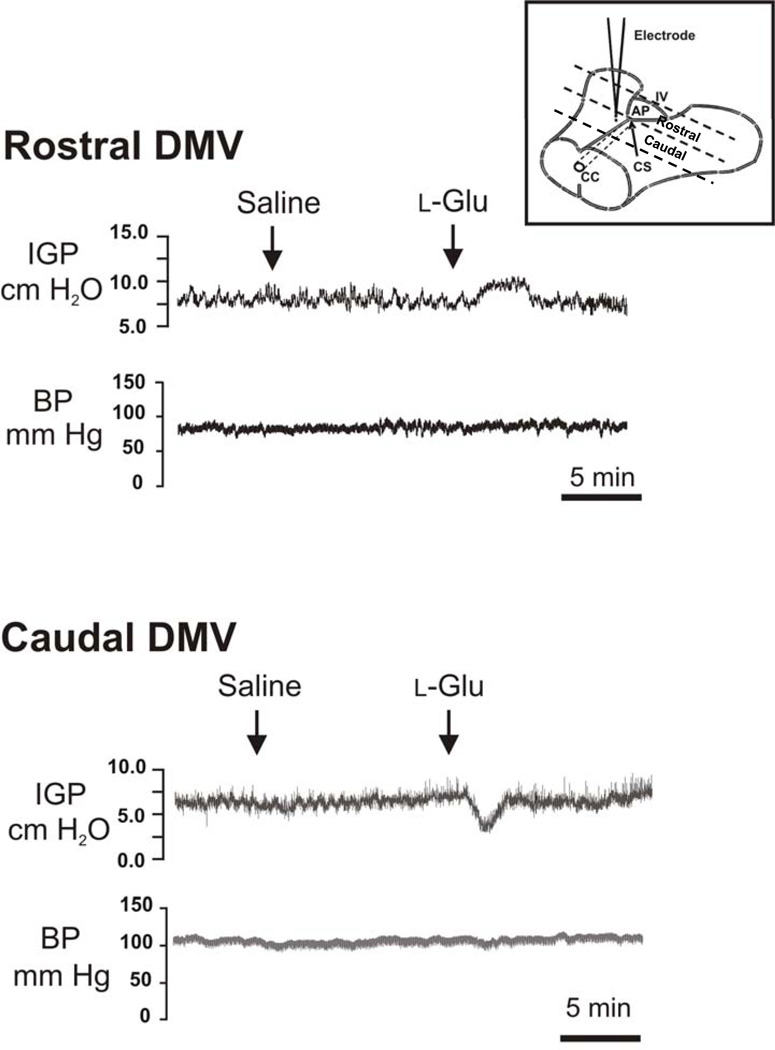
Microinjection of l-Glu into the rostral and caudal parts of the DMV evoked opposite changes in intragastric pressure. As shown in Fig. 1, microinjection of l-Glu (10 pmol, 20 nL) into the rostral part of the DMV caused a transient increase in intragastric pressure without affecting the mean arterial pressure. By contrast, microinjection of the same dose of l-Glu into the caudal part of the DMV evoked a decrease in intragastric pressure resulting in intragastric relaxation (Fig. 1). Again, these changes occurred without any change in mean arterial pressure. The ability of l-Glu to cause contraction or relaxation appeared to be transient and the intragastric pressure returned to basal values within 1–5 min. In the controls, microinjection of 20 nL saline into the rostral or caudal parts of the DMV did not cause any significant changes in intragastric pressure or arterial blood pressure.
Figure 1. Effects of microinjection of l-Glu into the DMV on intragastric pressure (IGP).
Microinjection of l-Glu into the rostral part of the DMV (0.3 mm rostral to the calamus scriptorius at the level of the area postrema) caused an increase in intragastric pressure, whereas microinjection into the caudal part of the nucleus (at a level 0.1 mm posterior to the calamus scriptorius) caused a decrease in intragastric pressure. Blood pressure (BP) did not change significantly. Microinjection of saline into the same site in the brain stem did not evoke noticeable changes in intragastric pressure or blood pressure. Insert, schematic drawing of the landmarks on the brain stem surface to indicate the location of the rostral and caudal part of the DMV. CC, central cannel; CS, the calamus scriptorius; AP, area postrema; IV, fouth ventricle.
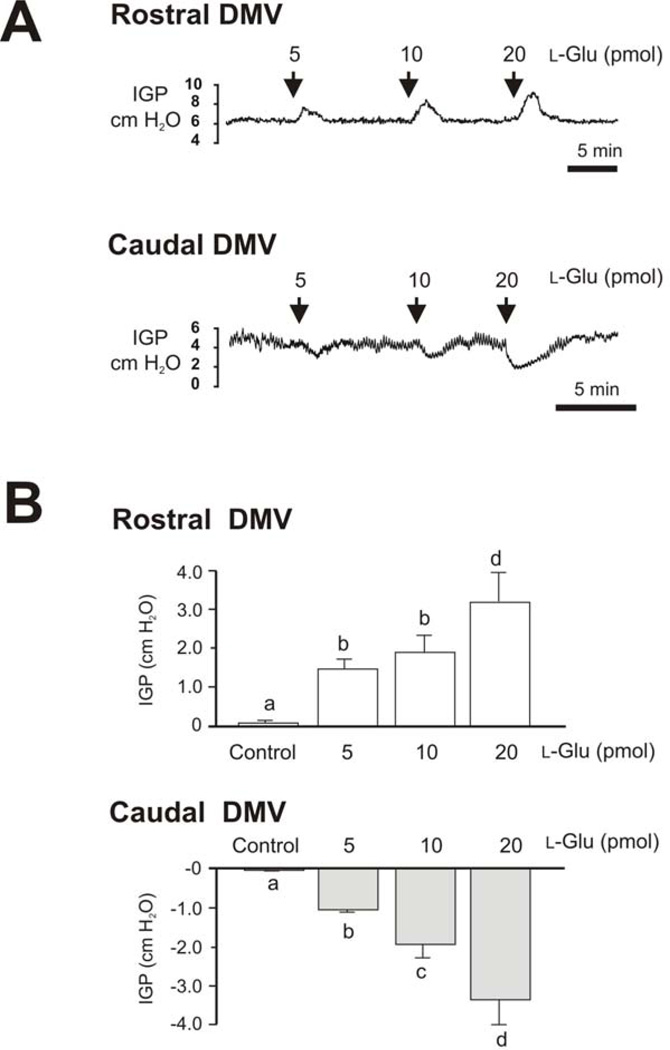
The effect of microinjection of l-Glu into the rostral or caudal parts of the DMV appeared to be dose related. Representative intragastric pressure recordings in response to microinjection of different doses of l-Glu into the rostral and caudal parts of the DMV are shown in Fig. 2. Microinjections of 5, 10, and 20 pmol l-Glu (20 nL) into the rostral DMV caused respective dose-related increases in intragastric pressure of 1.43 ± 0.3, 1.91 ± 0.1, and 3.2 ± 0.6 cm H2O (n=7, P < 0.05, Fig. 2). In contrast, microinjections of the same doses of l-Glu (20 nL) into the caudal part of the DMV produced dose-related decreases in intragastric pressure of −1.04 ± 0.07, −1.9 ± 0.2, and −3.8 ± 1.0 cm H2O, respectively (n=6, P < 0.05, Fig. 2).
Figure 2. Dose-related responses of intragastric pressure (IGP) to microinjection of l-Glu into the DMV.
A. Representative recordings of intragastric pressure (IGP) show a dose-related response to microinjections of l-Glu into the DMV. l-Glu produced gastric contractions when injected into the rostral part of the DMV and gastric relaxations when injected into the caudal part of the nucleus.
B. Bar chart shows dose-related responses of intragastric pressure (IGP) to microinjection of l-Glu into the rostral and caudal DMV and saline control. Each bar represents mean±SE of 5 to 8 experiments. Each bar issignificantly different from each other based on ANOVA one-way analysis of variance followed by student Newman Keuls multiple-comparisons test (P<0.001).
Pharmacological studies of the effect of l-Glu on intragastric pressure
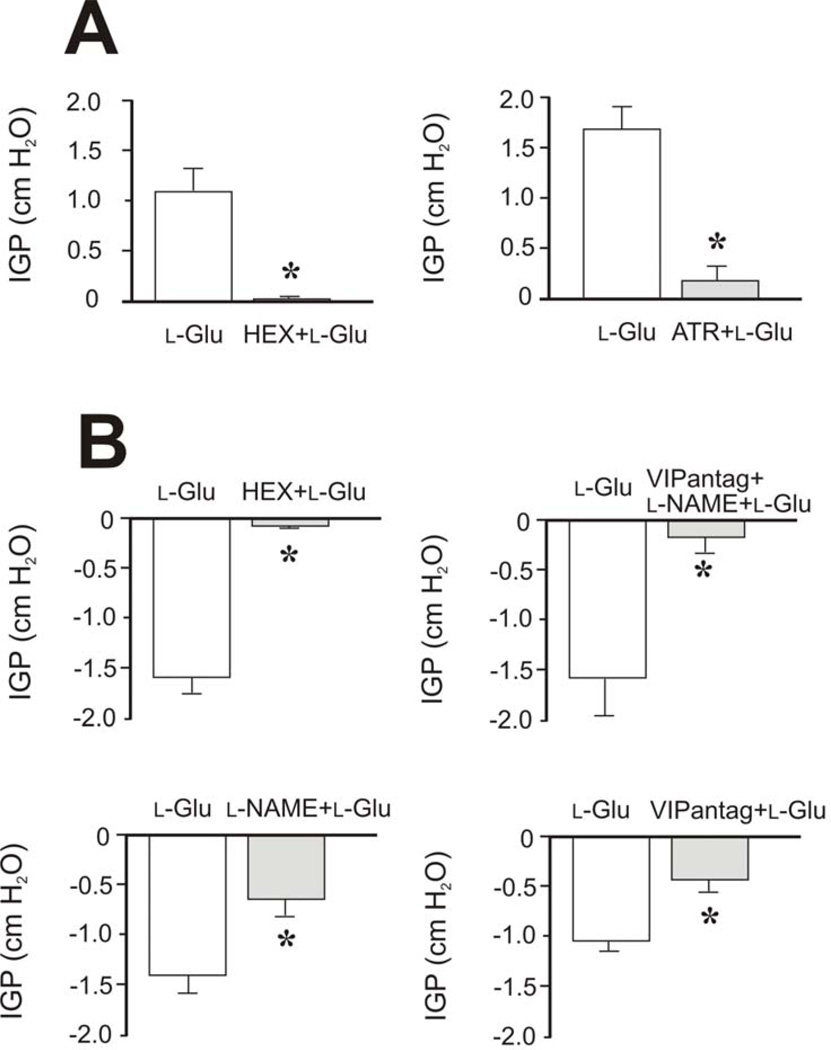
Further studies were performed to determine the neural pathways activated by microinjection of l-Glu into the rostral and caudal parts of the DMV. Intravenous administration (i.v.) of hexamethonium (10 mg/kg) completely prevented gastric contraction evoked by l-Glu (10 pmol) microinjected into the rostral part of the DMV (reduction by 93.3±4.2% compared to control, n = 4) and gastric relaxation evoked by injection of the same dose of l-Glu into the caudal part of the DMV (reduction by 93.5±2.9% compared to control, n = 5) (Fig. 3). This suggests that l-Glu acts on the DMV to mediate gastric contraction and relaxation via preganglionic cholinergic pathways.
Figure 3. Effects of hexamethonium, atropine, l-NAME, and VIP antagonist on l-Glu–induced changes in intragastric pressure.
Microinjections of l-Glu (10 pmol, 20 nL) were administered into the rostral (A) and caudal (B) DMV. A: Pretreatment with hexamethonium (HEX, 10 mg/kg, i.v.), a ganglionic nicotinic blocking agent, blocked l-Glu–induced gastric contraction (n=4) (left panel). This suggests that microinjection of l-Glu into the rostral part of the DMV evokes gastric contraction via preganglionic cholinergic pathways. Pretreatment with atropine (ATR, 0.5 mg/kg, i.v.) markedly inhibited the l-Glu–induced increase in intragastric pressure (n=5) (right panel), suggesting that microinjection of l-Glu into the rostral part of the DMV evokes gastric contraction mainly via intragastric cholinergic neurons. B: Pretreatment with hexamethonium (HEX, 10 mg/kg, i.v.) blocked l-Glu–induced gastric relaxation (n=5), indicating that gastric relaxation evoked by microinjection of l-Glu into the caudal part of the DMV is mediated via preganglionic cholinergic pathways. Administration of l-NAME (10 mg/kg, i.v.), a potent inhibitor of nitric oxide synthesis, partially inhibited gastric relaxation induced by microinjection of l-Glu into the caudal part of the DMV (n=7). Similarly, pretreatment with VIP antagonist (VIPantag, [p-choloro-d-Phe6, Leu17] VIP, 30 nmol/kg) also reduced gastric relaxation evoked by l-Glu (n=7). Combined administration of l-NAME and VIP antagonist completely blocked gastric relaxation (n=4). These findings suggest that gastric relaxation evoked by l-Glu injected into the caudal part of the DMV is mediated by intragastric nitric oxide synthase (nNOS)– and VIP-containing neurons. *P < 0.05 compared to l-Glu administration.
Ipsilateral vagotomy at the cervical level abolished gastric contraction or relaxation evoked by microinjection of l-Glu into the rostral (3/3) or caudal (3/3) DMV, confirming that the effects of l-Glu are mediated by the vagus nerve. In separate studies, we demonstrated that atropine (0.5 mg/kg, i.v.) inhibited gastric contraction evoked by microinjection of l-Glu (10 pmol) by 85.5± 4.5% (n = 5, P < 0.05) (Fig. 3). On the other hand, gastric relaxation evoked by microinjection of l-Glu (10 pmol) into the caudal part of the DMV was reduced either by pretreatment with l-NAME (10 mg/kg, i.v.) (52.5±11.9%, n = 7, P < 0.05) or VIP antagonist (10−6 M) (56.3±14.9%, n = 7, P < 0.05) (Fig. 3). Combined administration of l-NAME and VIP antagonist markedly inhibited gastric relaxation evoked by l-Glu (87.8±4.3%, n = 4, P < 0.05) (Fig. 3). These observations suggest that l-Glu–induced gastric relaxation was mainly mediated by the release of NO and VIP in the gastric myenteric neurons. The locations of the microinjection sites were determined by postmortem examination as described in Materials and Methods (Fig. 4 and Fig. 5).
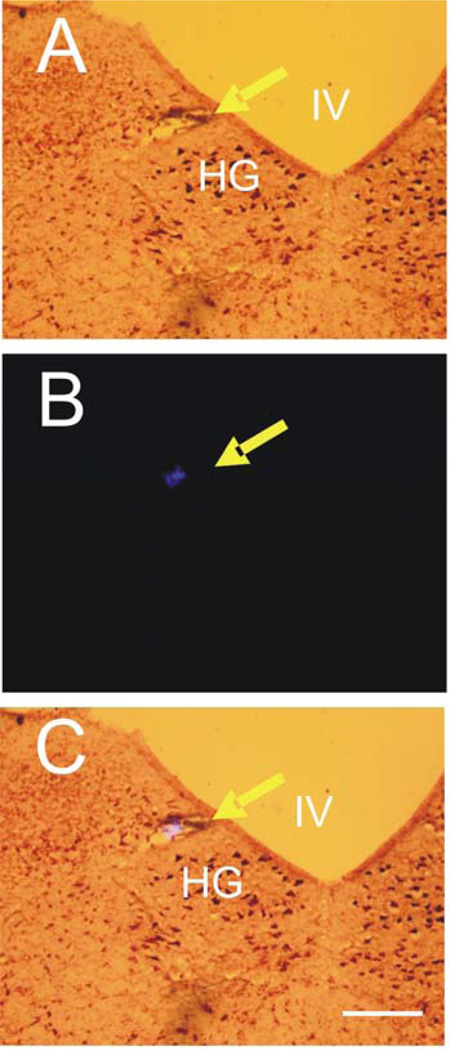
Figure 4. Histological identification of the microinjection sites in the medulla oblongata.
The injection sites of l-Glu identified by the deposit of fluorescent microbeads which were injected at the same sites of l-Glu microinjection at the end of the experiment. A: Brain stem section stained with neutral red. B: Computer image of fluorescent microbeads. C: Image of brain stem section overlapped with computer image of fluorescent microbeads. IV, fourth ventricle; HG, hypoglossal nucleus. Arrow indicates site of microinjection. Calibration bar: 500 µm.
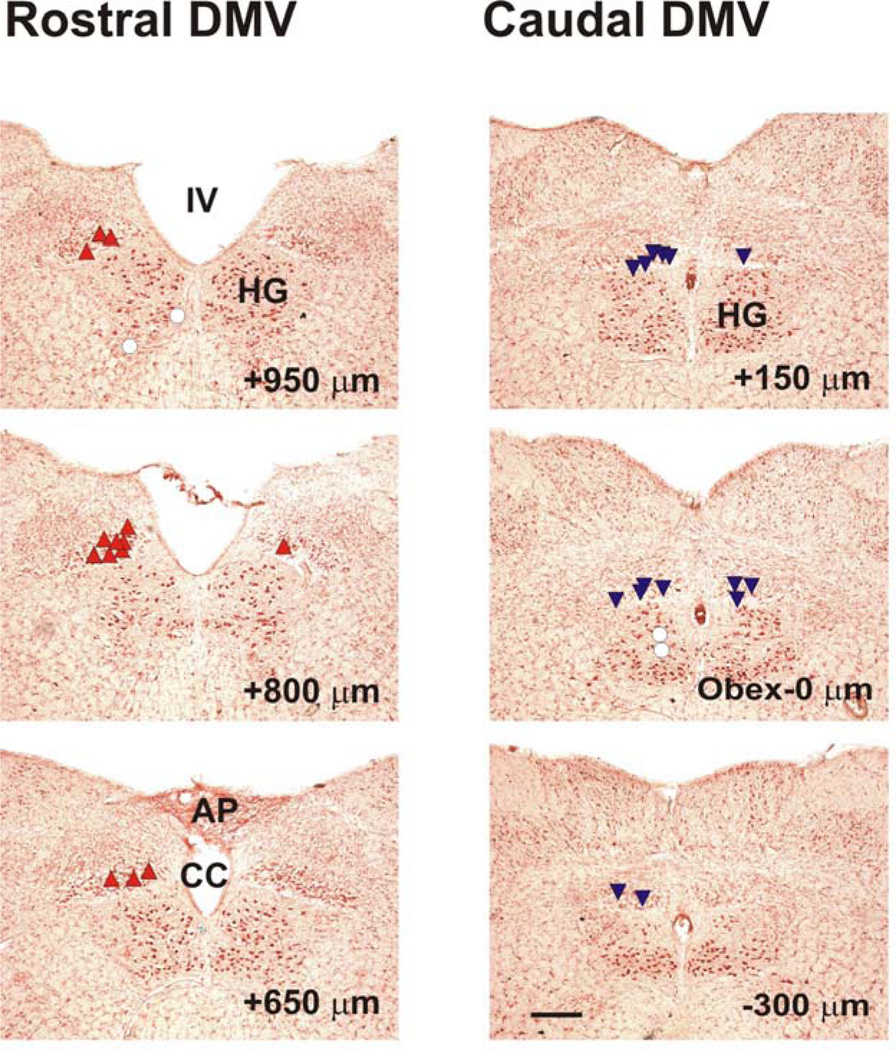
Figure 5. Distribution of sites of l-Glu microinjection on coronal sections of the medulla oblongata.
The sites where microinjections of L-Glu produced an increase in intragastric pressure are labeled with upright triangles (σ). The sites with no response or a slight response to injection of l-Glu are labeled with open circles (μ). The sites which produced a decrease in intragastric pressure are labeled with inverted triangles (τ). The distance (in µm) of each section from the obex is indicated in the lower right corner of each section. IV, fourth ventricle; HG, hypoglossal nucleus; AP, area postrema; CC, central channel. Calibration bar: 300 µm.
Differential activation of intragastric ChAT- and nNOS/VIP-containing neurons by microinjection of l-Glu into the rostral and caudal DMV
Staining of c-Fos immunoreactivity combined with double or triple labeling of ChAT, nNOS, and/or VIP antibody were performed to identify intragastric myenteric neurons activated by injection of l-Glu into the rostral or caudal parts of the DMV. Immunohistochemical detection of protein products of the immediate early gene c-Fos in the intragastric myenteric neurons was used to identify the activation of postganglionic neurons in the stomach. c-Fos immunoreactivity was detected in the nuclei of intragastric myenteric neurons containing ChAT, nNOS, or VIP (Fig. 6). Stimulation of the rostral part of the DMV by l-Glu predominantly activated cholinergic neurons, as c-Fos expression was mainly detected in intragastric ChAT-containing neurons (Fig. 6, 7). In contrast, microinjection of l-Glu into the caudal part of the DMV, which caused gastric relaxation, stimulated c-Fos expression in neurons containing nNOS and VIP but not ChAT (Fig.6, 7). ChAT and nNOS immunoreactive neurons were not colocalized in the same group of intragastric myenteric neurons.
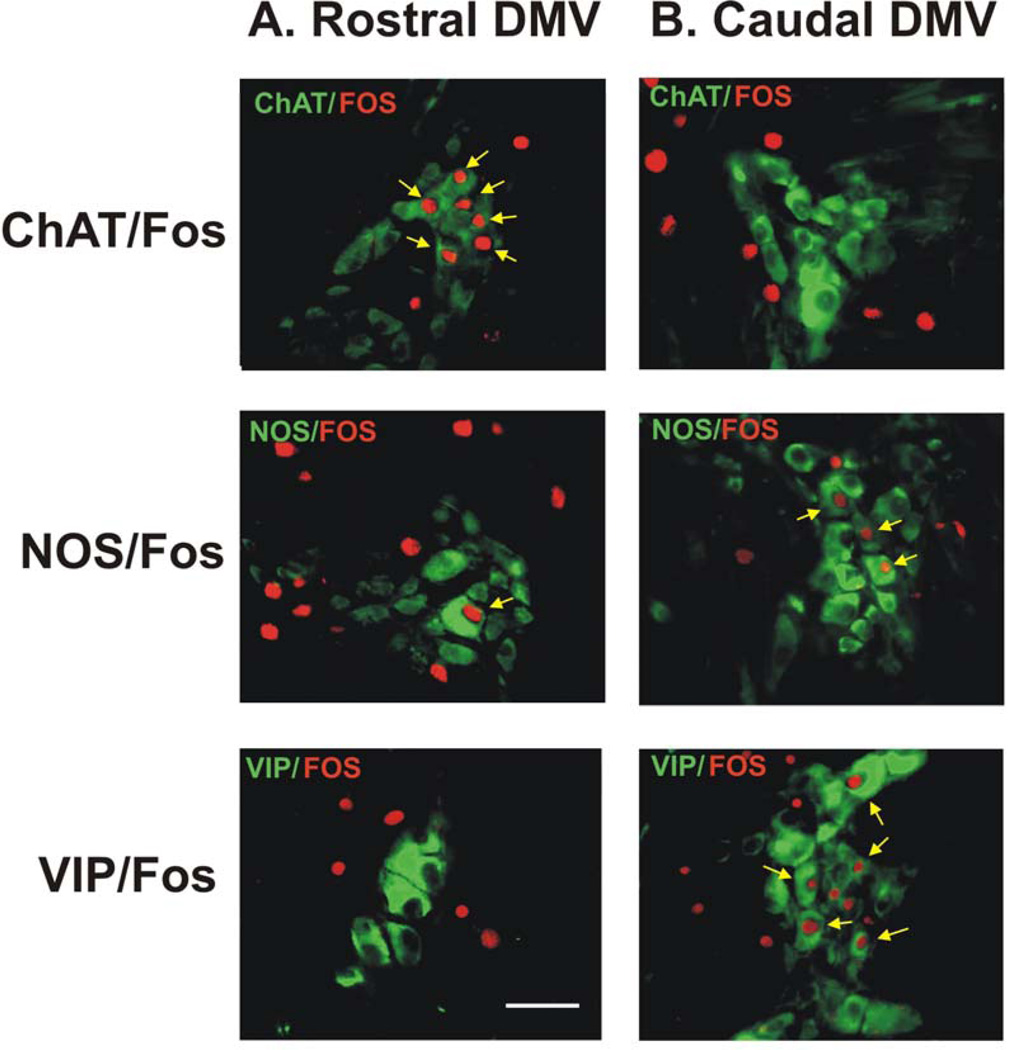
Figure 6. Immunohistochemical demonstration of double labeling for c-Fos and choline acetyltransferase (ChAT), neuronal nitric oxide synthase (nNOS), or VIP in intragastric myenteric ganglionic neurons in flat sections of gastric wall of rats.
Stimulation of DMV neurons in the rostral part of the nucleus (panel A) induced Fos immunoreactivity (Cy3, red) in intragastric myenteric neurons that mainly contain ChAT but not nNOS or VIP. In contrast, stimulation of DMV neurons in the caudal part of the nucleus (panel B) induced FOS immunoreactivity in intragastric myenteric neurons that mainly contain nNOS and VIP but not ChAT. Arrows indicate c-Fos–positive ChAT-, nNOS-, or VIP-containing neurons. Calibration bar: 50 µm.
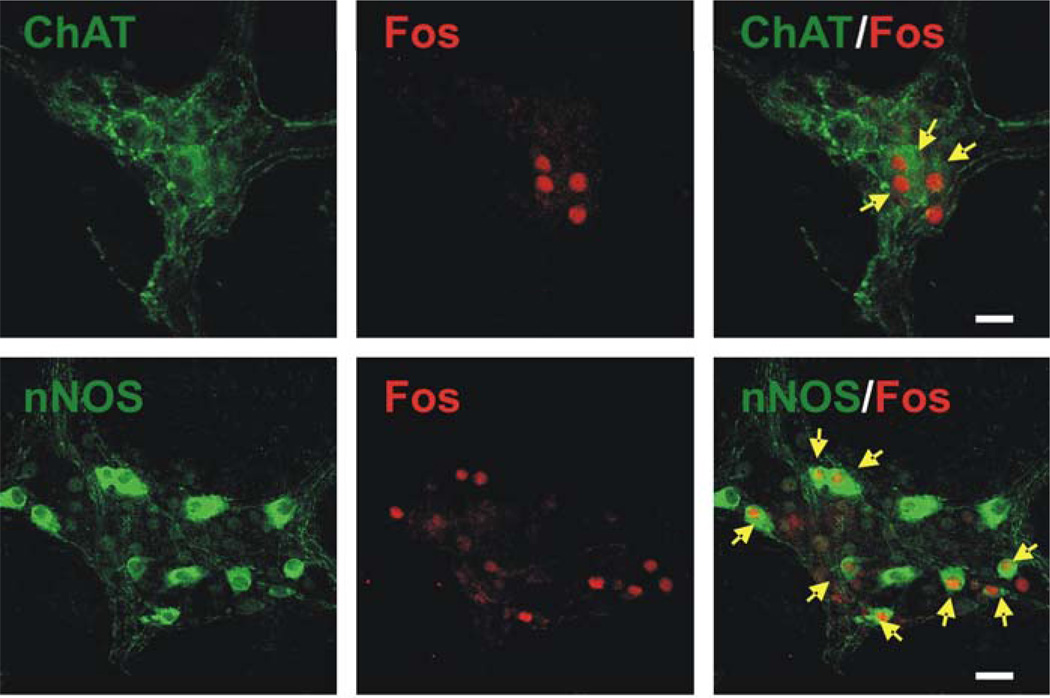
Figure 7. Immunohistochemical demonstration of double labeling for c-Fos and choline acetyltransferase (ChAT) or nitric oxide synthase (nNOS) in intragastric myenteric ganglionic neurons in whole mount preparation.
Stimulation of DMV neurons in the rostral part of the nucleus (upper panel) induced Fos immunoreactivity (Cy3, red) in intragastric myenteric neurons that mainly contain ChAT. In contrast, stimulation of DMV neurons in the caudal part of the nucleus (lower panel) induced FOS immunoreactivity in intragastric myenteric neurons that mainly contain nNOS. Arrows indicate c-Fos–positive ChAT- or nNOS containing neurons. Calibration bar: 50 µm.
Quantitative analysis of intragastric myenteric neurons that expressed ChAT, nNOS, or VIP immunoreactivities and were c-Fos–positive after l-Glu administration was performed; the data are summarized in Table 1. ChAT immunoreactivity in response to microinjection of l-Glu in the rostral part of the DMV was evident in 88.2% of c-Fos–positive neurons. On the other hand, 81.2% and 39.4% of all c-Fos–positive intragastric myenteric neurons expressed nNOS and VIP immunoreactivities, respectively, in response to microinjection of l-Glu into the caudal DMV (Table 1).
Table 1.
c-Fos Immunoreactive Neurons in Intragastric Ganglia Following Microinjection of L-Glu into the DMV
| Rostral | Caudal | |
|---|---|---|
| ChAT+Fos / Fos | 908/1029 (88.2%)* | 203/1197 (16.9%) |
| NOS+Fos / Fos | 94/1287 (7.3%) | 1138/1402 (81.2%)* |
| VIP+Fos / Fos | 20/696 (2.8%) | 264/670 (39.4%)* |
P<0.05
In the control group, very few c-Fos–positive neurons were observed in the stomach wall contralateral to the microinjection site. Similarly, only a few c-Fos–positive neurons were found in saline-injected group.
Discussion
It has been suggested that the excitation of vagal motor neurons that increases contractility acts by stimulation of cholinergic postganglionic enteric neurons in the stomach, whereas excitation of vagal neurons that inhibit gastric motility is thought to result from the activation of vagal neurons that synapse with postganglionic non-adrenergic, non-cholinergic (NANC) intragastric motor neurons (5,38). Our results confirmed and extended these observations by clearly showing that first, spatial organization in the rat DMV controls gastric contraction and relaxation. Depending on the location of the microinjection, l-Glu caused gastric contraction when injected into the rostral part of the DMV and relaxation when injected into the caudal part of the nucleus. Second, l-Glu activates DMV cholinergic preganglionic neurons that synapse with intragastric cholinergic neurons or nitrergic/VIPergic neurons in the gastric wall to mediate gastric contraction and relaxation, respectively.
In this study we employed urethane as anesthetic since it has been used successfully to investigate the effect of DMV stimulation on gastric motor function in rats (29). However it is recognized that urethane may have adverse effects on the CNS which can complicate interpretation of data. To clarify this issue we have repeated some of our motility studies in rats anesthetized with a mixture of xylacaine and ketamine and found that they produced similar results (data not shown) (29).
In addition to cholinergic neurons, nNOS-containing neurons are present in the DMV and found primarily in the most rostral end, although some have been identified at a site in the DMV caudal to the obex (9, 14). These NOS-containing neurons in the DMV (14) innervate the lower esophageal sphincter (10) and stomach (41) via the vagus nerve. Consequently, it has been proposed that nitrergic neurons in the DMV are involved in gastric relaxation (14, 42). In the present study, the inhibitory responses of the stomach evoked by l-Glu injected into the caudal part of the DMV were blocked by hexamethonium, a nicotinic receptor antagonist. This suggests that projections of the cholinergic neurons in the DMV synapse with myenteric neurons in the stomach. Studies with l-NAME and a VIP antagonist further delineate the nature of these postsynaptic pathways in the gastric wall. The neurons in the caudal part of the DMV are likely cholinergic, synapsing with NANC-, NO-, and VIP-containing intragastric myenteric neurons to mediate gastric relaxation. Since most DMV neurons contain glutamate receptors (2, 12), microinjection of l-Glu would excite neurons in this nucleus, including cholinergic and NOS-containing neurons. However, because hexamethonium blocked glutamate-induced gastric activities, nitrergic neurons in the DMV are unlikely to play a role, if any, in glutamate-induced gastric relaxation. Alternatively, the strength of the stimulation evoked by the doses of l-Glu used in this study was not strong enough to excite those NOS-containing neurons.
l-Glu stimulation of the rostral part of the DMV produced gastric contraction. Immunocytochemistry studies revealed that c-Fos expression was mainly found in intragastric ChAT-containing neurons (88%). However, c-Fos expression was also detected in some NOS-containing (7.3%) and some VIP-containing (2.8%) intragastric myenteric neurons, which indicates that some of the NANC neurons were activated during the stimulation. Indeed, it has been reported that stimulation of neurons in the rostral part of the DMV may evoke minor gastric relaxation (15). This finding may be explained by our observations that a group of neurons in the rostral part of the DMV may have a synaptic connection with intragastric NANC neurons producing relaxation. However, the excitatory effects were dominant when neurons in the rostral part of the DMV were activated by l-Glu, overriding the minor inhibitory effects of NANC neurons.
l-Glu stimulation of the caudal part of the DMV produced gastric relaxation. Our immunocytochemistry studies revealed that c-Fos expression was mainly found in nNOS- (81%) and VIP- (39%) containing neurons. Pharmacological study demonstrated equal contribution of NO and VIP (NO vs. VIP: 47.1% vs. 43.6%) to mediate gastric relaxation, in response to l-Glu stimulation at a dose of 10 pmol. It is conceivable that not all the intragastric NOS-containing neurons innervate gastric muscle. Some of these neurons may innervate blood vessels or secretory cells. Interestingly, c-Fos expression was also found in some ChAT-containing neurons (16.9%) when l-Glu was injected into the caudal part of the DMV. In the present study, l-Glu–induced gastric relaxation was blocked by hexamethonium. The intragastric pressure remained at baseline after hexamethonium treatment. In the guinea pig, gastric cholinergic and nitrergic neurons are mostly separated and colocalization has not been documented (39). It was reported that 10% to 12% of intragastric cholinergic neurons contain VIP (28, 39). In human rectum there is an even greater degree of colocalization between ChAT and VIP neurons (28). Our quantitative data was obtained using a double immunostaining technique. Consequently, ChAT-positive neurons could also contain VIP. Takahashi and Owyang (36) showed that optimal NO and VIP release occurred at different frequencies of electrical stimulation. Release of NO occurred at a lower intensity and VIP release at a higher intensity in response to vagal stimulation. It is conceivable that l-Glu stimulation of the caudal part of the DMV selectively evoked VIP release from ChAT/VIP neurons. Future experiments involving triple staining of c-Fos with VIP and ChAT will address this issue.
There is ample evidence that neurons in the solitary tract nucleus utilize either GABA or glutamate as neurotransmitters to mediate inhibitory and excitatory effects on the DMV neurons (37). Sivaro et al (31) demonstrated that although there is no direct evidence for tonic glutamatergic inputs to the DMV, administration of GABA receptor antagonist induced gastric contraction via a glutamatergic pathway since kyneurenic acid abolished gastric contractions induced by bicuculline. One possible explanation is that tonic GABA inputs act on the presynaptic GABA receptor to inhibit glutamate excitatory inputs to DMV neurons. Our demonstration that neurons of the DMV is highly sensitive to L-Glu stimulation indicates that glutamate can directly stimulate the DMV to modulate gastric motility via the vagal pathways. It has been shown that stimulation of the nucleus of the solitary tract by l-Glu causes a change in blood pressure along with changes in intragastric pressure (32,33). Elevated blood pressure alone may also cause changes in gastric pressure. In a dog study, Semba et al. (26) observed that a gastric excitatory response was accompanied by a decrease in blood pressure, whereas a gastric inhibitory response was accompanied by an increase in blood pressure. In our studies, blood pressure remained unchanged during microinjection of l-Glu into the DMV (Fig. 1). Our data demonstrate that l-Glu–induced changes in gastric motility were eliminated by ipsilateral vagotomy. This strongly suggests that l-Glu–induced changes in gastric motility are caused by stimulation of neurons in the DMV rather than stimulation of the nucleus of the solitary tract or evoked by other secondary effects such as changes in blood pressure.
In summary, we have demonstrated that depending on the location of the stimulation (i.e. rostral or caudal) l-Glu may activate DMV neurons to mediate gastric contraction and relaxation. Cholinergic neurons in the gastric corpus mainly respond to l-Glu stimulation of neurons in the rostral part of the DMV; both nitrergic and VIPergic neurons are responsive to l-Glu stimulation in the caudal part of the DMV producing gastric relaxation. This spatial organization of the DMV cholinergic neurons provides the structural basis for differential regulation of DMV neurons projecting to the stomach. Different groups of glutamate-containing neurons from the brain stem or higher centers projecting to the DMV may regulate gastric contraction or relaxation depending on whether they synapse with cholinergic neurons in the rostral or caudal part of DMV.
Acknowledgments
This study was supported by the American Diabetes Association Grant 1-06-JF-58 to SY Zhou and the National Institute of Diabetes and Digestive and Kidney Diseases Grants P30-DK34933, DK-48419, DK-58913, and DK-061423 to C. Owyang.
References
- 1.Agoston DV, Conlon JM, Whittaker VP. Selective depletion of the acetylcholine and vasoactive intestinal polypeptide of the guinea-pig myenteric plexus by differential mobilization of distinct transmitter pools. Exp Brain Res. 1988;72:535–542. doi: 10.1007/BF00250599. [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol. 1998;402:75–92. [PubMed] [Google Scholar]
- 3.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260(1 Pt 2):R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 4.Boeckxstaens GE, Pelckmans PA, De Man JG, Bult H, Herman AG, Van Maercke YM. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch Int Pharmacodyn Ther. 1992;318:107–115. [PubMed] [Google Scholar]
- 5.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what’s new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira M, Singh A, Dretchen KL, Kellar KJ, Gillis RA. Brainstem nicotinic receptor subtypes that influence intragastric and arterial blood pressures. J Pharmacol Exp Ther. 2000;294:230–238. [PubMed] [Google Scholar]
- 7.Feng HS, Lynn RB, Han J, Brooks FP. Gastric effects of TRH analogue and bicuculline injected into dorsal motor vagal nucleus in cats. Am J Physiol. 1990;259(2 Pt 1):G321–G326. doi: 10.1152/ajpgi.1990.259.2.G321. 1990. [DOI] [PubMed] [Google Scholar]
- 8.Grabauskas G, Zhou SY, Das S, Lu Y, Owyang C, Moises HC. Prolactin-releasing peptide affects gastric motor function in rat by modulating synaptic transmission in the dorsal vagal complex. J Physiol. 2004;561:821–839. doi: 10.1113/jphysiol.2004.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo JJ, Browning KN, Rogers RC, Travagli RA. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2001;280:G361–G367. doi: 10.1152/ajpgi.2001.280.3.G361. [DOI] [PubMed] [Google Scholar]
- 10.Hyland NP, Abrahams TP, Fuchs K, Burmeister MA, Hornby PJ. Organization and neurochemistry of vagal preganglionic neurons innervating the lower esophageal sphincter in ferrets. J Comp Neurol. 2001;430:222–234. doi: 10.1002/1096-9861(20010205)430:2<222::aid-cne1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Ohga A, Ohta T. Gastric relaxation and vasoactive intestinal peptide output in response to reflex vagal stimulation in the dog. J Physiol. 1988;404:683–693. doi: 10.1113/jphysiol.1988.sp017313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler JP, Baude A. Distribution of AMPA receptor subunits GluR1-4 in the dorsal vagal complex of the rat: a light and electron microscope immunocytochemical study. Synapse. 1999;34:55–67. doi: 10.1002/(SICI)1098-2396(199910)34:1<55::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Krowicki ZK, Burmeister MA, Berthoud HR, Scullion RT, Fuchs K, Hornby PJ. Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am J Physiol Gastrointest Liver Physiol. 2002;283:G465–G472. doi: 10.1152/ajpgi.00264.2001. [DOI] [PubMed] [Google Scholar]
- 14.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Krowicki KZ, Sivarao VD, Abrahams PT, Hornby JP. Excitation of dorsal motor vagal neurons evokes non-nicotinic receptor-mediated gastric relaxation. J Auton Nerv Syst. 1999;77:83–89. [PubMed] [Google Scholar]
- 16.Leslie RA, Gwyn DG, Hopkins DA. The central distribution of the cervical vagus nerve and gastric afferent and efferent projections in the rat. Brain Res Bull. 1982;8:37–43. doi: 10.1016/0361-9230(82)90025-9. 3. [DOI] [PubMed] [Google Scholar]
- 17.Li CG, Rand MJ. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Owyang C. Secretin at physiological doses inhibits gastric motility via a vagal afferent pathway. Am J Physiol. 1995;268:G1012–G1026. doi: 10.1152/ajpgi.1995.268.6.G1012. [DOI] [PubMed] [Google Scholar]
- 19.Lynn RB, Feng HS, Han J, Brooks FP. Gastric effects of thyrotropin-releasing hormone microinjected into the dorsal vagal nucleus in cats. Life Sci. 1991;48:1247–1254. doi: 10.1016/0024-3205(91)90519-h. [DOI] [PubMed] [Google Scholar]
- 20.Monroe MJ, Hornby PJ, Partosoedarso ER. Central vagal stimulation evokes gastric volume changes in mice: a novel technique using a miniaturized barostat. Neurogastroenterol Motil. 2004;16:3–4. doi: 10.1046/j.1365-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 22.Raybould HE, Jakobsen LJ, Novin D, Tache Y. TRH stimulation and l-glutamic acid inhibition of proximal gastric motor activity in the rat dorsal vagal complex. Brain Res. 1989;495:319–328. doi: 10.1016/0006-8993(89)90224-2. [DOI] [PubMed] [Google Scholar]
- 23.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- 25.Rossiter CD, Norman WP, Jain M, Hornby PJ, Benjamin S, Gillis RA. Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol. 1990;259:G899–G906. doi: 10.1152/ajpgi.1990.259.6.G899. [DOI] [PubMed] [Google Scholar]
- 26.Semba T, Kimura N, Fuji K. Bulbar influence of gastric motility. Jpn J Physiol. 1969;19:521–533. doi: 10.2170/jjphysiol.19.521. [DOI] [PubMed] [Google Scholar]
- 27.Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res. 1997;778:302–308. doi: 10.1016/s0006-8993(97)01058-5. [DOI] [PubMed] [Google Scholar]
- 28.Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn’s disease. Neurogastroenterol Motil. 2001;13:255–264. doi: 10.1046/j.1365-2982.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- 29.Shi M, Jones AR, Ferreira M, Sahibzada N, Gillis RA, Verbalis JG. Glucose does not activate nonadrenergic, noncholinergic inhibitory neurons in the rat stomach. Am J Physiol Regul Integr Comp Physiol. 2004;288:R742–R750. doi: 10.1152/ajpregu.00561.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sivarao DV, Krowicki ZK, Abrahams TP, Hornby PJ. Vagally-regulated gastric motor activity: evidence for kainate and NMDA receptor mediation. Eur J Pharmacol. 1999;368:173–182. doi: 10.1016/s0014-2999(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 31.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABA-A receptors in rat hindbrain nuclei controlling gastric function. Neurogastroenterol Motil. 1999;368:173–182. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 32.Spencer SE, Talman WT. Central modulation of gastric pressure by substance P: a comparison with glutamate and acetylcholine. Brain Res. 1986;385:371–374. doi: 10.1016/0006-8993(86)91085-1. [DOI] [PubMed] [Google Scholar]
- 33.Spencer SE, Talman WT. Modulation of gastric and arterial pressure by nucleus tractus solitarius in rat. Am J Physiol. 1986;250:R996–R1002. doi: 10.1152/ajpregu.1986.250.6.R996. [DOI] [PubMed] [Google Scholar]
- 34.Sykes RM, Spyer KM, Izzo PN. Demonstration of glutamate immunoreactivity in vagal sensory afferents in the nucleus tractus solitarius of the rat. Brain Res. 1997;762:1–11. doi: 10.1016/s0006-8993(97)00368-5. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 38.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Ann Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanden Berghe P, Coulie B, Tack J, Mawe GM, Schemann M, Janssens J. Neurochemical coding of myenteric neurons in the guinea-pig antrum. Cell Tissue Res. 1999;297:81–90. doi: 10.1007/s004410051335. [DOI] [PubMed] [Google Scholar]
- 40.Yokotani K, Okuma Y, Nakamura K, Osumi Y. Release of endogenous acetylcholine from a vascularly perfused rat stomach in vitro; inhibition by M3 muscarinic autoreceptors and alpha-2 adrenoceptors. J Pharmacol Exp Ther. 1993;266:1190–1195. [PubMed] [Google Scholar]
- 41.Zheng H, Berthoud HR. Functional vagal input to gastric myenteric plexus as assessed by vagal stimulation-induced Fos expression. Am J Physiol Gastrointest Liver Physiol. 2000;279:G73–G81. doi: 10.1152/ajpgi.2000.279.1.G73. [DOI] [PubMed] [Google Scholar]
- 42.Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience. 1999;90:685–694. doi: 10.1016/s0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhou S-Y, Lu YX, Owyang C. l-Glutamate mediates gastric contraction and relaxation by activating different subpopulations of cholinergic neurons in the vagal dorsal motor nucleus.(abst) Gastroenterology. 2001;120:A533. [Google Scholar]