Abstract
Background
Articular cartilage defects are most often caused by trauma and osteoarthritis and less commonly by metabolic disorders of the subchondral bone, such as osteonecrosis and osteochondritis dissecans. Such defects do not heal spontaneously in adults and can lead to secondary osteoarthritis. Medications are indicated for symptomatic relief. Slow-acting drugs in osteoarthritis (SADOA), such as glucosamine and chondroitin, are thought to prevent cartilage degeneration. Reconstructive surgical treatment strategies aim to form a repair tissue or to unload compartments of the joint with articular cartilage damage.
Methods
In this article, we selectively review the pertinent literature, focusing on original publications of the past 5 years and older standard texts. Particular attention is paid to guidelines and clinical studies with a high level of evidence, along with review articles, clinical trials, and book chapters.
Results
There have been only a few randomized trials of medical versus surgical treatments. Pharmacological therapies are now available that are intended to treat the cartilage defect per se, rather than the associated symptoms, yet none of them has yet been shown to slow or reverse the progression of cartilage destruction. Surgical débridement of cartilage does not prevent the progression of osteoarthritis and is thus not recommended as the sole treatment. Marrow-stimulating procedures and osteochondral grafts are indicated for small focal articular cartilage defects, while autologous chondrocyte implantationis mainly indicated for larger cartilage defects. These surgical reconstructive techniques play a lesser role in the treatment of osteoarthritis. Osteotomy near the knee joint is indicated for axial realignment when unilateral osteoarthritis of the knee causes axis deviation.
Conclusion
Surgical reconstructive techniques can improve joint function and thereby postpone the need for replacement of the articular surface with an artificial joint.
Hyaline articular cartilage enables the physiological functioning of joints (e1– e3). Osteoarthritis, trauma, and disorders of the subchondral bone—such as osteochondritis dissecans (OCD) or osteonecrosis, which secondarily affect the articular cartilage—may cause defects of the cartilage (e4– e7). In spite of their different etiology, the clinical end stage is identical: reduced articular function, manifesting as pain and impaired movement. After complete loss of the articular cartilage, endoprosthetic surface replacement is often the only remaining therapeutic option (e8). Reconstructive joint-preserving treatments are therefore of high clinical relevance (e9).
This article provides an overview of medical and surgical approaches to cartilage reconstruction, which are indicated if cartilage defects remain symptomatic in spite of conservative and medical treatment. Since several different disorders result in cartilage loss, we will not exclusively be focusing on the topic of osteoarthritis (e10). This article will neither reflect on aspects of prevention (e11), nor the wide range of physical-physiotherapeutic measures (e12)—which constitute essential components within the multimodal therapeutic approach—nor rheumatic disorders (e13– e16), owing to the complexity of these areas. Neither will we discuss current topics—such as the application of chondrogenic factors as proteins (e17) or genes (e18), tissue engineering of cartilage in bioreactors (e19), testing of biocomposites with improved scaffolds (e20), or preclinical pharmacological therapies (e21).
Definitions
Chondral defects are limited to the cartilage (1, e5, e9). Osteochondral defects extend into the subchondral bone (1, e5, e9). Since regeneration means the identical reconstruction of the original articular cartilage, repair results in a distinct, potentially inferior, form of cartilage (1, e5, e9).
Epidemiology
Osteoarthritis is the most common articular disorder: some 10% of men older than 60 develop osteoarthritis (e22– e24). A prospective study of 1000 arthroscopies of the knee joint found cartilage defects in 61% of cases, 44% of these due to osteoarthritis, 28% due to focal cartilage defects, and 2% due to OCD (e25). In 3% of all patients over the age of 50, knee pain is due to osteonecrosis (e26).
Etiology
A multitude of disorders can lead to articular cartilage defects (Table 1). Circumscribed cartilage defects whereby the surrounding cartilage remains in a normal condition often arise as a result of trauma or OCD (e5, e9). Osteoarthritis is characterized by areas of poorly delineated defects. Primary osteoarthritis is a complex pathology in whose genesis genetic, biomechanical, and biochemical factors have a role (e27– e29). It may also be caused by secondary—for example, traumatic—defects to the articular cartilage (e30). OCD is a potentially reversible disorder primarily of the subchondral bone (e4, e5). If it extends to the articular cartilage, then an osteochondral defect may develop (e4, e5, e31, e32). Osteonecrosis arises from bone infarction that causes an osteochondral defect (e33– e38). The important question, why cartilage defects in adults do not regenerate by themselves, has not been answered in spite of many studies. Possible reasons include the lacking blood supply to the bone, aggressive proliferation of synovial cells, and insufficient signals to promote regeneration, and/or early activation of catabolic signaling cascades (e1, e29).
Table 1. Common disorders that cause articular cartilage defects.
| Disorder | Frequency | Etiology | Site of origin | Treatment of cartilage defects | ||
| Conservative | Surgical | |||||
| Traumatic cartilage defect | ++ | Primary | Shear forces, compression | Cartilage | Physical therapy, temporary load relief, NSAIDs | Age, joint, and stage dependent; refixation, micro- fracture surgery, OCT, ACI, (e2, e5) |
| Osteoarthritis | +++ | Primary | Multifactorial, impaired cartilage metabolism, biochemical, biomecha- nical, genetic factors (e2, e3, e66, e67), polyarthritis | Cartilage(subchondral bone?) | Physical therapy, temporary load relief, NSAIDs, SADOA, intra-articular injections: corticoids, hyaluronic acid, (2, 3, 8) | Débridement alone not indicated (20, e67); Marrow stimulation techniques in circumscribed defects, corrective osteotomy, endoprosthesis (e2, e4– e6, e8, e104) |
| Secondary | Infections, trauma, obesity, joint mechanics impaired (mechanical leg axis malalignment, dysplasia, posttraumatic) | |||||
| Osteochondrosis dissecans | + | Primary | Impaired microcirculation, microtrauma; subsequently, an osteochondral fragment may be detached as a loose body, and an osteochondraldefect results. Most commonly affected age groups: children and adolescents | Subchondral bone | Physical therapy, temporary load relief, NSAIDs (e5) | Age, joint, and stage dependent. Refixation, retrograde and antegrade drilling, microfracture surgery, ACI, OCT, spongiosaplasty (e22, e23, e30– e34) |
| Osteonecrosis | + | Primary(Ahlbäck’s disease, SPONK) | Disrupted perfusion of subchondral bone, microtrauma (e24, e26); subsequent subchondral fracture with collapse of the covering cartilage triggers development of osteochondral defect. Most commonly affected age group: > 60 years, mostly affects women. | Subchondral bone | Physical therapy, temporary load relief, NSAIDs | Débridement , microfracture, retrograde and antegrade drilling, spongiosaplasty , endoprosthesis (e2, e29) |
| Secondary(SON) | Corticoid therapy, Caisson disease, alcohol abuse, trauma, Gaucher’s disease, SLE, radiotherapy (e12, e27, e111– e113) | |||||
| Chronic polyarthritis | ++ | Primary | Chronic inflammatory disorders of the synovial membrane, autoimmune origin | Synovial membrane | Physical therapy, NSAIDs , immunosuppressants (methotrexate, TNF-alpha antagonists, corticoids) (e10) | Synovectomy, radiosynoviorthesis, endoprosthesis (e9– e12) |
ACI, autologous chondrocyte implantation; NSAIDs, non-steroidal anti-inflammatory drugs; OCT, osteochondral transplantation; SADOA, slow acting drugs in osteoarthritis; SLE, systemic Lupus erythematodes; SON, secondary osteonecrosis of the knee; SPONK, spontaneous osteonecrosis of the knee
Imaging diagnostics
Diagnosing focal, non-arthritic cartilage defects is difficult. The standard radiograph in two planes does not show chondral defects, while osteochondral defects are only visible after larger osseous fragments have become detached (e9). In order to diagnose osteoarthritis, radiological criteria (e39) and the size of the radiological joint space are used as direct indicators of cartilage thickness (e40, e41). For this purpose, the 45° weight bearing X-ray in the Rosenberg view is used. In this way, a narrowing of the joint space can be detected in those articular areas that are already damaged in the early stages of osteoarthritis and that bear weight when the knee is flexed (this is often undetectable when the knee is straightened in the anterior-posterior radiographic view) (e9, e41– e43).
In order to expose the defect, magnetic resonance imaging (MRI) has become the technique of choice. Increasingly, the high-resolution 3-Tesla MRI is used (e44-e46). Experimentally the cartilage volume is quantifiable, and in moderately severe or severe osteoarthritis it correlates to the narrowing of the joint space (e47). Bone marrow edema—as a sign of contusion—provides an important indirect indication of a cartilage defect. After cartilage reconstruction procedures (e44), MRI imaging protocols help to assess structural (Mocart assessment system) (e48) and biochemical parameters of the repair tissue (dGEMRIC and sodium imaging to assess the proteoglycan content; T2/T1rho(T1ρ) mapping to assess the collagen content and the fiber arrangement) (e44– e51).
These time consuming methods are not yet suitable for routine examinations.
The selection of additional approaches depends on the underlying pathology. Subchondral bone can be assessed by means of computed tomography (CT); CT arthrography enables precise assessments of the stability of the osteochondral fragment in OCD (e52).
Therapeutic principles
Pain reduction is the primary goal of medial therapy for all symptomatic cartilage defects (2, e53) in the context of a stepwise scheme, starting with paracetamol (acetaminophen) in mild pain, non-steroidal anti-inflammatory drugs (NSAIDs) in moderate pain, with opioids added on in case of severe pain. Intra-articular corticoids are indicated in the acute phase. Since their symptomatic effect has been confirmed (2, e53), we will not discuss them any further in this article.
Reconstructive surgical treatment aims to improve articular function and congruence as well as prevent osteoarthritic damage to intact areas of the cartilage. Table 1 shows the—mostly stage dependent—therapeutic options for traumatic cartilage defects (e4, e9), osteoarthritis (e4, e8– e10, e12), OCD (e31, e32, e54– e58), and osteonecrosis (e4, e38).
Medical treatment
Almost all of the treatments mentioned in this review article are used primarily for the treatment of osteoarthritis. The short follow-up periods remain a problem, in view of the slow progression of cartilage degeneration over time.
Causal pharmacological concepts for the treatment of osteoarthritis aim to slow down the degeneration process (2). The group of the slow acting drugs in osteoarthritis (SADOA) is divided into symptomatic slow acting drugs in osteoarthritis (SYSADOA), which have a symptomatic effect (improvement of joint function, pain reduction) and disease modifying osteoarthritis drugs (DMOADs). DMOADs are intended to stop the cartilage degeneration or even reverse it (Table 2). The Osteoarthritis Research Society International has published evidence based recommendations for the treatment of osteoarthritis of the knee and hip (gonarthrosis and coxarthrosis) (evidence level I, meta-analysis of 351 studies) (3).
Table 2. Overview of currently used substances for the treatment of osteoarthritis, and the evidence base.
| Substance | Application | Evidence base |
| Glucosamine* | p. o. | Contradictory; the GAIT-Studie did not show any symptomatic or structure-preserving effects (evidence level I) (4, 5). Structure-preserving effect reported in other studies (evidence level I) (e59, e62). |
| Chondroitin* | p. o. | Contradictory; the GAIT-Studie did not show any symptomatic or structure-preserving effects (evidence level I) (4, 5). Symptomatic and structure-preserving effects reported in another study (evidence level I) (6) |
| Diacerein* | p. o. | Mild symptomatic effect (evidence level I) (2, 7) |
| Doxycycline | p. o. | Indications of structure-preserving effects (evidence level I) (2) |
| IL-1 receptor antagonist | s. c., i. a | Primarily established for oral therapy of rheumatoid arthritis. Suitability for treatment of osteoarthritis is currently under investigation (2). |
| Fibroblast growth factor-18 | i. a. | In an osteoarthritis animal model, improved cartilage repair (e69); currently phase I and phase II studies of intra-articular injection for the treatment of osteoarthritis and traumatic cartilage defects (e70, e71) |
| Hyaluronic acid* | i. a. | Temporary symptomatic effect (8, e65– e67); no evidence of structure-preserving attributes |
| ICE inhibitors bisphosphonates calcitonin MMP inhibitors estrogen | p. o. p. o., i. v. i. v., i.m., s.c. p. o. p. o., TTS | Symptomatic structure-preserving effects have been described in individual studies. The data situation remains unclear, however, as controlled studies with large numbers of cases are lacking. |
Application methods: TTS, transcutaneous therapeutic system; p. o., per orem; i. a., intra-articular; i. v., intravenous; i. m., intramuscular; s. c., subcutaneous *SYSADOA (symptomatic slow acting drugs in osteoarthritis). ICE, Interleukin-1ß-converting-enzyme; MMP, matrix metalloproteinase; Confirmed clinical proof is so far lacking for the the classification of DMOADs (disease modifying osteoarthritis drugs), which we included in our article, which stop cartilage degeneration or even reverse it.
Glucosamine, a component of the cartilage matrix, has a mild anti-inflammatory effect when taken orally. Independent placebo controlled trials did not find any effect (e59); other studies showed a symptomatic effect (2). Two randomized controlled studies showed a mild, significant reduction in the joint space narrowing of the knee (e60, e61), but not in the hip joint (e62). The GAIT multicenter study found neither a reduction in the narrowing of the joint space nor any clinical improvement in pain and articular function after two years in patients with gonarthrosis (evidence level I) (4, 5).
The effects of chondroitin sulfate (component of cartilage matrix) regarding pain reduction, functional improvement, and cartilage preservation remain unclear. In patients with osteoarthritis of the knee, an improvement in symptoms and a reduction in the narrowing of the joint space on radiography were described after two years (6, e63), but the GAIT Study did not find any differences between the verum and placebo groups (evidence level I for both) (4, 5, e64).
The orally administered drug diacerin (1,8-diacetoxy-3-carboxy anthraquinone, not licensed in Germany) inhibits the proinflammatory cytokine interleukin-1 (IL-1). It seems unlikely that it affects the degeneration of the cartilage (2). A meta-analysis showed a mild symptomatic (anti-inflammatory) effect within the first six months (evidence level I) (7).
Intra-articular injection of hyaluronic acid (main component of the synovia) improves articular function and reduces pain compared with placebo (8). Clinically, its effect sets in later than that of intra-articular corticosteroids (which provide better pain reduction within the first two weeks) (e65). The symptomatic effect of hyaluronic acid is strongest 13 weeks after application (8). Although a better effectiveness has been suggested for hyaluronic acid preparations with a higher molecular weight (e66), no randomized clinical studies have so far confirmed this assumption. Moreover, when higher molecular weights were used, adverse effects were more common (swelling, pain) (e67). Confirmed data regarding the progression of osteoarthritis are lacking.
Fibroblast growth factor 18 (FGF-18) was found to stimulate the proliferation of chondrocytes and proteoglycan synthesis (e68) and to lead to improved cartilage repair in an animal model of osteoarthritis (e69). Current phase I and phase II studies are investigating intra-articular injection of recombinant FGF-18 for the treatment of osteoarthritis (e70) and trauma induced cartilage defects (e71).
Regarding causal therapy for osteoarthritis, further interesting therapeutic approaches exist (2), such as:
The selective inhibition of catabolic enzymes (for example, matrix metalloproteinases)
The selective inhibition of IL-1 and different signal transduction pathways (among others, MAPKinase -, NF-κB- signaling pathway), and
The intra-articular administration of thrombocyte-rich plasma (PRP) (e72).
It is also hypothesized that bisphosphonates, calcitonin, and estrogens may lead to a reduction of cartilage degeneration (2, e73).
For the treatment of osteonecrosis, osteoanabolic drugs—for example, the prostaglandin analogue iloprost—are under discussion, but thus far they do not have licensing approval. Clinically they may achieve pain reduction and functional improvement in the early stages (e74, e75) (evidence level IV). Randomized studies are lacking.
Surgical methods
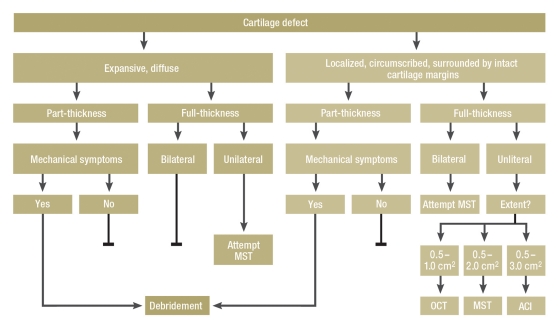
Reconstructive surgical approaches are indicated for cartilage defects that remain symptomatic in spite of sufficient conservative and medical treatment (Figure 1). Since they aim to prevent secondary processes they should be used at an early symptomatic stage. In selecting a suitable treatment method, the following parameters are important::
Figure 1.
Orientational flow chart for reconstructive surgical treatment for cartilage defects, depending on thickness and extent
Reconstructive surgical procedures are indicated in cartilage defects that remain symptomatic in spite of sufficient conservative and medical therapy. Further indicative parameters include localization, mechanical leg axis, knee instability, and the patient’s age. Mechanical symptoms include catching or locking. ACI, autologous chondrocyte implantation; MST, marrow stimulation techniques; OCT, osteochondral transplants
Etiology
Patient specific objectives (pain reduction, functional improvement)
Patient’s age
Body mass index (BMI)
Level of physical activity
Mechanical leg axis
Comorbidities (ligament injuries, meniscal injuries)
Size and location of defect
“Kissing” lesions.
Surgical options for focal cartilage defects include in particular marrow-stimulating procedures, osteochondral transplants, and autologous chondrocyte implantation(ACI). Osteotomy around the knee joint is indicated primarily in patients with unicompartmental gonarthrosis. Because of this multiplicity of approaches it is often difficult to compare the success rates of each surgical procedure with one another as well as with untreated defects. Equally unsatisfactory are the existing options for collecting quantitative data, since clinical assessment systems do not provide information on the biomechanical functionality of the repair tissue, as re-arthroscopy to collect a biopsy specimen from the repair tissue is controversial, and the correlation of histology and clinical symptoms is unclear. For this reason, more case studies (9– 18, e76, e77) have been reported than randomized controlled studies (18– 20, e78).
Débridement
Débridement of the articular cartilage entails smoothing the lesion and removing loose fragments in order to avoid the transmission of shear forces on intact layers of the cartilage. Since débridement does not avert the progression of osteoarthritis it is not recommended as the sole treatment (20, e78). It does, however, have a justifiable position in the therapy of activated osteoarthritis when mechanical symptoms (such as locking) are present, in clinically symptomatic meniscal lesions, and in synovialitis that is detritus-induced, which can lead to further degeneration of the joint (e8).
Marrow-stimulating procedures
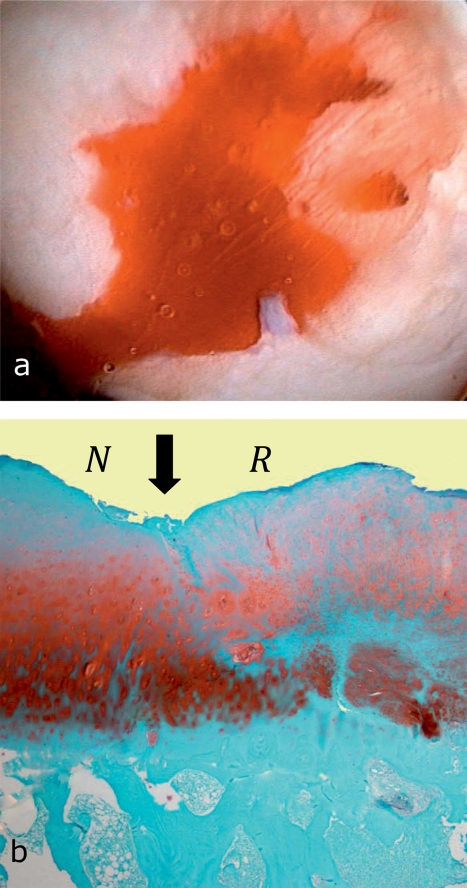
After perforation of the subchondral medullary cavity by means of microfracture surgery, Pridie drilling, or abrasion arthroplasty (e79– e81), a blood clot forms from bone marrow, on the basis of which the cartilage defect is filled with a repair tissue consisting of fibrocartilage (structurally and biomechanically inferior to hyaline articular cartilage) (Figure 2). Marrow-stimulating procedures are the first-line approach in circumscribed chondral defects (covering an area of less than 2–3 cm2) (e82). An independent prospective controlled study found improved clinical results after five years in 77% of patients (17). The size and number of defects (21), the patient’s level of physical activity, and the patient’s age are prognostically important (e82). Physically active patients younger than 30–40 years have better results than older, physically inactive patients (e83– e86). A case series in which, in addition to the microfracture surgery, a collagen membrane was inserted yielded better clinical results after three years compared with baseline (size of the lesion: 4.2 cm2) (e87). Randomized controlled studies are lacking. In addition to marrow-stimulating procedures, increased subchondral bone formation with subsequent thinning of the repair cartilage covering it has been described, as has the formation of intralesional osteophytes (e88-e90).
Figure 2.
Arthroscopic view of microfracture surgery and histological finding after microfracture surgery
Arthroscopic view in microfracture surgery: the cartilage defect was carefully débrided, the calcified cartilage meticulously removed, and stable walls created around the edges. After multiple microfractures have been applied with a microfracture chisel, threads of blood appear from the channels created by the microfracture—at low arthroscopic pump pressure—and form the basis for the subsequent repair tissue.
In the histology image after microfracture (Safranin O—fast green the original cartilage(left, N) shows signs of degeneration; the repair tissue (right, R) is characterized by round cells, characteristic for chondrocytes, and a proteoglycan-containing extracellular matrix. Arrow: integration of the repair tissue with the neighboring original hyaline cartilage.
Autologous chondrocyte transplantation
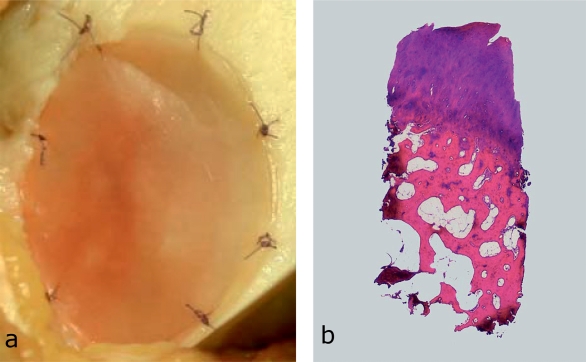
In classic autologous chondrocyte implantation (ACI), chondrocytes are isolated by means of cartilage biopsy, grown in cell culture, and then implanted into the defect, which has been covered with a periosteal flap (22). In the newer, matrix-associated transplantation methods, the chondrocytes are seeded in scaffolds as carrier substances (Figure 3) (e91– e93)—applying principles of tissue engineering. Their exact contribution to the repair tissue remains unknown. Full-thickness symptomatic cartilage defects (3–10 cm2) in the knee joint constitute the main indication (13, 23, e86, e94, e95). Cartilage defects in which other approaches have failed are a further indication (e9, e96– e98). ACI is not indicated to treat osteoarthritis (13, e98, e99).
Figure 3.
Intraoperative findings in matrix-associated autologous chondrocyte implantation (ACI) and osteochondral biopsy after matrix-associated ACI.
Intraoperative finding in matrix-associated autologous chondrocyte implantation. The chondrocytes are distributed in a biologically degradable matrix that inserted into the cartilage defect and fixated with resorbable sutures (size USP 6–0).
Osteochondral biopsy (hematoxylin and eosin) from a 39-year-old patient after ACI (primary diagnosis: traumatic cartilage injury, medial femoral condyle, 4.3 cm2). Clinically, the patient is asymptomtomatic. Histology shows a fibrocartilaginous repair tissue with early signs of degeneration of the superficial cartilage layer; hyaline cartilage is visible in the deeper layers of the repair tissue.
Compared with microfracture surgery (defect sizes 5.1 cm2 and 4.5 cm2) similar clinical and histological results were found after five years (18) (evidence level I). Younger patients in whom symptoms are of short duration and who had few prior surgical interventions showed the best results (e86, e100). A multicenter clinical observational study found after 10 years an improvement after ACI in 69% of all patients (12). A randomized study (evidence level II) comparing classic and matrix-associated ACI found no clinical differences two years postoperatively (24). Current studies give rise to the assumption that ACI compared with marrow-stimulating procedures results in better histological repair, although the clinical results are similar (14, 15, 19, e101). For large osteochondral defects (for example, in OCD), matrix-associated ACI with autologous bone graft (“sandwich technique”) is the procedure of choice (9, e4, e101, e102). Hypertrophy of the repair tissue and delamination of the periosteal flap—as has been observed in classic ACI—have become extremely rare (<1%) as a result of applying matrix-associated transplantation procedures (e103, e104).
Transplantation of adult mesenchymal stem cells
A recent cohort study (evidence level III) reported similar clinical results two years postoperatively for transplantation of autologous bone marrow-derived mesenchymal stem cells (MSC) as for ACI (lesion sizes 4.6 cm2 and 3.6 cm2) (e105). Case reports have shown fibrocartilaginous repair tissue (e106, e107). Intra-articular administration of MSC is under discussion for the treatment of osteoarthritis (e108). Clinical studies with higher evidence levels are needed before its use can be recommended.
Autologous osteochondral transplants
For this procedure, cartilage-bone cylinders from non-weight bearing areas are transplanted into a small osteochondral defect. In mosaicplasty, a larger lesion is filled in with multiple cylinders; it is also possible to transfer the posterior femoral condyle (e109– e112). In the medium term the results after single osteochondral transplantation have been good in 80–80% of cases; with regard to the knee, they were more successful for the femorotibial joint than the femoropatellar joint (e113, e114). A prospective randomized study showed better clinical-functional and MRI results after three years for osteochondral transplants than for microfracture surgery (e114). In a case series, transfer of the condyle bone showed an improvement after 5.5 years compared with baseline (evidence level IV) (e115). Osteochondral transplants are useful for small focal defects (less than 1.5 cm diameter), whereas for large lesions matrix-associated ACI with autologous bone graft is preferable to mosaicplasty because of the higher rate of complications associated with this technique (uneven surface, dislocation) and the multiple donor sites (9, e4, e102).
Redistribution of pathological loads by means of osteotomies
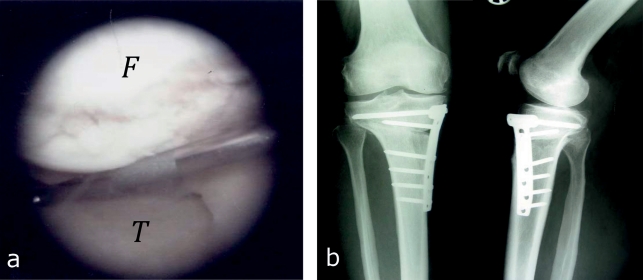
Osteotomies around the knee joint correct malalignment of the leg axis and are intended to prevent further cartilage degeneration by locally reducing pressure in the damaged joint compartment (e116– e120). The correction is usually performed on the frontal plane, however, any of the three planes can be corrected. The classical indication is the correction of genu varus deformity in cases of symptomatic early medial femoro-tibial osteoarthritis in the knee with an intact lateral femoro-tibial joint. In this scenario, open-wedge high tibial osteotomy performed in a biplanar technique and fixated with an internal plate fixator is increasingly gaining in importance (25) (Figure 4). Review articles have reported pain reduction and functional improvement in 90% of cases after correct patient selection (e121, e122). The ideal candidate is a younger patient (<50 years) with stable ligaments without a higher-degree of axial malalignment, a good range of motion, and optimal BMI (e121, e123, e124).
Figure 4.
Arthroscopic view of medial varus gonarthrosis and radiograph after open-wedge high tibial osteotomy
Arthroscopic view, medial compartment with osteoarthritic cartilage defects in the area of the medial femoral condyle (F) and the medial tibial plateau (T).
Radiograph after open-wedge high tibial osteotomy in medial valgus gonarthrosis. The osteotomy was performed in a biplanar technique: horizontal osteotomy of the posterior two thirds of the tibia is performed first; this is followed by an osteotomy with a 110 degrees cut behind the tuberosity parallel to the ventral tibial shaft. The result of the correction is fixated with a plate fixatior.
.
If the implantation of an endoprosthetic surface replacement is defined as an end point, the survival rates are 73% after five years and 52% after 10 years, a according to a meta-analysis (e124). A retrospective study (follow-up period 16.5 years) showed only a slight radiological progression of the medial gonarthrosis after high tibial osteotomy (e125). In the context of reconstructive cartilage surgery for focal defects, osteotomies are also performed during primary interventions to unload the affected compartment when axial malalignment is present or in case of revision surgery (with the aim to protect the repair tissue) (23, e97, e126).
Conclusion and outlook
The aim of medical therapy is to maintain joint function and delay the need for surgical measures. Medical therapy is—at least temporarily—able to maintain articular function in osteoarthritis while controlling the pain, independent of the stage of disease and at least temporarily. For DMOADs it has thus far not been shown whether they slow down osteoarthritic cartilage degeneration or even stop it.
Reconstructive surgical therapies result in cartilage repair. Marrow-stimulating procedures and osteochondral transplants are the first-line treatments for the clinically common small focal articular cartilage defects. ACI is unrivalled for the primary treatment of larger focal cartilage defects, and a secondary option after other reconstructive therapies have failed. ACI and marrow-stimulating procedures are therefore not competing with each other; rather, they complement each other. They have shown particularly good results in active patients younger than 40 years.
For the treatment of osteoarthritic cartilage defects, reconstructive surgical therapeutic approaches are of lesser importance. Osteotomies around the knee play a significant role in this setting as they remove weight of osteoarthritic areas of the joint and delay the need for endoprosthetic joint replacement. Orthopedic surgeons and trauma surgeons thus have a wide range of useful therapeutic options at their disposal (22, e127, e128).
Further controlled randomized clinical studies with longer follow-up periods that investigate the development of osteoarthritis as the ultimate parameter will result in improved cartilage reconstruction and joint preservation (e129).
Key Messages.
Pain reduction is the primary aim of medical therapy of all symptomatic cartilage defects..
In spite of causal pharmacological concepts, there are currently no medical drugs that stop or reverse the progression of cartilage destruction.
The aim of reconstructive-surgical treatment is an improvement in articular function and congruence, as well as the prevention of osteoarthritic damage to adjacent, intact areas of cartilage.
Marrow-stimulating procedures and osteochondral transplants are indicated for small focal articular cartilage defects; autologous cartilage implantation(ACI) is primarily indicated for larger cartilage defects.
Osteotomies near the knee joint are indicated in unilateral gonarthrosis with deviation of the leg axis.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Professor Madry is a clinical investigator in the randomized, double blind, placebo controlled, international, multicenter, clinical phase II study “AS902330 in Cartilage Injury Repair,” which studies the intraarticular effects of FGF-18 in cartilage defects and is funded by Merck Serono.
Dr Grün has shares in Orthogenics.
Professor Knutsen declares that no conflict of interest exists.
References
- 1.Noyes F, Stabler C. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–513. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 2.Steinmeyer J, Konttinen Y. Oral treatment options for degenerative joint disease-presence and future. Adv Drug Deliv Rev. 2006;58:168–211. doi: 10.1016/j.addr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Nuki G, Moskowitz R, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Sawitzke A, Shi H, Finco M, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58:3183–3191. doi: 10.1002/art.23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawitzke A, Shi H, Finco M, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459–1464. doi: 10.1136/ard.2009.120469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahan A, Uebelhart D, De Vathaire F, Delmas P, Reginster J. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524–533. doi: 10.1002/art.24255. [DOI] [PubMed] [Google Scholar]
- 7.Bartels E, Bliddal H, Schöndorff P, Altman R, Zhang W, Christensen R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2010;18:289–296. doi: 10.1016/j.joca.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD005321.pub2. CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochs BG, Müller-Horvat C, Albrecht D, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. Am J Sports Med. 2011;39:764–773. doi: 10.1177/0363546510388896. [DOI] [PubMed] [Google Scholar]
- 10.Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220–2233. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemeyer P, Lenz P, Kreuz PC. Chondrocyte-seeded type I/III collagen membrane for autologous chondrocyte transplantation: prospective 2-year results in patients with cartilage defects of the knee joint. Arthroscopy. 2010;26:1074–1082. doi: 10.1016/j.arthro.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Moseley J, Anderson A, Browne J, Mandelbaum B, et al. Long-term durability of autologous chondrocyte implantation: a multicenter observational study in US patients. Am J Sports Med. 2010;38:238–246. doi: 10.1177/0363546509348000. [DOI] [PubMed] [Google Scholar]
- 13.Behrens P, Bosch U, Bruns J, et al. Indikations- und Durchführungsempfehlungen der Arbeitsgemeinschaft „Geweberegeneration und Gewebeersatz“ zur Autologen Chondrozytentransplantation (ACT) Z Orthop Ihre Grenzgeb. 2004;142:529–539. doi: 10.1055/s-2004-832353. [DOI] [PubMed] [Google Scholar]
- 14.Vavken P, Samartzis D. Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthritis Cartilage. 2010;18:857–863. doi: 10.1016/j.joca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 16.Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33–41. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 17.Nehrer S, Dorotka R, Domayer S, Stelzeneder D, Kotz R. Treatment of full-thickness chondral defects with hyalograft C in the knee: a prospective clinical case series with 2 to 7 years’ follow-up. Am J Sports Med. 2009;37(Suppl 1):81S–87S. doi: 10.1177/0363546509350704. [DOI] [PubMed] [Google Scholar]
- 18.Knutsen G, Drogset J, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at 5 years. J Bone Joint Surg Am. 2007;89-A:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 19.Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S–19S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 20.Kirkley A, Birmingham T, Litchfield R, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097–1107. doi: 10.1056/NEJMoa0708333. [DOI] [PubMed] [Google Scholar]
- 21.Soltheim E, Oyen J, Hegna J, Austgulen O, Harlem T, Strand T. Microfracture treatment of single or multiple articular cartilage defects of the knee: a 5-year median follow-up of 110 patients. Knee Sports Traumatol Arthrosc. 2010;18:504–508. doi: 10.1007/s00167-009-0974-y. [DOI] [PubMed] [Google Scholar]
- 22.Brittberg M, Peterson L, Sjögren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85-A(Suppl 3):109–115. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- 23.Gomoll AH, Farr J, Gillogly SD, Kercher J, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470–2490. [PubMed] [Google Scholar]
- 24.Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 25.Niemeyer P, Koestler W, Kaehny C, et al. Two-year results of open-wedge high tibial osteotomy with fixation by medial plate fixator for medial compartment arthritis with varus malalignment of the knee. Arthroscopy. 2008;24:796–804. doi: 10.1016/j.arthro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- e1.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- e2.Gurlt E. Gelenkkrankheiten. Berlin: Reimer; 1853. Veränderung der Gelenkknorpel. [Google Scholar]
- e3.Hueter C. Klinik der Gelenkkrankheiten mit Einschluss der Orthopaedie. Leipzig: Vogel. 1870 [Google Scholar]
- e4.Pape D, Filardo G, Kon E, van Dijk C, Madry H. Disease-specific clinical problems associated with the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:448–462. doi: 10.1007/s00167-010-1052-1. [DOI] [PubMed] [Google Scholar]
- e5.Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- e6.Gies T. Über die Heilung von Knorpelwunden. Dtsch Z Chir. 1882;18 [Google Scholar]
- e7.Otte P. Die Regenerationsunfähigkeit des Gelenkknorpels. Z -Orthop. 1958;90:299–303. [PubMed] [Google Scholar]
- e8.Lützner J, Kasten P, Günther KP, Kirschner S. Surgical options for patients with osteoarthritis of the knee. Nat Rev Rheumatol. 2009;5:309–316. doi: 10.1038/nrrheum.2009.88. [DOI] [PubMed] [Google Scholar]
- e9.Madry H. Operative und rekonstruktive Behandlung. In: Kohn D, editor. Orthopädie und orthopädische Chrurgie. Knie. Stuttgart, New York: Thieme; 2005. 367 pp. [Google Scholar]
- e10.Michael JWP, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Ratzlaff CR, Liang MH. New developments in osteoarthritis. Prevention of injury-related knee osteoarthritis: opportunities for the primary and secondary prevention of knee osteoarthritis. Arthritis Res Ther. 2010;12 doi: 10.1186/ar3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Feeley BT, Gallo RA, Sherman S, Williams RJ. Management of osteoarthritis of the knee in the active patient. J Am Acad Orthop Surg. 2010;18:406–416. doi: 10.5435/00124635-201007000-00003. [DOI] [PubMed] [Google Scholar]
- e13.van der Zant FM, Boer RO, Moolenburgh JD, Jahangier ZN, Bijlsma JW, Jacobs JW. Radiation synovectomy with (90)Yttrium, (186)Rhenium and (169)Erbium: a systematic literature review with meta-analyses. Clin Exp Rheumatol. 2009;27:130–139. [PubMed] [Google Scholar]
- e14.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- e15.Trieb K, Hofstaetter SG. Treatment strategies in surgery for rheumatoid arthritis. Eur J Radiol. 2009;71:204–210. doi: 10.1016/j.ejrad.2009.04.050. [DOI] [PubMed] [Google Scholar]
- e16.Mäkelä KT, Eskelinen A, Pulkkinen P, Virolainen P, Paavolainen P, Remes V. Cemented versus cementless total hip replacements in patients fifty-five years of age or older with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93:178–186. doi: 10.2106/JBJS.I.01283. [DOI] [PubMed] [Google Scholar]
- e17.Sellers RS, Zhang R, Glasson SS, et al. Repair of articular carti-lage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) J Bone Joint Surg Am. 2000;82:151–160. doi: 10.2106/00004623-200002000-00001. [DOI] [PubMed] [Google Scholar]
- e18.Madry H, Orth P, Cucchiarini M. Gene therapy for cartilage repair. Cartilage. 2011 doi: 10.1177/1947603510392914. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- e20.Stoddart MJ, Grad S, Eglin D, Alini M. Cells and biomaterials in cartilage tissue engineering. Regen Med. 2009;4:81–98. doi: 10.2217/17460751.4.1.81. [DOI] [PubMed] [Google Scholar]
- e21.Beyer C, Schett G. Pharmacotherapy: concepts of pathogenesis and emerging treatments. Novel targets in bone and cartilage. Best Pract Res Clin Rheumatol. 2010;24:489–496. doi: 10.1016/j.berh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- e22.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953–957. doi: 10.1016/j.joca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- e24.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- e25.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular carti-lage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- e26.Pape D, Seil R, Fritsch E, Rupp S, Kohn D. Prevalence of spontaneous osteonecrosis of the medial femoral condyle in elderly patients. Knee Surg Sports Traumatol Arthrosc. 2002;10:233–240. doi: 10.1007/s00167-002-0285-z. [DOI] [PubMed] [Google Scholar]
- e27.Aigner T, Söder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis–structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3:391–399. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- e28.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531–559. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- e29.Goldring MB, Goldring SR. Articular cartilage and subchondral -bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- e30.Lotz MK. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12 doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e31.Lützner J, Mettelsiefen J, Günther KP, Thielemann F. Treatment of osteochondritis dissecans of the knee joint [Therapie der Osteochondrosis dissecans des Kniegelenkes] Orthopade. 2007;36:871–879. doi: 10.1007/s00132-007-1130-3. [DOI] [PubMed] [Google Scholar]
- e32.Petersen JP, Steinhagen J, Catala-Lehnen P, Bruns J. Osteochondrosis dissecans des Kniegelenkes. Z Orthop Ihre Grenzgeb. 2006;144:R63–R76. doi: 10.1055/s-2006-924323. [DOI] [PubMed] [Google Scholar]
- e33.Ahlback S. Osteoarthrosis of the knee. A radiographic investiga-tion. Acta Radiol. 1968;227:7–72. [PubMed] [Google Scholar]
- e34.Lotke P, Ecker M. Osteonecrosis of the knee. Orthop Clin North Am. 1985;16:797–808. [PubMed] [Google Scholar]
- e35.Cruess R. Osteonecrosis of bone. Current concepts as to etiology and pathogenesis. Clin Orthop Relat Res. 1986;208:30–39. [PubMed] [Google Scholar]
- e36.Pape D, Lorbach O, Anagnostakos K, Kohn D. Die Osteonekrose des postarthroskopischen Kniegelenks. Orthopade. 2008;37:1099–1100. doi: 10.1007/s00132-008-1303-8. [DOI] [PubMed] [Google Scholar]
- e37.Weldon D. The effects of corticosteroids on bone: osteonecrosis (avascular necrosis of the bone) Ann Allergy Asthma Immunol. 2009;103:91–97. doi: 10.1016/S1081-1206(10)60159-7. [DOI] [PubMed] [Google Scholar]
- e38.Zywiel MG, McGrath MS, Seyler TM, Marker DR, Bonutti PM, Mont MA. Osteonecrosis of the knee: a review of three disorders. Orthop Clin North Am. 2009;40:193–211. doi: 10.1016/j.ocl.2008.10.010. [DOI] [PubMed] [Google Scholar]
- e39.Kellgren J, Lawrence J. Radiological assessment of osteo–arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e40.Altman RD, Bloch DA, Dougados M, et al. Measurement of structural progression in osteoarthritis of the hip: the Barcelona consensus group. Osteoarthritis Cartilage. 2004;12:515–524. doi: 10.1016/j.joca.2004.04.004. [DOI] [PubMed] [Google Scholar]
- e41.Merle-Vincent F, Vignon E, Brandt K, et al. Superiority of the Lyon schuss view over the standing anteroposterior view for detecting joint space narrowing, especially in the lateral tibiofemoral compartment, in early knee osteoarthritis. Ann Rheum Dis. 2007;66:747–753. doi: 10.1136/ard.2006.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e42.Hunter DJ, Buck R, Vignon E, et al. Relation of regional articular cartilage morphometry and meniscal position by MRI to joint -space width in knee radiographs. Osteoarthritis Cartilage. 2009;17:1170–1176. doi: 10.1016/j.joca.2009.04.001. [DOI] [PubMed] [Google Scholar]
- e43.Le Graverand MP, Buck RJ, Wyman BT, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3.0 Tesla MRI and Lyon-Schuss radiography. Ann Rheum Dis. 2010;69:155–162. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- e44.Trattnig S, Winalski CS, Marlovits S, Jurvelin JS, Welsch GH, Potter HG. Magnetic resonance imaging of cartilage repair: a -review. Cartilage. 2011;2:5–26. doi: 10.1177/1947603509360209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e45.Schoth F, Kraemer N, Niendorf T, Hohl C, Gunther RW, Krombach GA. Comparison of image quality in magnetic resonance imaging of the knee at 1.5 and 3.0 Tesla using 32-channel receiver coils. Eur Radiol 200. 18:2258–2264. doi: 10.1007/s00330-008-0972-3. [DOI] [PubMed] [Google Scholar]
- e46.Stahl R, Luke A, Li X, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients–a 30-Tesla MRI study. Eur Radiol. 2009;19:132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- e47.Eckstein F, Benichou O, Wirth W, et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: Data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–1225. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e48.Marlovits S, Singer P, Zeller P, et al. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- e49.Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage le-sions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943–949. doi: 10.1177/0363546509358266. [DOI] [PubMed] [Google Scholar]
- e50.Kon E, Di Martino A, Filardo G, et al. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.04.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- e51.Crema MD, Roemer FW, Marra MD. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research1. Radiographics. 2011;31:37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e52.Menetrey J, Unno-Veith F, Madry H, Van Breuseghem I. Epidemiology and imaging of the subchondral bone in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18:463–471. doi: 10.1007/s00167-010-1053-0. [DOI] [PubMed] [Google Scholar]
- e53.Altman RD. Early management of osteoarthritis. Am J Manag -Care. 2010;16(Suppl Management):S41–S47. [PubMed] [Google Scholar]
- e54.Kocher M, Tucker R, Ganley T, Flynn J. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- e55.Kocher MS, Czarnecki JJ, Andersen JS, Micheli LJ. Internal fixa-tion of juvenile osteochondritis dissecans lesions of the knee. Am J Sports Med. 2007;35:712–718. doi: 10.1177/0363546506296608. [DOI] [PubMed] [Google Scholar]
- e56.Ojala R, Kerimaa P, Lakovaara M, et al. MRI-guided percutaneous retrograde drilling of osteochondritis dissecans of the knee -Skeletal Radiol. 2011;40:765–770. doi: 10.1007/s00256-011-1118-2. [DOI] [PubMed] [Google Scholar]
- e57.Wall E, Vourazeris J, Myer G, Emery K, et al. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am. 2008;90:2655–2664. doi: 10.2106/JBJS.G.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e58.Donaldson LD, Wojtys EM. Extraarticular drilling for stable osteochondritis dissecans in the skeletally immature knee. J Pediatr Orthop. 2008;28:831–835. doi: 10.1097/BPO.0b013e31818ee248. [DOI] [PubMed] [Google Scholar]
- e59.Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, -chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c4675. c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e60.Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a rando-m-ised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- e61.Pavelká K, Gatterová J, Olejarová M, et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, -randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113–2123. doi: 10.1001/archinte.162.18.2113. [DOI] [PubMed] [Google Scholar]
- e62.Rozendaal RM, Koes BW, van Osch GJ, et al. Effect of glucosa-mine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148:268–277. doi: 10.7326/0003-4819-148-4-200802190-00005. [DOI] [PubMed] [Google Scholar]
- e63.Hochberg MC, Zhan M, Langenberg P. The rate of decline of joint space width in patients with osteoarthritis of the knee: a systematic review and meta-analysis of randomized placebo-controlled trials of chondroitin sulfate. Curr Med Res Opin. 2008;24:3029–3035. doi: 10.1185/03007990802434932. [DOI] [PubMed] [Google Scholar]
- e64.Miller KL, Clegg DO. Glucosamine and chondroitin sulfate. Rheum Dis Clin North Am. 2011;37:103–118. doi: 10.1016/j.rdc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- e65.Bannuru R, Natov N, Obadan I, Price L, Schmid C, McAlindon T. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704–1711. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]
- e66.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–3121. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- e67.Reichenbach S, Blank S, Rutjes AW, et al. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 2007;57:1410–1418. doi: 10.1002/art.23103. [DOI] [PubMed] [Google Scholar]
- e68.Beyer C, Schett G. Pharmacotherapy: concepts of pathogenesis and emerging treatments. Novel targets in bone and cartilage. Best Pract Res Clin Rheumatol. 2010;24:489–496. doi: 10.1016/j.berh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- e69.Moore EE, Bendele AM, Thompson DL, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- e70.ClinicalTrials.gov A multicenter study of rhFGF 18 in patients with knee osteoarthritis not requiring surgery. www.clinicaltrials.gov/ct2/show/NCT01033994?intr=%22AS902330%22&rank=3.
- e71.ClinicalTrials.gov AS902330 in cartilage injury repair (CIR) - clinicaltrials.gov/ct2/show/NCT01066871?intr=%22AS902330%22&rank=1.
- e72.Sánchez M, Anitua E, Azofra J, et al. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–913. [PubMed] [Google Scholar]
- e73.Bingham C, Buckland-Wright J, Garnero P, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- e74.Kraenzlin ME, Graf C, Meier C, Kraenzlin C, Friedrich NF. Possible beneficial effect of bisphosphonates in osteonecrosis of the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1638–1644. doi: 10.1007/s00167-010-1106-4. [DOI] [PubMed] [Google Scholar]
- e75.Jäger M, Zilkens C, Bittersohl B, et al. Efficiency of iloprost treatment for osseous malperfusion. Int Orthop. 2011;35:761–765. doi: 10.1007/s00264-010-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e76.Kon E, Verdonk P, Condello V, et al. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med. 2009;37(Suppl 1):156S–166S. doi: 10.1177/0363546509351649. [DOI] [PubMed] [Google Scholar]
- e77.Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010;468:147–157. doi: 10.1007/s11999-009-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e78.Moseley J, O’Malley K, Petersen N, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- e79.Steadman J, Rodkey W, Rodrigo J. Microfracture: surgical -technique and rehabilitation to treat chondral defects. Clin Orthop -Relat Res. 2001;391:362–369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- e80.Pridie KH. A Method of Resurfacing Knee Joints. J Bone Joint Surg Br. 1959;41-B:618–619. [Google Scholar]
- e81.Johnson L. Arthroscopic abrasion arthroplasty historical and -pathologic perspective: present status. Arthroscopy. 1986;2:54–69. doi: 10.1016/s0749-8063(86)80012-3. [DOI] [PubMed] [Google Scholar]
- e82.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- e83.Kreuz P, Steinwachs M, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments of the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- e84.Kreuz P, Erggelet C, Steinwachs M, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180–1186. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- e85.Bekkers J, Inklaar M, Saris D. Treatment selection in articular cartilage lesions of the knee. A systemic review. Am J Sports Med. 2009;37(Supplement 1):148S–55S. doi: 10.1177/0363546509351143. [DOI] [PubMed] [Google Scholar]
- e86.Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220–2233. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e87.Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456–1464. doi: 10.1007/s00167-010-1042-3. [DOI] [PubMed] [Google Scholar]
- e88.Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18:434–447. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e89.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic -analysis. Am J Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- e90.Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170–1179. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- e91.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- e92.Niemeyer P, Lenz P, Kreuz PC, et al. Chondrocyte-seeded type I/III collagen membrane for autologous chondrocyte transplantation: prospective 2-year results in patients with cartilage defects of the knee joint. Arthroscopy. 2010;26:1074–1082. doi: 10.1016/j.arthro.2009.12.028. [DOI] [PubMed] [Google Scholar]
- e93.Gaissmaier C, Koh JL, Weise K, Mollenhauer JA. Future perspec-tives of articular cartilage repair. Injury. 2008;39(Suppl 1):S114–S120. doi: 10.1016/j.injury.2008.01.033. [DOI] [PubMed] [Google Scholar]
- e94.Niemeyer P, Köstler W, Salzmann GM, Lenz P, Kreuz PC, Südkamp NP. Autologous chondrocyte implantation for treatment of focal cartilage defects in patients age 40 years and older: A matched-pair analysis with 2-year follow-up. Am J Sports Med. 2010;38:2410–2416. doi: 10.1177/0363546510376742. [DOI] [PubMed] [Google Scholar]
- e95.Moseley JB, Jr, Anderson AF, Browne JE, et al. Long-term durability of autologous chondrocyte implantation: a multicenter, observational study in US patients. Am J Sports Med. 2010;38:238–246. doi: 10.1177/0363546509348000. [DOI] [PubMed] [Google Scholar]
- e96.Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. In-creased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902–908. doi: 10.1177/0363546508330137. [DOI] [PubMed] [Google Scholar]
- e97.Brittberg M. Brittberg M, Imhoff A, Madry H, Mandelbaum B, editors. Failed articular cartilage repair: What to do? Current concepts in cartilage repair. Guildford, UK In: (eds.):DJO Publications. 2010:165–172. [Google Scholar]
- e98.Madry H, Pape D. Autologous chondrocyte transplantation -[Autologe Chondrozytentransplantation] Orthopade. 2008;37:756–763. doi: 10.1007/s00132-008-1270-0. [DOI] [PubMed] [Google Scholar]
- e99.Nehrer S, Dorotka R, Domayer S, Stelzeneder D, Kotz R. Treatment of full-thickness chondral defects with hyalograft C in the knee: a prospective clinical case series with 2 to 7 years’ follow-up. Am J Sports Med. 2009;37(Suppl 1):81S–87S. doi: 10.1177/0363546509350704. [DOI] [PubMed] [Google Scholar]
- e100.Harris JD, Brophy RH, Siston RA, Flanigan DC. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26:841–852. doi: 10.1016/j.arthro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- e101.Vavken P, Samartzis D. Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthritis Cartilage. 2010;18:857–863. doi: 10.1016/j.joca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- e102.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment Schallberger of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A(Suppl 2):17–24. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- e103.Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage le-sions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943–949. doi: 10.1177/0363546509358266. [DOI] [PubMed] [Google Scholar]
- e104.Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation - A systematic review. Osteoarthritis Cartilage. 2011;19:779–791. doi: 10.1016/j.joca.2011.02.010. [DOI] [PubMed] [Google Scholar]
- e105.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- e106.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- e107.Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cart-ilage. 2007;15:226–231. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- e108.Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- e109.Paul J, Sagstetter A, Kriner M, Imhoff AB, Spang J, Hinterwimmer S. Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J Bone Joint Surg Am. 2009;91:1683–1688. doi: 10.2106/JBJS.H.00429. [DOI] [PubMed] [Google Scholar]
- e110.Safran MR, Seiber K. The evidence for surgical repair of articular cartilage in the knee. J Am Acad Orthop Surg. 2010;18:259–266. doi: 10.5435/00124635-201005000-00002. [DOI] [PubMed] [Google Scholar]
- e111.Ferkel RD, Scranton PE, Stone JW, Kern BS. Surgical treatment of osteochondral lesions of the talus. Instr Course Lect. 2010;59:387–404. [PubMed] [Google Scholar]
- e112.Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- e113.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. J Bone Joint Surg. 2003;85-A:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- e114.Gudas R, Kalesinskas R, Kimtys V, et al. A prospective random-ized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066–1075. doi: 10.1016/j.arthro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- e115.Braun S, Minzlaff P, Hollweck R, Wörtler K, Imhoff A. The 55-years results of MegaOATS - autologous transfer of the posterior femoral condyle: a case series study. Arthritis Res Ther. 2008;10 doi: 10.1186/ar2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e116.Fujisawa Y, Masuhara K, Shiomi S. The effect of high tibial -osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop Clin North Am. 1979;10:585–608. [PubMed] [Google Scholar]
- e117.Wakabayashi S, Akizuki S, Takizawa T, Yasukawa Y. A comparison of the healing potential of fibrillated cartilage versus eburnated bone in osteoarthritic knees after high tibial ostetomy: An arthroscopic study with 1-year follow-up. Arthroscopy. 2002;18:272–278. doi: 10.1053/jars.2002.30488. [DOI] [PubMed] [Google Scholar]
- e118.Akizuki S, Yasukawa Y, Takizawa T. Does arthroscopic abrasion arthroplasty promote cartilage regeneration in osteoarthritic knees with eburnation? A prospective study of high tibial osteotomy with abrasion arthroplasty versus high tibial osteotomy alone. Arthroscopy. 1997;13:9–17. doi: 10.1016/s0749-8063(97)90204-8. [DOI] [PubMed] [Google Scholar]
- e119.Kanamiya T, Naito M, Hara M, Yoshimura I. The influences of biomechanical factors on cartilage regeneration after high tibial osteotomy for knees with medial compartment osteoarthritis: clinical and arthroscopic observations. Arthroscopy. 2002;18:725–729. doi: 10.1053/jars.2002.35258. [DOI] [PubMed] [Google Scholar]
- e120.Koshino T, Wada S, Ara Y, Saito T. Regeneration of degenerated articular cartilage after high tibial osteotomy for medial compartment osteoarthritis of the knee. Knee. 2003;10:229–236. doi: 10.1016/s0968-0160(03)00005-x. [DOI] [PubMed] [Google Scholar]
- e121.Hofmann S, Lobenhoffer P, Staubli A, van Heerwaarden R. Osteotomien am Kniegelenk bei Monokompartmentarthrose. Orthopade. 2009;8:755–769. doi: 10.1007/s00132-009-1458-y. [DOI] [PubMed] [Google Scholar]
- e122.Birmingham TB, Giffin JR, Chesworth BM, et al. Medial opening wedge high tibial osteotomy: a prospective study of gait, and patient-reported outcomes. Arthritis Rheum. 2009;61:648–657. doi: 10.1002/art.24466. [DOI] [PubMed] [Google Scholar]
- e123.Bonnin M, Chambat P. Der Stellenwert der valgisierenden, zuklappenden Tibiakopfosteotomie bei der medialen Gonarthrose. Orthopade. 2004;33:135–142. doi: 10.1007/s00132-003-0586-z. [DOI] [PubMed] [Google Scholar]
- e124.Brinkman JM, Lobenhoffer P, Agneskirchner JD, Staubli AE, Wymenga AB, van Heerwaarden RJ. Osteotomies around the knee: patient selection, stability of fixation and bone healing in high -tibial osteotomies. J Bone Joint Surg Br. 2008;90:1548–1557. doi: 10.1302/0301-620X.90B12.21198. [DOI] [PubMed] [Google Scholar]
- e125.Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High -tibial valgus osteotomy in unicompartimental osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19:122–127. doi: 10.1007/s00167-010-1256-4. [DOI] [PubMed] [Google Scholar]
- e126.Brittberg M. Unloading of the repaired cartilage lesion: why, when and how. In: Brittberg M, Imhoff A, Madry H, Mandelbaum B, editors. Current Concepts in Cartilage Repair. Guildford, UK: DJO Publications; 2010. pp. 99–106. [Google Scholar]
- e127.Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-010-1764-z. PMID: 21240578. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- e128.Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. Instr Course Lect. 2010;59:181–204. [PubMed] [Google Scholar]
- e129.Mankin HJ. Chondrocyte transplantation-one answer to an old question. N Engl J Med. 1994;331:940–941. doi: 10.1056/NEJM199410063311410. [DOI] [PubMed] [Google Scholar]