Abstract
Advances in mass spectrometry (MS) have encouraged interest in its deployment in urine biomarker studies, but success has been limited. Urine exosomes have been proposed as an ideal source of biomarkers for renal disease. However, the abundant urinary protein, uromodulin, cofractionates with exosomes during isolation and represents a practical contaminant that limits MS sensitivity. Uromodulin depletion has been attempted but is labor- and time-intensive and may remove important protein biomarkers. We describe the application of an exclusion list (ExL) of uromodulin-related peptide ions, coupled with high-sensitivity mass spectrometric analysis, to increase the depth of coverage of the urinary exosomal proteome. Urine exosomal protein samples from healthy volunteers were subjected to tandem MS and abundant uromodulin peptides identified. Samples were run for a second time, while excluding these uromodulin peptides from fragmentation to allow identification of peptides from lower-abundance proteins. Uromodulin exclusion was performed in addition to dynamic exclusion. Results from these two procedures revealed 222 distinct proteins from conventional analysis, compared with 254 proteins after uromodulin exclusion, of which 188 were common to both methods. By unmasking a previously unidentified protein set, adding the ExL increased overall protein identifications by 29.7% to a total of 288 proteins. A fixed ExL, used in combination with conventional methods, effectively increases the depth of urinary exosomal proteins identified by MS, reducing the need for uromodulin depletion.
Keywords: mass spectrometry, urine, human, microvesicle, Tamm-Horsfall protein
INTRODUCTION
Recent technological advances in mass spectrometry (MS) have enabled large-scale biomarker studies in a variety of fields.1 This has been especially true in nephrology, where urine provides an ideal source of biomarkers.2 It can be obtained noninvasively in relatively large quantities, with no special requirements other than a suitable receptacle. Despite this, few urinary biomarkers have yet entered clinical practice, and clinicians remain largely limited to the use of urine indicator strips and microscopy. This paradox may be partly explained by the size, complexity, dynamic range, and variability of the urinary proteome; challenges in standardizing sample preparation; and the presence of abundant urinary proteins such as uromodulin.
In health, 30% of urinary peptides and proteins are derived from plasma and enter the urine by traversing the glomerular filtration barrier. The remaining 70% arise in the renal tract and may be derived from renal tubular epithelium, urothelium, or the prostate or seminal vesicles. This complex composition of the urine proteome impedes identification of kidney-specific biomarkers.
Exosomes are nanovesicles derived from the endocytic pathway, formed as luminal vesicles of the multivesicular body, and released into the extracellular space by a variety of cell types.3 Recent identification of renal tubular epithelium-derived exosomes in urine has brought the expectation of a less-complex and variable, more kidney-specific protein fraction for biomarker discovery.4 Indeed, exosomes are particularly suitable for biomarker discovery as a result of their stability during purification and the relative ease with which they can be isolated from urine.5,6 Urine exosome proteins have already been proposed as potential biomarkers for a variety of kidney diseases.7–10
Uromodulin is the most-abundant urinary protein in health, excreted in quantities of 20–70 mg/day.11 It is a 68-kDa protein, synthesised exclusively in the ascending limb of the loop of Henlé, is heavily glycosylated, and polymerizes into high MW filaments in urine.12,13 Urine exosomes may be isolated by a variety of methods,5,12,14 but uromodulin cofractionates with exosomes with each of these, and the presence of such a highly abundant protein limits protein coverage achieved by MS.15,16 To overcome this, some investigators have attempted uromodulin depletion by thermochemical methods4 or by floating exosomes on a sucrose gradient.17 However, such methods may lead to loss or alteration of abundance of target proteins,18 may exclude exosomal subpopulations,17 and are labor- and time-intensive.
Abundant proteins in complex samples represent a common obstacle to identification of less-abundant proteins during tandem MS, which uses two mass spectrometers in a series, identifying intense peptides in the first (MS1) for characterization by the second (MS2). As only the most-intense peptide ions in MS1 are selected for fractionation in MS2, coeluting peptides of lower abundance are often not characterized, whereas abundant peptides are characterized repeatedly and redundantly. One common approach to this problem is the use of exclusion lists (ExLs), of which “dynamic exclusion” (DE) is most-widely used. In DE, the most intense, repeatedly observed precursor peptide ions are added sequentially to a list of mass-to-charge ratio (m/z) values, which are excluded from further characterization for a predetermined interval immediately following the initial observation, known as exclusion time. Effective exclusion requires filtering out a narrow m/z range spanning the relevant m/z (reference mass) value. This exclusion window is expressed as the percentage-of-the-reference-mass (PRM) interval. In contrast, “fixed exclusion” (FE) refers to a more-rudimentary approach, which relies on the continuous exclusion of a prespecified PRM window for the duration of an experiment.
Here, we report a novel application of an ExL-based approach to increase the depth of protein coverage during MS, while retaining uromodulin within samples, which uses dynamic and FE.
MATERIALS AND METHODS
Exosomes were isolated from 360 ml of 10 healthy volunteer urine samples, according to established methods,4,5 with some modifications. Subjects urinated directly into a container with protease inhibitors, including PMSF (500 μl 0.5 M solution), leupeptin (450 μg), and sodium azide (15 mL of a 100-mM solution). Urine was centrifuged within 30 min of collection (Beckman Avanti J26-XP centrifuge, JA-17 fixed-angle rotor, polyallomer 50 mL centrifuge bottles, Beckman Coulter, Brea, CA, USA) for 20 min at 17,000 g. The supernatant was passed through a 22-μm filter and ultracentrifuged for 135 min at 235,000 g and 4°C (Beckman Optima L-100 XP VAC ultracentrifuge, Ti45 fixed-angle titanium rotor, 70 mL polycarbonate ultracentrifuge bottles, Beckman Coulter). Each ultracentrifugation pellet was suspended in 50 μl isolation solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6) and pooled with the other pellets from the same urine sample. This work was approved by the Cambridge Local Research Ethics Review committee.
Protein from resuspended exosomal pellets was concentrated by precipitation. Samples were added to 5 vol 100% 0.1 M ammonium acetate, incubated at −20°C overnight, and then centrifuged at 3000 g for 10 min at 4°C. The pellet was washed with 80% ammonium acetate, centrifuged at 3000 g for 10 min at 4°C, resuspended in 80% acetone, and centrifuged at 3000 g for 15 min at 4°C. The acetone was removed and the pellet desiccated under a Speedvac for 3 min. Protein content of each pooled sample was quantified using a Bio-Rad Bradford protein-binding colorimetric assay (Bio-Rad, Hercules, CA, USA). Protein pellets were stored at −80°C until use.
For MS, protein pellets were suspended in Laemmli sample buffer19 and incubated at 95°C. Protein (50 μg) from each sample was loaded on a 12% SDS-polyacrylamide gel, and gels were stained with colloidal Coomassie blue; each gel track sliced into 28 equal segments. Proteins were reduced, alkylated, and in-gel-digested with trypsin.
The uromodulin ExL was generated in two stages. First, trypsin digests from each of 10 samples were subjected to liquid chromatography (LC)-MS/MS (using DE), hereafter referred to as the unfiltered run. From these runs, all uromodulin-derived peptides were identified. The ExL was populated with m/z values corresponding to the most-frequently observed uromodulin peptides. Second, the optimal settings for FE of uromodulin were defined trough performing a number of runs with a variety of exclusion window settings, with the objective of maximizing the additional yield and minimizing redundancy with the ExL. Results from these experiments are summarized in Supplemental Fig. 1. Finally, the optimal settings were applied for FE of uromodulin, as an overlay to conventional DE (Table 1), in a second LC-MS/MS run of each of 10 samples.
TABLE 1.
ExL Settings
|
Conventional run |
ExL run |
||
|---|---|---|---|
| DE | DE | FE (uromodulin) | |
| ExL size | 50 | 50 | 20 |
| Exclusion duration(s) | 120 | 120 | Continuous |
| PRM | −1.5 to 1.5 | −1.5 to 1.5 | −0.07 to 0.09a |
aFor FE of uromodulin peptides, the continuous use of a PRM exclusion window similar to that used in DE resulted in an unacceptably low number of protein identifications (data not shown). This was circumvented by narrowing the exclusion window markedly as shown.
LC-MS/MS was performed using an Eksigent NanoLC-1D Plus (Eksigent Technologies, Dublin, CA, USA) HPLC system and an LTQ Orbitrap MS (ThermoFisher, Waltham, MA, USA). Peptides were separated by reverse-phase chromatography [flow rate 300 nL/min; LC Packings PepMap100 column (C18, 75 μM i.d.×150 mm, 3 μM particle size), Dionex, Sunnyvale, CA, USA]. Peptides were loaded onto a precolumn [Dionex Acclaim PepMap100 (C18, 5 μM particle size, 100A, 300 μM i.d.×5 mm)] from the autosampler (0.1% formic acid, 5 min, flow rate of 10 μL/min). Peptides were eluted onto the analytical column by switching the 10 port valve. The gradient for solvents A (water+0.1% formic acid) and B (acetonitrile+0.1% formic acid) was 5–50% B over 40 min. A nanospray source (New Objective, Woburn, MA, USA) was used for electrospray ionization. m/z values of eluting ions were measured in the Orbitrap mass analyzer with a mass range of 350–1600 and the resolution set at 7500. Peptides were fragmented by collision-induced dissociation.
MS data were processed using Sequest Bioworks Browser (Version 3.3.1 SP1, ThermoFisher) to generate MS/MS peak lists. Combined peak-list files were submitted to the MASCOT search algorithm (Version 2.2.1, Matrix Science, London, UK) and searched against the International Protein Index-Human database, Version 4.3. Spectra were rescored using MASCOT Percolator, a machine learning tool that minimizes false discoveries and incorporates target decoy searching.20 Protein identification required two or more unique peptides,21 with a false-discovery rate of 0.1. To establish whether proteins had been identified previously in urinary exosomes, gene names were searched against the ExoCarta exosomal protein database.22
Data were analyzed with Stata SE v11.2 (StataCorp, College Station, TX, USA). Data are presented as mean (sd) or median (interquartile range) as appropriate. Peptide counts were compared with the Wilcoxon matched-pairs signed-ranks test. Proportions were compared using the χ2 test.
RESULTS
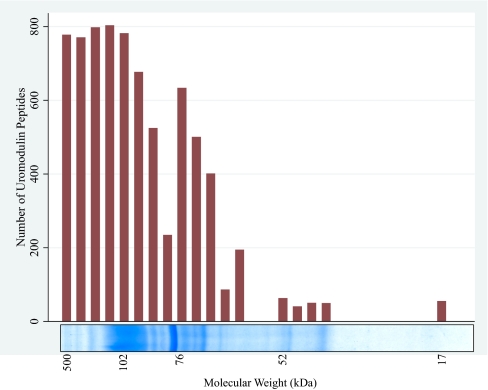
In unfiltered runs, uromodulin was the most-abundant protein present. We identified a total of 5332 spectra corresponding to uromodulin peptides, representing 37 distinct peptide sequences (Table 2 and Fig. 1).
TABLE 2.
Uromodulin Peptides Identified by Conventional and ExL Methods
| Peptide sequence | ExL peptide | Conventional method |
Excluded m/z | ExL |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide count | Mean peptide score | Charge state observed |
Number of oxidations |
Peptide count | Mean peptide score | Charge state observed |
Number of oxidations |
|||||||||||
| 1 | 2 | 3 | 0 | 1 | 2 | 1 | 2 | 3 | 0 | 1 | 2 | |||||||
| TALQPMVSALNIR | YES | 714 | 707.41 | — | 638 | 76 | 469 | 245 | — | 707.41 | 53 | 55.7 | — | 32 | 21 | 33 | 20 | — |
| DSTIQVVENGESSQGR | YES | 624 | 853.41 | — | 471 | 154 | 624 | — | — | 853.41 | 316 | 67.9 | — | 79 | 237 | 316 | — | — |
| VFMYLSDSR | YES | 560 | 559.27 | 2 | 558 | — | 432 | 128 | — | 559.27 | 22 | 32.7 | 1 | 21 | — | 9 | 13 | — |
| DWVSVVTPAR | YES | 527 | 565.304 | — | 527 | — | 527 | — | — | 565.304 | 5 | 43.9 | 2 | 3 | — | 5 | — | — |
| TLDEYWR | YES | 469 | 491.735 | — | 469 | — | 469 | — | — | 491.735 | 15 | 23 | 15 | — | — | 15 | — | — |
| MALFQTPSYTQPY QGSSVTLSTEAFL YVGTMLDGGDLSR | YES | 460 | 1416.02 | — | — | 460 | 158 | 225 | 77 | 1416.02 | 7 | 34.5 | — | — | 7 | 3 | 1 | 3 |
| VGGTGMFTVRa | YES | 411 | 520.76 | — | 411 | — | 209 | 202 | — | 520.76 | 572 | 67.3 | — | 572 | — | 1 | 572 | — |
| YFIIQDR | YES | 315 | 477.753 | — | 315 | — | 315 | — | — | 477.753 | 5 | 25.6 | 4 | 1 | — | 5 | — | — |
| FSVQMFRa | YES | 226 | 457.72 | — | 226 | — | 40 | 186 | — | 457.72 | 478 | 33.4 | — | 478 | — | 478 | — | — |
| FVGQGGAR | YES | 207 | 396.21 | — | 207 | — | 207 | — | — | 396.21 | 8 | 38.8 | — | 8 | — | 8 | — | — |
| SLGFDKVFMYLSDSR | YES | 193 | 882.94 | — | 99 | 94 | 107 | 86 | — | 882.94 | 58 | 49.8 | — | 22 | 36 | 15 | 43 | — |
| DNRDWVSVVTPAR | YES | 148 | 757.89 | — | 97 | 51 | 148 | — | — | 757.89 | 0 | — | — | — | — | — | — | — |
| LECGANDMK | YES | 98 | 527.233 | — | 98 | — | 29 | 69 | — | 527.233 | 27 | 49.2 | — | 27 | — | 25 | 2 | — |
| INFACSYPLDMK | YES | 79 | 737.84 | — | 79 | — | 25 | 54 | — | 737.84 | 12 | 66.9 | — | 12 | — | — | 12 | — |
| STEYGEGYACDTDLR | YES | 77 | 868.86 | — | 77 | — | 77 | — | — | 868.86 | 9 | 64.6 | — | 9 | — | 9 | — | — |
| MAETCVPVLRa | YES | 75 | 596.302 | — | 75 | — | 51 | 24 | — | 596.302 | 151 | 70 | — | 151 | — | — | 151 | — |
| DGPCGTVLTR | YES | 59 | 538.265 | — | 59 | — | 59 | — | — | 538.265 | 0 | — | — | — | — | — | — | — |
| FAGNYDLVYLHCE VYLCDTMNEK | YES | 25 | 957.42 | — | — | 25 | 6 | 19 | — | 957.42 | 18 | 66.8 | — | — | 18 | — | 18 | — |
| CSGFNDR | YES | 20 | 428.175 | — | 20 | — | 20 | — | — | 428.175 | 40 | 47.2 | — | 40 | — | 40 | — | — |
| CSGFNDRDNR | YES | 12 | 620.76 | — | 9 | 3 | 12 | — | — | 620.76 | 10 | 26 | — | 2 | 8 | 10 | — | — |
| SLGFDK | NO | 8 | 666.443 | 8 | — | — | 8 | — | — | 666.443 | 4 | 27.2 | 4 | — | — | 4 | — | — |
| VLNLGPITR | NO | 4 | 491.809 | — | 4 | — | 4 | — | — | 491.809 | 6 | 43.6 | — | 6 | — | 6 | — | — |
| VSLKTALQPMVSAL NIRVGGTGMFTVR | NO | 2 | 1197.25 | — | 1 | 1 | — | 1 | 1 | 1197.25 | 0 | — | — | — | — | — | — | — |
| FVGQGGARMAETC VPVLRCNTAAPMW LNGTHPSSDEGIVSR | NO | 2 | 1482.33 | — | 2 | — | — | 1 | — | 1482.33 | 0 | — | — | — | — | — | — | — |
| WHCQCK | NO | 2 | 459.69 | — | 2 | — | 2 | — | — | 459.69 | 2 | 15.5 | — | 2 | — | 2 | — | — |
| TALQPMVSALNIR VGGTGMFTVR | NO | 2 | 1218.09 | — | 2 | — | — | 2 | — | 1218.09 | 0 | — | — | — | — | — | — | — |
| NETHATYSNTLYL ADEIIIR | NO | 2 | 779.757 | — | — | 2 | 2 | — | — | 779.757 | 0 | — | — | — | — | — | — | — |
| VSLKTALQPMVSA LNIR | NO | 2 | 614.362 | — | — | 2 | 2 | — | — | 614.362 | 0 | — | — | — | — | — | — | — |
| WHCQCKQDFNITDI SLLEHR | NO | 1 | 1300.58 | — | 1 | — | 1 | — | — | 1300.58 | 0 | — | — | — | — | — | — | — |
| GQPSLTWMLMVVVA SWFITTAATDTSEA RTKYNCPAR | NO | 1 | 1398.34 | — | — | 1 | 1 | — | — | 1398.34 | 0 | — | — | — | — | — | — | — |
| VFMYLSDSRCSGF NDRDNR | NO | 1 | 1170.51 | — | 1 | — | 1 | — | — | 1170.51 | 0 | — | — | — | — | — | — | — |
| VWLPLLLSATLTLTFQ | NO | 1 | 908.065 | — | 1 | — | 1 | — | — | 908.065 | 0 | — | — | — | — | — | — | — |
| GQPSLTWMLMVVV ASWFITTAATDTSEAR | NO | 1 | 1067.56 | — | — | 1 | 1 | — | — | 1067.56 | 0 | — | — | — | — | — | — | — |
| FAGNYDLVYLHCE VYLCDTMNEKCKP TCSGTRFR | NO | 1 | 1407.89 | — | — | 1 | — | 1 | — | 1407.89 | 0 | — | — | — | — | — | — | — |
| DSTIQVVENGESS QGRFSVQMFR | NO | 1 | 1300.8 | — | 1 | — | 1 | — | — | 1300.8 | 0 | — | — | — | — | — | — | — |
| CKPTCSGTR | NO | 1 | 533.747 | — | 1 | — | 1 | — | — | 533.747 | 0 | — | — | — | — | — | — | — |
| SGSVIDQSR | NO | 1 | 474.257 | — | 1 | — | 1 | — | — | 474.257 | 16 | 49 | — | 16 | — | 16 | — | — |
| FRSGSVIDQSR | NO | 0 | 626.31 | — | — | — | — | — | — | 626.31 | 2 | 25.3 | — | 2 | — | 2 | — | — |
| YFIIQDRCPHTR | NO | 0 | 803.55 | — | — | — | — | — | — | 803.55 | 2 | 10 | — | 2 | — | 2 | — | — |
| VSLGK | NO | 0 | 502.3 | — | — | — | — | — | — | 502.3 | 12 | 18.5 | 12 | — | — | 12 | — | — |
| DLNIK | NO | 0 | 602.36 | — | — | — | — | — | — | 602.36 | 23 | 30.4 | 23 | — | — | 23 | — | — |
aPeptides showing a paradoxical increase after exclusion.
For each peptide, only the most abundant form, in terms of charge and number of oxidations, was excluded. The characteristics of the excluded species appear in boldface type in the columns denoting charge state and number of oxidations. No methylated peptides were identified. For each of the peptides showing a paradoxical increase after exclusion, the increase was driven by nonexcluded species of that peptide.
FIGURE 1.
Gel appearance of uromodulin peptides, which were most abundant in gel slices at or above 100 kDa, but some were identified from gel slices across the entire MW range.
As exclusion windows filter not only the m/z for which they are designed, but also any other peptide ions for which the m/z falls within the exclusion window, its application must take into account two important trade-offs: 1) setting the window too wide will result in exclusion of a multitude of “desirable” peptides; too narrow and the undesirable peptide may not be consistently excluded; and 2) excluding infrequently observed uromodulin peptides will result in failure to detect any peptides, for which the m/z falls within the exclusion window, without significant gains in protein coverage; failure to exclude frequently observed peptides leaves the problem of their preferential characterization in MS2 unresolved. We balanced the first of these trade-offs by exploring a number of PRM window widths (Supplemental Fig. 1) to identify the optimal PRM exclusion window. The second was not formally explored but was addressed by arbitrarily selecting the 20 most-frequently observed of 37 uromodulin peptides for exclusion. For this set of 20 abundant uromodulin peptides (peptide count 12–714), ions representing their most-abundant charge and oxidation states were used to generate a list of m/z values for the ExL. For each peptide, where different species of the same peptide occurred (by charge or oxidation), the m/z for only the most-abundant species was excluded. The 17 remaining lower-abundance peptides (peptide count 1–8) were not excluded (Table 2).
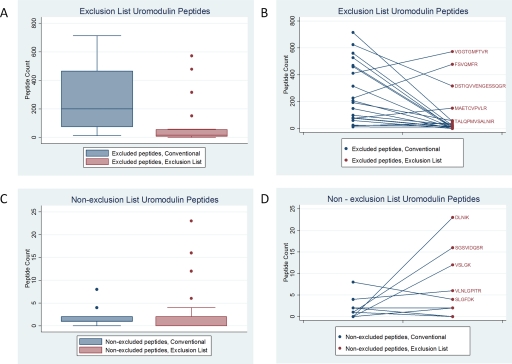
First, we asked if the ExL was effective in reducing the number of uromodulin-derived precursor ions selected for MS2. We compared uromodulin peptide counts for excluded and nonexcluded uromodulin peptides in the conventional and ExL datasets. The ExL significantly reduced the median peptide count of the 20 excluded peptides from 200 (12–714) to 16 (0–572; P=0.001), although three excluded peptides increased paradoxically (Fig. 2). For these three peptides (VGGTGMFTVR, FSVQMFR, and MAETCVPVLR), multiple oxidation states had been identified during conventional runs. Only the most-abundant species of these peptides had been excluded, thus allowing nonexcluded species of VGGTGMFTVR, FSVQMFR, and MAETCVPVLR to be observed with increased frequency. Indeed, all nonexcluded uromodulin peptides were observed with greater frequency with the ExL, showing an overall increase from 0 (0–2) to 17 (7–56; Fig. 2), although this difference was not statistically significant (P=0.61). The ExL reduced the overall number of MS/MS events triggered by uromodulin peptides by 73.2%. Correspondingly, the coverage ratio for uromodulin reduced from 56.8% to 37.7% with application of the ExL (Fig. 3).
FIGURE 2.
Uromodulin peptide counts without and with exclusion. (A and B) Excluded uromodulin peptides and (C and D) uromodulin peptides that were not excluded. Although A and C show the distribution of peptide counts, with dots representing statistical outliers, B and D demonstrate the change in counts for each individual peptide without and with the ExL. Excluded uromodulin peptides (A and B) were reduced significantly by application of the ExL, although three peptides paradoxically increased. Nonexcluded uromodulin peptide counts (C and D) were not significantly different after ExL application.
FIGURE 3.
Uromodulin protein coverage ratio. (A) Uromodulin peptides identified during conventional runs covered 56.8% of the protein sequence. This reduced to 37.7% after applying the ExL (C). Excluded peptides covered 34.8% of the protein sequence (B).
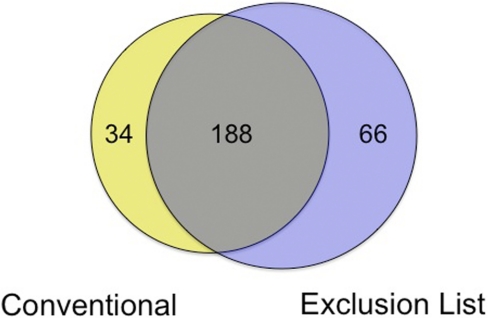
Next, we asked if the ExL improved the depth of exosomal protein coverage. Using conventional methods, a total of 222 distinct proteins was identified from 10 separate samples; after running each of these a second time with application of the ExL, 254 proteins were identified. These two protein sets shared 188 proteins in common (Fig. 4). The uromodulin ExL method failed to identify 34 (15.3%) proteins identified by the conventional method, as a result of nonuromodulin peptides falling within the uromodulin exclusion windows. However, the ExL unmasked the presence of an additional protein set of 66 proteins, increasing the overall number of identifications by 29.7% to 288 proteins. Of proteins identified exclusively by the ExL, a significantly greater proportion (53%) included newly identified urinary exosomal proteins, compared with 37% of those identified exclusively by the conventional approach (P=0.03).
FIGURE 4.
Overlap of proteins identified without and with the ExL. Application of the ExL unmasked 66 proteins not seen with conventional analysis. Not all proteins identified by conventional methods were also evident with the ExL, demonstrating the complementary rather than substitutive nature of the ExL.
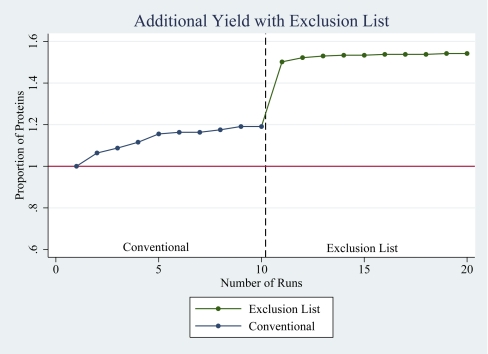
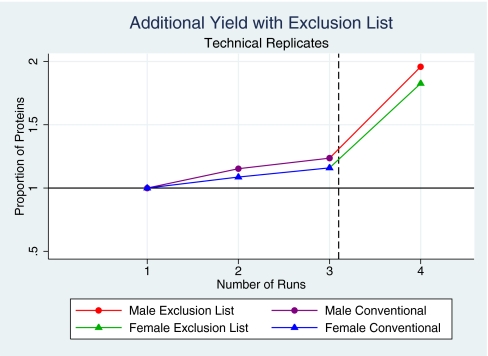
We asked if the additional proteins revealed with application of the ExL could be explained simply by the effect of performing repeated runs23 or whether this was more specifically attributable to the ExL. We addressed this by considering the effects of the ExL on protein identifications from biological and analytical replicates, respectively. First, we considered the number of additional proteins identified from each of 10 biological replicate runs. Consistent with previous reports,23 repeated runs resulted in an increase in the number of proteins identified. Redundancy increased with each run, and the incremental identification of new proteins decreased from 6% from the second run to nil from the 10th and final run. Despite this progression to complete redundancy, a further 10 replicate runs using the ExL demonstrated a further and marked increase in new protein identifications (Fig. 5). Second, we assessed the impact of the ExL on analytical replicates by performing three consecutive conventional runs on the same sample for one male (M) and one female (F) subject and compared the protein yield from each of these triplicate runs with the yield from the corresponding ExL runs. The second and third runs increased the overall number of proteins identified from the first conventional run by 15% (M) and 9% (F) and by 6% (M) and 7% (F), respectively. A subsequent run with addition of the ExL yielded an additional increase of 72% (M) and 66.7% (F; Fig. 6). Together, these data confirm that the unmasking effect of the ExL exceeds the gains obtained from repeated conventional runs.
FIGURE 5.
Incremental increase in protein yield with repeated runs. Repeated conventional runs on biological replicates increase the number of new protein identifications, although redundancy increases with an increasing number of runs. Application of the ExL unmasks an additional set of previously unseen proteins.
FIGURE 6.
Comparison of the uromodulin ExL with repeated conventional analysis. Consecutive analytical replicate runs on each of two samples reveal a small, incremental increase with each consecutive run. Application of the ExL yields a large, additional increment.
DISCUSSION
We report improved depth of urine exosomal protein coverage through the use of FE of abundant uromodulin-derived peptides in addition to and following conventional analysis with DE. Rather than representing a substitute, this approach is complementary to conventional methods. Although the principle of FE is not new,24,25 it has, to our knowledge, not been used for uromodulin specifically or for urine proteomics in general nor has it been used in addition to DE rather than in substitution.
Our method reduces the need for physical removal of uromodulin, the most-abundant urinary protein in nonproteinuric subjects, which cofractionates with exosomes during isolation by differential centrifugation4,9,17 or with the use of nanomembrane concentrators.6 Pisitkun and colleagues4 used thermochemical manipulation of exosomal samples by incubation at 95°C with DTT, proposing that this allows nonpolymerized uromodulin to be removed in the ultracentrifugation supernatant. However, in our hands, this approach reduced the amount of uromodulin in the exosomal pellet only marginally (Supplemental Fig. 2). Furthermore, as exosomes adhere to uromodulin,12 its removal may lead to a loss of exosomal material. More recently, the same group suggested amending the isolation protocol to add DTTduring the low-speed centrifugation, thus transferring even more uromodulin to the exosomal pellet in an attempt to maximize exosomal yield and to allow the use of uromodulin as a standardization factor.12 Although this method may allow standardization of exosomal excretion rates, it would increase obfuscation during MS of the exosomal proteome by uromodulin.
In contrast, Hogan and colleagues17 used flotation of exosomes on a density gradient to isolate a subpopulation of polycystin-1-positive exosomes depleted of uromodulin. Although effectively reducing the uromodulin contamination of this subpopulation of exosomes, this approach instead concentrates uromodulin with other exosomal fractions, confounding their analysis.
In plasma, low-abundance protein coverage is improved by depleting abundant proteins using commercially available immunoadsorption kits. Theoretically, uromodulin could also be removed by immunoadsorption. To our knowledge, this approach has not been reported, but the propensity for exosomes to adhere to uromodulin fibrils suggests that this method would also lead to a loss of exosomal material.
The use of the ExL enabled us to detect more low-abundance peptides and proteins by effectively reducing uromodulin-induced MS/MS switching events. The observed paradoxical increase of three excluded peptides shows that the exclusion of a m/z for one oxidation state, in the presence of another relatively abundant oxidation state, may allow the nonexcluded m/z for that peptide to be observed more frequently. For example, in the case of VGGTGMFTVR, the propensity to select the methionine-oxidized VGGTGMFTVR for MS2 may be a result of the exclusion of a window for INFACSYPLDMK, which falls very close to methionine-oxidized VGGTGM FTVR, thus allowing preferential selection of methionine-oxidized VGGTGMFTVR for MS2 (Table 2). It is possible that filtering out m/z for more than one oxidation state where neither is dominant, perhaps across multiple MS runs where experimental material is not limited, might result in an even greater protein yield with uromodulin exclusion than that reported here. For certain excluded peptides where multiple charge and oxidation states exist, for example MALFQTPSYTQPYQGSSVTLSTEAFLY VGTMLDGGDLSR, exclusion of the most abundant of these also resulted in a reduction of a nonexcluded species. This may be explained by the additional use of DE, which after FE of the most-abundant peptides, instead selected less-abundant species for DE.
The ExL increased the overall number of proteins identified by almost 30%. Although repeated runs are known to increase identifications of lower-abundance proteins, redundancy also increases, whereas additional identifications decrease for each additional run performed. As we have demonstrated from performing conventional runs on 10 samples, there are no additional gains from performing further replicates. These data are consistent with findings reported by Durr and colleagues,23 who identified <5% more proteins after 10 replicate runs. Despite complete redundancy after running 10 replicates, the additional 30% increase in protein identifications was achieved simply by applying the ExL to the same 10 samples. Secondly, from additional triplicate (analytical replicate) runs on each of two subjects, we demonstrated low redundancy and large gains from a single, additional ExL run (Fig. 5). Taken together, these findings demonstrate significant gains from using the ExL, which far exceed the yield expected from repeated conventional runs.
Other than reducing the need for time- and labor-intensive sample preparations, our uromodulin ExL approach has other notable strengths. First, once the exclusion m/z values have been identified, its application is straightforward. Second, although markedly increasing the depth of protein coverage, uromodulin retention also allows its use for standardization. However, it does require duplicate MS runs and should not be used as a stand-alone method.
FE is not universally successful. When applied to complex samples with multiple abundant proteins, FE can fail to increase protein identifications.26 However, urine exosomal samples are particularly suited to FE, as uromodulin represents a single-predominant and highly abundant exclusion target. An ExL for uromodulin therefore provides an effective parallel approach to conventional LC-MS/MS to improve the depth of protein coverage without uromodulin depletion and should be used in tandem with the conventional approach.
ACKNOWLEDGMENT
T.F.H. is supported by an Action Medical Research Training Fellowship and holds a Raymond and Beverly Sackler Research Studentship. P.D.C. is supported by a Biotechnology and Biological Sciences Research Council Research Training Fellowship. This work is also supported by the Wellcome Trust (awards 088489 to F.E.K. and 079895 to Cambridge Institute for Medical Research) and the Cambridge National Institute for Health Research Biomedical Research Centre.
Footnotes
The authors declare that they have no conflict of interest or financial disclosures.
REFERENCES
- 1. Cox J, Mann M. Is proteomics the new genomics? Cell 2007;130:395–398 [DOI] [PubMed] [Google Scholar]
- 2. Knepper MA. Common sense approaches to urinary biomarker study design. J Am Soc Nephrol 2009;20:1175–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic 2008;9:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 2004;101:13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou H, Yuen PS, Pisitkun T, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 2006;69:1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheruvanky A, Zhou H, Pisitkun T, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol 2007;292:F1657–F1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou H, Pisitkun T, Aponte A, et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int 2006;70:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell PJ, Welton J, Staffurth J, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med 2009;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzales PA, Pisitkun T, Hoffert JD, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 2009;20:363–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteomics 2010;4:416–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lhotta K. Uromodulin and chronic kidney disease. Kidney Blood Press Res 2010;33:393–398 [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int 2010;77:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Rooijen JJ, Voskamp AF, Kamerling JP, Vliegenthart JF. Glycosylation sites and site-specific glycosylation in human Tamm-Horsfall glycoprotein. Glycobiology 1999;9:21–30 [DOI] [PubMed] [Google Scholar]
- 14. Merchant ML, Powell DW, Wilkey DW, et al. Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics Clin Appl 2010;4:84–96 [DOI] [PubMed] [Google Scholar]
- 15. Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 2002;1:845–867 [DOI] [PubMed] [Google Scholar]
- 16. Thongboonkerd V, McLeish KR, Arthur JM, Klein JB. Proteomic analysis of normal human urinary proteins isolated by acetone precipitation or ultracentrifugation. Kidney Int 2002;62:1461–1469 [DOI] [PubMed] [Google Scholar]
- 17. Hogan MC, Manganelli L, Woollard JR, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 2009;20:278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simpson RJ. Purifying Proteins for Proteomics: A Laboratory Manual, 2nd ed Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Books, 2004 [Google Scholar]
- 19. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685 [DOI] [PubMed] [Google Scholar]
- 20. Brosch M, Yu L, Hubbard T, Choudhary J. Accurate and sensitive peptide identification with mascot percolator. J Proteome Res 2009;8:3176–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradshaw RA, Burlingame AL, Carr S, Aebersold R. Reporting protein identification data: the next generation of guidelines. Mol Cell Proteomics 2006;5:787–788 [DOI] [PubMed] [Google Scholar]
- 22. Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 2009;9:4997–5000 [DOI] [PubMed] [Google Scholar]
- 23. Durr E, Yu J, Krasinska KM, et al. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 2004;22:985–992 [DOI] [PubMed] [Google Scholar]
- 24. Scherl A, Francois P, Converset V, et al. Nonredundant mass spectrometry: a strategy to integrate mass spectrometry acquisition and analysis. Proteomics 2004;4:917–927 [DOI] [PubMed] [Google Scholar]
- 25. Mi W, Wang J, Ying WT, Jia W, Cai Y, Qian XH. A tandem mass spectrometry acquisition strategy based on exclusion of precursor ions. Chinese J Anal Chem 2010;38:241–244 [Google Scholar]
- 26. Pellitteri-Hahn MC, Warren MC, Didier DN, et al. Improved mass spectrometric proteomic profiling of the secretome of rat vascular endothelial cells. J Proteome Res 2006;5:2861– 2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.