Abstract
For reasons unknown, temporomandibular disorder (TMD) can manifest as localized pain or in conjunction with widespread pain. We evaluated relationships between cytokines and TMD without or with widespread palpation tenderness (TMD−WPT or TMD+WPT, respectively), at protein, transcription factory activity, and gene levels. Additionally, we evaluated the relationship between cytokines and intermediate phenotypes characteristic of TMD and WPT. In a case-control study of 344 females, blood samples were analyzed for levels of 22 cytokines and activity of 48 transcription factors. Intermediate phenotypes were measured by quantitative sensory testing and questionnaires asking about pain, health, and psychological status. Single nucleotide polymorphisms (SNPs) coding cytokines and transcription factors were genotyped. TMD−WPT cases had elevated protein levels of pro-inflammatory cytokine MCP-1 and anti-inflammatory cytokine IL-1ra, whereas TMD+WPT cases had elevated levels of pro-inflammatory cytokine IL-8. MCP-1, IL-1ra, and IL-8 were differentially associated with experimental pain, self-rated pain, self-rated health, and psychological phenotypes. TMD−WPT and TMD+WPT cases had inhibited transcription activity of the anti-inflammatory cytokine TGFβ1. Interactions were observed between TGFβ1 and IL-8 SNPs: an additional copy of the TGFβ1 rs2241719 minor T allele was associated with twice the odds of TMD+WPT among individuals homozygous for the IL-8 rs4073 major A allele and half the odds of TMD+WPT among individuals heterozygous for rs4073. These results demonstrate how pro- and anti-inflammatory cytokines contribute to the pathophysiology of TMD and WPT in genetically-susceptible people. Furthermore, they identify MCP-1, IL-1ra, IL-8, and TGFβ1 as potential diagnostic markers and therapeutic targets for pain in patients with TMD.
Keywords: Epistasis, Gene polymorphism, Interleukin-1 receptor antagonist (IL-1ra), Interleukin-8 (IL-8), Monocyte chemotactic protein-1 (MCP-1), Transforming growth factor β1 (TGFβ1)
1. Introduction
Temporomandibular disorders (TMD) elicit pain in temporomandibular joints and masticatory muscles and occur frequently in the population. In 2002, 6% of women and 3% of men in the US reported TMD-like pain [42]. Many TMD patients also suffer from widespread pain, characterized by bilateral pain in multiple body areas extending beyond the head and face [4; 37; 38; 45]. One study found that 62% of females referred to a tertiary care clinic for TMD reported widespread pain [91]. Both TMD and widespread pain are complex chronic pain conditions characterized by a mosaic of intermediate phenotypes associated with altered pain processing and psychological status, usually in the absence of tissue damage [25]. Identification of biological mediators linked to these conditions is needed to better understand their pathophysiology and to develop effective treatments.
An emerging literature suggests that cytokines contribute to pathophysiology of TMD and WBP. Cytokines are small intracellular regulatory proteins secreted by immune cells in the periphery and neurons and glia in the central nervous system [53; 62]. In an acute setting, pro-inflammatory cytokines (e.g., macrophage inflammatory protein-1 and interleukin-8) confer survival advantage by promoting immune responses that limit tissue damage and initiate remodelling [8; 27; 33]. However, persistent elevations result in tissue pathology and altered nociceptor function. Pro-inflammatory cytokine levels are elevated in TM joints of TMD patients [46; 60; 69; 89] and circulating blood of patients with widespread pain [7; 36; 86; 93; 100]. Additionally, elevated pro-inflammatory cytokine levels are correlated with greater pain sensitivity [51; 69; 84; 89], perceived stress [59], and depressed mood [58], which are phenotypes characteristic of TMD and widespread pain.
While pro-inflammatory cytokines play a definitive role in pain induction, little is known about their contribution to chronic pain conditions. To date, most studies of TMD have focused on local effects of cytokines within TM joints; however, it is highly plausible that more generalized systemic processes contribute to TMD and related disorders. Pro-inflammatory cytokines may drive the transition from acute to chronic pain by activating transcription of genes influencing biological pathways that regulate pain and psychological status [63; 68; 70; 90]. An additional layer of complexity is added when considering the contribution of genetic polymorphisms influencing cytokine synthesis and release. More work is needed to understand the complex nature of these biological, psychophysiological, and genetic relationships.
This study sought to elucidate contributions of cytokines to TMD without and with widespread palpation tenderness (WPT) at gene, transcript, and protein levels as well as contributions of cytokines to intermediate phenotypes characteristic of TMD and WPT. We hypothesized that cytokine protein levels and gene regulatory (i.e., transcription factor) profiles would be shifted towards a pro-inflammatory state among cases and that this shift would coincide with increased pain, poorer self-rated health, and psychological distress. Additionally, we hypothesized that polymorphisms in relevant cytokine and transcription factor genes would contribute to case status. To test these hypotheses, we determined associations of 1) circulating cytokines with case status and intermediate phenotypes, 2) gene regulatory pathways with case status, and 3) relevant cytokine and transcription factor gene polymorphisms with case status.
2. Materials and Methods
This study was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill (UNC-CH). Written informed consent was obtained from all study participants.
2.1 Study design and study participants
This study uses data from all 344 people in whom circulating cytokines were measured as part of case-control study of TMD. Between 2005 and 2009, female volunteers were recruited from the Chapel Hill, NC area using advertisements placed in local newspapers and flyers posted in and around the UNC Health Center. This included tertiary care clinics that provide treatment for people with chronic pain. The intention was to enroll 200 females with chronic TMD and 200 females without TMD, providing sufficient statistical power to address the study’s primary aims regarding genetic influences on TMD, assuming odds ratios of at least 2.3, and with Bonferroni adjustment for testing 7 targeted genetic variants.
Both cases and controls were self-identified white females aged 18–60 years who provided signed informed consent for study procedures including biological samples. Enrollment was limited to females, because they have higher prevalence of TMD than men, and to whites to avoid problems of population stratification in assessing genetic associations. While there are statistical methods that can adjust for population stratification in genetic association studies, restricting studies to a single racial group was the preferred approach at the time this study was designed [26]. Exclusion criteria were self-reported history of one or more of 14 health-related conditions: diabetes; drug or alcohol abuse; heart disease hypertension not controlled by medication; hyperthyroidism; lupus erythematosis; psychiatric illness requiring hospitalization; respiratory disease (other than asthma) not controlled by medication; rheumatoid arthritis; kidney failure or dialysis; epilepsy; current chemotherapy or radiation treatment; pregnant or nursing mothers; facial injury or surgery; current orthodontic treatment.
To be enrolled as cases, study participants had to report facial pain for at least five days during the previous two weeks and be diagnosed with TMD arthralgia or myalgia during a standardized clinical examination that used the Research Diagnostic Criteria for TMD [28]. In the examination, myalgia was diagnosed when pain was reported during digital palpation of ≥ 3 of 8 muscle groups (masseter, temporalis, submandibular, lateral pterygoid, each palpated bilaterally) and arthralgia was diagnosed when pain was reported during digital palpation of either TM joint. Digital palpation of muscle and joint cranial sites was performed using a force of 0.45 kg.
TMD cases were sub-classified according to presence or absence of WPT. WPT represents a useful marker of widespread pain because it evaluates aspects of both tenderness and distress characteristic of chronic widespread musculoskeletal pain conditions [34]. For example, Pfau et al (2009) found that TMD patients with ≥11 bodily tender points closely resembled fibromyalgia patients with respect to experimental pain sensitivity [75]. For this analysis, WPT was based on reports of tenderness during the examiners’ digital palpation of 18 bodily sites: lower sternocleidomastoid, lower cervical, trapezius, supraspinatus, second rib, lateral epicondyle, gluteal, greater trochanter, and knee (all palpated bilaterally). Digital palpation of those bodily sites was done using a force of 1.4 kg. WPT was classified as present when tenderness to palpation was reported in ≥ 6 of those sites, with ≥ 2 sites eliciting pain bilaterally. Self-reported history of bodily pain did not contribute to the classification of WPT.
Controls reported no history of orofacial pain within the preceding six months and no prior diagnosis for TMD. Additionally, their examination confirmed that they did not have TMD arthralgia or myalgia. Using these criteria, 396 females were enrolled into the case-control study [81]: 115 TMD−WPT cases, 84 TMD+WPT cases, and 197 controls. The numbers of subjects used for analysis of the phenotypes and genotypes (N=344) and for analysis of gene expression (N=195) are described in Table S1.
2.2 Assessment of psychological and self-rated health phenotypes
Prior to their clinical examination, study participants completed the short form McGill Pain Questionnaire (MPQ), Pennebaker Index of Limbic Languidness (PILL), Perceived Stress Scale (PSS), Short Form 12 version 2 (SF12v2), Profile of Mood States- Bi-Polar (POMS), and State-Trait Anxiety Inventory (STAIY-1 and STAIY-2). The MPQ contains 11 verbal descriptors assessing sensory components of pain, 5 descriptors of affective components of pain, and 1 question about pain intensity [61]. Responses on four-point ordinal scales were summed to compute scores for each component. The PILL asks about the frequency of 54 somatic symptoms such as itchy eyes, pimples, and dizziness [74]. Frequency was recorded on a five-point Likert scale ranging from “never or almost never” to “more than once a week” and the sum of all 54 responses was used as an index of somatic awareness. The PSS asks 10 questions about responses to and perceived control over daily stressors [16]. Responses were recorded on a five-point scale ranging from “never” to “very often”. A single summary score was computed by first reverse-coding responses to questions regarding control and then summing all ten items. The SF-12v2 evaluated self-rated health using 12 questions about physical and mental health. Published scoring algorithms [96] were used to compute scales for six domains: global health rating, physical functioning, physical roles, emotional functioning, emotional roles, and pain interference. Each scale ranged from 0 to 100, with higher values signifying better function and health. The POMS consists of 72 questions about mood-related states that were used to compute a global score of mood [55]. The STAIY-1 contains 20 statements evaluating levels of state anxiety [87], yielding a single summary score.
2.3 Assessment of clinical and experimental pain
Following their clinical examination, study participants completed a 45 minute session of quantitative sensory testing where they rated responses to three, standardized noxious stimuli. First, threshold and tolerance to thermal pain were assessed on the forearm by applying a thermode that increased in temperature at a rate of 0.5°C/sec. Threshold was defined as the temperature at which pain was first reported, while tolerance was defined as the highest temperature that could be tolerated (with an upper limit of 50°C). Second, ratings of pain in response to 10 repeated thermal stimuli of 50°C applied to the right hand were evaluated [76]. Stimuli of 0.5 sec duration were repeated once every three seconds and pain was rated on a 0–100 numerical rating scale (NRS). Plots of each subject’s pattern of response at each temperature were used to derive two summary measures of temporal “windup” (highest NRS response minus lowest NRS response and slope of a linear regression line fitted for the first five NRS responses) and two summary measures of thermal sensitivity (first NRS and sum of all NRS responses). Third, pressure pain thresholds in response to a hand-held pressure algometer [43] were assessed bliaterally at two extracranial sites: trapezius muscles and the lateral epicondyle. Pressure threshold at each site was recorded as the algometer loading (kg) at which pain was first detected.
2.4 Assessment of circulating cytokine protein levels
During the clinic visit, study participants’ whole blood was collected into EDTA-coated vacutainer tubes and placed on ice. Whole blood was centrifuged at a speed of 1520 rcf for 10 minutes at 4°C to isolate plasma, which was aliquoted into cryotubes, fast-frozen using liquid nitrogen, and stored at −80°C. Plasma samples were then thawed on ice and applied to a standard multiplex platform to simultaneously measure levels of 22 cytokines. The panel of 22 cytokines (Fluorokine MAP Multiplex Human Cytokine Panel A- R&D Systems; Minneapolis, MN) included the following: monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), macrophage inflammatory protein-1β (MIP-1β), regulated upon activation normal t-cell expressed and secreted (RANTES), epithelial-derived neutrophil-activating peptide 78 (ENA-78), fibroblast growth factor basic (FGF basic), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-1 receptor antagonist (IL-1ra), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-17 (IL-17), tumor necrosis factor α (TNFα), thrombopoietin (Tpo), and vascular endothelial growth factor (VEGF). In brief, plasma samples from 344 study participants were separately incubated with a set of color-coded beads that were pre-coated with primary antibodies specific to each cytokine. Samples were then incubated with a corresponding set of specific biotinylated secondary antibodies followed by streptavidin-phycoerythrin conjugate. Finally, color-coded beads were read by a Luminex dual laser analyzer that detects the specific analyte and the magnitude of the phycoerythrin signal in order to determine levels of individual cytokines. Standards were measured in duplicate and then the mean fluorescent intensity was calculated for each sample. For a comprehensive list of detection thresholds for each cytokine, please refer to the following URL: http://www.rndsystems.com/product_detail_objectname_fmaphumansample.aspx.
2.5 Assessment of gene regulatory pathways
Duplicate plasma samples were analyzed for variation in gene regulation using a novel cellular biosensor system called FACTORIAL (Attagene, Inc; Morrisville, NC) [79]. FACTORIAL was developed to assess the activity of multiple transcription factors simultaneously, generating a transcription factor activity profile that represents a stable and sustained cell signature to distinguish pathological conditions. The system comprises a library of 52 reporter constructs (48 linked to specific gene regulatory pathways and 4 internal controls) that are evaluated according to their transcription rates. All reporters produce essentially identical messages that are subjected to processing, which generates a spectrum of distinguishable fragments that are analyzed quantitatively. The homogeneity of the reporter library affords inherently uniform detection conditions for all reporters and provides repeatability, accuracy and robustness of assessment. For a comprehensive list of the reporter constructs and internal controls, please refer to the following URL: www.attagene.com/technology.php.
Plasma samples were pooled from TMD−WPT cases, TMD+WPT cases, and healthy controls and applied to cells in culture. To obtain a comprehensive set of possible cellular responses that distinguish TMD−WPT and TMD+WPT from controls, we employed 4 cell types of different tissue origin that would likely express different subsets of receptors. Human teratocarcinoma stem cells (PA-1 cells) were selected because they express a larger number of receptors and exhibit greater diversity in signaling compared to differentiated cells possessing fewer specialized pathways [99]. These cells express a wide variety of growth factor/cytokine receptors including GM-CSF, IL-1, IL-4, VEGF, TNFα, and TGFβ [6; 9; 15; 32; 64]. Undifferentiated human neuroblastoma cells (SH-SY5Y cells) were selected as they express pain-relevant receptors and molecules (e.g., opioids and their receptors) [95] and posses native neuronal machinery and response characteristics [72; 92]. Human hepatoma cells (HepG2 cells) and human glioma cells (U87MG cells) were selected because they provide model systems within which to study cytokine-relevant inflammatory pathways [12; 48; 50]. Twenty-four hours after transfection, cells were supplied with fresh medium (either 1% or 10% fetal bovine serum; FBS) and incubated with or without inducer (10% human plasma sample) for either 4 hours to gain information about primary, immediate transcriptional responses or for 24 hours to reveal slower primary responses and secondary responses. All experiments were performed in triplicate. The reporter cDNAs were amplified by PCR using a pair of primers common for all reporters. The PCR products were labeled with a fluorescent dye and digested by the HpaI. The digest produced a spectrum of fluorescently labeled DNA fragments of different lengths that were resolved by capillary electrophoresis (CE) and detected as separate fluorescent peaks. The capillary electrophoresis data were processed using Attagraph™ software to generate 32 independent transcription reporter construct profiles (4 cell types × 2 FBS concentrations × 2 time points × 2 inducer plasma samples from TMD−WPT or TMD+WPT cases normalized to responses generated using plasma samples from healthy controls).
2.6 Genotyping
DNA extracted from whole blood collected from study participants was genotyped by Beckman-Coulter Genomics (Morrisville, NC) using the Algynomics Pain Research Panel (http://www.beckmangenomics.com/genomic_services/genotyping/pain_research_panel.html). This platform is a dedicated array for the targeted assessment of genes involved in acute and chronic pain conditions, utilizing the Affymetrix MegAllele platform. The panel genotypes SNPs in candidate genes whose protein products are linked to biological pathways that influence pain transmission, inflammatory response, or psychological state. This analysis was limited to SNPs in genes corresponding to the cytokine proteins and transcription factors found to be associated with case status. These included 4 SNPs in the MCP-1 gene locus (rs2857656, rs4586, rs13900, and rs1080327), 9 SNPs in the IL-1ra gene locus (rs2234676, rs1794065, rs3181052, rs419598, rs315952, rs315951, rs4252041, rs9005, and rs315946), 4 SNPs in the IL-8 gene locus (rs4073, rs2227307, rs2227306, and rs4694637), and 3 SNPs in the TGFβ1 gene locus (rs2241719, rs4803455, and rs1982072).
Genotyping and SNP calls were checked for quality and filtered using utilities implemented in PLINK version 1.07 [77]. All 301 samples exhibited Caucasian ancestry and an overall genotype call rate < 95%. All 22 SNPs exhibited a call rate > 95%, minor allele frequency > 1% in either case or control groups, and agreement across repeated samples > 98%.
2.7 Data analysis
2.7.1 Association of circulating cytokine protein levels with case status and intermediate phenotypes
The statistical analysis first evaluated unadjusted associations between cytokines and case-status using one way analysis of variance (SAS GLM procedure), testing the null hypothesis that the mean concentration of each cytokine was equivalent in TMD−WPT cases, TMD+WPT cases, and controls. Analysis of variance was appropriate because skewness and kurtosis of cytokine distributions was not extreme (absolute values were 1.0 or less) and the least squares methods are robust for sample sizes of this magnitude [57]. Bonferroni adjustment was used to determine a critical P-value of P<0.002, therefore adjusting for multiple tests of 11 cytokines and the two pairs of case-control comparisons (case-case comparisons were not tested). This yielded 3 cytokines that were then tested in a multivariable generalized logit model (SAS LOGISTIC procedure) to determine combined effects of cytokines on case-status. Parameter estimates from the final model were used to compute predicted probabilities of each type of case, and the data were plotted to depict effects of those cytokines and intermediate phenotypes that distinguished between the two types of case status.
The same three cytokines were tested for associations with 16 intermediate phenotypes: trapezius pressure pain threshold; lateral epicondyle pressure pain threshold; thermal pain threshold; rating of first thermal pulse from thermal windup procedure; net change in 0–100 NRS rating during thermal windup procedure; PILL somatization scale; MPQ affective component of pain; MPQ sensory component of pain; MPQ present pain intensity; POMS global mood scale; perceived stress scale; STAIY-1 state anxiety scale; SF12 mental health scale; SF12 physical function scale; SF12 role emotional scale; SF12 role physical scale. A threshold of P<0.003 was used, equivalent to Bonferroni adjustment for 16 intermediate phenotypes.
2.7.2 Association of gene regulatory pathways with case status
The number of inductions or inhibitions in pooled samples collected from TMD−WPT and TMD+WPT cases relative to healthy controls was calculated for each transcription reporter construct. Fold-induction and fold-inhibition were defined as endpoint activities of ≥1.15 and ≤0.85, respectively, relative to healthy controls. Activities between 0.85 and 1.15 were excluded as they fell within the reported normal range of variation for the assay. A two-tailed binomial probability test was then performed to identify gene regulatory pathways that were consistently induced or inhibited over random chance, assuming each sample had an equal chance of being induced or inhibited. Under this assumption, pathways with reporter constructs that were induced or inhibited in more than 22 of 32 profiles met the two-tailed binomial significance level of 0.05.
2.7.3 Association of cytokine SNPs with case status
The analysis of genetic associations began by identifying SNPs that had different allele frequencies in TMD−WPT and TMD+WPT relative to controls. Detection of SNPs associated with case status was performed using univariate logistic regression in Plink version 1.07 (http://pngu.mgh.harvard.edu/purcell/plink) [77], where the log odds of being a case was modeled using each SNP from the IL-1ra (9), IL-8 (4), MCP-1 (4), and TGFβ1 (3) gene loci as predictor variables. SNPs were represented by the number of copies (0, 1 or 2) of the minor allele. Thus, parameter estimates from the models represent the change in log odds for case status associated with each additional copy of that SNP’s minor allele. P-values were calculated from the Wald Chi-square statistics, with P. Bonferroni correction of P-values was performed using the number of SNPs in each gene locus K, such that αbonferroni = 0.05/K.
Potential interactions were then investigated between TGFβ1 SNPs and SNPs in the MCP-1, IL1-ra and IL-8 gene loci using the GENMOD procedure in SAS. This was a genotype-based test, where each SNP was modeled as the number of copies of its minor allele. Log odds of case status was modeled with three covariates: the main effect of one SNP, the main effect of the second SNP, and an interaction term for both SNPs. This interaction term measures the degree to which the effect of one SNP on the log odds of case status varies according to the second SNP. The main effects for each SNP are defined similarly to the univariate analysis, and the interaction term was the product of the main effects. Bonferroni correction of P-values for the interaction term was applied to adjust for the total number of pair-wise tests performed for each gene locus.
3. Results
Descriptive statistics for study participants are presented in Supplementary Table 1.
3.1 Circulating cytokine protein levels and case status
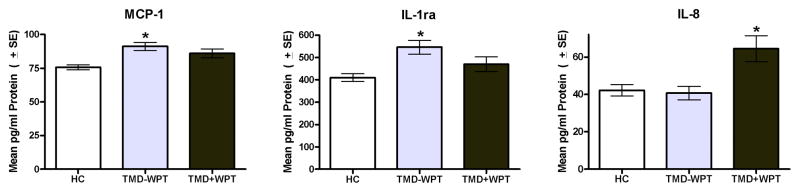
Analysis of variance models that tested for variation in circulating cytokine protein levels between controls and TMD subgroups revealed statistically significant differences for MCP-1, IL-1ra and IL-8, and the pattern varied noticeably for each one (Fig. 1). Levels of MCP-1 and IL-1ra were significantly elevated in TMD−WPT cases, but not in TMD+WPT cases relative to controls. In contrast, levels of IL-8 were significantly elevated in TMD+WPT cases, but not in TMD−WPT cases relative to controls. Levels of MIP-1β were also elevated in TMD+WPT cases (P<0.01), however pairwise comparisons with controls failed to reach the critical value of P<0.002. Of the remaining 18 cytokines, 7 failed to exhibit differences between groups (ENA-78, FGF basic, G-CSF, IL-6, TNFα, Tpo, and VEGF) and 11 were undetectable (MIP-1α, RANTES, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-10, and IL-17).
Fig. 1.
Circulating cytokine protein signatures differ among TMD−WPT and TMD+WPT cases. MCP-1 and IL-1ra are TMD-specific as their levels are elevated in TMD−WPT, but not in TMD+WPT cases. IL-8 is TMD+WPT-specific as its levels are elevated in TMD+WPT, but not in TMD−WPT cases. N = 344. Data are Mean ± SEM. *P<0.002, the critical P-value after Bonferroni correction testing for differences between each case group versus the control group.
3.2 Multivariable association of circulating cytokine protein levels with case status
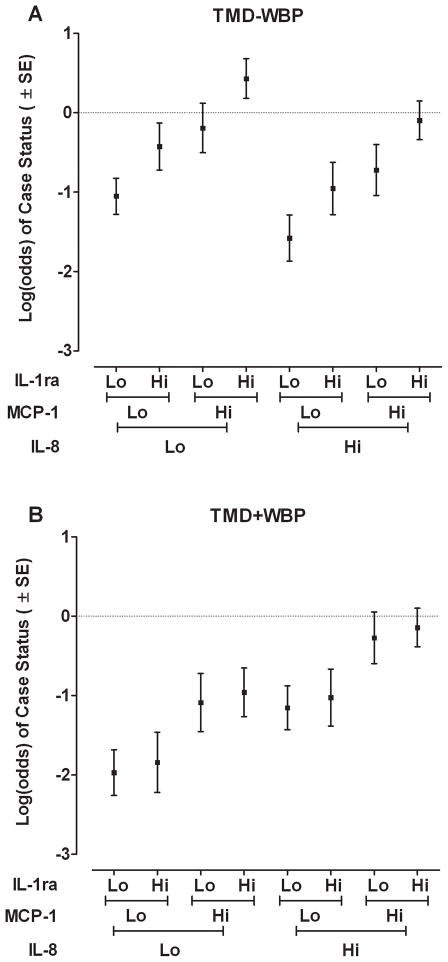
The multivariable analysis showed independent contributions of all three cytokines, and the effects were selective for the two case-classifications (Table 1). MCP-1 and IL-1ra were significantly associated with TMD−WPT. The odds ratios of 1.5 for MCP-1 and 1.4 for IL-1ra signified that an increase of one standard deviation in concentration of each cytokine was associated with an increase of approximately 50% in odds of TMD−WPT. MCP-1 and IL-8 were significantly associated with TMD+WPT. An increase of one standard deviation in concentration of either cytokine was associated with an increase of approximately 50% in odds of TMD+WPT. The association between MCP-1 and TMD+WPT was not observed in the bivariate analysis shown at Fig 1, likely because the multivariable model removed potential confounding effects of other cytokines. These effects can be visualized in Fig. 2, which uses linear predictors from the model in Table 1 to show how cytokines were differentially associated with the two case-classifications relative to controls. On the log scale, high levels of MCP-1 or IL-1ra independently increased odds of TMD−WPT, with no independent contribution of IL-8. In contrast, high levels of MCP-1 and IL-8 increased odds of TMD+WPT, with no independent contribution of IL-1ra.
Table 1.
Multivariable generalized logit model predicting odds of TMD−WPT and TMD+WPT
| Predictor variable* | Parameter | Odds ratio | ||||
|---|---|---|---|---|---|---|
| Outcome | Estimate | SE | P-value† | Estimate | 95%CI | |
| IL-8 | TMD + WBP | 0.41 | 0.14 | 0.000 | 1.5 | (1.1, 2.0) |
| TMD − WBP | −0.26 | 0.15 | 0.77 | (0.58, 1.0) | ||
| MCP-1 | TMD+ WBP | 0.44 | 0.18 | 0.005 | 1.6 | (1.1, 2.2) |
| TMD − WBP | 0.43 | 0.15 | 1.5 | (1.1, 2.1) | ||
| IL-1ra | TMD + WBP | 0.06 | 0.17 | 0.083 | 1.1 | (0.77, 1.5) |
| TMD − WBP | 0.31 | 0.14 | 1.4 | (1.0, 1.8) | ||
| Intercept | TMD + WBP | −1.0 | 0.16 | n/a | n/a | |
| TMD − WBP | −0.57 | 0.13 | n/a | |||
Predictor variables were all transformed to unit normal deviates. Odds ratios therefore represent relative change in odds of outcome associated with one standard deviation unit change in predictor variable.
P-value is from 2 df likelihood ratio test of null hypothesis that predictor variable is unrelated to both outcomes.
Model is from 344 females: 175 controls, 66 cases of tempormandibular disorder with widespread palpation tenderness (TMD+WPT) and 103 cases of tempormandibular disorder without widespread palpation tenderness (TMD−WPT). For overall model, likelihood ratio Chi-square=44.7, 6 df, P<0.0001.
Fig. 2.
Circulating cytokine levels are differentially associated with log-odds of TMD−WPT and TMD+WPT. (A) Both MCP-1 and IL-1ra are independently associated with log-odds of TMD−WPT, whereas IL-8 is not. (B) In contrast, IL-8 and MCP-1 are associated with odds of TMD+WPT, while IL-1ra is not. Predicted odds, plotted on the log scale, was calculated for each case classification relative to controls using parameter estimates from a multivariable generalized logit model shown in Table 3 (N=344 females). Cytokine protein levels were modelled as continuous variables, each transformed to unit normal deviates. “Lo” signifies a value of one standard deviation below the mean for the cytokine and “Hi” signifies a value of one standard deviation above the mean for the cytokine.
3.3 Circulating cytokine protein levels and intermediate phenotypes
When the same three circulating cytokines were compared with intermediate phenotypes, distinctive patterns of association again were observed (Table 2). Levels of MCP-1, IL-1ra, and/or IL-8 were significantly associated with 9 of the 16 intermediate phenotypes measuring sensitivity to experimental pain, self-rated pain, self-rated health, and psychological status. MCP-1 was significantly associated with self-rated pain, perceived stress, self-rated physical function and physical/emotional health, and somatic awareness. Similarly, IL-1ra was significantly associated with self-rated physical function and somatic awareness. In contrast, IL-8 was strongly associated with experimental pressure pain thresholds, but not with self-rated pain, self-rated health, or psychological status. Not shown in Table 2 are the seven intermediate phenotypes that showed no significant association (ie. P>0.003) with any of the cytokines based on the same univariate tests for association: self-rated mental health (SF-12v2), role limitation due to mental health, mood (POMS scale), anxiety (STAIY-1), and all three measures of sensitivity to thermal experimental pain..
Table 2.
Standardized regression coefficients for relationships between cytokines and intermediate phenotypes
| Intermediate phenotype | Cytokine | ||
|---|---|---|---|
| MCP-1 | IL-1ra | IL-8 | |
| Pressure pain threshold on trapezius (QST) | 0.00 | 0.03 | −0.27* |
| Pressure pain threshold on lateral epicondyle (QST) | 0.01 | 0.01 | −0.29* |
| Affective component of pain (MPQ) | 0.22* | 0.06 | 0.00 |
| Sensory components of pain (MPQ) | 0.17* | 0.03 | 0.01 |
| Intensity of current pain (MPQ) | 0.27* | 0.09 | 0.02 |
| Somatic awarness (PILL) | 0.18* | 0.17* | 0.01 |
| Psychological stress (PSS) | 0.17* | 0.13 | −0.08 |
| Physical functioning (SF12v2) | −0.23* | −0.21* | 0.07 |
| Role limitations due to physical health (SF12v2) | −0.19* | −0.13 | 0.02 |
QST = Quantitative Sensory Testing, MPQ = McGill Pain Questionnaire, PILL = Pennebaker Inventory of Limbic Languidness, PSS = Perceived Stress Scale, SF12v2 = Short Form 12 version 2. Data = standardized β coefficient. Not listed are seven intermediate phenotypes that were not signficantly associated (P>0.0035) with any of the cytokines: thermal pain threshold, thermal pain first pulse rating, thermal pain windup, profile of mood states global score, state anxiety score mental health and role limitation due to mental health.
P<0.003, equivalent to Bonferroni correction for 16 intermediate phenotypes.
3.4 Gene regulatory pathways and case status
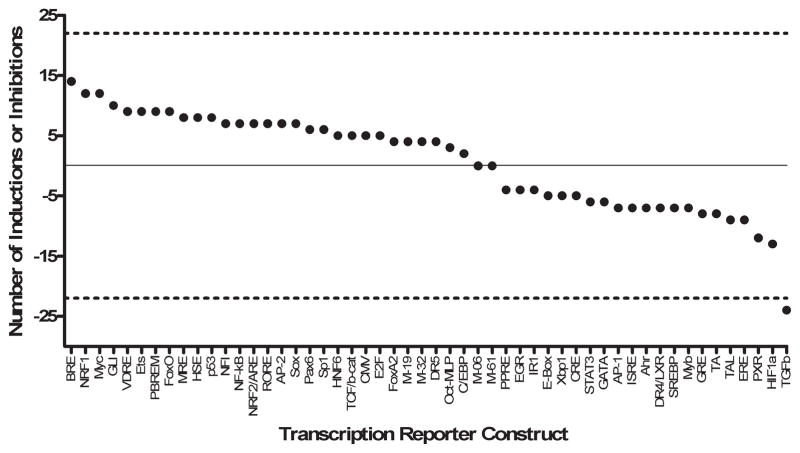
Findings from the FACTORIAL analysis of gene regulation showed that transcription factor activity profiles did not significantly differ across cell type or experimental condition. Furthermore, transcription factor activity profiles remained consistent between TMD−WPT and TMD+WPT cases. For that reason, fold-change in transcription factor activities for TMD−WPT and TMD+WPT across cell type and experimental condition were considered together (for a total of 32 activity profiles per transcription reporter construct). TGFβ1 activity was consistently inhibited in cases relative to controls in 24 out of 32 profiles (Figure 3). Fold-inductions or fold-inhibitions among the remaining 47 transcription reporter constructs did not differ between cases and controls. Fold-change for the four internal controls (M-06, M-19, M-32, and M-61) was at or close to 0. Thus, TGFβ1 represents a stable and sustained marker of gene regulatory events that are altered in females with TMD−WPT and TMD+WPT. Limited availability of biological samples did not allow us to test transcription factor activity for individual study participants and, thus, precluded us from studying a causative link between TGFβ1 activity and cytokine levels.
Fig. 3.
TGFβ1 transcription activity is altered among TMD−WPT and TMD+WPT cases. Fold-change in transcription factor activities for TMD−WPT and TMD+WPT cases across cell type and experimental condition are shown. TGFβ1 activity was consistently inhibited in cases relative to controls in 24 activity profiles. Fold-inductions or fold-inhibitions among the remaining 47 transcription reporter constructs did not differ between cases and controls. Data are the number of fold-inductions (values above 0) or fold-inhibitions (values below 0) out of 32 activity profiles per transcription factor in TMD−WPT and TMD+WPT cases relative to healthy controls. The solid line set at 0 represents no change relative to controls, while the dashed lines set at 22 and −22 represent cut-offs corresponding to the number of inductions or inhibitions expected by chance based on a two-tailed binomial probability test. The value for TGFβ1 fell outside the boundaries of the chance expectation, and therefore is statistically significant (P<0.05).
3.5 Interaction between IL-8 and TGFβ1 gene polymorphisms and case status
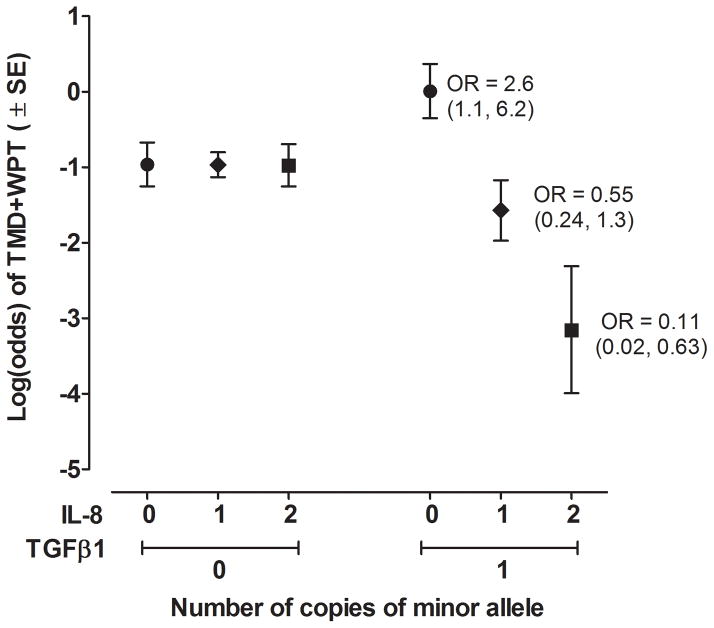
When evaluated individually in univariate logistic regression models, none of the 22 MCP-1, IL1-ra, IL-8, or TGFβ1 SNPs were significantly associated with odds of TMD−WPT or TMD+WPT relative to controls. Within the IL-8 gene locus, however, two SNPs (rs4073, rs2227307) had significant interactions with TGFβ1 SNP rs2241719 in affecting case status (Table 3). Each of these IL-8 SNPs had similar interaction effects with rs2241719, likely because they are in high linkage disequilibrium with one another (pair-wise R2 > 0.95). The rs4073*rs2241719 interaction had the greatest effect on TMD+WPT case status (adjusted P=0.03). Among females having no copies of the minor T allele of the TGFβ1 SNP rs2241719, there was little variation in log odds of TMD+WPT according to the number of copies of the A allele of IL-8 SNP rs4073 (Figure 4). However, among females with one copy of the minor T allele of TGFβ1 SNP rs2241719, there was a large decrease in log odds of TMD+WPT associated with each additional copy of the minor allele of IL-8 SNP rs4073. As a consequence, the odds ratio for TMD+WPT associated with an additional copy of the minor T allele of TGFβ1 SNP rs2241719 was 2.6 (95%CI = 1.1, 2.6) in females with 0 copies of the minor A allele of IL-8 SNP rs4073, whereas the odds ratio was 0.54 (95%CI = 0.2, 1.6) in females with 1 copy of the minor A allele of IL-8 SNP rs4073. No significant interactions were found between MCP-1, IL1-ra and/or IL-8 SNPs and TGFβ1 SNPs with TMD−WPT case status.
Table 3.
TMD+WPT interaction model parameter estimates for IL-8 and TGFβ1 SNPs and SNP interactions
| IL-8 SNP | TGFβ1 SNP | Interaction Term | IL-8 Main Effect | P-value | TGFβ1 Main Effect | P-value | Interaction Term | P-value |
|---|---|---|---|---|---|---|---|---|
| rs4073 | rs2241719 | rs4073* rs2241719 | −0.005 | 0.9827 | 0.97 | 0.0260 | −1.58 | 0.0037 |
| rs2227307 | rs2241719 | rs2227307* rs2241719 | −0.021 | 0.9297 | 0.94 | 0.0315 | −1.57 | 0.0041 |
Data are from binary logistic regression models for N=66 TMD+WPT cases and 175 controls.
Fig. 4.
IL-8 and TGFβ1 SNPs interact to affect log-odds of TMD+WPT. Predicted log-odds of TMD+WPT is from a logistic regression model testing for effects of IL-8 and TGFβ1 SNPs, each modeled as the number of copies of the minor alllele and the interaction between the two SNPs (N=344 females). Contrasting effects of IL-8 on case status were apparent. Log-odds of TMD+WPT was not associated with the number of copies of the minor A allele of IL-8 rs4073 among heterozygotes of the major A allele of TGFβ1 rs2241719. In contrast, log-odds of TMD+WPT was markedly reduced with each additional copy of the minor A allele of IL-8 rs4073 among heterozygotes of the major A allele of TGFβ1 rs2241719. Likewise, contrasting effects of TGFβ1 on case status were apparent. Among females with 0 copies of the minor A allele of IL-8 rs4073 (●), an additional copy of the minor T allele of TGFβ1 rs2241719 was associated with an increase of 0.97 in the log-odds of TMD+WPT. This is equivalent to an odds ratio of 2.6 (95%CI = 1.6, 6.2) for the association of the T allele of TGFβ1 with TMD+WPT among homozygotes for the major A allele of IL-8 rs4073. In contrast, among females with 1 copy of the minor A allele of IL-8 rs4073 (◆), an additional copy of the minor T allele of TGFβ1 rs2241719 was associated with a decrease of 0.60 in the log-odds of TMD+WPT. This is equivalent to an odds ratio of 0.55 (95%CI = 0.24, 1.3) for the association of the T allele of TGFβ1 with TMD+WPT among heterozygotes for IL-8 rs4073. Among females with 2 copies of the minor A allele of IL-8 rs4073 (■), there was a corresponding reduction of 2.2 in log-odds of TMD+WPT, equivalent to an odds ratio of 0.11 (95% CI = 0.02, 1.3). Only eight individuals were homozygous for the minor allele T of TGFβ1 rs2241719, so data for that genotype are not plotted.
4. Discussion
Consistent with our hypothesis, females with localized and widespread manifestations of chronic pain differed from pain-free controls with respect to their cytokine profiles measured at different levels of the pathway from gene to protein.
4.1 Circulating cytokine protein levels
TMD−WPT cases had elevated levels of the pro-inflammatory cytokine MCP-1 and the anti-inflammatory cytokine IL-1ra. In contrast, TMD+WPT cases had elevated levels of the pro-inflammatory cytokine IL-8 with no compensatory increases in IL-1ra, thus indicating a more severe pro-/anti-inflammatory imbalance among TMD+WPT cases.
We believe this is the first study to show increased concentrations of circulating MCP-1 levels in individuals with TMD. Bivariate analysis showed an association between MCP-1 and TMD−WPT, while multivariable modelling, which removes potential confounding effects of other cytokines, revealed an association between MCP-1 and TMD+WPT as well as TMD−WPT. MCP-1, a member of the CC chemokine family, has potent chemotactic activity for monocytes and macrophages which express its receptor CC chemokine receptor-2 (CCR2) [31]. MCP-1 is expressed on sensory neurons and its expression in both neurons and peripheral tissues is upregulated following local inflammation [44]. In animal models, administration of MCP-1 produces mechanical and thermal hyperalgesia [3; 78] and stimulates release of calcitonin gene-related peptide (CGRP), a key molecule involved in pain transmission [78]. Based on their findings that MCP-1 was elevated in TM joint fluid of TMD patients, Ogura and colleagues proposed that MCP-1 contributes to TMD by promoting recruitment of mononuclear cells into inflamed synovial tissues [69]. MCP-1 has also been observed in individuals with chronic systemic inflammation [49; 80], supporting our findings and suggesting that a generalized pro-inflammatory state might contribute to painful TMD.
Our finding of elevated circulating IL-8 levels in TMD+WPT cases is consistent with results from clinical studies showing that fibromyalgia patients exhibit elevated plasma or sera IL-8 levels [7; 36; 47; 71; 93; 94] and that IL-8 levels are positively correlated with symptom duration [93]. IL-8 is a member of the CXC chemokine family well known for its ability to regulate inflammatory processes [65]. Upon release from activated macrophages and endothelial cells, IL-8 binds to CXC receptor 1 (CXCR1) and CXC receptor 2 (CXCR2) on neutrophil cell surfaces to stimulate cell migration and release of additional pro-inflammatory mediators [66]. IL-8 also represents a neuroimmune link to pain. Administration of IL-8 produces dose-dependent hyperalgesia, which is mediated by sympathetic amines that sensitize nociceptors [3; 17; 20]. In animals, administration of anti-IL-8 serum [17; 18] or an allosteric inhibitor of CXCR1 and CXCR2 receptors [19] reduces the severity of persistent pain. In fibromyalgia patients, biopsychosocial and exercise therapies that reduce circulating IL-8 levels, also reduce pain [71; 94].
Abnormalities in levels of pro-inflammatory cytokines among cases were accompanied by alterations in levels of anti-inflammatory cytokines. Individuals with TMD−WPT had elevated levels of the anti-inflammatory cytokine IL-1ra, while those with TMD+WPT did not. IL-1ra is a member of the IL-1 cytokine family that serves as an endogenous negative-feedback regulator to control potentially pathologic inflammatory events [5]. In response to acute-phase proteins and other cytokines, IL-1ra is secreted by monocytes, macrophages, and neutrophils to block activity of IL-1 pathways involved in inflammation and pain [5]. In animals, IL-1ra blocks the development of IL-8-induced mechanical hyperalgesia [23]. In vitro studies have further shown that IL-1ra suppresses the expression of IL-8 [82], which may explain two of our findings: a lack of elevated IL-8 levels in TMD−WPT cases with an intact IL-1ra response; and presence of elevated IL-8 levels in TMD+WPT cases with an impaired IL-1ra response. Clinical studies of TMD have shown that elevated plasma IL-1ra levels are associated with reductions in pain [52]. The impaired IL-1ra response among TMD+WPT cases, suggests that therapies enhancing IL-1ra levels may prove especially efficacious for individuals with widespread pain.
Cytokines were also differentially associated with intermediate phenotypes characteristic of TMD and WPT. MCP-1 was associated with self-rated pain, self-rated physical function, and psychological status. Similarly, IL-1ra was associated with self-rated physical function and psychological status. In contrast, IL-8 was associated with experimental pressure pain thresholds. Thus, MCP-1/IL-1ra and IL-8 may differentially contribute to localized and widespread pain, respectively, through influencing distinct pathways involved in either self-rated health or pain processing. The finding that IL-8 was exclusively associated with pressure pain is in line with that of Gur and colleagues who found that IL-8, but not IL-1β or IL-6, is associated with evoked pressure pain, but not clinical pain, physical functioning, or depression [36].
4.2 Cytokine transcription factor activity
Together, these results suggest that low levels of IL-1ra relative to high levels of proinflammatory cytokines lead to pain manifesting in several anatomical locations and to poorer self-rated health. Imbalances in levels of pro- and anti-inflammatory cytokines may drive chronic pain by altering the relative expression of genes linked to relevant biological pathways [63; 68; 70; 90]. Here, FACTORIAL analysis of gene regulatory pathways indicated that TGFβ1 activity was consistently inhibited in both TMD−WPT and TMD+WPT cases relative to controls. TGFβ1 is an anti-inflammatory cytokine which also has pleiotropic effects on cellular functions such as proliferation, differentiation, and tissue repair [10; 41; 56]. Pro-inflammatory cytokines stimulate synthesis and release of TGFβ1 from macrophages in the periphery and glial cells in the central nervous system, which in turn can block immune cell proliferation, free radical induction, and additional pro-inflammatory cytokine release [21; 29; 54; 88]. A protective role for TGFβ1 in both the development and maintenance of chronic pain was recently demonstrated by Echeverry and colleagues who found that TGFβ1 administration prior to or following peripheral nerve injury normalized pain behavior in rats [29]. Furthermore, TGFβ1 administration suppressed activation of microglia and astrocytes, minimized neuronal damage by decreasing the expression of MCP-1, and reduced the expression of local pro-inflammatory cytokines.
TGFβ1 signalling is mediated through activation of the Smad family of proteins, which translocate into the nucleus to regulate expression of target genes, including MCP-1 and IL-8 [1; 24; 35]. While the net effect of TGFβ1 signaling is to govern the resolution of inflammation [83], the specific effects of TGFβ1/Smad signalling on gene expression differ depending on factors such as cell type, differentiation state, and local cytokine milieu. Thus, inhibited TGFβ1 activity may represent a common marker of impaired Smad gene regulatory events in localized and widespread pain, while patterns of MCP-1, IL-1ra, and IL-8 levels distinguish pro-inflammatory processes specific to either TMD−WPT or TMD+WPT.
4.4 Cytokine genotype
An additional layer of complexity is added when considering the contribution of genetic variability. Genetic polymorphisms that influence the synthesis and release of cytokines [22; 39; 97; 98] have been associated with complex chronic pain conditions such as low back pain, irritable bowel syndrome, and vulvar vestibulitis [30; 73; 85]. In the present study, we identified SNPs in IL-8 and TGFβ1 genes that interacted to affect odds of TMD+WPT. Specifically, the minor T allele of TGFβ1 rs2241719 either increased or decreased odds of TMD+WPT, depending on the number of copies of the minor A allele of IL-8 rs4073.
The minor rs4073 A IL-8 allele is a common variant (allele frequency = 0.489) located 251 base pairs upstream of the transcription start site. Individuals homozygous for rs4073 A allele exhibit increased IL-8 production [40] and increased risk of diabetic neuropathy [2]. The minor rs2241719 A TGFβ1 allele (allele frequency = 0.154) is located in the 3′ untranslated region (UTR) of the gene. Variation in 3′ untranslated regions can alter translation efficiency, RNA secondary structure, RNA stability, and RNA-protein interactions [13; 14]. While rs2241719 has not been examined in previous genetic association studies or functional assays, it is located near rs2241718 which has been linked to the pathogenesis of chronic obstructive pulmonary disease [11]. Thus, rs2241719 may represent either a functional SNP or a marker for rs2241718 or another functional SNP.
Others have observed epistatic interactions between IL-8 and mutations located in molecular pathways related to the ones seen here. For example, the protective effect of IL-8 rs4073 T on risk of inflammatory eye disease was significantly increased by an exonic SNP in the gene encoding matrix metallopeptidase 9 (MMP9), a functionally-related collagenase involved in inflammation and tissue remodelling [67]. Thus, the epistatic interaction observed between IL-8 and TGFβ1 genes likely reflects biological interactions between their products as described above. Future studies aimed at understanding this and other epistatic interactions will help us further understand the immunopathology underlying chronic pain conditions such as TMD and WPT.
5. Conclusion
In conclusion, these findings illustrate how localized and anatomically widespread patterns of chronic pain are associated with distinctive profiles of inflammatory biomarkers at protein, transcription factor activity, and gene levels. The anti-inflammatory cytokine IL1-ra was associated specifically with localized TMD, while the pro-inflammatory cytokine IL-8 was associated specifically with TMD+WPT. This pattern, together with differential contributions to intermediate phenotypes, suggests that cytokines might serve as useful diagnostic markers to distinguish between etiologically-distinct manifestations of chronic pain. Furthermore, these results suggest that therapeutics able to normalize levels of MCP-1 and IL-8 as well as enhance IL-1ra and TGFβ1 signaling could be beneficial for treating chronic pain. Finally, the epistatic interactions observed between IL-8 and TGFβ1 highlight the possibility that such therapies might need to be personalized according patients’ genetic susceptibility to pathologic immune responses.
Supplementary Material
Acknowledgments
We thank David Barrow in the General Clinical Research Center’s Bioanalytical Core Laboratory at UNC for overseeing the measurement of cytokine levels in participants’ samples. Additionally, we thank Sergei Makarov and Alex Medvedev at Attagene Inc. for applying their novel transcription factor activity assay to our participants’ samples as well as for insightful discussions related to TGFβ1 gene regulation. This work was funded by the NIH/NICHHD K12 HD052191 to A.N., the NIH/NIDCR R01 DE016558 to L.D., and the NIH/NINDS PO1 NS045685 to A.N., G.S., L.D., and W.M.
Footnotes
Statement of conflict of interest. This study includes results from genotyping that was performed using the Algynomics Pain Research Panel. Algynomics Inc. is a company providing research services in personalized pain medication and diagnostics. Five authors have interests in Algynomics Inc. which may be relevant in determining conflicts of interest. Specifically, Drs. Slade, Diatchenko, Smith, Maixner and Nackly hold shares and/or stock options in Algynomics Inc. Also, Drs. Diatchenko and Maixner are Office-holders in Algyonomics Inc. All other authors report no known conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham S, Sawaya BE, Safak M, Batuman O, Khalili K, Amini S. Regulation of MCP-1 gene transcription by Smads and HIV-1 Tat in human glial cells. Virology. 2003;309(2):196–202. doi: 10.1016/s0042-6822(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia TS, Khullar M, Ahuja M, Kohli HS, Bhansali A, Mohan V, Venkatesan R, Rai TS, Sud K, Singal PK. Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One. 2009;4(4):e5168. doi: 10.1371/journal.pone.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn DK, Lee KR, Lee HJ, Kim SK, Choi HS, Lim EJ, Park JS. Intracisternal administration of chemokines facilitated formalin-induced behavioral responses in the orofacial area of freely moving rats. Brain Res Bull. 2005;66(1):50–58. doi: 10.1016/j.brainresbull.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Allerbring M, Haegerstam G. Characteristics of patients with chronic idiopathic orofacial pain. A retrospective study. Acta Odontol Scand. 1993;51(1):53–58. doi: 10.3109/00016359309041148. [DOI] [PubMed] [Google Scholar]
- 5.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 7.Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, Ciapparelli A, Dell’Osso L, Bombardieri S. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25(2):225–230. [PubMed] [Google Scholar]
- 8.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165(6):728–737. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 9.Bermudez Y, Yang H, Saunders BO, Cheng JQ, Nicosia SV, Kruk PA. VEGF- and LPA-induced telomerase in human ovarian cancer cells is Sp1-dependent. Gynecol Oncol. 2007;106(3):526–537. doi: 10.1016/j.ygyno.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Bottner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75(6):2227–2240. doi: 10.1046/j.1471-4159.2000.0752227.x. [DOI] [PubMed] [Google Scholar]
- 11.Celedon JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, DeMeo DL, Reilly JJ, Kwiatkowski DJ, Chapman HA, Laird N, Sylvia JS, Hernandez M, Speizer FE, Weiss ST, Silverman EK. The transforming growth factor-beta1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD) Hum Mol Genet. 2004;13(15):1649–1656. doi: 10.1093/hmg/ddh171. [DOI] [PubMed] [Google Scholar]
- 12.Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J Clin Neurosci. 2005;12(8):930–933. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Chen GL, Vallender EJ, Miller GM. Functional characterization of the human TPH2 5′ regulatory region: untranslated region and polymorphisms modulate gene expression in vitro. Hum Genet. 2008;122(6):645–657. doi: 10.1007/s00439-007-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JM, Ferec C, Cooper DN. A systematic analysis of disease-associated variants in the 3′ regulatory regions of human protein-coding genes II: the importance of mRNA secondary structure in assessing the functionality of 3′ UTR variants. Hum Genet. 2006;120(3):301–333. doi: 10.1007/s00439-006-0218-x. [DOI] [PubMed] [Google Scholar]
- 15.Cimoli G, Russo P, Billi G, Mariani GL, Rovini E, Venturini M. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on human ovarian cancer cells. Jpn J Cancer Res. 1991;82(11):1196–1198. doi: 10.1111/j.1349-7006.1991.tb01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 17.Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol. 1991;104(3):765–767. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunha JM, Sachs D, Canetti CA, Poole S, Ferreira SH, Cunha FQ. The critical role of leukotriene B4 in antigen-induced mechanical hyperalgesia in immunised rats. Br J Pharmacol. 2003;139(6):1135–1145. doi: 10.1038/sj.bjp.0705346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha TM, Barsante MM, Guerrero AT, Verri WA, Jr, Ferreira SH, Coelho FM, Bertini R, Di Giacinto C, Allegretti M, Cunha FQ, Teixeira MM. Treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR1/2, diminishes neutrophil influx and inflammatory hypernociception in mice. Br J Pharmacol. 2008;154(2):460–470. doi: 10.1038/bjp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha TM, Verri WA, Jr, Poole S, Parada CA, Cunha FQ, Ferreira SH. Pain facilitation by proinflammatory cytokine actions at peripheral nerve terminals. In: deLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. Seattle, WA: IASP Press; 2007. pp. 67–83. [Google Scholar]
- 21.da Cunha A, Vitkovic L. Transforming growth factor-beta 1 (TGF-beta 1) expression and regulation in rat cortical astrocytes. J Neuroimmunol. 1992;36(2–3):157–169. doi: 10.1016/0165-5728(92)90047-o. [DOI] [PubMed] [Google Scholar]
- 22.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99(2):303–310. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis AJ, Perkins MN. The involvement of bradykinin B1 and B2 receptor mechanisms in cytokine-induced mechanical hyperalgesia in the rat. Br J Pharmacol. 1994;113(1):63–68. doi: 10.1111/j.1476-5381.1994.tb16174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol. 2002;71(5):731–740. [PubMed] [Google Scholar]
- 25.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123(3):226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Diatchenko L, Slade GD, Nackley AG, Maixner W. Responses to Drs. Kim and Dionne regarding comments on Diatchenko, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain 2006;125:216–24. Pain. 2007;129(3):366–370. doi: 10.1016/j.pain.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA, Kluger MJ, Oppenheim JJ, Powanda MC. The Physiological and Pathological Effects of Cytokines Liss. New York: John Wiley & Sons Inc; 1990. [Google Scholar]
- 28.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6(4):301–355. [PubMed] [Google Scholar]
- 29.Echeverry S, Shi XQ, Haw A, Liu H, Zhang ZW, Zhang J. Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol Pain. 2009;5:16. doi: 10.1186/1744-8069-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster DC, Sazenski TM, Stodgell CJ. Impact of genetic variation in interleukin-1 receptor antagonist and melanocortin-1 receptor genes on vulvar vestibulitis syndrome. J Reprod Med. 2004;49(7):503–509. [PubMed] [Google Scholar]
- 31.Gangur V, Birmingham NP, Thanesvorakul S. Chemokines in health and disease. Vet Immunol Immunopathol. 2002;86(3–4):127–136. doi: 10.1016/s0165-2427(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 32.Gatanaga M, Grosen EA, Burger RA, Granger GA, Gatanaga T. Release of soluble TNF/LT receptors from a human ovarian tumor cell line (PA-1) by stimulation with cytokines in vitro. Lymphokine Cytokine Res. 1993;12(4):249–253. [PubMed] [Google Scholar]
- 33.Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7(11):1083–1103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- 34.Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17(4):593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 35.Gupta J, Robbins J, Jilling T, Seth P. TGFbeta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011;11(3):311–316. doi: 10.4161/cbt.11.3.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gur A, Karakoc M, Nas K, Remzi, Cevik, Denli A, Sarac J. Cytokines and depression in cases with fibromyalgia. J Rheumatol. 2002;29(2):358–361. [PubMed] [Google Scholar]
- 37.Hagberg C. General musculoskeletal complaints in a group of patients with craniomandibular disorders (CMD). A case control study. Swed Dent J. 1991;15(4):179–185. [PubMed] [Google Scholar]
- 38.Hagberg C, Hagberg M, Kopp S. Musculoskeletal symptoms and psychosocial factors among patients with craniomandibular disorders. Acta Odontol Scand. 1994;52(3):170–177. doi: 10.3109/00016359409027592. [DOI] [PubMed] [Google Scholar]
- 39.Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TW, Breedveld FC, Seymour AB. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis Rheum. 2004;50(6):1976–1983. doi: 10.1002/art.20310. [DOI] [PubMed] [Google Scholar]
- 40.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55(12):1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isong U, Gansky S, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain. 2008;22(4):317–322. [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeger B, Reeves JL. Quantification of changes in myofascial trigger point sensitivity with the pressure algometer following passive stretch. Pain. 1986;27(2):203–210. doi: 10.1016/0304-3959(86)90211-3. [DOI] [PubMed] [Google Scholar]
- 44.Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport. 2008;19(2):183–186. doi: 10.1097/WNR.0b013e3282f3c781. [DOI] [PubMed] [Google Scholar]
- 45.John MT, Miglioretti DL, LeResche L, Von Korff M, Critchlow CW. Widespread pain as a risk factor for dysfunctional temporomandibular disorder pain. Pain. 2003;102(3):257–263. doi: 10.1016/S0304-3959(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 46.Kaneyama K, Segami N, Nishimura M, Suzuki T, Sato J. Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders. Br J Oral Maxillofac Surg. 2002;40(5):418–423. [PubMed] [Google Scholar]
- 47.Kim SK, Kim KS, Lee YS, Park SH, Choe JY. Arterial stiffness and proinflammatory cytokines in fibromyalgia syndrome. Clin Exp Rheumatol. 2010;28(6 Suppl 63):S71–77. [PubMed] [Google Scholar]
- 48.Kim TK, Lee JS, Oh SY, Jin X, Choi YJ, Lee TH, Lee E, Choi YK, You S, Chung YG, Lee JB, DePinho RA, Chin L, Kim H. Direct transcriptional activation of promyelocytic leukemia protein by IFN regulatory factor 3 induces the p53-dependent growth inhibition of cancer cells. Cancer Res. 2007;67(23):11133–11140. doi: 10.1158/0008-5472.CAN-07-1342. [DOI] [PubMed] [Google Scholar]
- 49.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koj A, Jura J. Complex analysis of genes involved in the inflammatory response: interleukin-1-induced differential transcriptome of cultured human hepatoma HepG2 cells. Acta Biochim Pol. 2003;50(3):573–582. [PubMed] [Google Scholar]
- 51.Kopp S. The influence of neuropeptides, serotonin, and interleukin 1beta on temporomandibular joint pain and inflammation. J Oral Maxillofac Surg. 1998;56(2):189–191. doi: 10.1016/s0278-2391(98)90867-9. [DOI] [PubMed] [Google Scholar]
- 52.Kopp S, Alstergren P, Ernestam S, Nordahl S, Morin P, Bratt J. Reduction of temporomandibular joint pain after treatment with a combination of methotrexate and infliximab is associated with changes in synovial fluid and plasma cytokines in rheumatoid arthritis. Cells Tissues Organs. 2005;180(1):22–30. doi: 10.1159/000086195. [DOI] [PubMed] [Google Scholar]
- 53.Kress M, Sommer C. Neuroimmunology and Pain: Peripheral Effects of Proinflammatory Cytokines. In: Brune K, Handwerker HO, editors. Hyperalgesia: Molecular Mechanisms and Clinical Implications. Vol. 30. IASP Press; 2004. pp. 57–65. [Google Scholar]
- 54.Lindholm D, Castren E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117(2):395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorr M, Mcnair DM. Profie of Mood States-Bipolar form (POMS-BI) San Diego, CA: Educational and Industrial Testing Service; 1988. [Google Scholar]
- 56.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171(5):2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 57.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 58.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 59.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto K, Honda K, Ohshima M, Yamaguchi Y, Nakajima I, Micke P, Otsuka K. Cytokine profile in synovial fluid from patients with internal derangement of the temporomandibular joint: a preliminary study. Dentomaxillofac Radiol. 2006;35(6):432–441. doi: 10.1259/dmfr/77288976. [DOI] [PubMed] [Google Scholar]
- 61.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 62.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morales MEP, Gereau RWI. Cytokine regulation of ion channels in the pain pathway. In: deLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. Seattle, WA: IASP Press; 2007. pp. 67–83. [Google Scholar]
- 64.Murata T, Obiri NI, Puri RK. Human ovarian-carcinoma cell lines express IL-4 and IL-13 receptors: comparison between IL-4- and IL-13-induced signal transduction. Int J Cancer. 1997;70(2):230–240. doi: 10.1002/(sici)1097-0215(19970117)70:2<230::aid-ijc15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 65.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol. 1997;34(4):311–318. [PubMed] [Google Scholar]
- 66.Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183(5):3425–3432. doi: 10.4049/jimmunol.0900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natividad A, Hull J, Luoni G, Holland M, Rockett K, Joof H, Burton M, Mabey D, Kwiatkowski D, Bailey R. Innate immunity in ocular Chlamydia trachomatis infection: contribution of IL8 and CSF2 gene variants to risk of trachomatous scarring in Gambians. BMC Med Genet. 2009;10:138. doi: 10.1186/1471-2350-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogura N, Akutsu M, Tobe M, Sakamaki H, Abiko Y, Kondoh T. Microarray analysis of IL-1beta-stimulated chemokine genes in synovial fibroblasts from human TMJ. J Oral Pathol Med. 2007;36(4):223–228. doi: 10.1111/j.1600-0714.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 69.Ogura N, Satoh K, Akutsu M, Tobe M, Kuyama K, Kuboyama N, Sakamaki H, Kujiraoka H, Kondoh T. MCP-1 production in temporomandibular joint inflammation. J Dent Res. 2010;89(10):1117–1122. doi: 10.1177/0022034510376041. [DOI] [PubMed] [Google Scholar]
- 70.Ogura N, Tobe M, Sakamaki H, Nagura H, Abiko Y, Kondoh T. Tumor necrosis factor-alpha increases chemokine gene expression and production in synovial fibroblasts from human temporomandibular joint. J Oral Pathol Med. 2005;34(6):357–363. doi: 10.1111/j.1600-0714.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 71.Ortega E, Garcia JJ, Bote ME, Martin-Cordero L, Escalante Y, Saavedra JM, Northoff H, Giraldo E. Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exerc Immunol Rev. 2009;15:42–65. [PubMed] [Google Scholar]
- 72.Pahlman S, Mamaeva S, Meyerson G, Mattsson ME, Bjelfman C, Ortoft E, Hammerling U. Human neuroblastoma cells in culture: a model for neuronal cell differentiation and function. Acta Physiol Scand Suppl. 1990;592:25–37. [PubMed] [Google Scholar]
- 73.Park MI, Camilleri M. Genetics and genotypes in irritable bowel syndrome: implications for diagnosis and treatment. Gastroenterol Clin North Am. 2005;34(2):305–317. doi: 10.1016/j.gtc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- 75.Pfau DB, Rolke R, Nickel R, Treede RD, Daublaender M. Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and Fibromyalgia Syndrome. Pain. 2009;147(1–3):72–83. doi: 10.1016/j.pain.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 77.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82(1):51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- 79.Romanov S, Medvedev A, Gambarian M, Poltoratskaya N, Moeser M, Medvedeva L, Diatchenko L, Makarov S. Homogeneous reporter system enables quantitative functional assessment of multiple transcription factors. Nat Methods. 2008;5(3):253–260. doi: 10.1038/nmeth.1186. [DOI] [PubMed] [Google Scholar]
- 80.Romero-Sanchez C, Tsou HK, Jan MS, Wong RH, Chang IC, Londono J, Valle-Onate R, Siew Howe H, Yu D, Leung BP, Wei JC. Serum Monocyte Chemotactic Protein-1 Concentrations Distinguish Patients With Ankylosing Spondylitis From Patients With Mechanical Low Back Pain. J Spinal Disord Tech. 2010 doi: 10.1097/BSD.0b013e3181e15cc8. [DOI] [PubMed] [Google Scholar]
- 81.Sanders AE, Slade G, Maixner W, Nackley AG, Diatchenko L. Excess Risk of Temporomandibular Disorder Associated with Cigarette Smoking in Young Adults. Journal of Pain. 2011 doi: 10.1016/j.jpain.2011.08.003. Accepted for publication 16 August 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwarz YA, Amin RS, Stark JM, Trapnell BC, Wilmott RW. Interleukin-1 receptor antagonist inhibits interleukin-8 expression in A549 respiratory epithelial cells infected in vitro with a replication-deficient recombinant adenovirus vector. Am J Respir Cell Mol Biol. 1999;21(3):388–394. doi: 10.1165/ajrcmb.21.3.3549. [DOI] [PubMed] [Google Scholar]
- 83.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 84.Shafer DM, Assael L, White LB, Rossomando EF. Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. J Oral Maxillofac Surg. 1994;52(8):786–791. doi: 10.1016/0278-2391(94)90217-8. discussion 791–782. [DOI] [PubMed] [Google Scholar]
- 85.Solovieva S, Leino-Arjas P, Saarela J, Luoma K, Raininko R, Riihimaki H. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109(1–2):8–19. doi: 10.1016/j.pain.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Sommer C, Hauser W, Gerhold K, Joraschky P, Petzke F, Tolle T, Uceyler N, Winkelmann A, Thieme K. Etiology and pathophysiology of fibromyalgia syndrome and chronic widespread pain. Schmerz. 2008;22(3):267–282. doi: 10.1007/s00482-008-0672-6. [DOI] [PubMed] [Google Scholar]
- 87.Spielberger C, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y1) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 88.Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151(4):2150–2158. [PubMed] [Google Scholar]
- 89.Takahashi T, Kondoh T, Fukuda M, Yamazaki Y, Toyosaki T, Suzuki R. Proinflammatory cytokines detectable in synovial fluids from patients with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(2):135–141. doi: 10.1016/s1079-2104(98)90415-2. [DOI] [PubMed] [Google Scholar]
- 90.Tobe M, Ogura N, Abiko Y, Nagura H. Interleukin-1beta stimulates interleukin-8 production and gene expression in synovial cells from human temporomandibular joint. J Oral Maxillofac Surg. 2002;60(7):741–747. doi: 10.1053/joms.2002.33239. [DOI] [PubMed] [Google Scholar]
- 91.Turp JC, Kowalski CJ, O’Leary N, Stohler CS. Pain maps from facial pain patients indicate a broad pain geography. J Dent Res. 1998;77(6):1465–1472. doi: 10.1177/00220345980770061101. [DOI] [PubMed] [Google Scholar]
- 92.Vaughan PF, Peers C, Walker JH. The use of the human neuroblastoma SH-SY5Y to study the effect of second messengers on noradrenaline release. Gen Pharmacol. 1995;26(6):1191–1201. doi: 10.1016/0306-3623(94)00312-b. [DOI] [PubMed] [Google Scholar]
- 93.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford) 2001;40(7):743–749. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Buchner M, Moser MT, Daniel V, Schiltenwolf M. The role of IL-8 in patients with fibromyalgia: a prospective longitudinal study of 6 months. Clin J Pain. 2009;25(1):1–4. doi: 10.1097/AJP.0b013e31817e13a3. [DOI] [PubMed] [Google Scholar]
- 95.Wang Z, Sadee W. Tolerance to morphine at the mu-opioid receptor differentially induced by cAMP-dependent protein kinase activation and morphine. Eur J Pharmacol. 2000;389(2–3):165–171. doi: 10.1016/s0014-2999(99)00881-x. [DOI] [PubMed] [Google Scholar]
- 96.Ware J, Kosinski M, Keller S. SF-12: how to score the SF-12 physical and mental health summary scales. Boston, Massachusetts: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 97.Warzocha K, Ribeiro P, Bienvenu J, Roy P, Charlot C, Rigal D, Coiffier B, Salles G. Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin’s lymphoma outcome. Blood. 1998;91(10):3574–3581. [PubMed] [Google Scholar]
- 98.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997;94(7):3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeuthen J, Norgaard JO, Avner P, Fellous M, Wartiovaara J, Vaheri A, Rosen A, Giovanella BC. Characterization of a human ovarian teratocarcinoma-derived cell line. Int J Cancer. 1980;25(1):19–32. doi: 10.1002/ijc.2910250104. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z, Cherryholmes G, Mao A, Marek C, Longmate J, Kalos M, Amand RP, Shively JE. High plasma levels of MCP-1 and eotaxin provide evidence for an immunological basis of fibromyalgia. Exp Biol Med (Maywood) 2008;233(9):1171–1180. doi: 10.3181/0712-RM-328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.