Liver transplantation is now an accepted therapy for end-stage liver disease.1 Organ preservation, transport, and subsequent transplantation involves a period of cold ischemia followed by reperfusion. Toxic oxygen radicals liberated at reperfusion can cause severe injury to the microvascular endothelial cell.2–6 It has recently been demonstrated that severe preservation injury to the endothelial cell results in an increased incidence of allograft rejection in liver transplantation.7 Although the exact reasons for this phenomenon are unclear, it is possible that endothelial cell injury may activate the neighboring Kupffer cell resulting in the production of inflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor (TNF).8 The IL-1 and TNF thus produced can initiate the rejection cascade leading ultimately to graft failure.9–14 An isolated perfused rat liver (IPRL) model was used to investigate whether reperfusion injury following cold preservation can activate the Kupffer cell as measured by increased production of IL-1 and TNF.
MATERIAL AND METHODS
Isolated Perfused Rat Liver Model
An IPRL model was done according to Rao et al. 15 Livers harvested from male Lewis rats (275–300 g) were preserved in cold UW solution for either (1) 24 hours, (2) 26 hours, (3) 48 hours, or (4) 50 hours (n = 6 each). At the end of the preservation period, the livers were flushed in a retrograde fashion through the infrahepatic IVC with 10 mL of cold lactated ringers, and the effluent was analyzed for content as described below. Separate experiments were done in which, following 24 and 48 hours of preservation, livers were perfused at 37°C for 2 hours (n = 6 each). Samples of the 2-hour perfusate solution as well as the washout perfusate from groups 1, 2, 3, and 4 were assayed for (1) purine nucleoside phosphorylase (PNP), an endothelial cell-specific enzyme, (2) superoxide anion (O2−),(3) serum glutamate pyruvate transaminase (SGPT), (4) serum glutamic oxaloacetic transaminase (SGOT), and (5) IL-1.
Assays
Endothelial cell function was assessed by measuring effluent levels of PNP.16 Free radical generation was analyzed by measuring levels of O2− 17 Hepatocyte preservation was assessed by measuring transaminase levels18 using a Technicon RA 500 autoanalyser (Technicon Instruments, Tarrytown, NY).
Levels of IL-1 in the perfusate were assayed using a radioim- munoassay kit from Medgenix (Belgium).19
Statistical Analysis
Data are expressed as mean ± SD. The statistical significance of differences between group mean was analyzed by the student t test. The alpha level has been adjusted for multiple comparisons using Bonferronis adjustment.20 The alpha leve per comparison was P < .05.
RESULTS
PNP
Levels of PNP at the end of 24, 26, 48, and 50 hours of cold preservation were 46.83 ± 4.69 U/L, 45.33 ± 10.42 U/L, 49.0± 3.41 U/L, and 55.83 ± 2.71 U/L respectively. These values were not significantly different from each other and were similar to control values. Levels of PNP at the end of 2 hours of reperfusion following 24 hours of cold ischemia were 71.94 ± 13.32 U/L. These values were significantly higher (P < .001) than those observed at the end of either 24 o r 26 hours of cold ischemia alone. Similarly, values at the end of reperfusion following 48 hours of cold ischemia, 14.9.69 ± 22.26 U/L, were significantly higher (P < .0001) than PNP values at the end of either 48 or 50 hours of cold ischemia alone. Endothelial cell injury was significantly greater (P < .0001) following reperfusion at the end of 48 hours of cold ischemia than at the end of 24 hours. Fig 1 presents values for PNP in our experiments.
Fig 1.
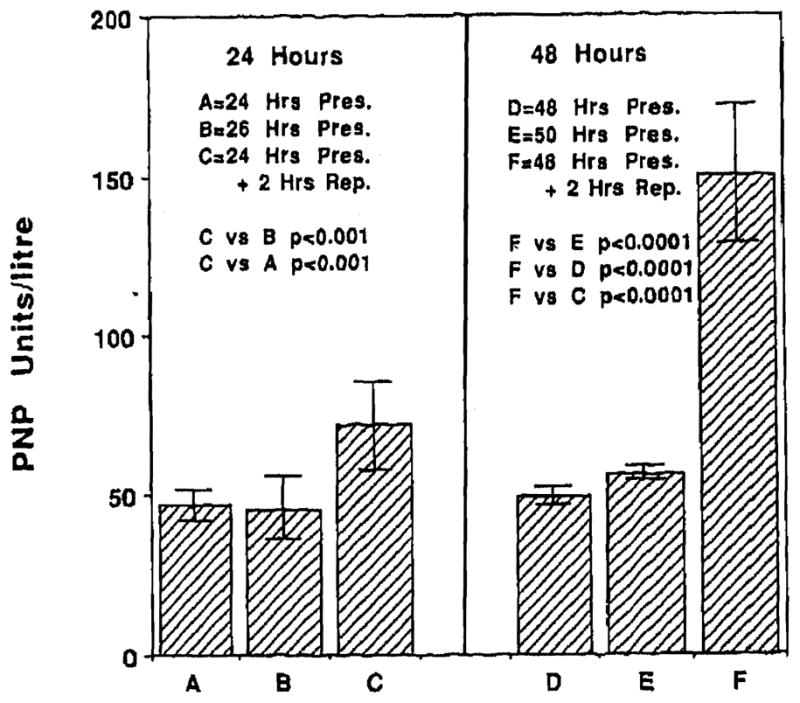
Levels of PNP at the end of 24, 26, 48, and 50 hours of cold preservation were similar to control levels and not different from each other. Reperfusion following either 24 hours (C) or 48 hours of cold preservation (F) resulted In significant injury to the microvascular endothelial cell (P < .001). Injury was more severe following 48 hours of cold preservation (F) as compared with 24 hours (P < .0001).
O2−
Superoxide levels at the end of 24, 26, 48, and 50 hours of cold preservation were respectively 0.33 ± 0.31, 0.33 ± 0.19, 0.51 ± 0.05, and 0.34 ± 0.09 nmol cytochrome-c reduced/mL respectively. These values were not significantly different from each other. Reperfusion following 24 and 48 hours of cold preservation resulted in superoxide levels of 1.06 ± 0.61 and 1.85 ±0.44 nmol cytochrome c reduced/mL respectively. These values were significantly higher (P <.001 each) than the corresponding values obtained at the end of cold preservation alone. Greater superoxide generation was observed following reperfusion at the end of 48 hours of cold preservation than at the end of 24 hours. Fig 2 presents the data for superoxide generation.
Fig 2.
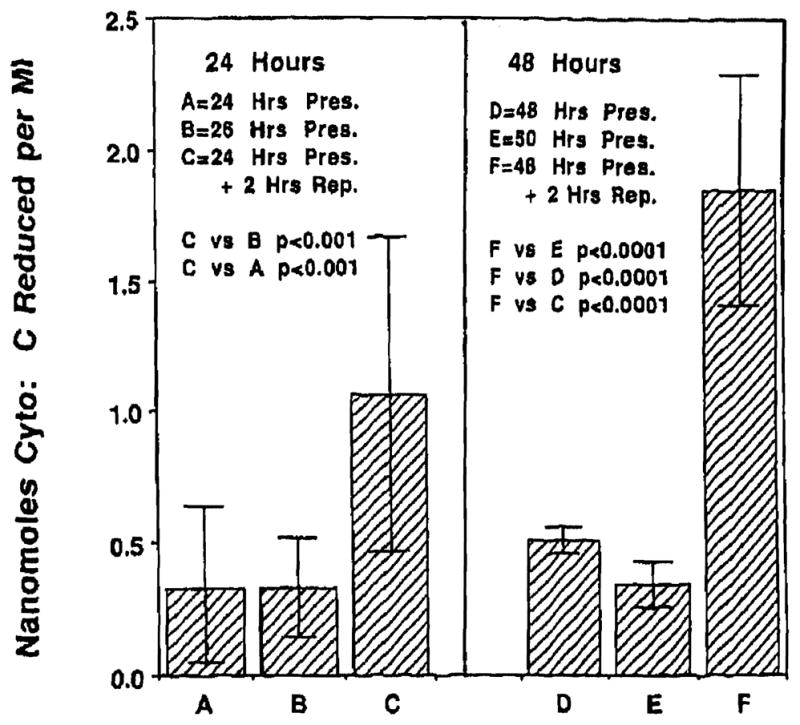
Reperfuslon following 24 or 48 hours of cold preservation resulted In a significant burst of superoxide generation (C and F). Levels of superoxide were significantly greater (P < .001, P < .0001) than at the end of cold ischemia alone. Significantly higher superoxide levels were observed on reperfusion following 48 hours (F) of preservation, than the levels found after 24 hours (C; P < .001).
Transaminases
SGOT
Levels of SGOT at the end of 24, 26, 48, and 50 hours of cold preservation were 68.42 ± 24.30 U, 68.33 ± 20.11 U, 88.83 ± 51.62 U, and 127.5 ± 19.15 U respectively. Reperfusion following 24 and 48 hours of cold preservation resulted in significantly higher (P < .001) levels of SGOT, of 221.31 ± 146.26 U, and 436.25 ± 248.87 U respectively. Hepatocellular injury was also significantly higher (P < .007) due to reperfusion at the end of 48 hours of cold preservation. Fig 3 presents the data for SGOT.
Fig 3.
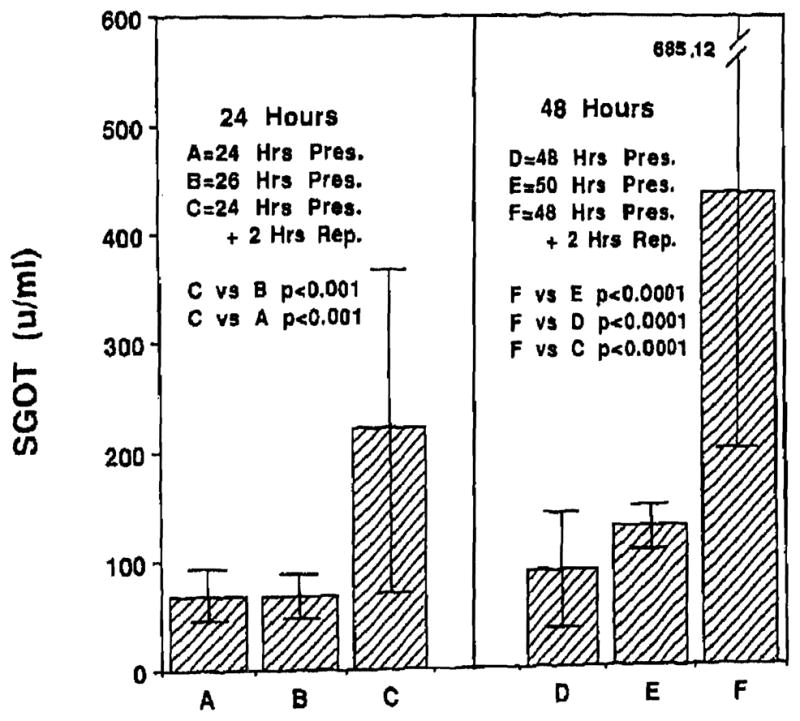
Reperfusion injury to the endothelial cell eventually resulted in hepatocellular injury. Hepatocellular injury as indicated by SGOT levels was more severe following 48 hours of cold Preservation (F) as compared with 24 hours (C; P < .007).
SGPT
Levels of SGPT at the end of 24, 26, 48, and 50 hours of cold preservation were 56.33 ± 24.41 U, 61.33 ± 21.52 U,82.83 ± 69.27 U, and 182.17 ± 14.48 U respectively. Reperfusion at the end of 24 and 48 hours of cold preservation resulted in SGPT levels of 118.56 ± 74.33 U and 414.31 ± 221.02 U respectively, which were higher than those obtained at the end of cold preservation alone. Fig 4 shows the data for SGPT.
Fig 4.
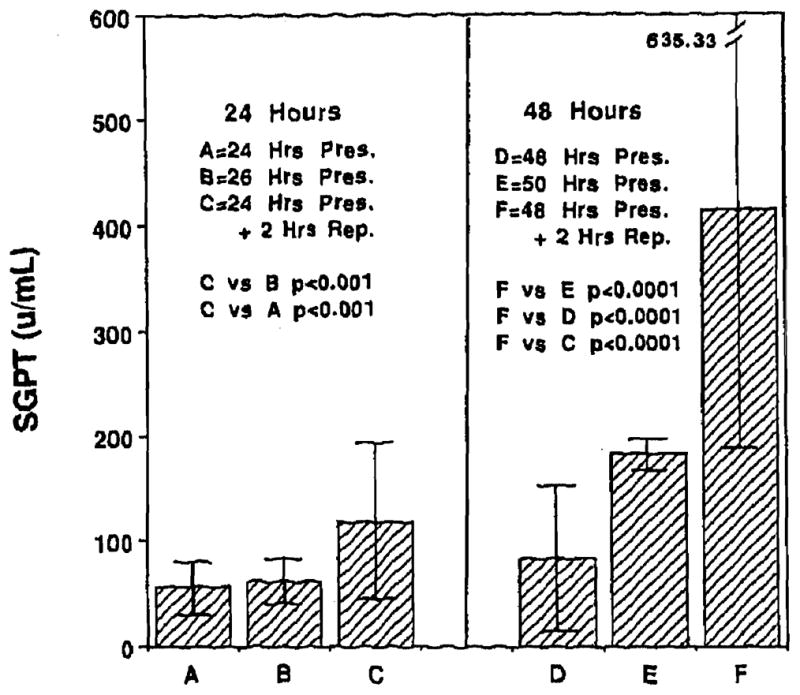
Levels of SGPT following 24 and 48 hours of cold preservation and reperfusion.
IL-1
Levels of IL-1 following 24, 26, 48, and 50 hours of cold preservation were respectively 0.81 ± 0.19 ng/mL, 0.77 ± 0.14 ng/mL, 0.55 ± 0.16 ng/mL, and 0.61 ± 0.09 ng/rnL respectively. Reperfusion following 24 and 48 hours of cold preservation resulted in IL-1 levels of 1.04 ± 0.18 ng/mL and 0.89 ± 0.18 ng/mL respectively. These values were significantly higher (P < .05) than IL-1 values at the end of cold preservation alone. Fig 5 presents the data for IL-1.
Fig 5.
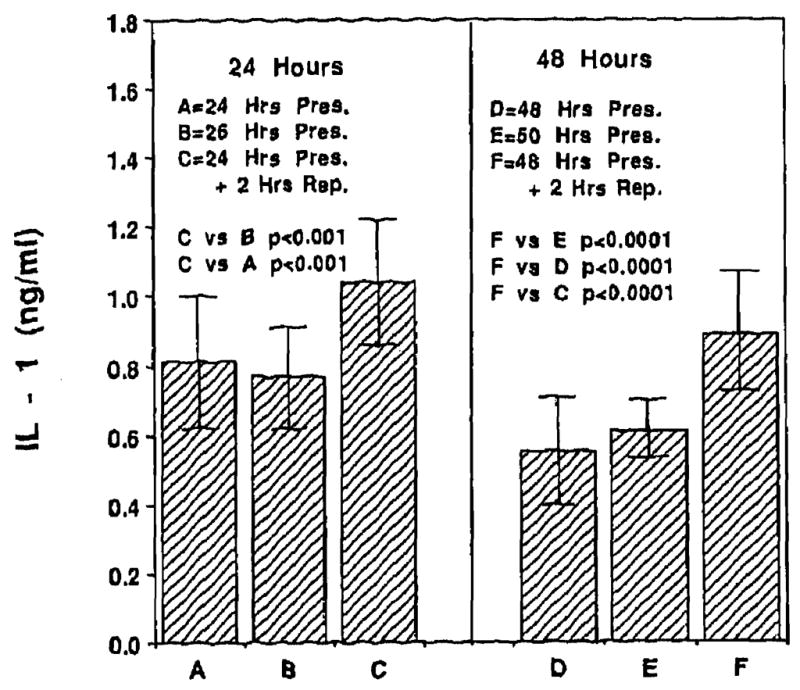
Reperfusion injury to the endothelial cell was accompanied by a significant degree of Kupffer cell activation as measured by IL-1 levels. Levels of IL-1 at the end of cold preservation alone were significantly lower (P < .05) than those following reperfusion.
DISCUSSION
Reperfusion following cold preservation resulted in significant injury to the microvascular endothelial cell as reflected by elevated levels of PNP, an endothelial cell- specific enzyme16 (Fig 1). This injury was more severe on reperfusion following 48 hours of cold preservation as compared with 24 hours of preservation, and was not evident after cold preservation alone. Our data is in agreement with that of Caldwell-Kenkel et al21 and Thurman et al22 and contrary to that of McKeown et al and Holloway et al23,24 who demonstrated injury to the sinusoidal endothelial cell following cold preservation alone. Toxic oxygen radicals liberated at reperfusion have been implicated in the injury to the endothelial cell.4–6,15 In our experiments endothelial cell injury was accompanied by the generation of toxic oxygen radicals (Fig 2). Irreversible endothelial injury eventually resulted in severe injury to the hepatocyte (Figs 3 and 4).
Reperfusion injury following cold preservation resulted in significant activation of Kupffer cells as reflected by elevated levels of IL-1 (Fig 5). Levels of TNF were also significantly elevated (data not shown). Again, elevated levels of IL-1 and TNF were observed only after reperfusion following cold ischemia. Our data is in complete agreement with that of LeMasters et al25 and Colleti et al.26 Howard et al7 have recently demonstrated that severe preservation injury results in an increased incidence of cellular rejection. Activated Kupffer cells following reperfusion can further release toxic oxygen radicals, perpetuating endothelial cell injury. This injury can result in the recruitment of inflammatory cells into the allograft to clean up the injury, Inflammatory cells thus attracted, including lymphocytes and macrophages, could activate the immune response with synchronous expression of histocompatibility antigens in the allograft, increasing its antigenicity.
In conclusion, our data suggest that reperfusion injury following cold ischemia injures the endothelial cell and activates the Kupffer cell with a self-perpetuating injury. It is attractive to speculate that this injury may upregulate the antigenicity of the allograft.
References
- 1.Van Thiel DH, Schade RR, Hakala TR, et al. Hepatology. 1984;4:66. doi: 10.1002/hep.1840040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parks D, Bulkley GB, Granger DN, et al. Gastroenterology. 1982;82:9. [PubMed] [Google Scholar]
- 3.Granger DN, Rutili G, McCord JM. Gastroenterology. 1987;81:22. [PubMed] [Google Scholar]
- 4.Adkison D, Hollwart ME, Benoit JN, et al. Acta Physiol Scand. 1986;126:101. [PubMed] [Google Scholar]
- 5.McCord JM. N Engl J Med. 1985;312:159. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 6.Marubayashi S, Dohi K, Ochi K, et al. Surgery. 1986;184:191. [PubMed] [Google Scholar]
- 7.Howard TK, Klintmalm GBG, Cofer JB, et al. Transplantation. 1990;49:103. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Wardle EN. Liver. 1987;7:63. doi: 10.1111/j.1600-0676.1987.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Pober JS, Gimbrone MA. Proc Natl Acad Sci USA. 1982;79:6641. doi: 10.1073/pnas.79.21.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pober JS, Gimbrone MA, Cotran RS, et al. J Exp Med. 1983;157:1339. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bevilacqua MP, Pober JS, Wheeler ME, et al. J Clin Invest. 1985;76:2003. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins T, Lapierre LA, Fiers W, et al. Proc Natl Acad Sci USA. 1986;83:446. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevilacqua MO, Pober JS, Mendrick DL, et al. Proc Natl Acad Sci USA. 1987;84:9238. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pober JS, Gimbrone MA, Lapierre LA. J Immunol. 1986;137:1893. [PubMed] [Google Scholar]
- 15.Rao PN, Walsh TP, Makowka L, et al. Transplantation. 1990;49:193. [Google Scholar]
- 16.Rao PN, Walsh TR, Makowka L, et al. Hepatology. 1990;11:193. doi: 10.1002/hep.1840110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCord JM, Fridovich I. J Biol Chem. 1969;244:6049. [PubMed] [Google Scholar]
- 18.Ontell SJ, Makowka LM, Ove P, et al. Gastroenterology. 1988;95:1617. doi: 10.1016/s0016-5085(88)80086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon JG, Dinarello CA. Science. 1985;227:1247. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- 20.Milliken GA, Johnson DE. Designed Experiments. Vol. 1. Belmont, CA: Wadsworth; 1981. Analysis of Messy Data. [Google Scholar]
- 21.Caldwell-Kenkel J, Thurman RG, LeMasters JJ. Transplantation. 1988;45:834. [PubMed] [Google Scholar]
- 22.Thurman RG, Marzi I, Seiz G, et al. Transplantation [Google Scholar]
- 23.McKeown CMB, Edwards V, Phillips MJ, et al. Transplantation. 1989;48:178. [Google Scholar]
- 24.Holloway CMB, Harvey PRC, Mullen JBM. Transplantation. 1989;48:178. doi: 10.1097/00007890-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 25.LeMasters JJ, Caldwell Kenkel J, Cumin RT, et al. In: Cells of the Hepatic Sinusoid. Wisse E, Knook DL, editors. Vol. 2. Kupffer Cell Foundation; Rijswijk, The Netherlands: 1989. p. 277. [Google Scholar]
- 26.Colleti LM, Remick DG, Burtch GD, et al. J Clin Invest. 1990;85:1936. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]


