Abstract
Reliable and objective markers of neuronal function and pathology that can directly assess the effects of neuroprotective treatments in the brain are urgently needed for clinical trials in neurodegenerative diseases. Here we assessed the sensitivity of high field proton magnetic resonance spectroscopy (1H MRS) to monitor reversal of neurodegeneration by taking advantage of a well characterized conditional mouse model of spinocerebellar ataxia type 1 (SCA1), where the cerebellar pathology and ataxic phenotype are reversible by doxycycline administration. Transgene expression was suppressed by feeding the mice with chow that contains doxycycline from 6 – 12 weeks of age in an early stage group and from 12 – 24 weeks in a mid-stage group. Cerebellar neurochemical profiles of treated and untreated conditional mice were measured at 9.4 tesla (T) before and after treatment and compared to those of wild type (WT) controls, as well as to histology measures (molecular layer thickness in the primary fissure and a global pathological severity score). Concentrations of N-acetylaspartate (NAA) and myo-inositol in the treated mice trended towards normalization to WT levels in both the early and mid-stage groups. The NAA-to-myo-inositol ratio was significantly different between the treated vs. untreated SCA1 mice and demonstrated partial reversal to WT values both at early and mid-stage, consistent with the histological measures. Taurine and total creatine levels were completely normalized in early and mid-stage treatment groups, respectively. The MRS markers were a more sensitive measure of treatment response than the histological measures from the same volume-of-interest in the early stage group. NAA, myo-inositol and taurine levels were significantly correlated with the histology measures in data combined from all groups. These data demonstrate that MRS markers reliably detect rescue from neuronal pathology and imply that the neurochemical levels measured by MRS accurately reflect treatment efficacy. Therefore this study presents an important step in validating MRS biomarkers as potential surrogate markers to evaluate therapeutics in pre-clinical and clinical trials in SCA1.
Keywords: SCA1, mouse model, MRS, cerebellum, neurodegeneration
Introduction
Neuroimaging methodologies are attractive because of their potential to objectively and non-invasively evaluate the effects of therapies in brain disorders. Among these, volumetric MRI has been widely used to measure cerebral atrophy and to monitor progressive neurodegeneration because the relationship between atrophic changes and neuron loss is well established (Allegrini and Sauer, 1992, Mueller et al., 2006). On the other hand, the neurodegenerative process starts with neuronal dysfunction, and neuroprotective agents are likely to be most effective in rescuing neuronal function and stopping/reversing disease progression before substantial neuronal loss occurs (DeKosky and Marek, 2003). Hence, neuroimaging methods that can detect neuronal dysfunction and its reversal are needed in this period. One such method is proton magnetic resonance spectroscopy (1H MRS), which detects concentrations of endogenous neurochemicals. We and others have shown that neurochemical alterations are present in the absence of neuronal loss (Jenkins et al., 2000, Niessen et al., 2007, Öz et al., 2010b) and that such alterations correlate with functional aspects of pathology, such as plaque load in Alzheimer’s disease (Choi et al., 2010, Kantarci et al., 2008, von Kienlin et al., 2005) and dendritic atrophy in the cerebellum in ataxia (Öz et al., 2010b). Therefore this method presents great potential for detection of early neurochemical abnormalities and the reversal of these upon treatment. Indeed, studies with patients demonstrated the potential to monitor therapy response in the brain by MRS (Bender et al., 2005, Kalra and Arnold, 2004, Öz et al., 2005). However, it is unclear in such human studies if the reversal of abnormal neurochemical levels reflects a reversal of the underlying pathology or a temporary response of cellular biochemistry to the tested treatment. These can be differentiated by correlating the MR measures to pathological findings in the same brains in animal models that reproduce the pathology and the phenotype of the human disease.
We previously demonstrated that multiple neurochemical alterations can be reliably and pre-symptomatically detected in a well-characterized transgenic model of spinocerebellar ataxia type 1 (SCA1) (Öz et al., 2010b). SCA1 is a hereditary, polyglutamine-induced neurodegenerative disorder that results in loss of motor coordination primarily caused by cerebellar Purkinje cell (PC) dysfunction and loss (Orr et al., 1993). In the transgenic SCA1 model we studied previously by MRS, the ataxic phenotype develops as a result of neuronal dysfunction and dendritic atrophy, rather than neuronal loss (Clark et al., 1997), which makes the model ideal for delineating the neurochemical correlates of PC dysfunction and pathology. In this model, a subset of the altered neurochemicals, specifically N-acetylaspartate, myo-inositol and glutamate, correlated robustly with the molecular layer thickness and a semi-quantitative pathological severity score (Öz et al., 2010b). Furthermore, the levels of the same neurochemicals were also altered in patients with SCA1 and correlated with their clinical status as assessed by a validated ataxia scale (Öz et al., 2010a). Because these neurochemical levels seem to reflect the neuronal dysfunction/pathology in SCA1 both in patients and the transgenic mouse model, we hypothesized that they would also serve as reliable markers for reversal of neuronal pathology with therapy. To test this hypothesis in a well-controlled fashion, we took advantage of a conditional transgenic SCA1 mouse model where the expression of the mutant ataxin-1 protein was put under doxycycline regulation such that its expression can be turned on and off at will (Zu et al., 2004). These mice overexpress the mutant human ataxin-1 protein with an 82 glutamine stretch. Suppression of transgene expression by doxycycline reverses the neurological phenotype and pathology in this model at early and mid-stages of disease and even at late disease stage albeit to a smaller extent (Zu et al., 2004).
To assess the sensitivity of MRS biomarkers to reversal of pathology, we obtained cerebellar neurochemical profiles of conditional SCA1[82Q] mice before and after treatment with doxycycline to suppress mutant ataxin-1 expression. We compared their profiles to littermates that were not treated, as well as to wild type (WT) controls. We further assessed the cerebella of the mice by histology and evaluated correlations between neurochemical levels and quantitative pathology measures in the same brains.
Materials and Methods
Study design
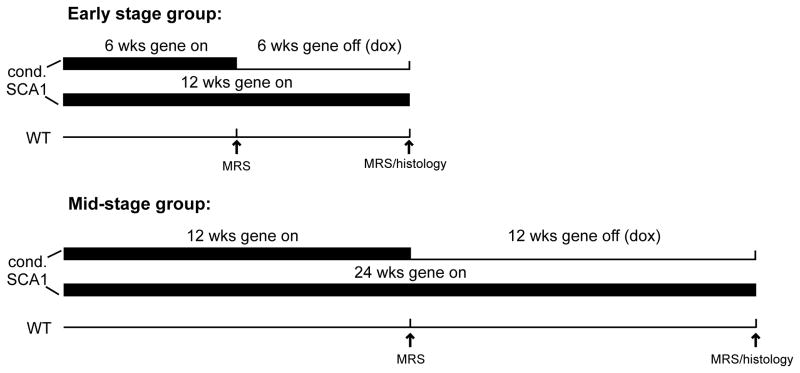
A total of 41 conditional SCA1[82Q] transgenic mice were studied. Mice were obtained from 11 different litters to introduce inter-litter variability. Mice were divided into two groups: Those in the early stage group were treated with doxycycline from 6 to 12 weeks of age to suppress transgene expression and those in the mid-stage group were treated with doxycycline from 12 to 24 weeks of age (Fig. 1). These ages were selected based on the previously characterized pathological progression in these mice (Zu et al., 2004). In the early stage group (N = 19, all males), 9 mice were scanned before treatment began at 6 weeks and after treatment ended at 12 weeks, 9 mice were untreated littermate controls and scanned at 6 and 12 weeks, and 1 untreated mouse was scanned only at 6 weeks. In the mid-stage group (N = 22, 18 males, 4 females), 10 mice were scanned before treatment began at 12 weeks and after treatment ended at 24 weeks, 9 mice were untreated littermate controls and scanned at 12 and 24 weeks, and 3 untreated mice were scanned only at 12 weeks. All mice were sacrificed for histological evaluation after the last MR scan.
Figure 1.
Study design. Transgene expression in the conditional SCA1[82Q] mice was suppressed with doxycycline administration from 6–12 weeks in the early stage treatment group and from 12–24 weeks in the mid-stage treatment group. Two sets of controls were studied at the same time points: Transgenic, untreated littermates and wild type (FVB) mice. The gene on (bold line) and gene off (thin line) periods are shown.
Mice from the background strain (FVB) were used as WT controls. Because the previous investigation (that demonstrated the behavioral and pathological outcomes of suppressing transgene expression in the conditional SCA1 mice) compared the conditional SCA1 mice to untreated FVB mice (Zu et al., 2004), our previously published data from untreated FVB mice (obtained by utilizing identical methods in a concurrent but separate experiment (Öz et al., 2010b)) were used as WT control concentrations. These included 13 FVB mice (all males) scanned at 6 weeks, 15 FVB mice (13 males, 2 females) scanned at 12 weeks and 9 FVB mice (7 males, 2 females) scanned at 24 weeks. In addition, to assess if doxycycline treatment alone has a major effect on the neurochemical profile, 4 of the male FVB control mice were treated with doxycyline from after the 6 week scan until their 12 week scan and 4 other male FVB mice were treated with doxycyline from after the 12 week scan until their 24 week scan; data from their post-treatment scans were not included among the WT control concentrations.
Female mice in similar numbers per group (two per each of the WT, treated and untreated SCA1 groups at mid-stage) were included in the study due to availability at the desired ages and because prior MRS studies did not find an influence of gender on neurochemical levels (Nagae-Poetscher et al., 2004, Pouwels and Frahm, 1998).
Doxycycline administration
Mice were fed with chow that contains 200 mg/kg of doxycycline (“Dox Diet” from Bio-Serve, Frenchtown, NJ) to suppress transgene expression. To ensure that a high dosage was achieved immediately, they were also administered doxycycline in their drinking water (2 mg/mL doxycycline in a 5% sucrose solution) for the first 2 days of study. With this protocol the mice received approximately 33 mg of doxycycline per kg of body weight per day from the chow and an additional ~530 mg/kg/day from the water for the first 2 days of treatment.
Animal preparation for MR scanning
All experiments were performed according to procedures approved by the University of Minnesota Institutional Animal Care and Use Committee. Animals were induced with 3–4% isoflurane and a 1:1 mixture of O2:N2O. Spontaneously breathing mice were fixed in a custom built mouse holder and maintained anesthetized with 1.5–2% isoflurane while monitoring body temperature and respiration rate (SA Instruments, Inc) to ensure unchanging physiological status. Body temperature was maintained at 36–37°C with a circulating warm water system and a heating fan that received feedback from the rectal temperature probe. The typical scanning time for each animal was approximately 50 min – 1 h.
MR protocol
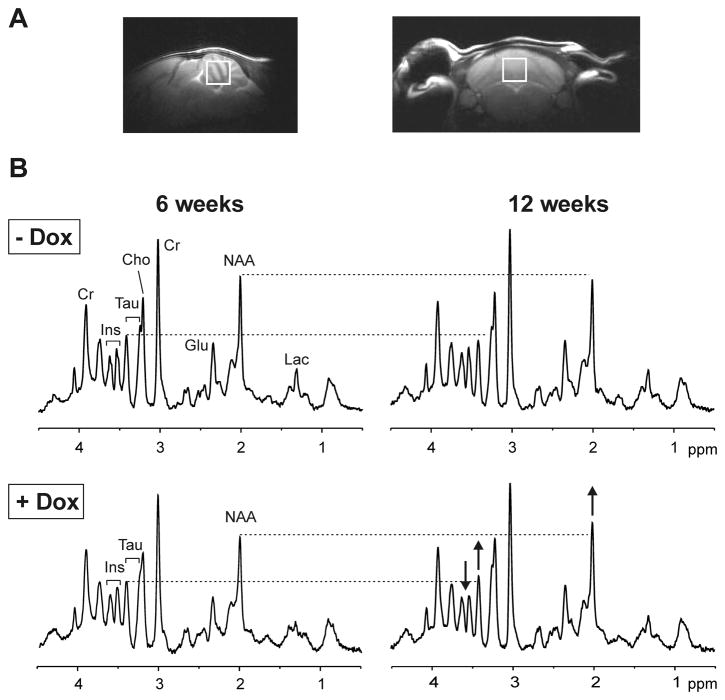
The MR scanning protocol was identical to our prior study (Öz et al., 2010b). Briefly, studies were performed with a quadrature surface RF coil on a 9.4 T/31 cm magnet (Magnex Scientific, Abingdon, UK) interfaced to a Varian INOVA console (Varian, Inc., Palo Alto, CA, USA). A 5–7 μL volume-of-interest (VOI) centered on the midline in the cerebellum (Fig. 2A) was selected based on coronal and sagittal multi-slice images obtained with a rapid acquisition with relaxation enhancement (RARE) sequence (Hennig et al., 1986). The VOI was placed such that it only contained cerebellar tissue (Fig. 2A). In addition, the VOI was placed consistently during longitudinal studies by using anatomical landmarks. Similar to our prior study (Öz et al., 2010b), if necessary, the size of the VOI was reduced in SCA1[82Q] mice in the follow-up scan to cover the same cerebellar region and avoid cerebrospinal fluid (CSF). Unlike the prior study, this was necessary only in a few animals because cerebellar atrophy was minor in this conditional SCA1 model of early pathology. All first- and second-order shims were adjusted using FASTMAP with echo-planar readout (Gruetter and Tkáč, 2000). Localized 1H MR spectra were acquired with a short-echo localization by adiabatic selective refocusing (LASER) sequence (TE = 15 ms, TR = 5 s, 256 averages) (Garwood and DelaBarre, 2001) combined with VAPOR (variable power RF pulses with optimized relaxation delays) water suppression (Tkáč et al., 1999). Unsuppressed water spectra acquired from the same VOI were used as a metabolite quantification reference. In addition, unsuppressed water spectra were acquired in a subset of animals at a series of TE values (TE = 5–400 ms; TR = 30 s for full relaxation) to evaluate CSF contribution to the VOI (Ernst et al., 1993, Öz et al., 2010a). No CSF contribution was found by fitting the integrals of these water spectra with a biexponential decay function, and hence no correction for CSF was necessary.
Figure 2.
Proton MRS in the conditional SCA1 mice. (A) Sagittal and coronal T2-weighted images showing the placement of the cerebellar volume-of-interest. (B) Localized proton MR spectra (LASER, TE = 15 ms, TR = 5 s) obtained from two conditional littermate SCA1 mice, one treated with doxycycline (+ Dox), the other untreated (− Dox), in the early stage group. Effects of doxycyline treatment on the spectrum of the treated mouse (bottom row) are shown with arrows indicating the direction of change. The spectra were processed identically, weighted with the same Gaussian function prior to Fourier transformation and scaled based on neurochemical concentrations obtained by LCModel. Prominent peaks in the spectra are marked: N-acetylaspartate (NAA), myo-inositol (Ins), taurine (tau), creatine (Cr), choline (Cho), glutamate (Glu), lactate (Lac).
Metabolite quantification
The contributions of individual metabolites to the spectra were quantified using an automated deconvolution program (LCModel) (Provencher, 1993) as described before using the same basis set consisting of 20 metabolites and an experimentally acquired macromolecule spectrum (Öz et al., 2010b). The criteria to select the reliable concentrations were based on Cramér-Rao lower bounds (CRLB) and were identical to our prior study (Öz et al., 2010b). Three metabolites (alanine, glycine and scyllo-inositol) were excluded from final analysis based on these criteria. If the correlation between two metabolites was consistently high (correlation coefficient < −0.5), their sum was reported rather than the individual values, as recommended by the LCModel manual (Provencher, 2001). Strong negative correlation was found in two cases, so that creatine + phosphocreatine (denoted tCr for total creatine) and glycerophosphocholine + phosphocholine (denoted tCho for total choline) were reported. Therefore 15 statistically uncorrelated concentrations were evaluated.
Histology
The histology was performed as described before (Öz et al., 2010b). Briefly, following sodium pentobarbital (100 mg/kg i.p.) anesthesia, mice were sacrificed by transcardial perfusion with pH 7.4 phosphate buffered saline followed by 10% formalin. Brains were postfixed in 10% formalin. Histology was performed in a blind fashion (regarding the treatment group and age of the animals) on paraffin-embedded sections using hematoxylin-and-eosin (H & E), and in some cases calbindin immunohistochemistry. The molecular layer (ML) thickness at the primary fissure and a semi-quantitative severity scale were used to quantify the extent of pathological progression because much of the pathology in these models is related to shrinkage of PCs rather than neuronal loss (Clark et al., 1997). The following severity scale was used: 0, no pathological changes; 0.5, near-normal but ML somewhat thinner; 1, mild changes including heterotopic PCs, vacuoles in PCs, thinning of ML, largely confined to the posterior lobules; 2, similar to 1 but more widespread; heterotopic PCs more numerous and often higher in the ML; 3, widespread ML thinning, numerous heterotopic PCs involving anterior lobules nearly as frequently as posterior, mild PC loss, primarily in the posterior lobules; 4, severe disorganization of cerebellar cortex with generalized severe atrophy of ML, frequent heterotopic PCs and PC loss.
Statistical analysis
Data from the different mouse groups were compared using two-way repeated measures ANOVA, with fixed effects for experimental group, week of scan, and their interaction, and a random effect for the repeated measurements on the same mouse. Analyses were done in SAS using PROC MIXED, assuming approximately normally distributed outcomes. Model contrasts of means were used to test group comparisons at each week; p-values were corrected for these multiple comparisons (2 comparisons before doxycycline treatment of the SCA1 mice, namely treated vs. untreated SCA1 mice and WT controls vs. all SCA1 mice, and 3 comparisons after treatment of the SCA1 mice, namely treated vs. untreated SCA1, treated SCA1 vs. WT controls and untreated SCA1 vs. WT controls, resulting in 5 total comparisons) using a step-down approach (Holm, 1979) for each metabolite (Table 1). We used the Holm method because it does not assume that the tests are independent of each other. Additional p-value correction across the multiple metabolites was not carried out. To explore the potential of gender influencing our conclusions, our contrasts were re-estimated, omitting the few female mice in the transgenic and control groups; similar results were obtained, and are not presented here. Spearman correlations were calculated to evaluate the relationship between neurochemical levels and histological severity scores and ML thickness measurements.
Table 1.
Statistical significance of comparisons of the treated and untreated conditional SCA1 and WT mice for the neurochemicals of interest; p-values corrected for multiple comparisons within each metabolite or metabolite ratio using the stepdown Bonferroni/Holm approach (Holm, 1979).
| Comparison | Age (wks) | NAA | myo-inositol | NAA/myo-inositol | taurine | tCr |
|---|---|---|---|---|---|---|
| Early stage group: | ||||||
| Treated SCA1 vs. untreated SCA1 | 6 | 0.6250 | 0.2088 | 0.2695 | 1.0000 | 1.0000 |
| SCA1 vs. WT | 6 | <0.0001 | <0.0001 | < 0.0001 | <0.0001 | 0.5794 |
| Treated SCA1 vs. untreated SCA1 | 12 | 0.1173 | 0.0298 | 0.0103 | 0.0002 | 0.7887 |
| Treated SCA1 vs. WT | 12 | 0.0382 | 0.0056 | 0.0001 | 1.0000 | 1.0000 |
| Untreated SCA1 vs. WT | 12 | 0.0004 | <0.0001 | < 0.0001 | <0.0001 | 0.5308 |
| Mid-stage group: | ||||||
| Treated SCA1 vs. untreated SCA1 | 12 | 0.2057 | 0.7565 | 0.3156 | 0.1725 | 1.0000 |
| SCA1 vs. WT | 12 | <0.0001 | <0.0001 | < 0.0001 | <0.0001 | 1.0000 |
| Treated SCA1 vs. untreated SCA1 | 24 | 0.1968 | 0.0672 | 0.0319 | 0.1437 | 0.0181 |
| Treated SCA1 vs. WT | 24 | 0.0012 | 0.0122 | < 0.0001 | 0.0006 | 1.0000 |
| Untreated SCA1 vs. WT | 24 | <0.0001 | <0.0001 | < 0.0001 | <0.0001 | 0.0369 |
Results
Longitudinal neurochemical alterations in conditional SCA1 mice vs. WT controls
To ensure that the cerebellar neurochemical levels of the conditional SCA1 mice randomly assigned to the treatment and no treatment groups were the same, their neurochemical profiles were compared for the baseline scan prior to treatment and no differences were found (Table 1). Therefore data from FVB mice were compared to all conditional SCA1 mice in the early stage group at 6 weeks and to all SCA1 mice in the mid-stage group at 12 weeks to identify differences between the conditional vs. WT mice at these time points. A comparison of untreated SCA1 vs. FVB mice at 24 weeks showed the neurochemical alterations in the conditional SCA1 mice at this time point. Based on this analysis the longitudinal neurochemical alterations in the untreated conditional SCA1 mice were as follows: N-acetylaspartate (NAA), taurine and myo-inositol were significantly different in the conditional SCA1 mice relative to WT controls at all ages (p < 0.0001), while GABA (p = 0.005) and tCr (p = 0.037) became abnormal only at 24 weeks with progressing disease. In addition, glutamine was different at 6 weeks (p = 0.03), N-acetylaspartylglutamate at 6 weeks (p = 0.02) and 24 weeks (p = 0.002) and tCho at 6 weeks (p = 0.02) and 12 weeks (p = 0.004) (Supplementary Fig. 1). The remaining 7 metabolites were not different between the conditional SCA1 mice and WT controls.
Effect of doxycycline administration on the neurochemical profile in conditional SCA1 mice
The effects of suppressing the mutant ataxin-1 expression with doxycycline were apparent in the MR spectra as a result of the high signal-to-noise and good resolution obtained consistently in the study. Figure 2B shows spectra obtained in two littermate mice from the early stage group. A slight decrease in NAA and taurine from 6 to 12 weeks was apparent in the untreated mouse (upper row) while an increase in NAA and taurine and a decrease in myo-inositol was detected in the mouse treated with doxycycline (lower row). Note the difference in the ratio of the myo-inositol and taurine peaks in the treated vs. untreated mice at 12 weeks.
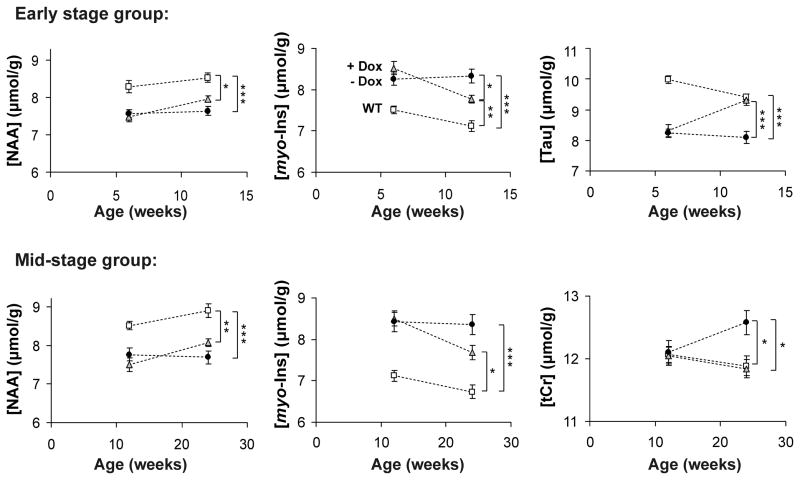
The levels of NAA and myo-inositol trended towards normalization to WT values in both the early and mid-stage groups, while taurine was completely normalized in the early stage group and tCr was completely normalized in the mid-stage group (Fig. 3). Myo-inositol and taurine levels were significantly different between the treated and untreated groups at the post-treatment scan in the early stage group and tCr levels were significantly different between the treated and untreated groups at the post-treatment scan in the mid-stage group (Fig. 3, Table 1). While the levels of NAA and myo-inositol were still different between the treated SCA1 mice vs. WT mice at the post-treatment scans, these differences were reduced relative to those between the untreated SCA1 vs. WT mice (Fig. 3, Table 1). To assess if the combined information from NAA and myo-inositol would heighten the sensitivity to group differences between treated and untreated SCA1 mice, we compared the NAA-to-myo-inositol ratio between groups. Indeed this ratio was significantly different between the treated vs. untreated SCA1 groups at the post-treatment scan both in the early stage and in the mid-stage group (Table 1).
Figure 3.
Time courses of neurochemicals that displayed reversal to wild type (WT) levels with doxycycline treatment. Mean metabolite concentrations ± SEM are shown from WT (open squares), treated SCA1[82Q] (gray triangles) and untreated SCA1[82Q] mice (black circles). N-acetylaspartate (NAA) and myo-inositol (myo-Ins) displayed partial reversal with treatment in both early and mid-stage groups, while taurine (Tau) and total creatine (tCr) levels were reversed completely to WT levels in the early and mid-stage groups, respectively. Statistically significant differences at the post-treatment scan denoted by: * p < 0.05, ** p < 0.01, *** p < 0.001; p-values corrected for multiple comparisons using the stepdown Bonferroni/Holm approach (Holm, 1979).
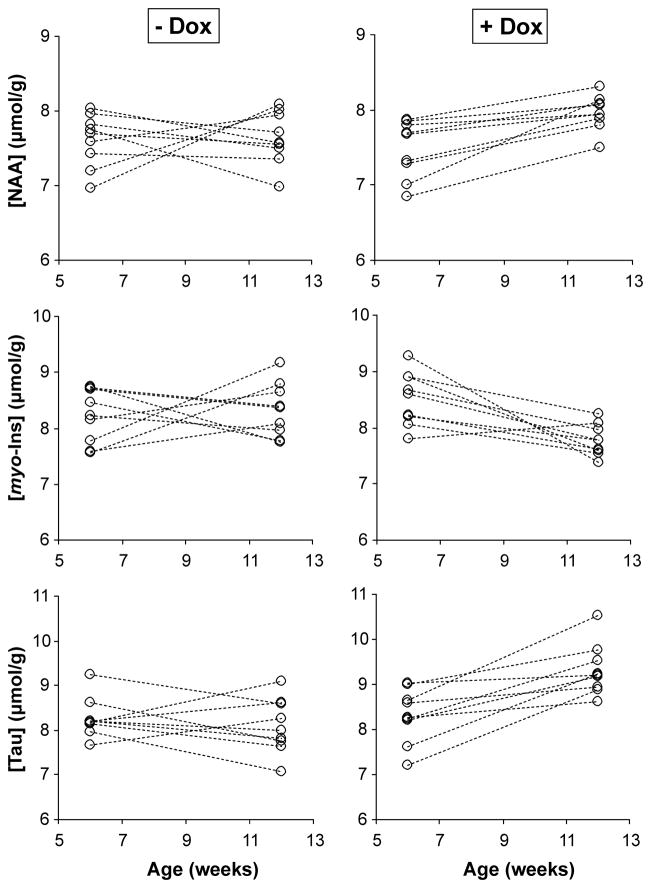
To demonstrate the potential of detecting treatment effects in individual mice, levels of NAA, myo-inositol and taurine from all SCA1 mice in the early stage group are shown in Figure 4. While there was overlap between the treated vs. untreated mice at 12 weeks, monitoring the change in all 3 metabolites demonstrated a clear difference between the groups. Namely, 8 of the 9 treated mice showed a change in all 3 metabolites in the expected direction, i.e. an increase in NAA and taurine and a decrease in myo-inositol (one mouse in the treatment group showed an increase in myo-inositol), whereas none of the mice in the untreated group showed a change in all 3 metabolites in the expected direction.
Figure 4.
N-acetylaspartate (NAA), myo-inositol (myo-Ins) and taurine (Tau) concentrations in individual SCA1 mice in the early stage group at 6 and 12 weeks. Left column: untreated mice, right column: doxycycline treated littermates.
Effect of doxycycline administration on pathology in conditional SCA1 mice
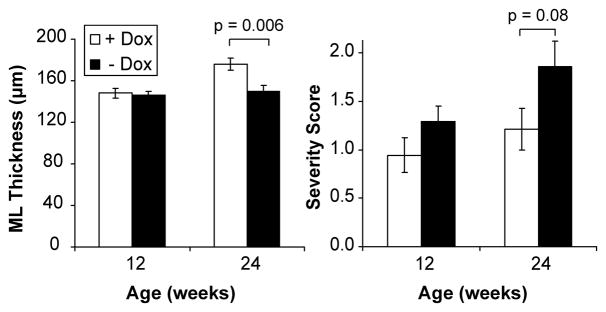
The partial reversal of the NAA-to-myo-inositol ratio in the treated conditional SCA1 mice was in agreement with pathological findings that also did not show full recovery. The molecular layer thickness in the primary fissure appeared unchanged with treatment in the early stage group, while the global severity score tended to be lower (Fig. 5). The molecular layer thickness in the treated mice in the mid-stage group was significantly higher than untreated mice and the severity score tended to decrease with treatment (Figs. 5 and 6). Therefore, the PC pathology tended to recover in the early stage group based on the global severity score and recovered significantly, but only partially, in the mid-stage group based on the molecular layer thickness. Note that histology data were available from 2 FVB mice at each time point, but were not included in Figure 5 due to the small sample size; however all 4 of these FVB mice had a severity score of 0 as expected.
Figure 5.
Histology findings in the early (at 12 weeks) and mid-stage (at 24 weeks) groups following doxycycline treatment (+ Dox) vs. no treatment (− Dox). Molecular layer thickness in the primary fissure and the global pathological severity score are shown. Error bars are SEM. p-values shown were obtained from an unpaired t-test comparison of the treated vs. untreated groups.
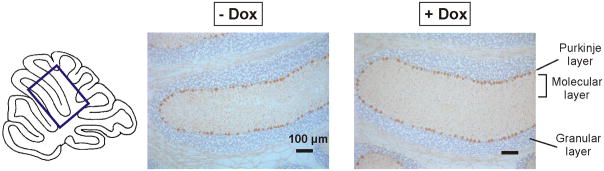
Figure 6.
Effect of doxycycline treatment on pathology in the mid-stage group. The area shown in the slides is indicated on the midsagittal cerebellum scheme. Slides obtained at 24 weeks of age from two conditional littermate SCA1 mice, one treated with doxycycline (+ Dox), the other untreated (− Dox), are shown. The molecular layer is thinner in the untreated mouse than the treated littermate. The sections are stained with calbindin immunochemistry and counterstained with hematoxylin. The scale bars indicate 100 μm.
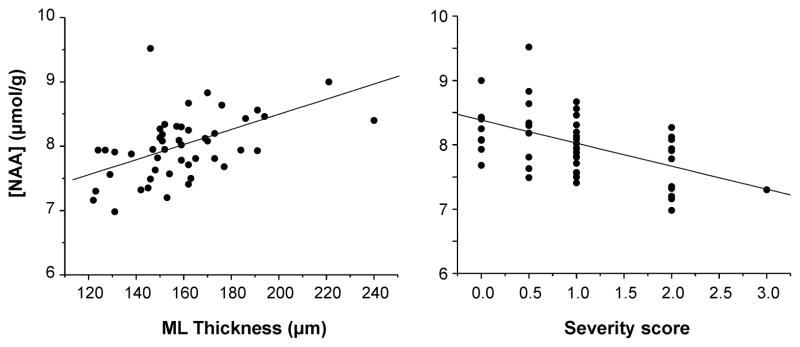
When data from all perfused mouse brains were combined, correlations between the NAA, myo-inositol and taurine levels and the pathology measures were detected (NAA with pathology measures shown in Fig. 7). Specifically, NAA and myo-inositol concentrations were significantly correlated with the molecular layer thickness (NAA: R = 0.49, p < 0.001, myo-inositol: R = − 0.35, p < 0.02) and NAA, myo-inositol and taurine concentrations significantly correlated with the pathological severity score (NAA: R = − 0.46, p = 0.001, myo-inositol: R = 0.45, p = 0.001, taurine: R = − 0.64, p < 0.0001).
Figure 7.
Correlation of MRS and histology findings. Cerebellar concentrations of NAA are significantly correlated with both molecular layer thickness in the primary fissure and the global pathological severity score.
Effect of doxycycline administration on the neurochemical profile in WT mice
A comparison of neurochemical levels in the cerebella of treated vs. untreated FVB mice at 12 and at 24 weeks (following 6 and 12 weeks of doxycycline administration, respectively) revealed only one statistically significant difference in aspartate at 24 weeks, a metabolite that was not different between SCA1 vs. FVB mice nor between treated and untreated SCA1 mice. Therefore, the doxycycline treatment alone did not substantially alter the levels of neurochemicals that were potential biomarkers in this study, suggesting that any alterations seen in the SCA1[82Q] mice upon doxycycline administration were due to its suppression of transgene expression and justifying the use of data from untreated FVB mice as WT controls.
Discussion
Here we demonstrated that neurochemical levels measurable by 1H MRS can be used to monitor reversal of neurodegeneration by taking advantage of a conditional SCA1 mouse model, where reversal of pathology and the ataxic phenotype can be induced by doxycycline administration (Zu et al., 2004). Based on their correlation with histological measures, the neurochemical levels accurately reflected treatment efficacy. Furthermore, these in vivo measures appeared to be a more sensitive measure than histology early-on based on the data obtained from the early disease stage group. The MRS method does not necessitate tracer injections as it measures endogenous concentrations, measurements can be repeated in the same subject with good test-retest reproducibility (Öz et al., 2010b), and the method can detect disease modifying vs. symptomatic treatment effects as indicated by the current study. The MRS biomarkers that correlated well with reversal of pathology here also show good correlations with clinical outcome in SCA1 (Öz et al., 2010a) and are sensitive to pre-symptomatic disease (Öz et al., 2010b). All of these constitute desired characteristics for surrogate markers in clinical trials (Mueller et al., 2006). Therefore this study presents an important step in validating the MRS biomarkers as potential surrogate markers to evaluate therapeutics in SCA1. Future therapeutic studies in SCA1 can take advantage of the conditional SCA1 model utilized here since it models early disease stage when neuroprotective agents are likely to be most effective. Note however that such agents may lead to more subtle disease reversal than possible with suppressing ataxin-1 expression, necessitating larger sample sizes than the current study to detect treatment effects.
Advantages of high field MRS for monitoring treatment response
While treatment response was monitored in the brain by MRS in a number of prior studies (Bender et al., 2005, Kalra and Arnold, 2004, Öz et al., 2005), very few studies attempted to validate that a reversal of neurochemical alterations indeed reflected reversal of pathology. In a study of a marmoset Parkinson’s disease model both the NAA-to-tCr ratio and immunohistochemistry confirmed the neuroprotective effect of modafinil (van Vlieta et al., 2008). Similarly, creatine supplementation in Huntington’s disease mouse models resulted both in increased striatal NAA-to-tCho and tCr-to-tCho ratios and in lessened atrophy of striatal neurons (Andreassen et al., 2001, Ferrante et al., 2000). However, correlations between the MRS and histology measures were not reported in these prior studies even though both sets of data were acquired in the same brains. Recently, a study that investigated the effects of non-steroidal anti-inflammatory drugs in a mouse model of Alzheimer’s disease reported a correlation between NAA levels and plaque burden (Choi et al., 2010).
The low variance in the current data afforded by the use of an ultra-high field scanner likely revealed significant MRS-histology correlations that may otherwise be obscured. Thus, the average test-retest coefficient of variation in the mouse cerebellum at 9.4 T using the current methodology is < 5% for the potential surrogate markers identified here (NAA, myo-inositol, taurine, tCr) (Öz et al., 2010b). In addition, the analysis in Figure 4 demonstrates the advantage of monitoring multiple MRS biomarkers simultaneously. Namely, 17 of 18 mice in the early stage group could be correctly assigned to the doxycycline treated vs. untreated groups by investigating the concentration change in 3 neurochemicals and assigning those mice that showed the expected response in all 3 to the treated group.
Progression of pathology and neurochemical alterations in conditional SCA1 mice
The disease progression in the conditional SCA1[82Q] mice was slower than in the non-conditional SCA1[82Q] line (Öz et al., 2010b). While the non-conditional SCA1[82Q] mice had pathological severity scores of 3 and 4 at 12–24 weeks of age (Öz et al., 2010b), the untreated conditional SCA1[82Q] mice were scored 1–2 at the same ages (except for one mouse that received a score of 3 at 24 weeks). In addition, the same neurochemicals (NAA, taurine, myo-inositol, tCr) were altered in the conditional and non-conditional SCA1 mice at a pathological severity score of 2. On the other hand, the conditional strain provided a closer look to the progression of the earliest markers of PC pathology. For example, tCr became significantly different only at 24 weeks in the conditional strain; therefore NAA, myo-inositol and taurine appear to be the earliest markers of cerebellar PC pathology in SCA1. Glutamate levels were not different in the conditional SCA1[82Q] mice, consistent with the prior observation that they became significantly different in the non-conditional SCA1[82Q] mice compared to controls only at a pathological severity score of 3 (Öz et al., 2010b). Therefore the time courses of the neurochemical changes as they relate to the pathological progression of disease were very consistent between the two mouse lines.
Among these early disease markers, NAA has been validated as a marker of neuronal dysfunction and loss (Clark, 1998). Our prior studies with the non-conditional SCA1 mice demonstrated that in this model, NAA is a marker of neuronal dysfunction and dendritic atrophy, rather than cell loss (Öz et al., 2010b). Changes in tCr likely indicate an energy imbalance which becomes prominent only at 24 weeks in the conditional SCA1 model. The other two primary markers identified in the current study, myo-inositol and taurine, have multiple functions in the cell, for example both are osmoregulators (Fisher et al., 2002, van Gelder, 1989, Wu and Prentice, 2010). The SCA1 mice demonstrate an elevation in myo-inositol and a deficit in taurine, suggesting that their abnormal levels are related to different roles of each metabolite. On the other hand, both metabolites show an increase over time with progressive neurodegeneration in the non-conditional SCA1 mice (Öz et al., 2010b), which may indeed be related to their osmoregulatory role. Similarly, myo-inositol and taurine decrease in parallel over time in WT animals (Fig. 3) (Öz et al., 2010b), which also may be related to their osmolyte role. Regarding other roles, myo-inositol is commonly considered a glial marker (Brand et al., 1993), however gliosis is not a prominent feature of the SCA1[82Q] mice (Öz et al., 2010b). Taurine, on the other hand, was demonstrated to be neuroprotective in some models of neurological insults (Chen et al., 2001, Taranukhin et al., 2010, Wu and Prentice, 2010) and appears critical for cerebellar development (Sturman et al., 1985), both of which may render a taurine deficit harmful in the SCA1 model. Also, the somata, dendrites, and dendritic spines of PCs, the primary site of pathology in the SCA1 model mice, contain the highest taurine levels in the cerebellar cortex (Ottersen, 1988), potentially explaining the taurine deficit. In contrast, some other mouse models of neurodegeneration display elevated taurine levels (Dedeoglu et al., 2004, Jenkins et al., 2000, Marjanska et al., 2005, Tkáč et al., 2007). Hence, the mechanistic reasons for altered metabolite levels may be different in each model and each neurodegenerative disease.
Effects of suppression of ataxin-1 transgene expression on neurochemistry and pathology
The molecular layer thickness and a global severity score were used in this study, rather than neuronal counting, to quantify progressive pathology because much of the pathology in these models is related to shrinkage of PCs rather than neuronal loss. Consequently, neurochemical concentrations were compared to histology scores in the same central cerebellar area based on anatomical landmarks, rather than using an exact MRS/histology co-registration approach. An exact correspondence of the two metrics was not attempted because the main goal of the study was to validate MRS as a measure of progressive disease and its reversal and not to validate MRS as an accurate measure of specific morphological changes, such as the molecular layer thickness, in SCA1. Along the same lines, only part of the MRS voxel contained pathologically affected tissue resulting in partial volume averaging relative to histology. On the other hand, the entire volume likely was biochemically abnormal. Therefore, when assessing the MRS vs. histology comparisons, it should be borne in mind that the two metrics measure different aspects of disease: MRS measures alterations in cellular physiology and biochemistry while histology measures the structural alterations (dendritic atrophy, vacuole formation, heterotopic PCs, etc.) that may occur following the biochemical changes.
In the mid-stage group, both histology and MRS measures (NAA and myo-inositol) indicated partial reversal of the PC pathology with doxycycline treatment (Figs. 3 and 5), consistent with prior observations in the conditional SCA1 mice at this disease stage (Zu et al., 2004). On the other hand, in the early stage group, the pathological measures were not significantly different between the treated and untreated groups following doxycycline administration (Fig. 5), while the MRS measures (myo-inositol, taurine and NAA-to-myo-inositol ratio) were (Fig. 3, Table 1). Therefore the MRS measures were more sensitive to the treatment effect in this early disease stage. Prior work on this model demonstrated complete reversal of pathology at early disease stage (Zu et al., 2004), however the molecular layer thickness was measured in that study in the posterior lobules of the cerebellum, which are affected earliest. Here we chose to measure the molecular layer thickness in the primary fissure (Fig. 6) because this area is included in the voxel for the MRS measurements, but the posterior lobules are not (Fig. 2A). Future magnetic resonance spectroscopic imaging (MRSI) studies may address regional differences in the level of neurodegeneration in the cerebellum in this model.
Consistent with partial reversal of early stage pathology in the posterior lobules in the current study, a trend for reversal with doxycycline was observed based on the global severity score (where the animals with the lower scores primarily had pathology in the posterior lobules, see Materials and Methods), but not based on the primary fissure molecular layer thickness. In addition, the prior study (Zu et al., 2004) showed more subtle changes in pathology in this early stage, such as proximal dendritic pruning and loss of dendritic spines, which were reversed by doxycycline. The standard H&E staining in the current study would not have revealed these subtle alterations and their reversal, but the MRS markers appear sensitive to the biochemical effects of such subtle pathology and their reversal. Lastly, a higher doxycycline dose (~530 mg/kg/day) was used in this prior study for the duration of doxycycline treatment. Therefore, the transgene expression might have been partially suppressed with the lower dose doxycycline chow (~33 mg/kg/day) used here, leading to partial reversal of pathology. The rationale in using the chow was that high dose doxycycline had to be administered in sucrose solution, the long term administration of which may cause diabetes. We have recently demonstrated that doxycycline in sucrose solution can be administered for up to 14 weeks without causing diabetes; therefore further studies will be able to test the effects of administering higher doses of doxycycline on the neurochemical markers.
The doxycycline administration did result in complete reversal of abnormal taurine levels to control values in the early stage group and completely prevented tCr levels from becoming abnormal at 24 weeks in the mid-stage group. Therefore, while NAA and myo-inositol accurately reflected the progressive neurodegenerative process, taurine and tCr likely reflected an early biochemical response to the neurodegenerative process, which may have been completely reversed by suppressing the transgene expression. Interestingly, the taurine deficit was reversed with doxycycline treatment only in early, but not mid-stage disease (only a trend for partial reversal was observed at mid-stage, Supplementary Fig. 1). This was consistent with attainment of an irreversible level of cellular dysfunction within the molecular layer, i.e. with a decrease in the ability to recover PC and motor function with progressive disease (Zu et al., 2004).
Comparison to patient data and potential clinical impact
Among the potential markers identified here, NAA, myo-inositol and their ratio will likely be the more robust surrogate markers of reversal of pathology in clinical trials because they correlate with progressive pathology (Öz et al., 2010b) and its reversal, as well as the clinical status in patients (Öz et al., 2010a). Consistently, the NAA-to-myo-inositol ratio differentiates patients with SCA1 from controls with very high specificity and sensitivity (Öz et al., 2010a). Glutamate is expected to be useful for monitoring treatment response in patients with later stage disease because its concentrations correlate with progressive pathology (Öz et al., 2010b) and clinical status (Öz et al., 2010a), but change after NAA, myo-inositol, tCr and taurine. Higher tCr levels were also observed in patients with SCA1 (Öz et al., 2010a) as in the SCA1 mice, but did not correlate with the pathological measures. Therefore the interpretation of a treatment response of tCr levels will require further investigation. Lastly, taurine may not be a very reliable marker in patients because its concentrations are much lower in humans than rodents.
From a practical perspective, the cost of MR technology as a surrogate measure relative to clinical outcome measures typically utilized in trials for neurodegenerative diseases may be considered prohibitive at first sight. However, non-invasive MR imaging markers may in fact result in savings when sample sizes needed in clinical trials are considered. For example, 260 patients are needed in each group for a 2-arm interventional trial that aims to reduce disease progression by 50% in one year in SCAs when the validated Scale for the Assessment and Rating of Ataxia (SARA) is used as the outcome measure (Schmitz-Hubsch et al., 2010). We estimate that only 20–45 patients (depending on the within-person correlation) would be needed per arm of the trial to detect a comparable change by MRS in one year with the same power as in the SARA study based on our prior data acquired in the human cerebellum at 4T (Öz et al., 2010a, Öz and Tkáč, 2011). Considering the major expenses in a trial are personnel time, coordination and patient travel (which are comparable for MR scanning and the clinical scales), this can result in a reduction of trial cost despite the need for more specialized equipment for MR scanning.
Limitations and future
A limitation of the current study was that the level of suppression of transgene expression by doxycycline was not assessed by quantifying the mRNA levels for mutant ataxin-1. Future studies will need to investigate the response of the MRS biomarkers to different levels of suppression of transgene expression. Availability of the MRS technology at other institutions and hospitals is another current limitation; however high field (3T) scanners are now available at almost all major medical schools and clinical research centers, i.e. the likely locations for SCA clinical trials. We recently demonstrated that the MRS methods utilized in our mouse model studies are also feasible at a clinical scanner in the human brain and provide extended neurochemical profiles from the cerebellum (Emir et al., 2011). Therefore, we anticipate that it will be possible to utilize the methodology validated here in clinical trials in the near future.
In conclusion, the current study demonstrated total normalization of some MRS measures and partial normalization of others in a treatment model of neurodegeneration, while changes on pathological scores remained modest. As a measure of altered cellular physiology MRS has likely advantages over structural pathological/neurodegeneration measures in its ability to identify reversibility in neurodegenerative diseases and presents great potential for use in future pre-clinical and clinical trials.
Supplementary Material
Cerebellar neurochemical profiles of the wild type (WT) (open bars), doxycycline treated SCA1[82Q] (gray bars), and untreated SCA1[82Q] mice (black bars) in the early stage (A) and mid-stage groups (B). Error bars shown are SEM. Metabolites that are significantly different (p<0.05) in treated and untreated SCA1[82Q] mice compared to the WT controls are marked with a star. This comparison was between the WT mice and the combined (treated + untreated) SCA1[82Q] mice for the 6 week data in the early stage and 12 week data in the mid-stage groups (the pooled SCA1 groups that showed significant differences compared to WT controls are shown by brackets) because no differences were found between the treated and untreated SCA1[82Q] mice prior to doxycycline treatment.
Highlights.
Neurodegeneration is reversible with doxycycline in a conditional SCA1 mouse model.
We studied this model by high field magnetic resonance spectroscopy and histology.
Abnormal neurochemical levels normalized upon doxycycline treatment.
They correlated significantly with semi-quantitative measures of pathology.
MRS biomarkers can be used to monitor reversal of neurodegeneration.
Acknowledgments
We thank the staff of the Center for MR Research for maintaining and supporting the NMR system, Orion Rainwater and Bob Ehlenfeldt for maintaining the mouse colonies and LuAnn Anderson for expert technical help with histology. This work was supported by the National Institute of Neurological Disorders and Stroke grants R21 NS060253 (G.Ö.) and R01 NS022920 (H.T.O.) and a grant from the Bob Allison Ataxia Research Center (G.Ö.). The Center for MR Research is supported by National Center for Research Resources (NCRR) biotechnology research resource grant P41 RR008079, Neuroscience Center Core Blueprint Award P30 NS057091 and the WM Keck Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allegrini PR, Sauer D. Application of magnetic resonance imaging to the measurement of neurodegeneration in rat brain: MRI data correlate strongly with histology and enzymatic analysis. Magn Reson Imaging. 1992;10:773–778. doi: 10.1016/0730-725x(92)90411-r. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, Bender J, Weindl A, Dose M, Gasser T, Klopstock T. Creatine supplementation lowers brain glutamate levels in Huntington’s disease. J Neurol. 2005;252:36–41. doi: 10.1007/s00415-005-0595-4. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ, Hsu CC, Faiman MD, Schloss JV, Wu JY. Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J Neurosci Res. 2001;66:612–619. doi: 10.1002/jnr.10027. [DOI] [PubMed] [Google Scholar]
- Choi JK, Jenkins BG, Carreras I, Kaymakcalan S, Cormier K, Kowall NW, Dedeoglu A. Anti-inflammatory treatment in AD mice protects against neuronal pathology. Exp Neurol. 2010;223:377–384. doi: 10.1016/j.expneurol.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–7395. doi: 10.1523/JNEUROSCI.17-19-07385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Choi JK, Cormier K, Kowall NW, Jenkins BG. Magnetic resonance spectroscopic analysis of Alzheimer’s disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 2004;1012:60–65. doi: 10.1016/j.brainres.2004.02.079. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- Emir UE, Auerbach EJ, Van De Moortele PF, Marjańska M, Ugurbil K, Terpstra M, Tkáč I, Öz G. Regional neurochemical profiles in the human brain measured by 1H MRS at 7 tesla using local B1 shimming. NMR Biomed. 2011 doi: 10.1002/nbm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson. 1993;102:1–8. [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002;82:736–754. doi: 10.1046/j.1471-4159.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- Jenkins BG, Klivenyi P, Kustermann E, Andreassen OA, Ferrante RJ, Rosen BR, Beal MF. Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington’s disease mice. J Neurochem. 2000;74:2108–2119. doi: 10.1046/j.1471-4159.2000.0742108.x. [DOI] [PubMed] [Google Scholar]
- Kalra S, Arnold DL. ALS surrogate markers. MRS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(Suppl 1):111–114. doi: 10.1080/17434470410019861. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Knopman DS, Dickson DW, Parisi JE, Whitwell JL, Weigand SD, Josephs KA, Boeve BF, Petersen RC, Jack CR., Jr Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248:210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanska M, Curran GL, Wengenack TM, Henry PG, Bliss RL, Poduslo JF, Jack CR, Jr, Ugurbil K, Garwood M. Monitoring disease progression in transgenic mouse models of Alzheimer’s disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2005;102:11906–11910. doi: 10.1073/pnas.0505513102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Weiner MW. Evaluation of treatment effects in Alzheimer’s and other neurodegenerative diseases by MRI and MRS. NMR Biomed. 2006;19:655–668. doi: 10.1002/nbm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae-Poetscher LM, Bonekamp D, Barker PB, Brant LJ, Kaufmann WE, Horska A. Asymmetry and gender effect in functionally lateralized cortical regions: a proton MRS imaging study. J Magn Reson Imaging. 2004;19:27–33. doi: 10.1002/jmri.10429. [DOI] [PubMed] [Google Scholar]
- Niessen HG, Debska-Vielhaber G, Sander K, Angenstein F, Ludolph AC, Hilfert L, Willker W, Leibfritz D, Heinze HJ, Kunz WS, Vielhaber S. Metabolic progression markers of neurodegeneration in the transgenic G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:1669–1677. doi: 10.1111/j.1460-9568.2007.05415.x. [DOI] [PubMed] [Google Scholar]
- Orr HT, Chung MY, Banfi S, Kwiatkowski TJ, Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LP, Zoghbi HY. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Quantitative assessment of taurine-like immunoreactivity in different cell types and processes in rat cerebellum: an electronmicroscopic study based on a postembedding immunogold labelling procedure. Anat Embryol (Berl) 1988;178:407–421. doi: 10.1007/BF00306047. [DOI] [PubMed] [Google Scholar]
- Öz G, Hutter D, Tkáč I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010a;25:1253–1261. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Nelson CD, Koski DM, Henry PG, Marjańska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010b;30:3831–3838. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn Reson Med. 2011;65:901–910. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Tkáč I, Charnas LR, Choi IY, Bjoraker KJ, Shapiro EG, Gruetter R. Assessment of adrenoleukodystrophy lesions by high field MRS in non-sedated pediatric patients. Neurology. 2005;64:434–441. doi: 10.1212/01.WNL.0000150906.52208.E7. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. LCModel & LCMgui User’s Manual. 2001. [Google Scholar]
- Schmitz-Hübsch T, Fimmers R, Rakowicz M, Rola R, Zdzienicka E, Fancellu R, Mariotti C, Linnemann C, Schöls L, Timmann D, Filla A, Salvatore E, Infante J, Giunti P, Labrum R, Kremer B, van de Warrenburg BP, Baliko L, Melegh B, Depondt C, Schulz J, du Montcel ST, Klockgether T. Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology. 2010;74:678–684. doi: 10.1212/WNL.0b013e3181d1a6c9. [DOI] [PubMed] [Google Scholar]
- Sturman JA, Moretz RC, French JH, Wisniewski HM. Postnatal taurine deficiency in the kitten results in a persistence of the cerebellar external granule cell layer: correction by taurine feeding. J Neurosci Res. 1985;13:521–528. doi: 10.1002/jnr.490130407. [DOI] [PubMed] [Google Scholar]
- Taranukhin AG, Taranukhina EY, Saransaari P, Podkletnova IM, Pelto-Huikko M, Oja SS. Neuroprotection by taurine in ethanol-induced apoptosis in the developing cerebellum. J Biomed Sci. 2010;17(Suppl 1):S12. doi: 10.1186/1423-0127-17-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkáč I, Dubinsky JM, Keene CD, Gruetter R, Low WC. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H NMR spectroscopy. J Neurochem. 2007;100:1397–1406. doi: 10.1111/j.1471-4159.2006.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkáč I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- van Gelder NM. Brain taurine content as a function of cerebral metabolic rate: osmotic regulation of glucose derived water production. Neurochem Res. 1989;14:495–497. doi: 10.1007/BF00964908. [DOI] [PubMed] [Google Scholar]
- van Vlieta SA, Blezer EL, Jongsma MJ, Vanwersch RA, Olivier B, Philippens IH. Exploring the neuroprotective effects of modafinil in a marmoset Parkinson model with immunohistochemistry, magnetic resonance imaging and spectroscopy. Brain Res. 2008;1189:219–228. doi: 10.1016/j.brainres.2007.10.059. [DOI] [PubMed] [Google Scholar]
- von Kienlin M, Kunnecke B, Metzger F, Steiner G, Richards JG, Ozmen L, Jacobsen H, Loetscher H. Altered metabolic profile in the frontal cortex of PS2APP transgenic mice, monitored throughout their life span. Neurobiol Dis. 2005;18:32–39. doi: 10.1016/j.nbd.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wu JY, Prentice H. Role of taurine in the central nervous system. J Biomed Sci. 2010;17(Suppl 1):S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–8861. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cerebellar neurochemical profiles of the wild type (WT) (open bars), doxycycline treated SCA1[82Q] (gray bars), and untreated SCA1[82Q] mice (black bars) in the early stage (A) and mid-stage groups (B). Error bars shown are SEM. Metabolites that are significantly different (p<0.05) in treated and untreated SCA1[82Q] mice compared to the WT controls are marked with a star. This comparison was between the WT mice and the combined (treated + untreated) SCA1[82Q] mice for the 6 week data in the early stage and 12 week data in the mid-stage groups (the pooled SCA1 groups that showed significant differences compared to WT controls are shown by brackets) because no differences were found between the treated and untreated SCA1[82Q] mice prior to doxycycline treatment.