Abstract
The use of a completely diverting portacaval shunt for the purpose of lowering the serum concentration of cholesterol and low-density lipoproteins is reviewed and a recommendation provided that such a shunt for hyperlipidemia should be considered for type II individuals.
It has been more than four years since completely diverting portacaval shunt was first performed clinically1 for the purpose of lowering the serum concentration of cholesterol and low-density lipoproteins (LDL). Since then, we have treated two more patients with this method. An additional nine patients who have undergone the same procedure in other centers have been documented in the literature.2–7
These 12 cases represent less than one half of the true total. Through an informal registry that has been kept at the National Institutes of Health, Bethesda, Md, it is known that more than 30 portacaval shunts have been carried out for the treatment of hyperlipidemia, with results that are in general agreement with the smaller sample that is accessible through the formal literature.
RATIONALE OF PORTAL DIVERSION
The reasons for proposing portal diversion to lower serum lipid levels were simpler than the justifications and explanations that have become possible in hindsight. At the time we saw our first patient with homozygous type II hyperlipidemia, we had been engaged in a study of children with glycogen storage disease who were treated with end-to-side portacaval shunt. Patients with type I glycogenosis (glucose-6-phosphatase deficiency) have elevated serum lipid levels of all classes. They were noted to have an immediate and permanent remission of these abnormalities after portacaval shunt.8 The pragmatic question was asked: would the same effect occur after portal diversion in patients with hyperlipidemia? Mostly on this basis, the operation was carried out. Support for the idea was provided from our own laboratory research, but the relevance of this animal work to humans, and particularly to homozygous type II hyperlipidemia, was not known, since the etiology of this autosomal dominant inherited disorder is by no means understood. All three of our patients had homozygous type II hyperlipidemia.
Goldstein and Brown9 have suggested that in type II hyperlipidemia there may be an absence of, or defect in, the cell surface receptor sites that normally bind and transport LDL cholesterol into the cell. Because cholesterol then does not enter the cell, they suggest that there is an absence of the normal feedback suppression of cholesterol synthesis.
Whatever its cause, the homozygous form of type II hyperlipoproteinemia has a shockingly poor prognosis if there is not a good response to medicinal therapy. Lipid-rich deposits are laid down in widely separated superficial and deep parts of the body, including blood vessels, where premature atherosclerosis is the consequence. Cardiac valves are similarly affected, with aortic stenosis being particularly common. The patients usually die of cardiovascular complications before the age of 20 years, despite all conventional therapy. The gravity of this situation justified the trial of a new approach, which was carried out under the appropriate conditions of informed consent.
EFFECTS OF CLINICAL PORTAL DIVERSION
Colorado Cases
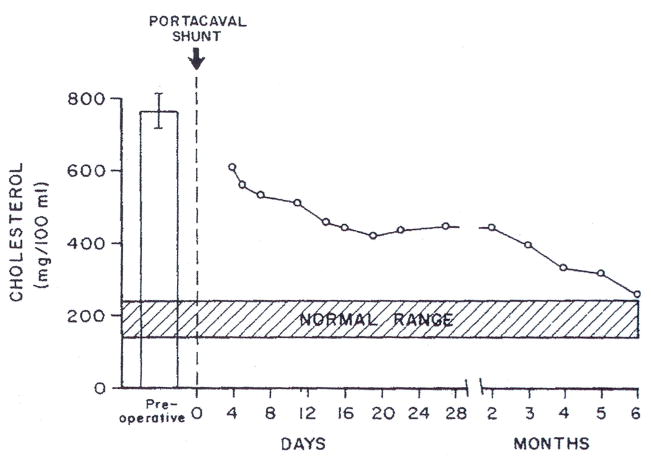
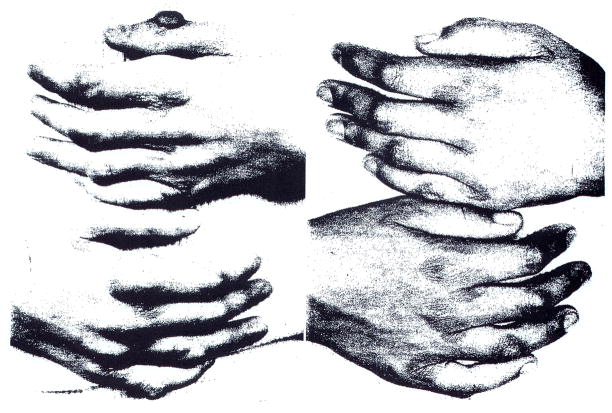
Our first patient, an 11-year-old girl, had suffered a major myocardial infarction about two months before undergoing portacaval shunt. After the portal diversion, her serum cholesterol values fell from about 800 mg/100 ml to levels that were consistently below 400 mg/100 ml (Fig 1). Unsightly xanthomas began to resorb from visible subcutaneous and tendinous locations (Fig 2). Attacks of preexisting angina pectoris became less frequent and finally stopped. By cardiac catheterization 16 months after the end-to-side portacaval shunt was performed, there was good evidence that reversal of aortic stenosis had occurred, with a diminution of the aortic valve gradient from 56 to 10 mm Hg.10 The coronary arteries were also thought to be less diseased than before, although three stenoses were still present. More than 1½ years after portacaval shunt, the girl died suddenly while returning home from school.
Fig 1.
Serum cholesterol concentrations after portacaval shunt in patient 1.
Fig 2.
Hands of patient 1 two weeks before (left) and 16 months after (right) portacaval shunt.
At autopsy, the residual coronary artery disease was confirmed. There was a large ventricular aneurysm. The conclusion was that death probably was caused by an acute cardiac arrhythmia.11 The patient should have undergone a coronary revascularization procedure, as was done in case 2. The decision against this was predicated on the hope that the coronary artery stenoses would spontaneously regress.
The portacaval shunt was widely patent. The liver, which weighed 618 gm, was grossly normal, and microscopically it was unchanged from the biopsy specimen that was obtained six months postoperatively. On light and electron microscopy, the most prominent findings were shrinkage of the hepatocyte size, depletion of rough endoplasmic reticulum, and the accumulation of intracytoplasmic lipid deposits. These changes are typical of those caused in the liver by portacaval shunt in all species studied so far.12 Hepatic function had not been changed by portacaval shunt as judged by standard liver function tests.
We have performed a portacaval shunt on two more patients with the same diagnosis (cases 2 and 3, Table). Patient 2 was 7 years old at the time of operation. She had preoperative serum cholesterol values that averaged 997 ± 47 (SD) mg/100 ml while on a very low cholesterol diet. Six months after the shunt, the cholesterol level measured in the same laboratory was 600 mg/100 ml (a 40% reduction), despite a relaxation of the diet. These values have continued to fall under careful medical management at Southwestern University Medical Center, Dallas. The cholesterol concentrations during the last year have been 450 ± 67 mg/100 ml. The xanthomas on her heels, elbows, and hands either have resorted completely or have greatly diminished in size.
Table 1.
Patients With Type II Hyperlipidemia Treated at the University of Colorado, Denver
| Patient/Age, yr | Date of Operation | Preshunt, mg/100 ml | Serum Cholesterol | Clinical Result | Comments | |
|---|---|---|---|---|---|---|
| Postshunt, mg/100 ml | Final Reduction, % | |||||
| 1/12 | 3/1/73 | 769 | 290 | 62 | Died 18½ mo postoperatively of cardiac arrest | Old myocardial infarct with ventricular aneurysm; residual stenoses of coronary arteries; xanthomas had disappeared. |
| 2/7 | 10/4/74 | 997 | 450 | 55 | Good | Underwent aortic & mitral valve replacement plus double coronary artery bypass 25 months after shunt; xanthomas nearly completely disappeared. |
| 3/8 | 8/5/75 | 1000 | 620 | 38 | Good | Xanthomas slowly disappearing |
This child also had aortic stenosis and angina pectoris. Because of persistence of her cardiac disease, she was treated at the Fort Worth (Tex) Children’s Hospital with aortic and mitral valve replacement plus double coronary artery bypass two years after portacaval shunt. The patient has returned to school with essentially unlimited activities. According to her physician, results of the operation were as follows:
Our therapeutic approach continues to be one of trying to get [her] cholesterol as low as possible with medication. I am totally convinced that the portacaval shunt was tremendously helpful to her. I doubt that we would have proceeded with the cardiac surgery if her cholesterol were still in the 900 to 1,000 mg% range. Her present cardiac difficulties are an extension of those already-present before the shunt surgery. Our main job now is to try and retard the development of atherosclerosis in her coronary bypass grafts (D. Bilheimer, MD, written communication, May 1977).
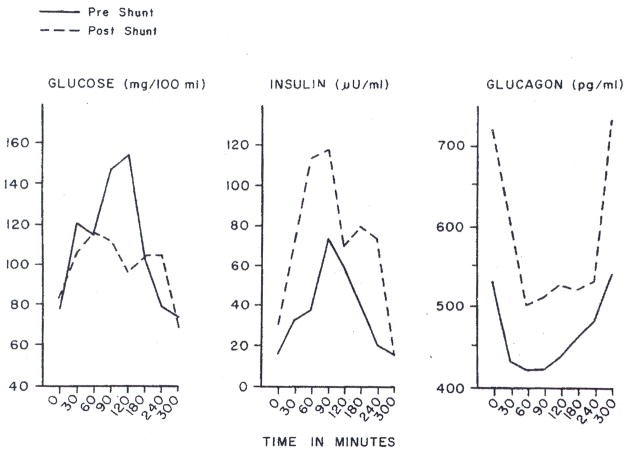
Our third patient has had a reduction in the serum cholesterol level from 1,000 to 600 to 650 mg/100 ml in the 21 months postoperatively (Table). Although the falls in cholesterol level were less striking than in our first two patients, visible xanthomas are slowly resorbing and the patient is asymptomatic almost two years postoperatively. Changes in peripheral insulin and glucagon content in these patients have been noted. Preoperative values were normal. Postoperatively, systemic venous insulin and glucagon values were both increased, especially the former (Fig 3).
Fig 3.
Systemic venous plasma glucose, insulin, and glucagon concentrations in patient 2 with hyperlipidemia, before and after portacaval shunt.
None of our three patients with hyperlipidemia has had any signs of hepatic encephalopathy. Nevertheless, the use of portacaval shunt to ameliorate hyperlipidemia accepts a trade-off of suboptimal conditions of liver perfusion in return for metabolic improvements that are derived from these suboptimal conditions. Realization of this fact has prompted us to recommend portacaval shunt for this inborn error only if there was a homozygous state. However, the freedom from encephalopathy in our patients suggests that symptomatic heterozygous patients who are refractory to medical therapy should also be considered for portal diversion. The patient of Weglicki et al7 probably had the heterozygous trait.
Other Reports
In the nine cases reported in the literature by others,2,7 the serum cholesterol level lowerings have been variable from patient to patient, as in our three cases. A significant fall was usually obtained if the shunt was patent. Cardiac symptoms were often relieved and resorption of xanthomas proceeded even if the falls in serum cholesterol concentration were not great. The latter finding has raised the possibility that cholesterol depots may keep the serum cholesterol concentrations high until the resorption is nearly complete. Failure to observe an antilipidemic effect has been associated with thrombosis of the shunt.5,6
Weglicki et al7 have used portacaval shunt and coronary artery bypass together in a young patient suffering from premature atherosclerosis. This kind of combined therapy will probably be used more frequently in the future, as was illustrated also by the treatment of our patient 2.
MECHANISM OF PORTAL DIVERSION EFFECT
The antilipidemic effect of portacaval shunt can be demonstrated with a normal starting point of serum cholesterol concentration just as easily as with a pathologically high concentration. This was first observed by Winter et al13 in normal dogs and confirmed in that species by Starzl et al,14 Coyle et al,15 Horak et al,16 and Guzman et al.17 The same has been seen in rats,18,19 pigs,20,21 and baboons.12
Studies in the dog14 and investigations in the rat,18,22 pig,20,21 and human23 have shown or suggested that a reduction in hepatic cholesterol or LDL synthesis or both is responsible at least in part for the falls in cholesterol levels. Only the reports of Coyle et al15 and Guzman et al17 in dogs have denied this. Other factors may contribute to the antilipidemic effect of portal diversion, as Ahrens24 and others14 have speculated.
Our experiments in dogs have suggested that the main reason for decreased hepatic cholesterol synthesis is deprivation of the liver of insulin.14 One consequence is a moderately severe liver injury of the kind described earlier in the autopsy findings of patient 2. Eaton25 has recently proposed that the changes in glucagon metabolism may be important in the antilipidemic effect of portal diversion. Further clarification of these mechanisms will be important if treatment alternatives to portacaval shunt are to be evolved.
Acknowledgments
This work was supported by research grants MRIS 8818-01 and 7227-01 from the Veterans Administration, by grants AM-17260 and AM-07772 from the National Institutes of Health, and by grants RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health. David Bilheimer MD, of Southwestern University Medical Center, Dallas referred patient 2 and subsequently cared for her. Robert McGehee, MD. of Fort Worth (Tex) Children’s Hospital carried out the cardiac operations on patient 2.
References
- 1.Starzl TE, Putnam CW, Chase HP, et al. Portacaval shunt in hyperlipoproteinaemia. Lancet. 1973;2:940–944. doi: 10.1016/s0140-6736(73)92599-3. [DOI] [PubMed] [Google Scholar]
- 2.Krogh L, Wickens JT. Portacaval shunt for hypercholesterolaemia. South Afr Med J. 1974;48:2302. [PubMed] [Google Scholar]
- 3.Stein EA, Mieny C, Spitz L, et al. Portacaval shunt in four patients with homozygous hypercholesterolemia. Lancet. 1975;1:832–835. doi: 10.1016/s0140-6736(75)93005-6. [DOI] [PubMed] [Google Scholar]
- 4.Russell D, Heimann KW, Levin SE, et al. Portacaval shunt for homozygous hypercholesterolemia. Lancet. 1976;2:1205. doi: 10.1016/s0140-6736(76)91726-8. [DOI] [PubMed] [Google Scholar]
- 5.Cywes S, Davies MRQ, Louw JH, et al. Portacaval shunt in two patients with homozygous type II hyperlipoproteinaemia. South Afr Med J. 1976;50:239–242. [PubMed] [Google Scholar]
- 6.Farriaux JP, Ribet M, Lebecq MF, et al. Traitement de I’hypercholestérolémie familiale de type II par anastomose portocave. Nouv Presse Med. 1976;5(34):2219–2221. [PubMed] [Google Scholar]
- 7.Weglicki WB, Ganda OP, Soeldner JS, et al. Portacaval diversion for severe hypercholesterolemia. Arch Surg. 1977;112:634–636. doi: 10.1001/archsurg.1977.01370050094016. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Putnam CW, Porter KA, et al. Portal diversion for the treatment of glycogen storage disease in humans. Ann Surg. 1973;178:525–539. doi: 10.1097/00000658-197310000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein JL, Brown MS. Familial hypercholesterolemia: Identification of a defect in the regulation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sri USA. 1973;70:2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Chase HP, Putnam CW, et al. Follow-up of patient with portacaval shunt for the treatment of hyperlipidemia. Lancet. 1974;2:714–715. doi: 10.1016/s0140-6736(74)93285-1. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Chase HP, Putnam CW, et al. Portacaval shunt in hyperlipidemia. Lancet. 1974;2:1263. doi: 10.1016/s0140-6736(74)90774-0. [DOI] [PubMed] [Google Scholar]
- 12.Putnam CW, Porter KA, Starzl TE. Hepatic encephalopathy and light and electron micrographic changes of the baboon liver after portal diversion. Ann Surg. 1976;184:155–161. doi: 10.1097/00000658-197608000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter IC, van Dolah HE, Crandell LA. Lowered serum lipid levels in the Eck fistula dog. Am J Physiol. 1941;133:566–571. [Google Scholar]
- 14.Starzl TE, Lee IY, Porter KA, et al. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg Gynecol Obstet. 1975;140:381–396. [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle JJ, Schwartz MZ, Marubbio AT, et al. The effect of portacaval shunt on plasma lipids and tissue cholesterol synthesis in the dog. Surgery. 1976;80:54–60. [PubMed] [Google Scholar]
- 16.Horak W, Gangl A, Funovics J, et al. Effect of portacaval shunt and arterialization of the liver on bile acid metabolism. Gastroenterology. 1975;69:338–341. [PubMed] [Google Scholar]
- 17.Guzman IJ, Coyle JJ, Schneider PD, et al. The effect of selective visceral caval shunt on plasma lipids and cholesterol dynamics. Surgery. to be published. [PubMed] [Google Scholar]
- 18.Edwards KDG, Herz R, Sealey JE, et al. Lowering of blood pressure, plasma renin substrate, cholesterol and triglyceride by portacaval anastomosis in rats fed on a 60% sucrose/5% lard diet. Clin Sci Mol Med. 1976;51:1455–1465. doi: 10.1042/cs051145s. [DOI] [PubMed] [Google Scholar]
- 19.Magide AA, Press CM, Myant NB, et al. The effect of portacaval anastomosis on plasma lipoprotein metabolism in rats. Biochim Biophys Acta. 1976;441:302–307. doi: 10.1016/0005-2760(76)90173-9. [DOI] [PubMed] [Google Scholar]
- 20.Chase HP, Morris T. Cholesterol metabolism following portacaval shunt in the pig. Atherosclerosis. 1976;24:141–148. doi: 10.1016/0021-9150(76)90071-x. [DOI] [PubMed] [Google Scholar]
- 21.Carew TE, Saik RP, Johansen KH, et al. Low density and high density lipoprotein turnover following portacaval shunt in swine. J Lipid Ret. 1976;17:441–450. [PubMed] [Google Scholar]
- 22.James J, Soeters PB, Fischer JE. In vivo demonstration of impaired cholesterol and fatty acid synthesis following portacaval shunt (PCS), abstracted. Gastroenterology. 1977;72:1075. [PubMed] [Google Scholar]
- 23.Bilheimer DW, Goldstein JL, Grundy SM, et al. Reduction in cholesterol and low density lipoprotein synthesis in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975;56:1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahrens EJ., Jr Homozygous hypercholesteraemia and the portacaval shunt: The need for a concerted attack by surgeons and clinical researchers. Lancet. 1974;2:449–451. doi: 10.1016/s0140-6736(74)91828-5. [DOI] [PubMed] [Google Scholar]
- 25.Eaton RP. Glucagon and lipoprotein. Metabolism. 1976;25:1415–1418. doi: 10.1016/s0026-0495(76)80154-0. [DOI] [PubMed] [Google Scholar]