Abstract
E2Fs are a family of transcription factors that regulate proliferation, differentiation and apoptosis in many cell types. E2F-1 is the prototypical E2F and the family member that has most often been implicated in also mediating apoptosis. To better understand the role of E2F-1 in mediating cardiomyocyte injury we initially analyzed E2F family member expression after ischemia/reperfusion (I/R) in vivo or simulated ischemia in vitro. I/R injury in vivo caused a 3.4-fold increase specifically in E2F-1 protein levels. Expression of other E2F family members did not change. To establish the role of E2F-1 in I/R we examined the response of germline deleted E2F-1 mice to I/R injury. Infarct size as a percentage of the area at risk was decreased 39.8% in E2F-1−/− mice compared to E2F-1+/+ controls. Interestingly, expression of classic, E2F-1 apoptotic target genes was not altered in E2F-1 null cardiomyocytes after I/R. However, upregulation of the primary member of the Forkhead family of transcription factors, FoxO-1a, was attenuated. Consistent, with a role for FoxO-1a as an important target of E2F-1 in I/R, a number of proapoptotic FoxO-1a target genes were also altered. These results suggest that E2F-1 and FoxO-1a belong to a complex transcriptional network that may modulate myocardial cell death during I/R injury.
INTRODUCTION
Cell cycle progression involves the tightly regulated transduction of mitogenic signals to cyclically expressed proteins known as cyclins to their catalytically active targets, the cyclin-dependent protein kinases (CDKs) [1,2]. Cell cycle proteins are re-expressed in dying cardiomyocytes in the adult heart particularly after ischemic injury [3] and have been implicated in regulating apoptotic signaling pathways initiated by hypoxia or ischemia [4,5]. We have previously demonstrated that genetic or pharmacological inhibition of CDK-2 activity during ischemia/reperfusion (I/R) injury not only resulted in a reduction in infarct size but also in a decrease in apoptotic myocytes [6]. The mechanisms by which cell cycle proteins lead to cell death and apoptosis specifically are just beginning to be characterized; however, E2F-1 is thought to be a key regulator. Hypoxia-inducedCDK-2 activity in cardiomyocytes has been reported to promote apoptosis through induction of E2F transcription factors [4]. To date, eight E2F family members have been identified and classified as either activating or repressing transcription [7]. E2Fs 1-3 activate the transcription of genes that promote progression through the cell cycle [8]. In contrast, E2Fs 4-6 are considered repressive proteins as their expression in G0/G1 cells is elevated and they actively inhibit E2F-responsive genes [8]. Although enhanced E2F-1 activity usually leads to proliferation, ectopic E2F-1 expression can also induce apoptosis in specific situations [9,10] and E2F-1 is the primary family member associated with programmed cell death [11]. Consistent with this, overexpression of E2F-1 in adult cardiac myocytes induces apoptosis [12], which has been linked to Bnip3 induction and activation of the intrinsic apoptosis pathway [13]. Overexpression of E2F-3 has been shown to induce apoptosis in cultured cardiac myocytes but whether it plays this role under more physiologically relevant conditions in vivo is unclear [14]. Further, it has been reported in nonmyocytes that E2F-3 induced apoptosis is associated with induction of E2F-1 and that the E2F-3-induced apoptosis is dependent on this E2F1 expression [15].
To better understand the role of E2F-1 in mediating cardiomyocyte death and to identify possible end effector molecules that facilitate E2F-1’s pro-apoptotic effect, we examined mice deficient for E2F-1 expression [16]. We demonstrate that I/R upregulates expression of E2F-1 both in vitro and in vivo. E2F-1−/− mice are phenotypically and histologically normal at baseline, but exhibit reduced infarct size in response to I/R injury. Surprisingly, neither expression of previously identified E2F-1 apoptotic gene targets nor Bnip3 were altered in E2F-1 null hearts acutely by I/R. However, we identified the noncanonical E2F-1 target, FoxO-1a, as a potential end effector in cardiomyocytes. FoxO family members are pleiotropic transcription factors that have been implicated in a number of cellular processes including differentiation, metabolism, DNA repair and apoptosis [17]. Interestingly, FoxO1 has previously been implicated in regulating apoptosis in cardiomyocytes [18]. More recently, investigators have shown the direct regulation of FoxO-1a by E2F-1 via ChIP analyses [19]. Taken together, we propose that E2F-1 and FoxO-1a regulate a complex transcription network, the output of which may have critical roles in mediating myocardial cell death during I/R injury.
MATERIALS AND METHODS
Animal studies
E2F-1 wildtype (E2F-1+/+) and null (E2F-1−/−) mice have been described previously [16] and were obtained from Jackson Laboratories. Mice were subjected to I/R injury as previously described [6]. Following a 30 minute (m) coronary artery occlusion, hearts were collected after 30m, 4hours (h), or 24h of reperfusion. Sham animals were subjected to the same procedure except the LAD was not occluded. Additional details regarding the I/R procedure may be found in Supplemental Data.
Infarct size assessment
Infarct size was calculated as percentage of myocardial infarction compared to the area at risk (AAR). The AAR was determined by perfusion of Evan’s blue dye (1% in PBS) into the aorta while the LAD was occluded at the site of the previous occlusion. Thus, all myocardial tissue was stained blue, except the AAR. The hearts were then cut into four to five transverse slices which were incubated with 1% TTC (in PBS) at 37°C for 25 min. The infarcted area is demarcated as a white area, while viable tissue stains red. The stained slices were fixed with 10% formaldehyde over night, and then were weighed and photographed under a microscope. The AAR and the infarct size were determined via planimetry using the NIH software Image J, and the degree of myocardial damage was calculated as percent of infarcted myocardium from the AAR [6].
Cardiomyocytes and histological analyses
Neonatal rat ventricular (NRVM) and adult mouse (ACM) cardiomyocytes were harvested as previously described [20-23]. Cardiomyocytes were treated where stated with either an adenovirus vector expressing GFP (control), LacZ (control), FoXO-1a (kindly provided by Dr. T.G. Unterman), or E2F-1 [12] at a multiplicity of infection of 50-100 (Ad-GFP, -LacZ, -FoXO-1a) and 10 (Ad-E2F-1) pfu/cell. For ischemia studies, 48h following viral infection, NRVMs or ACMs were placed in ischemic media (IM:106 mM NaCl, 4.4 mM KCl, 1.0 mM MgCl2, 38 mM NaHCO3, 2.5 mM CaCl2, 20 mM 2-deoxyglucose, and 1.0 mM NaCN, pH 6.6) for 45m, followed by 4h of reperfusion in serum starvation media (NRVM: 1:1 DMEM:199, 1.0% ITS (Insulin-Transferrin-Selenium, Sigma), 0.1% CD Lipid Concentrate, 1% Pen/Strep/L-Glutamine (PSG); ACM: MEM supplemented with 1.0% ITS, 1% PSG, 4mM NaHCO3, 10mM HEPES, 0.2% BSA, 25μM blebbistatin).
Cells were either collected and used for RNA and protein analyses or immediately fixed in ice-cold methanol and evidence of apoptosis was assessed by detection of nuclear DNA fragmentation by terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling [20] (TUNEL, Chemicon). Additional information regarding in vitro procedures may be found in Supplemental Data.
RNA and Protein Analysis
Total RNA was extracted (Trizol Reagent, Sigma) as per manufacturer’s instructions. Real-time quantitative PCR was conducted using the ABI PRISM 7700 Sequence Detection System; Taqman (Applied Biosystems, Foster City, CA) as previously described [24]. For semi-quantitative PCR analyses, all primers were cycled appropriately and resolved on 1% agarose gels. Primers sequences for all genes analyzed in the present study are available upon request.
Total and nuclear protein was extracted and Western blotting performed as previously described [21]. Cardiac mitochondria were isolated as discussed earlier and protein isolated by incubating mitochondria in lysis buffer (25 mM Tris-HCl pH 8, 250 mM NaCl, 0.5% Igepal CA630, 1 mM Na3VO4, 1mM NaF, 1mM PMSF, protein inhibitor pellet (Roche)). Protein expression was visualized using horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescence reagents (Amersham Biosciences, Sunnyvale, California). A list of all antibodies used may be found in Supplemental Data.
siRNA studies
NRVMs and ACMs were transfected with 125nM of FoxO-1a, E2F-1 or non-specific (NS) siRNA (rat and mouse where appropriate, Qiagen) with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s specifications. 48h after transfection, cells were either immediately harvested for molecular analyses, infected with virus where indicated or exposed to IM as described above. Additional information regarding siRNA methodology may be found in Supplemental Data.
Statistical Analyses
All data are presented as mean ± SEM. Results were compared by Student’s t-test or one-way analysis of variance and Tukey’s multiple comparison post-tests as indicated, using significance at a P value <0.05.
RESULTS
E2F-1 expression is increased in response to myocardial ischemia/reperfusion injury
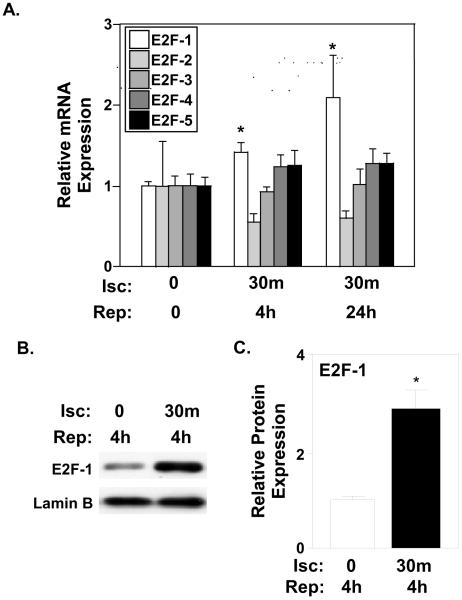
Minimal in vivo data exists characterizing the expression patterns of E2F-1 and E2F family members in myocardial tissue in response to an ischemic insult. To resolve this deficiency, we determined mRNA expression levels of E2F family members during I/R injury by real-time PCR analyses. E2F-1 mRNA increased 1.4- and 2.1-fold at 4h and 24h of reperfusion respectively compared to sham-operated hearts (Fig. 1A; P<0.05). E2F-2, -3, -4, and -5 mRNA was detectable in the mouse adult heart but no significant changes were noted in response to I/R. A corresponding 3-fold increase in E2F-1 protein was observed in wildtype mice after I/R. (Fig. 1C and B; P<0.002).
Figure 1. E2F-1 expression is increased in response to myocardial I/R injury.
A, Quantification via real-time PCR analyses revealed that myocardial E2F-1 mRNA was increased after 4h and 24h of reperfusion (n=4 per group), *P<0.05. No significant changes were noted in E2F-2, -3, -4, or -5 mRNA expression in response to I/R. B, Western blotting on nuclear ventricular lysates after 30 min ischemia and 4 hours of reperfusion in wildtype mice. C, Protein quantification revealed an increase in E2F-1 protein expression in wildtype hearts after reperfusion. Lamin B was used to normalize nuclear protein levels (n=4 per group), *P<0.002 for wildtype mice after 4h reperfusion compared to Sham-operated mice.
Ischemia/reperfusion injury is attenuated in E2F-1 null hearts
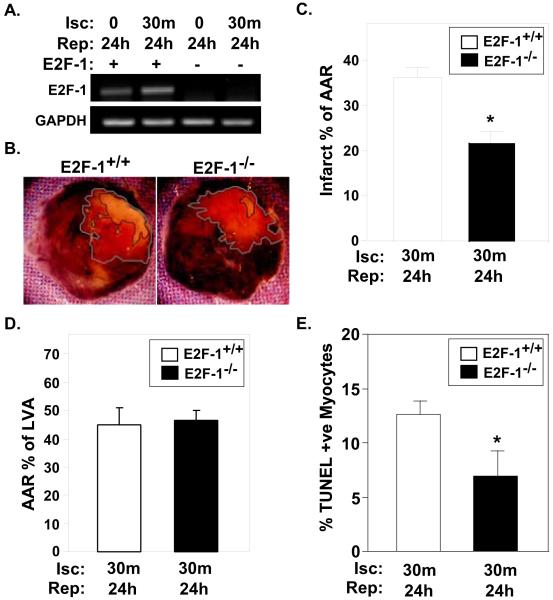
We have previously established that Rb, the negative regulator of E2F-1, is phosphorylated and inactivated in response to I/R injury [6]. This finding, taken with E2F-1’s increased expression would suggest that E2F-1 activity is significantly increased after I/R. To better understand the precise role of E2F-1 and to determine it’s involvement in myocardial damage after I/R, we subjected E2F-1+/+ and E2F-1−/− mice to I/R injury. E2F-1 null mice are viable and reproduce normally. Although adult E2F-1−/− mice have excess mature T cells due to a maturation stage-specific defect in thymocyte apoptosis [16], their hearts appeared histologically normal and cardiac function was indistinguishable from wildtype mice (Suppl. Fig. S1). As expected, E2F-1 mRNA was elevated in wildtype hearts after I/R but not detectable in E2F-1−/− animals (Fig. 2A). Infarct size (IFS, marked by black line) as a percentage of the area at risk (AAR, marked by grey line) was decreased by 39.8% in E2F-1−/− mice compared to E2F-1+/+ controls subjected to I/R (Fig. 2B and C; 21.9±2.6% E2F-1−/− vs. 36.4±2.2% E2F-1+/+, P<0.001), suggesting that E2F-1 exacerbates myocyte death after I/R. The difference in IFS between genotypes cannot be contributed to differences in vascularity, as we noted no difference in AAR with respect to total left ventricular area between E2F-1+/+ and E2F-1−/− mice (Fig. 2D). Consistent with E2F-1’s postulated role in apoptosis [9,10,11,16], the percentage of TUNEL positive nuclei in the border zone was 1.8-fold higher in E2F1+/+ ventricles compared to E2F-1−/− hearts after I/R injury (Fig. 2E; 12.65±1.23 vs. 6.98±2.30%, P< 0.05).
Figure 2. E2F-1 deficiency confers cardioprotection in response to I/R injury.
A, Representative E2F-1 mRNA expression obtained from semi-quantitative PCR analysis. As previously shown, E2F-1 mRNA expression is increased in wildtype mice after I/R injury. No E2F-1 mRNA expression was detected in E2F-null animals. E2F-1+/+ and E2F-1−/− mice were subjected to 30m coronary occlusion followed by 24h of reperfusion. B, Representative TTC-stained hearts from E2F-1+/+ and E2F-1−/− hearts after I/R injury are shown. C and D, A significant decrease in IFS was noted in E2F-null animals compared to wildtype controls (n=7 per group), P<0.001. No difference in the total area at risk with respect to total left ventricular area was seen between E2F-1+/+ and E2F-1−/− mice (D). E, The percentage of TUNEL positive nuclei were quantified on myocardial sections from the indicated genotypes and treatments. *P<0.05 E2F-1+/+ versus E2F-1−/− after I/R injury, (n=4 per group).
Expression of classic E2F-1 target genes are only modestly regulated after I/R injury
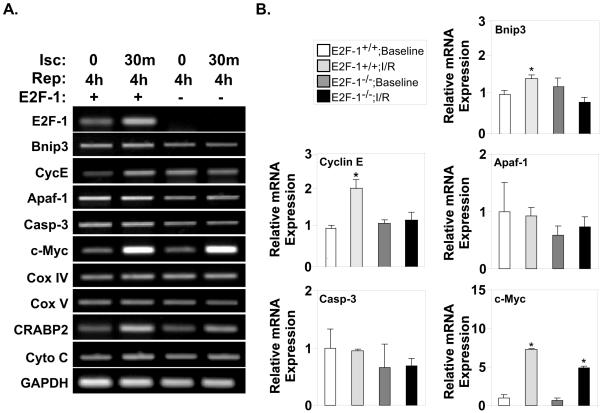
To better understand the downstream effectors that could mediate E2F-1’s effects on cell death, we examined a panel of known E2F-1 target genes in E2F-1+/+ and E2F-1−/− mice after I/R injury. There were relatively minor differences in expression of classic E2F-1 target genes between genotypes (Fig. 3A). Interestingly, Bnip3 mRNA, which has been reported to mediate E2F-1’s proapoptotic effects in neonatal cardiomyocytes [13,25] was only increased 1.4-fold at 4h after I/R (Fig. 3B; 1.00±0.12 vs. 1.4±0.14, P<0.05). Similarly, Bnip3 protein levels were minimally altered in E2F-1+/+ and E2F-1−/− mice after I/R injury (Suppl. Fig. S2). These results were confirmed in vitro on cultured cardiomyocytes (Suppl. Fig. S2). This result is consistent with reports that Bnip3 is expressed at high levels in adult myocardium [25] and only becomes significantly upregulated at late time points after I/R [26]. Thus, while Bnip3 may play a critical role in the apoptotic response, given the kinetics of its upregulation it is unlikely to account for E2F-1’s effects acutely. Cyclin E (CycE) mRNA levels were also increased 1.8-fold after I/R (Fig. 3B; 1.00±0.05 vs. 1.82±0.22, P<0.05) suggesting a positive feedback loop may exist where increases in CDK-2 inactivate Rb, further enhancing E2F-1 activity. There were no significant differences in reported E2F-1 apoptotic target genes caspase-3, APAF-1 or c-Myc.
Figure 3. Expression of classic E2F-1 target genes are not significantly regulated in myocardium after I/R injury.
A, Semi-quantitative PCR analyses of E2F-1-target genes was examined in E2F-1+/+ and E2F-1−/− mice after I/R injury. B, Quantification via real-time PCR analyses revealed Bnip3 and Cyclin E (CycE) mRNA levels were increased 1.4- and 1.8-fold respectively in wildtype myocardium after I/R but not in E2F-1-null animals (n=4 per group), P<0.05 and P<0.01, respectively. c-Myc mRNA level was increased dramatically in both wildtype and null myocardium after I/R injury, *P<0.01. No significant changes were noted in apoptotic peptidase activating factor -1 (Apaf-1), Caspase-3 (Casp-3) mRNA expression.
FoxO-1a is increased with I/R injury and regulated by E2F-1
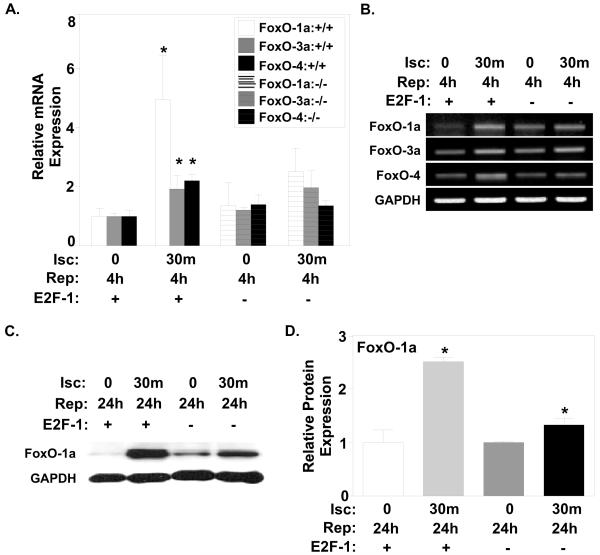
Since classic E2F-1 target genes were minimally regulated in our model of I/R, we sought to identify additional downstream effectors that might mediate E2F-1 effect on myocyte cell death. Since E2F-1 has been reported to upregulate FoxO-1a directly [19] and modulate its activity indirectly through activation of CDK-2 [27], we examined the expression of FoxO family members in both E2F-1+/+ and E2F-1−/− mice after I/R injury (Fig. 4A and B). Although both FoxO-3a and FoxO-4 mRNA levels were elevated ~2-fold in wildtype myocardium after I/R injury, FoxO-1a mRNA levels displayed the greatest increase after I/R (Fig. 4A; 1.00±0.26 vs. 4.96±1.49, P<0.05). This increase in FoxO-1a mRNA was not seen in E2F-null mice after I/R (Fig. 4A and B). The 2.5-fold increase in FoxO-1a protein expression in wildtype myocardium after 24h of reperfusion was attenuated in E2F-1−/− hearts (Fig. 4C and D; 1.00±0.02 vs. 1.33±0.13, P<0.05).
Figure 4. FoxO-1a is increased with I/R injury and regulated by E2F-1.
A, Quantification via real-time PCR analysis revealed all FoxO family members are increased after I/R. FoxO-1a mRNA expression displayed the largest increase after I/R. (n=4 per group), *P<0.05. The increase in all FoxO family members was abrogated in E2F-null mice after I/R. B, Representative FoxO-1a, -3, and -4, mRNA expression obtained from semi-quantitative PCR analyses. C, Western blot analysis of total FoxO-1a protein expression in E2F-1+/+ and E2F-1−/− myocardium after 24h of reperfusion. D, Protein quantification revealed an increase in FoxO-1a protein expression in both E2F-1+/+ and E2F-1−/− mice (n=3 per group), P<0.01 and P<0.05, respectively. GAPDH was used to normalize total protein levels.
FoxO-1a apoptotic targets are regulated in E2F-1+/+ and E2F-1−/− myocardium after I/R injury
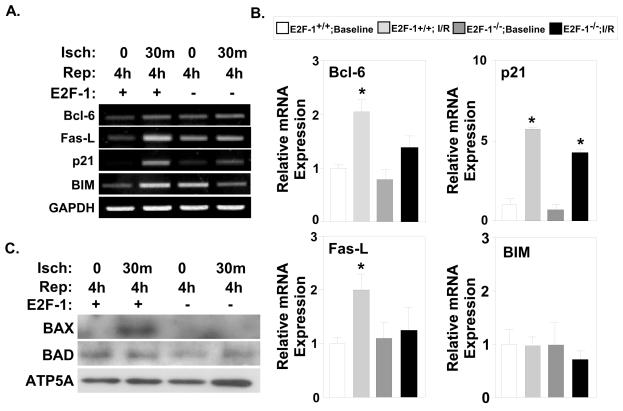
FoxO-1a is known to induce the expression of several genes involved in initiating apoptotic pathways [27,28]. Specifically, FoxO-1a is able to induce the expression of death receptor ligands, including the pro-apoptotic genes Fas ligand (FasL) and Bcl-6 which inhibits Bcl-2 family members [29,30]. In order to better understand the relationship between E2F-1 and FoxO-1a during I/R injury, we examined the expression of known FoxO-1a target genes in both E2F-1+/+ and E2F-1−/− mice (Fig. 5A). FasL and Bcl-6 mRNA were increased in wildtype myocardium after I/R injury (Fig. 5B; FasL 1.00±0.11 vs. 2.00±0.28, P<0.008; Bcl-6 1.00±0.06 vs. 2.05±0.22, P<0.002). In contrast, no increase in expression of Bcl-6 or FasL was noted in E2F-1 null myocardium after I/R. While FasL contributes to the extrinsic apoptotic pathway, Bcl-6 regulates genes in the intrinsic pathway, which effect mitochondrial translocation of pro-apoptotic factors. We examined mitochondrial levels of BAX and BAD in mitochondria isolated from E2F-1+/+ and E2F-1−/− hearts. Mitochondrial BAD protein levels were not significantly altered in both genotypes after I/R (Fig. 5C). However, while mitochondrial BAX was elevated in wildtype mice after I/R it was not detected in E2F-1−/− animals (Fig. 5C).
Figure 5. FoxO-1a gene targets are differentially regulated in E2F-1+/+ and E2F-1−/− myocardium after I/R injury.
A, Semi-quantitative PCR analyses of FoxO-1a target genes was examined in E2F-1+/+ and E2F-1−/− mice after I/R injury. B, Quantification via real-time PCR analyses revealed Bcl-6 and FasL mRNA levels were significantly increased in wildtype myocardium after I/R, but not in E2F-1-null animals, *P<0.005 and P<0.01, respectively. p21 mRNA level was increased dramatically in both wildtype and null myocardium after I/R injury, *P<0.01 (n=4 per group). C, Western blot analyses of BAX and BAD expression in cardiac mitochondrial protein extracts. ATP5A was used as a loading control. Fas ligand (FasL), Bcl-2-interacting mediator (BIM), Bcl-2 antagonist of cell death (BAD), Bcl-2-associated X protein (BAX).
FoxO-1a is a critical regulator of cardiomyocyte apoptosis in vitro
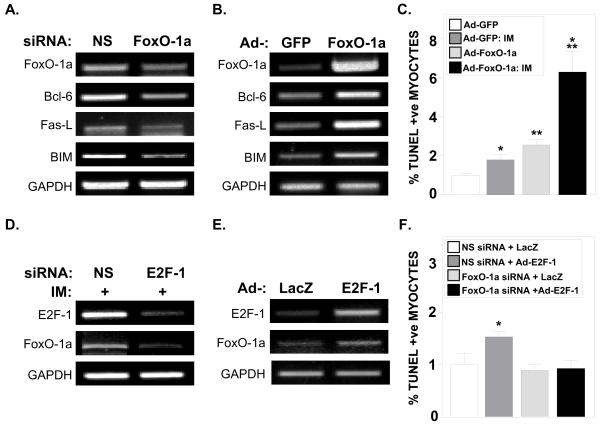
Given that the E2F-null mice are a germline deletion, non-cardiomyocytes could possibly be contributing to the gene changes we observed. Thus to confirm our in vivo findings, we examined the roles of E2F-1 and FoxO-1a in mediating apoptosis in both purified primary adult mouse cardiomyocytes (ACM) as well as neonatal rat ventricular myocytes (NRVM). After exposing ACMs to simulated ischemia media for 30m, followed by 4h of reperfusion, we noted an increase in mRNA expression of both E2F-1 and FoxO-1a (Suppl. Fig. S3). To confirm that FoxO-1a is able to directly activate FasL, Bcl-6 and BIM, we transfected ACMs with FoxO-1a siRNA. Silencing FoxO-1a led to a decrease in the expression of these three genes (Fig. 6A). Conversely, forced expression of FoxO-1a increased the expression of Bcl-6, FasL and BIM (Fig. 6B).
Figure 6. FoxO-1a is a critical regulator of cardiomyocyte apoptosis in vitro.
A, Semi-quantitative PCR analyses of Bcl-6, FasL and BIM mRNA in ACMs transfected with NS or FoxO-1a siRNA. B, Semi-quantitative PCR analyses of Bcl-6, FasL, and BIM mRNA in adult cardiomyocytes (ACMs) infected with control (Ad-GFP) or Ad-FoxO-1a virus. C, Neonatal rat ventricular myocytes (NRVMs) infected with Ad-GFP or Ad-FoxO-1a virus and exposed to ischemia media (IM). TUNEL positive nuclei were quantified after indicated treatments. *P<0.005 Ad-GFP vs. Ad-GFP+IM, **P<0.0001 Ad-GFP vs. Ad-FoxO-1a, ***P<0.001 Ad-FoxO-1a vs. Ad-FoxO-1a+IM infected NRVMs (data from representative experiment). D, Semi-quantitative PCR analyses of E2F-1 and FoxO-1a mRNA expression in ACMs treated with non-specific (NS) and E2F-1 siRNA and exposed to IM. E, Semi-quantitative PCR analyses of E2F-1 and FoxO-1a mRNA expression in ACMs infected with Ad-LacZ (control) or Ad-E2F-1 virus. F, NRVMs infected with Ad-LacZ or Ad-E2F-1 virus and transfected with NS or FoxO-1a siRNA where indicated. TUNEL positive nuclei were quantified after indicated treatments. *P<0.01 NS siRNA + Ad-LacZ vs. NS siRNA + Ad-E2F-1.
To clarify whether FoxO-1a could regulate cardiomyocyte death in vitro, we treated NRVMs with control vector (Ad-GFP) or Ad-FoxO-1a prior to simulated I/R. Exposure of control Ad-GFP infected NRVMs to simulated I/R led to a 79% increase in TUNEL+ve nuclei (Fig. 6C; 1.00±0.10 vs. 1.79±0.25, P<0.005). However, overexpression of FoxO-1a alone caused a 2.6-fold increase in apoptotic NRVMs as compared to Ad-GFP-infected cells (Fig. 6C; 1.00±0.10 vs. 2.55±0.26, P<0.0001). Apoptosis was further enhanced when simulated I/R was combined with Ad-FoxO-1a overexpression (Fig. 6C; 2.55±0.26 vs. 6.28±1.03, P<0.002).
To confirm that E2F-1 is necessary for the increase in FoxO-1a mRNA seen in response to ischemia, we transfected ACMs with either NS or E2F-1 siRNA, followed by exposure to simulated I/R. E2F-1 siRNA treatment reduced E2F-1 mRNA expression by 84% (siNS+IM: 1.03±0.05 vs. siE2F-1+IM: 0.16±0.14, P<0.003, n=3 for each group), which prevented the increase in FoxO-1a expression in response to simulated I/R (Fig. 6D). Overexpression of E2F-1 via Ad-E2F-1 infection led to increases in both E2F-1 and FoxO-1a mRNA expression (Fig. 6E). To demonstrate the obligate role of FoxO-1a during E2F-1-induced apoptosis, we transfected NRVMs with either control or FoxO-1a siRNA followed by Ad-E2F-1 infection and quantified TUNEL+ve nuclei. NRVMs treated with control siRNA exhibited a 53% increase in TUNEL+ve nuclei after Ad-E2F-1 infection compared to Ad-LacZ treated cells (Fig. 6F; 1.00±0.21 vs. 1.53±0.11, P<0.05). FoxO-1a siRNA transfection abolished the pro-apoptotic effect of Ad-E2F-1 in NRVMs as no change in TUNEL+ve nuclei was observed after Ad-E2F-1 infection (Fig. 6F).
DISCUSSION
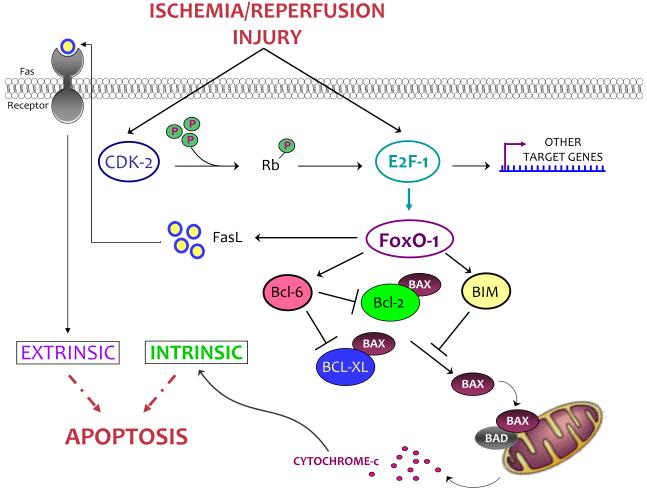
We have identified a novel transcriptional pathway that links E2F-1 to cardiac myocyte death acutely during I/R injury. Our data demonstrating a link between E2F-1 and FoxO-1a during I/R suggests that transcriptional networks are activated during myocardial I/R insults. We have demonstrated that both E2F-1 and FoxO-1a are elevated in response to I/R both in vivo and in vitro. Expression of FoxO-1a and its downstream target genes are diminished in E2F-1−/− mice following I/R injury in vivo as well as in vitro upon E2F-1 gene silencing. Forced expression of FoxO-1a is sufficient to induce apoptosis in vitro, which is further enhanced by simulated ischemia and reperfusion. Cardiomyocytes lacking FoxO-1a are protected from E2F-1-induced apoptosis supporting the idea that these two factors work in series and provides a model to account for E2F-1’s critical role in mediating myocardial I/R injury (Fig. 7). Despite previous work illustrating the ability of E2F-1 to regulate FoxO-1a promoter activity [19], the present study provides the first direct evidence demonstrating this relationship in the context of cardiovascular disease.
Figure 7. Schematic model of E2F-1-FoxO-1a transcriptional network after I/R.
In response to I/R, E2F-1 is upregulated and CDK-2 activity is increased leading to the phosphorylation of Rb and the enhancement of E2F-1 transcriptional activity. E2F-1 is a direct regulator of FoxO-1a leading to the activation of downstream proapoptotic genes. The coordinated activation of these proteins promotes apoptosis leading to cardiomyocyte death. Fas ligand (FasL), Bcl-2-interacting mediator (BIM), Bcl-2 antagonist of cell death (BAD), Bcl-2-associated X protein (BAX).
Ablation of E2F-1 expression leads to a decrease in infarct size after I/R injury, and we provide the first in vivo data elucidating the mechanism whereby E2F-1 might mediate cardiomyocyte death. Although E2F-1 is the E2F family member most associated with apoptosis, other E2Fs can also contribute [31] and apoptosis was not inhibited completely in E2F-1 null cardiac myocytes. However, neither E2F-2 or -3 expression increased after I/R nor was there a compensatory increase in E2F-2 expression in E2F-1−/− hearts after I/R injury (Suppl. Fig. S4). Thus the role of other E2F family members in mediating cardiac myocytes apoptosis after I/R injury is uncertain. The notion that E2F-1 may be critical for mediating cell death has been postulated for some time although the mechanisms have remained elusive. This was first proposed after the unexpected observation that aged E2F-1−/− mice develop tissue hyperplasia and tumors secondary to impaired apoptosis in vivo [16]. Likewise, deleting E2F-1 in Rb-deficient mice suppresses apoptosis and extends embryo survival [32]. Previous studies in other post-mitotic tissues have suggested an essential role for E2F-1 in mediating apoptosis as cortical neurons lacking E2F-1 were resistant to staurosporine-induced apoptosis in vitro [33] and E2F-1 null mice displayed decreased brain injury following focal ischemia [34]. Although numerous mechanisms have been proposed by which E2F-1 could mediate apoptosis, we noted very few alterations in the expression of classical E2F-1 target genes in our in vivo E2F-1 model. Recently it has been suggested that upregulation of Bnip3 accounts for E2F-1’s proapoptotic effects [13], however, Bnip3 is expressed at relatively high levels in the adult heart and is not regulated acutely with I/R so additional mechanisms are likely operational [25,26]. We observed a very modest increase in Bnip3 expression after I/R and were unable to induce Bnip3 mRNA expression after Ad-E2F-1 infection in either NRVM and ACMs (Suppl. Fig. S2). Both Bnip3 [25] and FoxO-1a [35], have been linked to autophagy in response to myocardial I/R [25]. However, this cannot explain the results we observed as the expression of known autophagy genes including, LC3, Gabarapl1, and Atg12 were indistinguishable between genotypes (Suppl. Fig. S6). Overexpression of E2F-1 in cardiac myocytes has also been reported to upregulate the pro-apoptotic gene p19ARF [14]. We saw no significant differences in expression of p19ARF after 4h of reperfusion suggesting it could not explain the difference in acute injury between genotypes (Suppl. Fig. S5A). Our data indicate that in E2F-1−/− mice, decreased levels of FoxO-1a are associated with decreased FasL, Bcl-6 and BIM induction. This lack of activation leads to the inability of BIM to target BAX and prevents its translocation to the mitochondria (Fig. 7).
The FoxO subfamily of Forkhead transcription factors (FoxO-1a, FoxO-3, FoxO-4 and FoxO-6) have emerged as critical regulators of gene expression, modulating apoptosis, cell cycle transition, cell differentiation, and glucose metabolism in many cell types [17,27,36]. The multiple family members and dichotomous effects of specific isoforms on processes like cell survival has often led to confusion about the true role of each isoform. For instance, they are substrates of Akt and have been implicated as negative regulators of cardiac hypertrophic growth [17,37]. However, cardioprotective signals that activate Akt-dependent survival pathways in the heart also result in enhanced phosphorylation and inactivation of FoxOs suggesting an important role in I/R injury [38]. Interestingly, CDK-2 is known to phosphorylate FoxO-1a during apoptosis [39], further linking growth pathways to the control of cell survival. Previous studies have shown that FoxO-1a and FoxO-3a expression is increased in the peri-infarct zone at 1 and 8 weeks post-MI respectively [40]; however, no data exists as to their regulation during acute periods of ischemia. Our data suggest they are actively involved in promoting apoptosis in both mitochondria-independent and -dependent apoptosis after I/R. FoxO-1a target genes Bcl-6 (mitochondria-dependent) and FasL (mitochondria-independent) are both elevated in response to I/R. FoxO-1a is required for E2F-1 induction of these genes, as silencing FoxO-1a is sufficient to block the increase of FasL, even after overexpression of E2F-1 in vitro (Suppl. Fig. S7).
Ischemia and signaling pathways activated during reperfusion are well known to regulate gene expression [41]; however, data supporting the importance of these transcriptional pathways in mediating pathological outcomes has been less forthcoming. Previous studies have demonstrated the importance of the JAK/STAT pathway in regulating the expression of stress-response genes and cardiomyocyte apoptosis [42]. More recent data has demonstrated that the JAK/STAT pathway also plays an important role in I/R injury [41,43]. Hypoxia-inducible factors have also been implicated in mediating acute cardiac myocyte injury in I/R [44,45]. We have proposed a novel transcriptional pathway mediated through E2F-1 and FoxO-1a that modulates the acute effects of ischemic injury. We provide evidence linking these two transcription factors in the setting of I/R both in vivo and in vitro. These data should contribute to the understanding of how transcriptional pathways mediate cardiomyocyte death during I/R injury.
Supplementary Material
Highlights.
E2F-1 regulates ischemia/reperfusion injury in vivo
FoxO1a is an E2F-1 target gene in cardiac muscle
Foxo-1a induces apoptosis in cardiac myocytes
ACKNOWLEDGEMENTS
We thank Dr. T.G. Unterman for kindly providing Ad-GFP and Ad-FoXO-1a viruses. This work was supported by the NIH (P01-HL080111 and R01-HL70748 to W.R.M.) as well as Laubisch and Cardiovascular Development Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none declared
REFERENCES
- [1].Byrnes KR, Faden AI. Role of cell cycle proteins in CNS injury. Neurochem Res. 2007;32(10):1799–1807. doi: 10.1007/s11064-007-9312-2. [DOI] [PubMed] [Google Scholar]
- [2].Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18(8):891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- [3].Reiss K, Cheng W, Giorando A, De Luca A, Li B, Kajstura J, Anversa P. Myocardial infarction is coupled with activation of cyclins and cyclin-dependent kinases in myocytes. Exp Cell Res. 1996;225(1):44–54. [PubMed] [Google Scholar]
- [4].Hauck L, Hansmann G, Dietz R, von Harsdorf R. Inhibition of hypoxia-induced apoptosis by modulation of retinoblastoma protein-dependent signaling in cardiomyocytes. Circ Res. 2002;91(9):782–789. doi: 10.1161/01.res.0000041030.98642.41. [DOI] [PubMed] [Google Scholar]
- [5].Adachi S, Ito H, Tamamori-Adachi M, Ono Y, Nozato T, Abe S, Ikeda M, Marumo F, Hiroe M. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res. 2001;88(4):408–414. doi: 10.1161/01.res.88.4.408. [DOI] [PubMed] [Google Scholar]
- [6].Liem DA, Zhao P, Angelis E, Chan SS, Zhang J, Wang G, Berthet C, Kaldis P, Ping P, MacLellan WR. Cyclin-dependent kinase 2 signaling regulates myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;102(10):1222–9. doi: 10.1016/j.yjmcc.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Iaquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. Curr Opin Cell Biol. 2007;19(6):649–657. doi: 10.1016/j.ceb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3(1):11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- [9].Qin XQ, Livingston DM, Kaelin WG, Jr., Adams PD. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci USA. 1994;91(23):10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shan B, Lee WH. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14(12):8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA. 1997;94(14):7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100(11):2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yurkova N, Shaw J, Blackie K, Weidman D, Jayas R, Flynn B, Kirshenbaum LA. The cell cycle factor E2F-1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circ Res. 2008;102(4):472–479. doi: 10.1161/CIRCRESAHA.107.164731. [DOI] [PubMed] [Google Scholar]
- [14].Ebelt H, Hufnagel N, Neuhaus P, Neuhaus H, Gajawada P, Simm A, Müller-Werdan U, Werdan K, Braun T. Divergent siblings: E2F2 and E2F4 but not E2F1 and E2F3 induce DNA synthesis in cardiomyocytes without activation of apoptosis. Circ Res. 2005;96(5):509–17. doi: 10.1161/01.RES.0000159705.17322.57. [DOI] [PubMed] [Google Scholar]
- [15].Lazzerini Denchi E, Helin K. E2F1 is crucial for E2F-dependent apoptosis. EMBO J. 2005;6(7):661–8. doi: 10.1038/sj.embor.7400452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr., Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85(4):549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- [17].Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- [18].Morris JB, Kenney B, Huynh H, Woodcock EA. Regulation of the proapoptotic factor FOXO1 (FKHR) in cardiomyocytes by growth factors and alpha1-adrenergic agonists. Endocrinology. 2005;146(10):4370–4376. doi: 10.1210/en.2005-0162. [DOI] [PubMed] [Google Scholar]
- [19].Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769(4):244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [20].Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM, MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J. 2006;25(16):3869–3879. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].MacLellan WR, Garcia A, Oh H, Frenkel P, Jordan MC, Roos KP, Schneider MD. Overlapping roles of pocket proteins in the myocardium are unmasked by germ line deletion of p130 plus heart-specific deletion of Rb. Mol Cell Biol. 2005;25(6):2486–2497. doi: 10.1128/MCB.25.6.2486-2497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pott C, Philipson KD, Goldhaber JI. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ Res. 2005;97(12):1288–1295. doi: 10.1161/01.RES.0000196563.84231.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- [24].Angelis E, Garcia A, Chan SS, Schenke-Layland K, Ren S, Goodfellow SJ, Jordan MC, Roos KP, White RJ, MacLellan WR. A cyclin D2-Rb pathway regulates cardiac myocyte size and RNA polymerase III after biomechanical stress in adult myocardium. Circ Res. 2008;102(10):1222–1229. doi: 10.1161/CIRCRESAHA.107.163550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14(1):146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- [26].Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of Bnip3 death pathways by calcium, phosphorylation, and hypoxia-reoxygenation. Antioxid Redox Signal. 2007;9(9):1309–1315. doi: 10.1089/ars.2007.1726. [DOI] [PubMed] [Google Scholar]
- [27].Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(15):2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- [28].Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73(6):689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- [29].Alikhani M, Alikhani Z, Graves DT. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. J Biol Chem. 2005;280(13):12096–12102. doi: 10.1074/jbc.M412171200. [DOI] [PubMed] [Google Scholar]
- [30].Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- [31].Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC, Helin K. CDC25A Phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol. 1999;19(9):6379–95. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsai KY, Hu Y, Macleod KF, Crowley D, Yamasaki L, Jacks T. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol Cell. 1998;2(3):293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- [33].Hou ST, Callaghan D, Fournier MC, Hill I, Kang L, Massie B, Morley P, Murray C, Rasquinha I, Slack R, MacManus JP. The transcription factor E2F1 modulates apoptosis of neurons. J Neurochem. 2000;75(1):91–100. doi: 10.1046/j.1471-4159.2000.0750091.x. [DOI] [PubMed] [Google Scholar]
- [34].MacManus JP, Koch CJ, Jian M, Walker T, Zurakowski B. Decreased brain infarct following focal ischemia in mice lacking the transcription factor E2F1. Neuroreport. 1999;10(13):2711–2714. doi: 10.1097/00001756-199909090-00004. [DOI] [PubMed] [Google Scholar]
- [35].Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284(41):28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- [37].Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114(11):1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Juhasz B, Thirunavukkarasu M, Pant R, Zhan L, Penumathsa SV, Secor ER, Jr., Srivastava S, Raychaudhuri U, Menon VP, Otani H, Thrall RS, Maulik N. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am J Physiol Heart Circ Physiol. 2008;294(3):H1365–H1370. doi: 10.1152/ajpheart.01005.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314(5797):294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- [40].Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res. 2008;102(2):e20–e35. doi: 10.1161/CIRCRESAHA.107.166744. [DOI] [PubMed] [Google Scholar]
- [41].Chi NC, Karliner JS. Molecular determinants of responses to myocardial ischemia/reperfusion injury: focus on hypoxia-inducible and heat shock factors. Cardiovasc Res. 2004;61(3):437–447. doi: 10.1016/j.cardiores.2003.11.033. [DOI] [PubMed] [Google Scholar]
- [42].Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275(14):10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- [43].Stephanou A. Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med. 2004;8(4):519–525. doi: 10.1111/j.1582-4934.2004.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Czibik G, Martinov V, Ruusalepp A, Sagave J, Skare O, Valen G. In vivo remote delivery of DNA encoding for hypoxia-inducible factor 1 alpha reduces myocardial infarct size. Clin Transl Sci. 2009;2(1):33–40. doi: 10.1111/j.1752-8062.2008.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Czibik G, Gravning J, Martinov V, Ishaq B, Knudsen E, Attramadal H, Valen G. Gene therapy with hypoxia-inducible factor 1 alpha in skeletal muscle is cardioprotective in vivo. Life Sci. 2011;88(11-12):543–550. doi: 10.1016/j.lfs.2011.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.