Abstract
A major development over the past two decades has been the realization that free radical induced lipid peroxidation and DNA damage are associated with major health problems, e.g. cancer and ageing. Plant-derived antioxidants are increasingly found beneficial in protecting against these diseases. Celtis australis L. and Celtis occidentalis L. are two plants that have a variety of uses in folk medicine but have not been evaluated before for their antioxidant and cytotoxic properties. Therefore, the extracts of both plants’ leaves were investigated for these activities, as well as isolation of the bioactive compounds responsible for the activities. Molecular structures of the compounds were elucidated by UV, HRESIMS, 1D (1H and 13C) and 2D (1H-13C HSQC and 1H-13C HMBC) NMR analyses. The ethanolic and aqueous extracts, n-butanol fractions and the isolated major compound were tested for their antioxidant activity using DPPH radical scavenging assay, xanthine oxidase-induced generation of superoxide radical and lipid peroxidation assay by thiobarbituric acid-reactive substances (TBARS) method using rat tissue homogenates. Cytotoxic activities were studied using standard MTT assay. A novel flavonoid C-triglycoside, 4‴-α-rhamnopyranosyl-2″-O-β-d-galactopyranosylvitexin, was isolated from both plants’ leaves, together with seven known flavonoids. The n-butanol fractions and the major compound 2″-O-β-galactopyranosylvitexin showed significant antioxidant activities, more pronounced than the tested standards BHT and dl-α-tocopherol in most tests. All extracts showed variable cytotoxic activities. This study provides strong evidence for the antioxidant and cytotoxic activities of the extracts of Celtis australis L. and Celtis occidentalis L. leaves, which were attributed to the polar n-butanol fractions and the major isolated flavonoid 2″-galactosylvitexin.
Keywords: Celtis australis L., Celtis occidentalis L., Flavonoid C-glycosides, DPPH, Xanthine oxidase, Lipid peroxidation, Cytotoxic activities
Introduction
Celtis australis L., Family Ulmaceae (Mediterranean hackberry), is a deciduous tree native to the Mediterranean region and southwestern Asia [1]. Decoctions of the leaves and fruits were used to astringe the mucous membrane in peptic ulcers, diarrhoea, and dysentery and as a remedy for heavy menstrual bleeding and colic [2, 3]. In Indian traditional medicine, the plant is an important remedy for bone fracture, contusions, sprains and joint pains [4].
Celtis occidentalis L., Family Ulmaceae (Hackberry, American Hackberry), is a medium-size deciduous tree native to North America. Native Americans used decoctions prepared from the bark as an aid in menses and to relieve sore throat and used the wood extract in treating jaundice [5, 6].
Previous investigations reported the isolation of acacetin 7-O-glucoside, isovitexin, cytisoside, 2″-α-rhamnopyranosylvitexin and 2″-α-rhamnopyranosyl-7-O-methylvitexin from the leaves of Celtis australis L. [7, 8], a sulphonated phenolic compound (celtisanin), apigenin, quercetin and quercetin-glucoside from the fruits [9] and a bacteriohopanoid from the barks [10]. Nothing was reported concerning Celtis occidentalis L.
Antioxidant and cytotoxic activities were reported before with other Celtis species e.g. Celtis philippinensis Blanco [11], Celtis africana Burm. f. [12], and Celtis iguanae (Jacq.) Sarg. [13]. According to the available literature, these activities were not investigated before in Celtis australis L. and Celtis occidentalis L. leaves.
Known isolated flavonoid C-glycosides e.g. vitexin, isovitexin, orientin and isoorientin were investigated before for their antioxidant activities [14–16].
Therefore, this study reports the isolation of a new flavonoid C-glycoside 8-(4‴-α-rhamnosyl-2″-O-β-d-galactopyranosylvitexin) along with seven known flavonoid glycosides (1–7). The antioxidant properties of the ethanolic and aqueous extracts were investigated since most of the herbal products available in the market are found in the form of tea bags or crude ethanolic extracts. Also the flavonoid-rich n-butanol fractions of the leaves as well as the major isolated flavonoid 6, were investigated for their antioxidant properties using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, xanthine oxidase-induced generation of superoxide radical assay and measurement of FeSO4/H2O2-stimulated lipid peroxidation in rat tissue homogenates. The cytotoxic activity against different human cell lines was also studied.
Results and Discussion
Phytochemical investigation
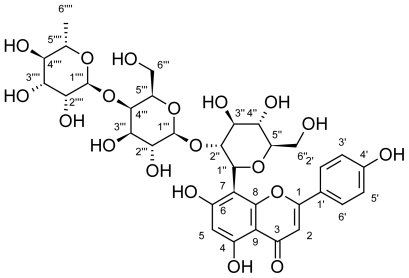
Compound 8 was obtained as a yellow powder. The positive ESI-MS spectrum gave a molecular ion at m/z 741 (M + H)+, whereas the negative ESI-MS (M – H) – ion at m/z 739. These data together with the 13C NMR spectrum showing 33 signals indicated the molecular formula to be C33H40O19. The UV spectrum showed characteristic flavone absorption at 272 and 330 nm [17]. The 1HNMR spectrum indicated the presence of an apigenin skeleton substituted at C-8 through C-C linkage confirmed by the appearance of H-6 at δ 6.21. The appearance of three anomeric proton signals suggested a triglycoside moiety. The first anomeric proton at δ 4.76, J=9.9 Hz was assigned to a β-d-gluco-pyranose, the second anomeric proton at δ 3.99, J=9 Hz was assigned to a β-d-galacto-pyranose sugar while an α-l-rhamnopyranose moiety was represented by an anomeric proton at δ 4.90 and a doublet at δ 1.19, J=6 Hz assigned for CH3-6″″ of the rhamnopyranose.
The positive-ion MS/MS of compound 8 showed fragment ion peak at m/z 594.3 (M+H – 146)+ assignable to the loss of a desoxyhexose moiety from the parent ion which was suggestive of a linear triglycoside moiety. The desoxyhexose identified as rhamnose was found to be attached at position C-4‴ of the galactose sugar evidenced by its downfield shifting to δ 67.2 together with the upfield shifting of the anomeric proton to δ 101.4. HBMC spectrum was used to identify the carbon and proton signals. Correlations appearing between H-1″/C-8 confirmed attachment of the sugar unit through C-linkage to C-8 of the apigenin moiety. Also, correlations between 2″-H/C-1‴ and 1‴-H/C-2″ confirmed the linkage of the galactose to C-2″ of glucose. All these data were consistent with the structure apigenin-8-C-(4‴-O-rhamnosyl-2″-galactosyl)-glucopyranoside, (4‴-α-l-rhamnosyl-2″-O-β-d-galactopyranosylvitexin). To the best of our knowledge, this is the first report for the isolation of this compound in nature.
Compounds 1 and 2 were identified as vitexin and orientin [18]; compounds 3 and 4 were identified as isovitexin and isoorientin [18–20]; compound 5 was identified as rutin [17, 21]. ESIMS and NMR analyses (1D [1H and 13C] and 2D [1H-13C HSQC and 1H-13C HMBC]) identified compound 6 as 2″-O-β-galactopyranosylvitexin and compound 7 as 2″-O-β-d-galactopyranosylorientin. Their data were compared with that reported in literature [22, 23]. Compounds (1–3 and 5–7) were isolated for the first time from genus Celtis. Both studied Celtis species showed a similar flavonoidal pattern differing only in the amount present.
In-vitro biological evaluation
DPPH radical scavenging activity [24]
All the tested samples had significant scavenging effects on the DPPH radical. The results recorded in table 2 indicated that 2″-O-β-galactopyranosylvitexin showed the highest activity among all the tested samples (84.8%), followed by the n-butanol fraction (70.3%) and the ethanolic extract (67.2%), and they were higher than α-tocopherol and BHT (66.5 and 55.3%, respectively). The results obtained for the n-butanol fractions and 2″-galactosylvitexin indicated that the flavonoidal content (C-flavonoid glycosides) is responsible for the scavenging effects, and this could be attributed to their hydrogen donating activity. In general, the phenolic OH is considered a scavenger of free radicals, and it consequently exhibits antioxidative activity [25].
Tab. 2.
Effects of the tested samples on the in vitro free radical generation
| Bioassay | DPPH % inhibition | Xanthine oxidasea IC50 (μM) |
|---|---|---|
| Ethanolic extract (CA) | 67.2 ± 2.10 | 76.8 ± 2.90 |
| Ethanolic extract (CO) | 58.5 ± 1.50 | 92.1 ± 3.55 |
| Aqueous extract (CA) | 55.6 ± 2.10 | 70.2 ± 2.18 |
| Aqueous extract (CO) | 48.5 ± 1.35 | 95.5 ± 3.70 |
| n-butanol fraction (CA) | 70.3 ± 2.20 | 27.2 ± 2.10 |
| n-butanol fraction (CO) | 65.9 ± 1.96 | 38.5 ± 1.78 |
| 2″-O-β-galactopyranosylvitexin | 84.8 ± 2.44 | 24.2 ± 1.95 |
| dl-α-tocopherol | 66.5 ± 2.75 | 78.5 + 2.88 |
| BHT | 55.3 ± 1.50 | 130.7 + 4.35 |
| Allopurinol | – | 18.0 ± 0.25 |
Uric acid production for controls was (61.0 ± 1.9 nmol/min).
All tested samples and positive controls were tested at 100 μg/mL.
Values are presented as mean ± SE of 3-test sample observation. P < 0.05 for all values.
CA = Celtis australis L.; CO = Celtis occidentalis L.
Xanthine oxidase-induced generation of superoxide radical [26]
The tested samples inhibited superoxide-induced reduction of nitroblue tetrazolium, which depends on direct inhibition of xanthine oxidase enzyme as shown in table 2. The major isolated flavonoid, 2″-O-β-galactopyranosylvitexin, showed the highest activity with IC50=24.2 μM, followed by the n-butanol fraction of both plants with IC50=27.2 μM and 38.5 μM, respectively.
Phenolic compounds are known to inhibit generation of the superoxide anion radical (O2•−) in the hypoxanthine-xanthine oxidase system. The radical scavenging action of these phenolic compounds is through the formation of stable free radicals, which contribute to the inhibitory effects on lipid peroxidation and participate in the inhibition of (O2•−) generation. Since the n-butanol fractions is rich in apigenin and luteolin derivatives e.g. isovitexin, orientin and isoorientin which were reported to have activities regarding inhibition of xanthine oxidase enzyme [16, 27]. Therefore, these fractions could be effective as natural antioxidants, through their double ability to inhibit xanthine oxidase activity and superoxide anion production, and could be considered an effective strategy in the treatment of inflammation.
FeSO4/H2O2-stimulated lipid peroxidation in rat tissue homogenate [28, 29]
For rat tissue homogenate (brain, heart and liver), the unstimulated control experiments i.e. the amount of thiobarbituric reactive substance (TBARS) formed in rat tissue homogenate (brain, heart and liver) were (0.44 ± 0.05, 0.25 ± 0.03 and 0.19 ± 0.02 nmol malondialdehyde, MDA/mg protein, respectively). After induction with FeSO4/H2O2, the amount of TBARS increased to 0.90 ± 2.55, 0.60 ± 2.16 and 0.52 ± 1.25 nmol malondialdehyde, MDA/mg protein, for brain, heart and liver, respectively. The tested samples significantly reduced malondialdehyde (MDA) formation in the presence of FeSO4-H2O2 in tissue homogenates indicating anti-lipid peroxidation activities. The inhibition percentages were in the range of (55.10–80.85%), (52.40–71.50%) and (43.90–78.25%) in brain, heart and liver rat tissue homogenates, respectively as recorded in table 3.
Tab. 3.
Inhibition effects of the tested samples on FeSO4/H2O2-stimulated lipid peroxidation (MDA production) in rat tissue homogenates in vitro
| Bioassay | Inhibition (%)a
|
||
|---|---|---|---|
| Sample | Brain | Heart | Liver |
| Ethanolic extract (CA)b | 80.85 ± 1.15 | 55.30 ± 1.80 | 68.30 ± 1.83 |
| Ethanolic extract (CO)b | 75.60 ± 1.97 | 50.22 ± 1.80 | 70.90 ± 2.15 |
| Aqueous extract (CA)b | 55.10 ± 2.28 | 52.40 ± 2.18 | 43.90 ± 2.25 |
| Aqueous extract (CO)b | 43.50 ± 1.55 | 48.75 ± 1.28 | 32.75 ± 1.80 |
| n-butanol fraction (CA)b | 66.18 ± 2.10 | 54.10 ± 1.80 | 49.75 ± 1.76 |
| n-butanol fraction (CO)b | 64.82 ± 2.05 | 60.05 ± 1.85 | 53.10 ± 1.80 |
| 2″-O-β-galactopyranosylvitexinc | 80.78 ± 3.12 | 71.50 ± 2.35 | 78.25 ± 3.10 |
| dl-α-tocopherolb | 62.90 ± 2.18 | 58.70 ± 1.80 | 71.10 ± 2.25 |
| BHTb | 52.48 ± 1.72 | 43.23 ± 1.65 | 53.20 ± 1.65 |
Values are presented as mean ± SE of 3-test sample observations, P < 0.05 for all values;
200 μg/mL;
100 μg/mL; CA = Celtis australis L.; CO = Celtis occidentalis L.
It was interesting to note that the inhibition effects produced by the tested samples were more pronounced for brain tissue homogenates than liver and heart tissue homogenates, which could be especially beneficial in treatment of Alzheimer’s disease in cases with oxidative stress due to elevated levels of TBARS [30]. The ethanolic extract and 2″-O-β-galactopyranosylvitexin showed the highest inhibition activity against FeSO4/H2O2-stimulated lipid peroxidation in brain rat tissue homogenate (80.85 and 80.78%), respectively, which was higher than both reference standards. Since dl-α-tocopherol is thought to be associated with lipid-rich membranes; its anti-oxidative ability is highly effective in protecting membranes against lipid peroxidation, as peroxyl and alkoxyl radicals. The data obtained from the present study indicates that the tested extracts have an anti-lipid peroxidative character with similar reaction mechanisms to those of dl-α-tocopherol.
Cytotoxic activity [31]
As shown in table 4, human hepatocellular carcinoma (HEP-G2), colon adenocarcinoma (COLO 205) and gastric carcinoma (NCI-N87) were the most sensitive of all cell lines examined to the activities of the extracts, followed by the ovary adenocarcinoma (NIH:OVCAR-3). The aqueous extract of Celtis occidentalis L. leaves showed the strongest activity against HEP-G2 (ED50= 18.60 μg/mL), while the plant’s ethanolic extract was most active against COLO 205 and NCI-N87 (ED50= 24.80 and 15.80 μg/mL, respectively).
Tab. 4.
ED50 (μg/mL) of the tested samples on the selected cell linesa
| Sample | HEP-G2 | CCRF-CEM | COLO 205 | NCI-N87 | NIH-OVAR-3 |
|---|---|---|---|---|---|
| Ethanolic extract (CA) | 26.10 ± 0.20 | > 100 | 25.65 ± 0.20 | 45.15 ± 0.25 | 77.65 ± 0.52 |
| Ethanolic extract (CO) | 39.85 ± 0.25 | > 100 | 24.80 ± 0.20 | 15.80 ± 0.05 | 68.50 ± 0.45 |
| Aqueous extract (CA) | 26.90 ± 0.20 | 77.50 ± 0.53 | 63.45 ± 0.40 | 35.10 ± 0.15 | 72.77 ± 0.48 |
| Aqueous extract (CO) | 18.60 ± 0.08 | 79.54 ± 0.47 | 26.60 ± 0.25 | 23.55 ± 0.10 | 76.10 ± 0.55 |
Values are presented as mean ± SE of 2 test sample observation, compared with that of control group (p < 0.05) for all values; HEP-G2…Human hepatocellular carcinoma; CCRF-CEM…leukemia carcinoma; COLO 205…colon adenocarcinoma; NIH-OVCAR-3…ovary adenocarcinoma; NCI-N87…gastric carcinoma; CA…Celtis australis L.; CO…Celtis occidentalis L.
Conclusions
This study provides strong evidence for the antioxidant activities of the leaves’ ethanolic extracts of Celtis australis L. and Celtis occidentalis L. that could be considered as two valuable medicinal plant species. The results obtained for the n-butanol fractions and the major isolated flavonoid, 2″-galactosylvitexin, indicated that the C-flavonoid glycosides in both studied plants are responsible for the scavenging effects through several mechanisms like hydrogen donating activity, inhibition of xanthine oxidase activity and superoxide anion production, as well as protection of membranes against lipid peroxidation. This could be potentially useful for the treatment of major free radical-induced degenerative diseases including brain dysfunction, inflammation, liver disorders and cancer. Further in-vivo studies could be done to support this point of view.
Experimental
Plant material
Celtis australis L. and Celtis occidentalis L. leaves were collected from El-Orman Botanical Garden and the Agricultural museum, Giza, Egypt. Dr. Mohamed El Gebaly, Taxonomist, National Research Center kindly verified identity of the plant material. Voucher specimens (G-02 and G-03) were kept in the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Egypt.
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), 2-thiobarbituric acid, ferrous sulphate, hydrogen peroxide, xanthine oxidase from bovine milk, allopurinol, nitroblue tetrazolium, 3-(4,5-dimethyl-1,3-thiazol-2-yl)-2,5-diphenyl-2H-tetrazol-3-ium bromide (MTT) and sodium 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonate (HEPES, for Kreb’s buffer) (Sigma, St. Louis, Mo, USA); coomassie plus protein assay reagent and albumin standard (Pierce, Rockford, IL, USA). dl-α-tocopherol (Nacalai, Tesuque, Tokyo, Japan); L-glutamine, 1% non-essential amino acids and 1% sodium pyruvate (Bio Whittaker, Walkersville, MD, USA). 10 % fetal bovine serum (FBS) (Gibco Br L, Rockville, MD, USA). TLC was performed on pre-coated sheets with silica gel F254 (Fluka, Sigma-Aldrich, Germany).Column chromatography was performed over silica gel H 60 for VLC (Sigma, St. Louis, Mo, USA.), sephadex LH-20 (Pharmacia Fine Chemicals, AB Uppsala, Sweden), polyamide and silica gel 60 (Fluka, Sigma-Aldrich, Germany). All other chemicals used were of analytical grade.
Animals
Male Wistar rats (250–300 g) were handled according to international regulations. They were allowed to take standard laboratory diet and water ad libitum, and the animals were maintained at 24 °C with 12 h light period.
Cell lines and culture media
Human hepatocellular carcinoma (HEP-G2), leukemia carcinoma (CCRF-CEM), colon adenocarcinoma (COLO 205), ovary adenocarcinoma (NIH:OVCAR-3) and gastric carcinoma (NCI-N87) cell lines were from the American Type Culture Collection (ATCC). Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island NY, USA). Eagle Minimum Essential Medium (EMEM) and Roswell Park Memorial Institute 1640 (RPMI) medium (Nissui Pharm. Co., Ltd., Tokyo, Japan); McCoy’s 5A modified medium (Sigma, St. Louis, Mo., USA).
Preparation of extracts and fractions
The air-dried powdered leaves of Celtis australis L. (1.5 kg) and Celtis occidentalis L. (1.2 kg) were exhaustively extracted with 95% ethanol by percolation to give 225 g and 200 g dry residue, respectively. An aliquot of the concentrated extracts (150 g, each) was suspended in distilled water and partitioned successively with petroleum ether (32 and 34 g), CH2Cl2 (2 and 2.5 g), EtOAc (4 and 3.1 g), and n-BuOH (40 and 21 g).
For the aqueous extract, 50 g of the air-dried powdered leaves was boiled with distilled water (3 times, each for 10 min). The extracts were then lyophilized to give 12 and 10 g, respectively.
Fractionation and separation of major components of Celtis australis L
The n-BuOH soluble fraction (20 g) was chromatographed on a silica gel H 60, VLC (Ø7 x 12.5 cm, 200 g) and eluted with CH2Cl2, gradients of CH2Cl2-EtOAc, EtOAc and gradients of EtOAc-MeOH up to pure MeOH. Fractions (200 mL, each) were collected and monitored by TLC. Similar fractions were pooled to give 6 collective fractions. Collective fraction 1 (2.1 g) was subjected to CC on polyamide using MeOH/H2O (20:80) v/v followed by CC on sephadex LH-20 using MeOH/(CH3)2CO/H2O (3:1:1) v /v/v to give compounds 1 (16 mg), 2 (7 mg) and 3 (60 mg). Collective fraction 2 (2 g) was purified by CC on silica gel 60 followed by CC on sephadex LH-20 using MeOH/(CH3)2CO/H2O (3:1:1) v/v/v to give compound 4 (20 mg). Collective fractions 3 (1.1 g) and 4 (4.2 g) were subjected to repeated CC on sephadex LH-20 using MeOH/H2O (50:50) v/v for elution to give compounds 5 (22 mg) and 6 (200 mg), respectively. Collective fraction 5 (750 mg) and 6 (1.5 g) were subjected to repeated CC on sephadex LH-20 using MeOH/(CH3)2CO/H2O (3:1:1) v/v/v to give compounds 7 (19 mg) and 8 (28 mg).
Fractionation and separation of major components of Celtis occidentalis L
The n-BuOH soluble fraction (15 g) was chromatographed on a silica gel H 60, VLC (Ø7 x 12.5 cm, 190 g) and eluted with CH2Cl2, gradients of CH2Cl2-EtOAc, EtOAc and gradients of EtOAc-MeOH up to pure MeOH. Fractions (200 mL) were collected and monitored by TLC. Similar fractions were pooled to give 5 collective fractions. Collective fractions 1 (0.7 g) and 2 (2.84 g) were subjected to CC on polyamide using MeOH/H2O (20:80) v/v followed by CC on sephadex LH-20 using MeOH/(CH3)2CO/H2O (3:1:1) v/v/v to give compounds 1 (5 mg), 2 (21 mg) and 3 (80 mg). Collective fraction 3 (1.89 g) and 4 (1.56 g) were subjected to repeated CC on sephadex LH-20 using MeOH/H2O (50:50) v/v for elution followed by CC on sephadex LH-20 using MeOH/(CH3)2CO/H2O (3:1:1) v/v/v to give compounds 4 (16 mg) and 5 (8 mg), respectively. Collective fraction 5 (2.1 g) was subjected to repeated CC on sephadex LH-20 using MeOH (100%) for elution followed by CC on sephadex LH-20 using MeOH/(CH3)2CO/H2O (3:1:1) v/v/v to give compounds 6 (22 mg), 7 (32 mg) and 8 (11 mg).
4‴-Rhamnosyl-2″-O-β-d-galactopyranosylvitexin (6-Deoxy-α-l-mannopyranosyl-(1→4)-β-d-galactopyranosyl-(1→2)-(1S)-1,5-anhydro- 1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]-d-glucitol, 8)
Yellow powder, mp: 289–291 °C. Rf: 0.21 (EtOAc/MeOH/H2O, 100:16:13 and 2 drops formic acid). UV/Vis λmax (MeOH) nm: 272, 302sh, 330; (NaOMe): 280, 326, 393; (AlCl3): 277, 304, 350, 382; (AlCl3/HCl): 278, 303, 344, 384; (NaOAc): 280, 310sh., 336sh., 390; (NaOAc/H3BO3): 274, 308, 351, 400sh. HR-ESI/MS: m/z = 739.2085 (M – H) (calcd 739.2091), m/z = 741.2202 (M + H) (calcd 741.2210). 1H and 13C NMR: Table 1.
Tab. 1.
1H and 13C NMR data of compound 8 (DMSO-d6, 300, 75 MHz)
| No. | Compound 8
|
No. | Compound 8
|
||
|---|---|---|---|---|---|
| δHa | δHa | No. | |||
| 2 | 163.7 | 3″ | 75.8 | ||
| 3 | 6.70, s | 102.5 | 4″ | 70.8 | |
| 4 | 182.0 | 5″ | 81.5 | ||
| 5 | 160.1 | 6″ | 61.1 | ||
| 6 | 6.21, s | 97.9 | 2″-O-Gal 1‴ | 3.99, d (9) | 101.4 |
| 7 | 162.4 | 2‴ | 72.3 | ||
| 8 | 104.3 | 3‴ | 74.0 | ||
| 9 | 156.3 | 4‴ | 67.2 | ||
| 10 | 103.9 | 5‴ | 75.0 | ||
| 1′ | 121.8 | 6‴ | 58.1 | ||
| 2′ | 8.01, d (8.7) | 128.8 | 4‴-O-Rha 1″″ | 4.90, s | 100.2 |
| 3′ | 6.89, d (9) | 115.8 | 2″″ | 70.4 | |
| 4′ | 160.9 | 3″″ | 70.6 | ||
| 5′ | 6.89, d (9) | 115.8 | 4″″ | 73.2 | |
| 6′ | 8.01, d (8.7) | 128.8 | 5″″ | 68.1 | |
| 8-C-Glc 1″ | 4.76, d (9.9) | 71.7 | 6″″ | 1.19, d (6) | 17.5 |
| 2″ | 78.9 | ||||
Coupling constants (J in Hertz) are in parentheses.
Equipment
Electrothermal 9100 equipment was used for melting point determination. UV spectra were measured using a Shimadzu UV 1650 PC spectrophotometer. ESI-MS (negative and positive modes) were determined on Thermo Finnigan (ion trap) equipment. NMR spectra were recorded in DMSO-d6 with a Varian Mercury instrument (1HNMR, 300 MHz; 13CNMR, 75 MHz). Absorbance was measured on a multi well scanning spectrophotometer (Dynex MR 5000, Chantilly, VA, USA).
In-vitro biological Evaluation
DPPH radical scavenging activity
The ability of the extracts to scavenge free radicals was determined according to the method of Hosny, et al 2002 [24]. In a 96-well plate, 10 μL of each sample or standard dissolved in ethanol (100 μg/mL) was added to 190 μL of 316 μM/mL DPPH solution. A blank was prepared using ethanol. After incubation at 30 °C for 30 min, the absorbance of each solution was measured at 517 nm. dl-α-tocopherol and BHT were used as positive controls. The scavenging activity of the samples was calculated as a percentage of free radical inhibition according to the formula:
where Ablank is the absorbance of the blank at zero time and Asample is the absorbance of the sample after 30 min. All experiments were carried out in triplicate.
Xanthine oxidase-induced generation of superoxide radical
The effect of the tested samples was measured according to the method described by Luis Gongora et al., 2003 [26] with slight modification. Superoxide was generated by oxidation of hypoxanthine (100 μM) with bovine milk xanthine oxidase in 1 mL of 10 mM KH2PO4-KOH buffer, pH 7.4, and was detected by the reduction of nitroblue tetrazolium (NBT) at 100 μM, followed spectrophotometrically at 560 nm. dl-α-tocopherol and BHT were used as positive controls. The effects of the test samples on enzyme activity was studied by measuring the uric acid formation from xanthine (2–25 μM) after 15 min incubation at 25 °C, while absorbance was measured at 295 nm using allopurinol as a reference standard. The inhibitory activity of the tested samples and isolated compound in terms of IC50, i.e. the concentration required for 50% inhibition of uric acid formation was calculated by linear regression analysis.
FeSO4/H2O2-stimulated lipid peroxidation in rat tissue homogenate [28, 29]
Male Wistar rats (250–300 g) were sacrificed, and the rat tissues (brain, heart and liver: 0.3–0.5 g) were rapidly removed and homogenized in 10 volumes of 15 mM Krebs buffer. Homogenates were centrifuged at 3000 x for 10 minutes at 4 °C to give supernatants containing (1.2 mg of protein/ ml; brain), (1.7 mg of protein/ ml; heart) and (2.5 mg of protein/ ml; liver) using Coomassie plus protein assay reagent and albumin standard as determined by the Bradford method [32]. During aerobic incubation of the tissue homogenates, MDA released reacts with thiobarbituric acid (TBA) to give a pink colour. The capability of the samples to inhibit MDA formation is used as a measure of their antioxidant activity. The pink colour complex of thiobarbituric acid reacting substance (TBARS) is measured at 532 nm for the test samples and positive standards (dl-α-tocopherol and BHT) (200 μg/mL), as well as, 2″-O-β-galactopyranosylvitexin (100 μg/mL). The results were expressed as nanomoles of MDA equivalents per milligram of protein of rat (brain, heart and liver) homogenates. All measurements were done in triplicate. The capability to inhibit MDA formation was calculated using the following equation:
Cytotoxic activity
Cell lines, HEP-G2, were cultured in DMEM containing 10% heat-inactivated FBS. CCRF-CEM and NCI-N87 cells were cultured in RPMI1640 medium supplemented with 1% L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate and 10% FBS. The COLO 205 human colon carcinoma cells were grown in McCoy’s 5A modified medium supplemented with 1% L-glutamine. NIH:OVCAR-3 cells were cultured in EMEM containing Earle’s salts heated and sublimated with amino acids and 10% FBS. All cell lines were cultured in T75 Falcon flasks in a humidified atmosphere with 5% CO2, at 37°C. Cellular viability was determined using the standard MTT colorimetric assay [31]. The assay is based on reduction of MTT by the mitochondrial dehydrogenase of viable cells to give a blue formazan product that can be measured spectrophotometrically at 550 nm. The 50 % effective dose (ED50) is calculated.
Statistical Analysis
All data were expressed as mean ± SE. Student’s t-test [33] was applied for detecting the significance of difference between each sample; P < 0.05 was taken as the level of significance.
Fig. 1.
Structure and atom numbering of compound 8.
Footnotes
Authors’ Statements
Competing Interests
The authors declare no conflict of interest.
Animal Rights
The institutional and international guide for the care and use of laboratory animals was followed. See the experimental part for details.
References
- 1.Quattrocchi U. CRC World Dictionary of Plant Names. Vol. 4. Boca Raton, Florida: CRC Press; 2000. pp. 468–469. [Google Scholar]
- 2.Chiej R. The Macdonald Encyclopedia of Medicinal Plants. Macdonald and Co (Publishers) Ltd; London and Sydney: 1988. p. 240. [Google Scholar]
- 3.Chevallier A. The Encyclopedia of Medicinal Plants. New York: DK Publishing Inc.; 1996. p. 183. [Google Scholar]
- 4.Gaur RD. Flora of district Garhwal North West Himalaya. Srinagar: Trans Media, Media House; 1999. p. 84. [Google Scholar]
- 5.Moerman DE. Native American Ethnobotany. Portland, Oregon, USA: Timber Press. Inc; 1986. [Google Scholar]
- 6.Lauriault J. Identification Guide to the Trees of Canada. Toronto: National Museum of Natural Sciences and Fitzhenry & Whiteside; 1989. http://www.pfaf.org/user/Plant.aspx?LatinName=Celtis+occidentalis. [Google Scholar]
- 7.Spitaler R, Gurschler S, Ellmerer E, Schubert B, Sgarbossa M, Zidorn C. Flavonoids from Celtis australis (Cannabaceae) Biochem Syst Ecol. 2009;37:120–121. http://dx.doi.org/10.1016/j.bse.2008.11.020. [Google Scholar]
- 8.Kaltenhauser M, Ellmerer EP, Zidorn C. Rhamnopyranosylvitexin derivatives from Celtis australis. J Serb Chem Soc. 2010;75:733–738. http://dx.doi.org/10.2298/JSC090817049K. [Google Scholar]
- 9.Badoni R, Semwal DK, Rawat U, Singh GJ. Celtisanin, a novel sulphonated phenolic from Celtis australis L. fruits. Nat Prod Res. 2010;13:1282–1286. doi: 10.1080/14786411003754306. http://dx.doi.org/10.1080/14786411003754306. [DOI] [PubMed] [Google Scholar]
- 10.Badoni R, Semwal DK, Badoni PP, Kothiyal SK, Rawat U. A novel bacteriohopanoid from Celtis australis L. bark. Chin Chem Letters. 2011;22:81–84. http://dx.doi.org/10.1016/j.cclet.2010.07.029. [Google Scholar]
- 11.Hwang BY, Chai HB, Kardono LS, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Cytotoxic triterpenes from the twigs of Celtis philippinensis. Phytochemistry. 2003;62:197–201. doi: 10.1016/s0031-9422(02)00520-4. http://dx.doi.org/10.1016/S0031-9422(02)00520-4. [DOI] [PubMed] [Google Scholar]
- 12.Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Celtis africana. Rec Nat Prod. 2009;3:23–31. [Google Scholar]
- 13.Schmeda-Hirschmann G, Feresin G, Tapia A, Higert N, Theoduloz C. Proximate composition and free radical scavenging activity of edible fruits from the Argentinian Yungas. J Sci Food Agric. 2005;85:1357–1364. http://dx.doi.org/10.1002/jsfa.2098. [Google Scholar]
- 14.Shibano M, Kakutani K, Taniguchi M, Yasuda M, Baba K. Antioxidant constituents in the dayflower (Commelina communis L.) and their α-glucosidase-inhibitory Activity. J Nat Med. 2008;62:349–353. doi: 10.1007/s11418-008-0244-1. http://dx.doi.org/10.1007/s11418-008-0244-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Lee BC, Kim JH, Sim GS, Lee DH, Lee KE, Yun YP, Pyo HB. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res. 2005;28:195–202. doi: 10.1007/BF02977715. http://dx.doi.org/10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- 16.Lin CM, Chen CT, Lee HH, Lin JK. Prevention of cellular ROS damage by isovitexin and related flavonoids. Planta Med. 2003;68:365–367. doi: 10.1055/s-2002-26753. http://dx.doi.org/10.1055/s-2002-26753. [DOI] [PubMed] [Google Scholar]
- 17.Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New York: Springer Verlag; 1970. [Google Scholar]
- 18.Leong AC, Kinjo Y, Tako M, Iwasaki H, Oku H, Tamaki H. Flavonoid glycosides in the shoot system of Okinawa Taumu (Colocasia esculenta S.) Food Chem. 2010;119:630–635. http://dx.doi.org/10.1016/j.foodchem.2009.07.004. [Google Scholar]
- 19.Zhou X, Peng J, Fan G, Wu Y. Isolation and purification of flavonoids glycosides from Trollius ledebouri using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. J Chromatogr A. 2005;109:216–221. doi: 10.1016/j.chroma.2005.07.064. http://dx.doi.org/10.1016/j.chroma.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Fan G, Hong Z, Chai Y, Wu Y. Preparative separation of isovitexin and isoorientin from Patrinia villosa Juss by high-speed counter-current chromatography. J Chromatogr A. 2005;1074:111–115. doi: 10.1016/j.chroma.2005.03.067. http://dx.doi.org/10.1016/j.chroma.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal PK. Carbon-13NMR of Flavonoids. New York: Elsevier; 1989. [Google Scholar]
- 22.Zou JH, Yang JS, Dong YS, Zhou L, Lin G. Flavone C-glycosides from flowers of Trollius ledebouri. Phytochemistry. 2005;66:1121–1125. doi: 10.1016/j.phytochem.2005.03.021. http://dx.doi.org/10.1016/j.phytochem.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Xiong Z, Ying X, Cui L, Zhu W, Li F. A rapid ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometric method for the qualitative and quantitative analysis of the constituents of the flower of Trollius ledibouri Reichb. Anal Chim Acta. 2006;580:170–180. doi: 10.1016/j.aca.2006.07.069. http://dx.doi.org/10.1016/j.aca.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 24.Hosny M, Johnson HA, Ueltschy AK, Rosazza JPN. Oxidation, reduction, and methylation of carnosic Acid by nocardia. J Nat Prod. 2002;65:1266–1269. doi: 10.1021/np020189n. http://dx.doi.org/10.1021/np020189n. [DOI] [PubMed] [Google Scholar]
- 25.Rice-Evans C, Miller NJ, Paganga O. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. http://dx.doi.org/10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 26.Gongora L, Mánez S, Giner RM, Recio MC, Schinella G, Rios JL. Inhibition of xanthine oxidase by phenolic conjugates of methylated quinic acid. Planta Med. 2003;69:396–401. doi: 10.1055/s-2003-39715. http://dx.doi.org/10.1055/s-2003-39715. [DOI] [PubMed] [Google Scholar]
- 27.Van Hoorn DEC, Nijveldt RJ, Paul AM, Van Leeuwen PAM, Zandrie Hofman Z, M’Rabet L, De Bont DBA, Van Norren K. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur J Pharm. 2002;451:111–118. doi: 10.1016/s0014-2999(02)02192-1. http://dx.doi.org/10.1016/S0014-2999(02)02192-1. [DOI] [PubMed] [Google Scholar]
- 28.Hino T, Kawanish S, Yasui H, Oka S, Sakurai H. HTHQ (1-O-hexyl-2,3,5-trimethylhydroquinone), an anti-lipid-peroxidative compound: its chemical and biochemical characterizations. Biochem Biophys Acta. 1998;1425:47–60. doi: 10.1016/s0304-4165(98)00050-6. http://dx.doi.org/10.1016/S0304-4165(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 29.Hosny M, Rosazza JP. Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J Agric Food Chem. 2002;50:5639–5645. doi: 10.1021/jf020503j. http://dx.doi.org/10.1021/jf020503j. [DOI] [PubMed] [Google Scholar]
- 30.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45:1594–1601. doi: 10.1212/wnl.45.8.1594. http://www.ncbi.nlm.nih.gov/pubmed/7644059. [DOI] [PubMed] [Google Scholar]
- 31.Ragab EA, Hosny M, Kadry HA, Ammar HA. Flavanone Glycosides from Gleditsia caspia. J Nat Prod. 2010;3:35–46. [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. http://dx.doi.org/10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Woodson RR. Probability and Mathematical Statistics. New York: Wiley; 1987. Statistical Methods for the Analysis of Biochemical Data Series; pp. 315–316. [Google Scholar]