Abstract
Analytical methods were developed for the identification of major degradation products of Ketoconazole, an antifungal agent. The stressed degradation of Ketoconazole drug substance was performed under acid, base, thermal, photo and oxidative stress conditions. The major degradation was observed under acid, base and oxidative stress conditions. The degradation study was performed on Inertsil ODS-3V, length 100 X diameter 4.6 mm, particle size 3 μm column using gradient method. These degradants were identified by LC-MS technique.
Keywords: Ketoconazole, Stress degradation, Hydrolysis degradent, Oxidative degradent, LC-MS
Introduction
Ketoconazole is an antifungal drug approved by the US FDA in 1981. Only a few analytical methods for the determination of the drug in biological samples and in the presence of other drugs have been reported [1–12]. The photodegradation behavior of Ketoconazole has been reported by Staub et al [13]. The drug substance is official in Ph. Eur. but the specified impurities are not mentioned. The present study deals with understanding the degradation behavior of Ketoconazole by subjecting it to acid, base, aqueous, thermal, photo and oxidative stress conditions. Furthermore, the two major degradation impurities observed under stressed condition were identified by LC-MS techniques, elemental analysis, NMR, and their structures were justified through mechanistic explanation.
Experimental
Material and reagents
Ketoconazole drug substance was obtained from Sharon Biomedichem (Navi Mumbai, India). All the chemicals and reagents, hydrochloric acid, sodium hydroxide, hydrogen peroxide (30 %), tetrabutylammonium hydrogen sulphate, acetonitrile and methanol were used of analytical grade, while a millipore milli Q plus water purification system (Milford, USA) was used to prepare distilled water (>18 μΩ).
Instruments
Integrated HPLC system, Ultimate 3000 manufactured by Dionex (Germany) was used for method development and method validation. This system consisted of a quaternary gradient pump, auto sampler, column oven and a photodiode array detector. PC installed Chromeleon software was used to record and to integrate the chromatograms. The analysis was carried out at ambient temperature. LCMS system, Agilent ion trap 6310 was used for mass fragmentation analysis. NMR experiments were recorded on Bruker 500 Mz spectrometer. Photostability studies were performed in a photostability chamber from Thermolab (India).
Chromatographic conditions
Analytical HPLC conditions
Inertsil ODS (Length: 100 mm, Diameter: 4.6 mm, Particle size: 3 μm) analytical column was used as a stationary phase. The flow rate was 2.0 ml min−1 and the detector was set at 220 nm. The volume of the sample solution injected was 10 μl. The gradient mobile phase consisted of Mobile phase A {(Acetonitrile: 3.4 g/l solution of tetrabutylammonium hydrogen sulphate (5:95 V/V)}: and Mobile phase B {(Acetonitrile: 3.4 g/l solution of tetra-butylammonium hydrogen sulphate (50:50 V/V)}. A membrane filter of 0.45 μm porosity was used to filter and degas the mobile phase. (Gradient program as mentioned in Tab. 1).
Tab. 1.
Mobile Phase gradient for HPLC chromatographic method
| Time (min) | Mobile phase A (% V/V) | Mobile phase B (& V/V) |
|---|---|---|
| 0 | 100 | 0 |
| 10 | 0 | 100 |
| 15 | 0 | 100 |
| 17 | 100 | 0 |
| 20 | 100 | 0 |
Analytical LC–MS conditions
Inertsil ODS (Length: 100 mm, Diameter: 4.6 mm, Particle size: 3 μm) analytical column was used as a stationary phase. The flow rate was 2.0 ml min−1 and the detector was set at 220 nm. The volume of the sample solution injected was 10 μL. The gradient mobile phase consisted of Mobile phase A (Water) and Mobile phase B (Acetonitrile). A membrane filter of 0.45 μm porosity was used to filter and degas the mobile phase. The gradient program as mentioned in Table 2. The LC-Mass condition was set using Nebulizer 50 PSI, dry gas temperature 350 degree and source ESI positive.
Tab. 2.
Mobile Phase gradient for LC-MS method
| Time (min) | Water (% V/V) | Acetonitrile (% V/V) |
|---|---|---|
| 0 | 100 | 0 |
| 20 | 0 | 100 |
| 30 | 0 | 100 |
Stress degradation of drug substance
Stress studies were carried out under acid, base, thermal, photo and oxidative stress conditions.
Acid Hydrolysis
250.0 mg of test sample + 2ml 1N HCl into 25 ml volumetric flask. Sample heated on boiling water bath at 100 deg, withdrawn at 2 min and 8 min, respectively, then neutralized with 1N NaOH solution and make up the volume to 25 ml with methanol. Pipette out 4 ml into 50 ml volumetric flask and dilute to volume with methanol.
One unknown degradation impurity was observed under acidic condition (Table 3 and figure 1c). In figure 1c, the main degradation product is unknown impurity at RRT 0.80.
Tab. 3.
Results of Acid degradation (1M HCl)
| Name of compounds | RRT | Sample “as such” | Initial | 4 hours at 25°C | 2 minutes heating at 100°C | 8 minutes heating at 100°C |
|---|---|---|---|---|---|---|
| Unknown | ~0.66 | ND | ND | 0.052 | ND | 0.085 |
| Unknown | ~0.72 | ND | ND | 0.105 | 0.042 | 0.080 |
| Unknown | ~0.76 | ND | ND | ND | 0.036 | ND |
| Unknown | ~0.80 | 0.005 | 0.018 | 0.878 | 5.778 | 22.122 |
| Ketoconazole | ~1.00 | 99.535 | 99.510 | 98.213 | 91.417 | 69.620 |
| Unknown | ~1.03 | 0.049 | 0.054 | 0.050 | 0.085 | 0.046 |
| Unknown | ~1.09 | ND | ND | ND | 0.045 | ND |
| Unknown | ~1.11 | ND | ND | 0.017 | 0.046 | 0.085 |
| Unknown | ~1.19 | 0.196 | 0.200 | 0.206 | 0.220 | 0.110 |
| Unknown | ~1.32 | 0.104 | 0.102 | 0.009 | 0.139 | ND |
| Unknown | ~1.38 | 0.114 | 0.110 | 0.106 | 0.126 | ND |
Fig. 1.
HPLC Chromatograms for Stressed conditions
Base Hydrolysis
250.0 mg of test sample + 2ml 1N NaOH into 25 ml volumetric flask. Sample heated on boiling water bath for 10 min and 30 min, respectively, then neutralized with 1N HCl solution and make up the volume to 25 ml with methanol. Pipette out 4 ml into 50 ml volumetric flask and dilute to volume with methanol.
One unknown degradation impurity was observed under basic condition which is the same as observed under acidic condition (Table 4 and figure 1d). In figure 1d, the main degradation product is unknown impurity at 0.80.
Tab. 4.
Results of Base degradation (1M NaOH)
| Name of compounds | RRT | Sample “as such” | Initial | 4 hours at 25°C | 10 minutes heating at 100°C | 30 minutes heating at 100°C |
|---|---|---|---|---|---|---|
| Unknown | ~0.66 | ND | 0.083 | 0.124 | 0.088 | ND |
| Unknown | ~0.72 | ND | 0.091 | 0.137 | 0.090 | 0.023 |
| Unknown | ~0.80 | 0.005 | 0.010 | 0.439 | 5.328 | 10.702 |
| Ketoconazole | ~1.00 | 99.535 | 99.310 | 98.055 | 92.172 | 88.821 |
| Unknown | ~1.03 | 0.049 | 0.076 | 0.136 | 0.079 | 0.109 |
| Unknown | ~1.09 | ND | ND | ND | ND | ND |
| Unknown | ~1.11 | ND | 0.014 | 0.004 | 0.161 | 0.163 |
| Unknown | ~1.19 | 0.196 | 0.200 | 0.212 | 0.100 | 0.037 |
| Unknown | ~1.32 | 0.104 | 0.099 | 0.114 | 0.094 | 0.130 |
| Unknown | ~1.38 | 0.114 | 0.111 | 0.120 | ND | ND |
Oxidation
250.0 mg of test sample + 2ml 30%H2O2 into 25 ml volumetric flask and heated for 10 min on boiling water bath. Make up the volume to 25 ml with methanol. Pipette out 4 ml into 50 ml volumetric flask and dilute to volume with methanol.
One unknown degradation impurity was observed under oxidative stress condition and it is different from the impurity observed under acidic/basic condition (Table 5 and figure 1e). In figure 1.e, the main degradation product is unknown impurity at RRT 0.72.
Tab. 5.
Results of Oxidative degradation (30% H2O2)
| Name of compounds | RRT | Sample “as such” | Initial | 4 hours at 25°C | 10 minutes heating at 100°C |
|---|---|---|---|---|---|
| Unknown | ~0.54 | ND | ND | ND | 0.219 |
| Unknown | ~0.56 | ND | ND | ND | 0.134 |
| Unknown | ~0.66 | ND | 0.125 | 0.149 | 0.084 |
| Unknown | ~0.72 | ND | 0.172 | 0.860 | 23.528 |
| Unknown | ~0.80 | 0.005 | 0.007 | 0.008 | 0.078 |
| Ketoconazole | ~1.00 | 99.535 | 99.185 | 98.478 | 74.995 |
| Unknown | ~1.03 | 0.049 | 0.124 | 0.116 | 0.500 |
| Unknown | ~1.19 | 0.196 | 0.213 | 0.212 | 0.174 |
| Unknown | ~1.32 | 0.104 | 0.012 | 0.010 | 0.047 |
| Unknown | ~1.38 | 0.114 | 0.124 | 0.124 | 0.122 |
Thermal
Test sample of Ketoconazole was subjected to thermal degradation by exposure to oven at 105°C for 24h and 60°C at 5 days and 10 days. 250.0 mg test sample of Ketoconazole were dissolved and diluted with methanol to 25 ml. Pipette out 4 ml into 50 ml volumetric flask and dilute to volume with methanol.
Photolysis
About 250.0 mg test sample of Ketoconazole is kept for UV degradation for 24hours at 254 nm wavelength and then dissolved and diluted with methanol to 25 ml. Pipette out 4 ml into 50 ml volumetric flask and dilute to volume with methanol. The drug substance was found stable under photo and thermal stress conditions as shown in below (Table 6, figure 1f and 1g).
Tab. 6.
Results of Thermal and UV degradation
| Name of compound | RRT | Sample “as such” | 105°C at 24 hours | 60°C at 5 days | 60°C at 10 days | 24 hours at 254 nm |
|---|---|---|---|---|---|---|
| Unknown | ~0.80 | 0.005 | 0.008 | 0.008 | 0.007 | 0.007 |
| Ketoconazole | ~1.00 | 99.535 | 99.576 | 99.578 | 99.561 | 99.536 |
| Unknown | ~1.03 | 0.049 | 0.046 | 0.050 | 0.055 | 0.053 |
| Unknown | ~1.19 | 0.196 | 0.179 | 0.183 | 0.190 | 0.195 |
| Unknown | ~1.32 | 0.104 | 0.103 | 0.093 | 0.102 | 0.097 |
| Unknown | ~1.38 | 0.114 | 0.085 | 0.084 | 0.083 | 0.109 |
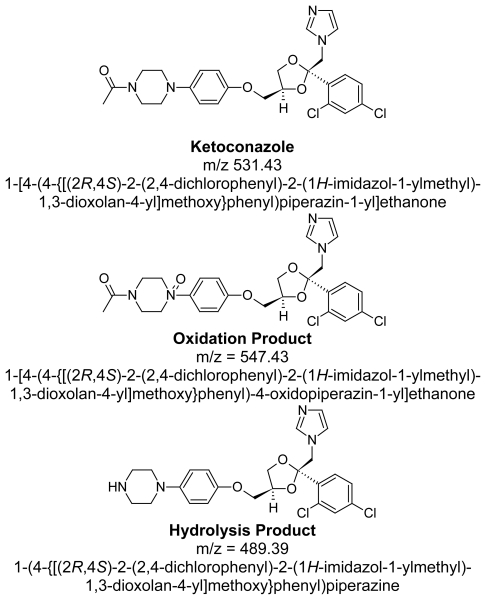
Preparation of Impurities
Hydrolysis degradent, Impurity D as per Ph. Eur., is synthesized in-house and identified by HPLC analysis, Mass spectrometer (figure 3b) and elemental analysis (figure 6b). Oxidative degradent is prepared in-house by degradation of Ketoconazole with 30% hydrogen peroxide by heating up to evaporate to dryness at 80°C. Ketoconazole gets converted to its N-oxide and identified by HPLC (figure 2b), LCMS analysis (figure 3c), NMR analysis (figure 5b) and Elemental analysis (figure 6c, 6d).
Fig. 3.
LC-Mass spectrum for Ketoconazole and degradent products
Fig. 6.
Elemental analysis for Ketoconazole and degradent products
Fig. 2.
HPLC Chromatograms for preparation Oxidative degradent
Fig. 5.
NMR Spectrum of Ketoconazole and N-Oxide
Elemental analysis
Elemental analysis (CHNO) of Ketoconazole, Hydrolysis degradent and Oxidative degradent performed and results shown below Table 7 and 8.
Tab. 7.
Results of Elemental analysis
| Ketoconazole MW 531.43 | Hydrolysis degradent MW 488.9 | Oxidative degradent MW 547.43 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Element | % Calc. | % Found | % Calc. | % Found | % Calc. | % Found |
| C | 58.71 | 58.87 | 58.91 | 58.84 | 56.994 | 58.378 |
| H | 5.27 | 5.38 | 5.32 | 5.35 | 5.115 | 5.254 |
| N | 10.54 | 10.55 | 11.45 | 10.62 | 10.230 | 10.092 |
| O | 12.04 | 12.03 | 9.82 | 10.03 | 14.614 | 14.066 |
Tab. 8.
No of atoms present in molecule
| Element | Ketoconazole | Hydrolysis degradent | Oxidative degradent |
|---|---|---|---|
| C | 26 | 24 | 26 |
| H | 28 | 26 | 28 |
| N | 4 | 4 | 4 |
| O | 4 | 3 | 5 |
| Observed Molecular formula | C26H28Cl2N4O4 | C24H26Cl2N4O3 | C26H28Cl2N4O5 |
NMR analysis
NMR analysis of Ketoconazole and the oxidative degradent were performed and the results are shown in Table 9.
Tab. 9.
Results of NMR analysis
| Ketoconazole | Oxidative degradent | |||
|---|---|---|---|---|
|
|
||||
| 1H δ (ppm) | No of protons | 1H δ (ppm) | No of protons | |
| 1 CH3 group | 2.134 | 2.939 | 2.103, 1.881 | 3.237 |
| 7 CH2 and 1 CH group | 2.988–3.897 | 15.075 | 3.200–4.794 | 15.525 |
| Aromatic protons | 6.777–7.660 | 9.757 | 6.826–7.841 | 9.796 |
Results and discussion
The degradation of Ketoconazole was performed under different stress conditions. Two major degradants are observed under stress degradation. One is hydrolysis product of Ketoconazole observed under acid/base condition and the other one is oxidative degradent observed under oxidative stress condition. The identification of oxidative degradent was achieved by LC-MS, NMR and Elemental analysis. The LC-MS data shows the mass 547.43 amu which exactly increase in the mass 16 amu from the Ketoconazole drug substance having mass 531.43 amu, which indicate the formation of N-oxide. LC-MS spectrums and fragmentation behavior of N-Oxide are given in figure 3c and 4b. Also, the elemental analysis of N-oxide shows the increase in oxygen atom (figure 6d), while in the case of hydrolysis decrease in oxygen atom compare to Ketoconazole. The NMR analysis of oxidative degradent shows the shifting of protons signal from their original position in Ketoconazole due to introduction electronegative oxygen atom (figure 5b).
Fig. 4.
Mass fragmentation behavior of Ketoconazole and N-Oxide
Hence, the formation of the oxidative degradation product from the drug as shown below is only due to the N-oxide formation at the piperazine ring. The lone pair at the nitrogen of the piperazine ring is more prone for oxidation to form an N-oxide. However, out of two nitrogen atoms, the electron pair on the nitrogen attached to the carbonyl group is participating in resonance delocalization with this group. Hence, the most possible N-Oxide at the nitrogen is at the one attached to the phenolic group (scheme 1).
Sch. 1.
Structure of elucidated compounds
Similar types of N-oxide degradents have been reported in the literature [14–16].
Conclusions
The Stress degradation on Ketoconazole was carried out under different acid, base, thermal, photo and oxidative stress conditions. The drug was found susceptible to acid, base and oxidative stress degradation. The unknown degradation products formed in the oxidative and hydrolysis stressed sample were identified using LC–MS and elemental analysis (CHNO). The investigations of oxidative and hydrolysis degradent will help to take proper care during selection of excipients in formulation, storage, packaging and handling of the drug product.
Acknowledgement
The authors are thankful to Mr. Lalit Mishra – Sharon Bio Medicine for their encouragement and support during the work.
Footnotes
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- 1.Vander Heyde Y, Nguyet AN, Detaevenier MR, Massart DL, Plaizier-Vercammen J. Simultaneous determination of ketoconazole and formaldehyde in a shampoo: liquid chromatography method development and validation. J Chromatogr A. 2002;958:191–201. doi: 10.1016/s0021-9673(02)00384-9. http://www.ncbi.nlm.nih.gov/pubmed/12134817. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen MN, Tallieu L, Plaizier-Vercammen J, Massart DL, Vander Heyden Y. Validation of an HPLC method on short columns to assay ketoconazole and formaldehyde in shampoo. J Pharm Biomed Anal. 2003;32:1–19. doi: 10.1016/s0731-7085(02)00640-4. http://dx.doi.org/10.1016/S0731-7085(02)00640-4. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Moety EM, Khattab FI, Kelani KM, AbouAl-Alamein AM. Chromatographic determination of clotrimazole, ketoconazole and fluconazole in pharmaceutical formulations. Farmaco. 2002;57:931–938. doi: 10.1016/s0014-827x(02)01270-3. http://dx.doi.org/10.1016/S0014-827X(02)01270-3. [DOI] [PubMed] [Google Scholar]
- 4.Vertzoni MV, Reppas C, Archontaki HA. Optimization and validation of a high-performance liquid chromatographic method with UV detection for the determination of ketoconazole in canine plasma. J Chromatogr B. 2006;839:62–67. doi: 10.1016/j.jchromb.2006.03.010. http://dx.doi.org/10.1016/j.jchromb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Velikinac I, Cudina O, Janković I, Agbaba D, Vladimirov S. Comparison of capillary zone electrophoresis and high performance liquid chromatography methods for quantitative determination of ketoconazole in drug formulations. Farmaco. 2004;59:419–424. doi: 10.1016/j.farmac.2003.11.019. http://dx.doi.org/10.1016/j.farmac.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Bernal JL, del Nozal MJ, Toribio L, Montequi MI, Nieto EM. Separation of ketoconazole enantiomers by chiral subcritical-fluid chromatography. J Biochem Biophys Methods. 2000;43:241–250. doi: 10.1016/s0165-022x(00)00060-9. http://dx.doi.org/10.1016/S0165-022X(00)00060-9. [DOI] [PubMed] [Google Scholar]
- 7.Yuen KH, Peh KK. Simple high-performance liquid chromatographic method for determination of ketoconazole in human plasma. J Chromatogr B. 1998;715:436–440. doi: 10.1016/s0378-4347(98)00253-9. http://dx.doi.org/10.1016/S0378-4347(98)00253-9. [DOI] [PubMed] [Google Scholar]
- 8.Bajad S, Johri RK, Singh K, Singh J, Bedi KL. Simple high-performance liquid chromatography method for the simultaneous determination of ketoconazole and piperine in rat plasma and hepatocyte culture. J Chromatogr A. 2002;949:43–47. doi: 10.1016/s0021-9673(01)01260-2. http://dx.doi.org/10.1016/S0021-9673(01)01260-2. [DOI] [PubMed] [Google Scholar]
- 9.Arranz P, Arranz A, Moreda JM, Cid A, Arranz JF. Stripping voltammetric and polarographic techniques for the determination of anti-fungal ketoconazole on the mercury electrode. J Pharm Biomed Anal. 2003;33:589–596. doi: 10.1016/s0731-7085(03)00247-4. http://dx.doi.org/10.1016/S0731-7085(03)00247-4. [DOI] [PubMed] [Google Scholar]
- 10.Giordani R, Trebaux J, Masi M, Regli P. Enhanced antifungal activity of ketoconazole by Euphorbia characias latex against Candida albicans. J Ethnopharmacol. 2001;78:1–5. doi: 10.1016/s0378-8741(01)00295-1. http://dx.doi.org/10.1016/S0378-8741(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 11.Bernal JL, del Nozal MJ, Toribio L, Montequi MI, Nieto EM. Separation of ketoconazole enantiomers by chiral subcritical-fluid chromatography. J Biochem Biophys Methods. 2000;43:241–250. doi: 10.1016/s0165-022x(00)00060-9. http://dx.doi.org/10.1016/S0165-022X(00)00060-9. [DOI] [PubMed] [Google Scholar]
- 12.de Bruijn P, Kehrer DF, Verweij J, Sparreboom A. Liquid chromatographic determination of ketoconazole, a potent inhibitor of CYP3A4-mediated metabolism. J Chromatogr B. 2001;753:395–400. doi: 10.1016/s0378-4347(00)00573-9. http://www.ncbi.nlm.nih.gov/pubmed/11334355. [DOI] [PubMed] [Google Scholar]
- 13.Staub I, Flores L, Gosmann G, Pohlmann A, Fröehlich PE, Schapoval EES, Bergold AM. Photostability Studies of Ketoconazole: Isolation and Structural Elucidation of the Main Photodegradation Products. Lat Am J Pharm. 2010;29:1100–1106. [Google Scholar]
- 14.Reddy GVR, Kumar AP, Reddy BV, Kumar P, Gauttam HD. Identification of degradation products in Aripiprazole tablets by LC-QToF mass spectrometry. Eur J Chem. 2010;1:20–27. http://dx.doi.org/10.5155/eurjchem.1.1.20-27.11. [Google Scholar]
- 15.Dyakonov T, Muir A, Nasri H, Toops D, Fatmi A. Isolation and characterization of cetirizine degradation product: mechanism of cetirizine oxidation. Pharm Res. 2010;27:1318–1324. doi: 10.1007/s11095-010-0114-x. http://dx.doi.org/10.1007/s11095-010-0114-x. [DOI] [PubMed] [Google Scholar]
- 16.Clement EM, Franklin M. Simultaneous measurement of zolmitriptan and its major metabolites N-desmethylzolmitriptan and zolmitriptan N-oxide in human plasma by high-performance liquid chromatography with coulometric detection. J Chromatogr B. 2002;766:339–343. doi: 10.1016/s0378-4347(01)00470-4. http://dx.doi.org/10.1016/S0378-4347(01)00470-4. [DOI] [PubMed] [Google Scholar]