Abstract
Objectives
The aim of this study was to test the efficacy of an antioxidant/anti-inflammatory supplement containing standardized lemon verbena (Aloysia triphylla, Lippia citriodora) extract and fish oil omega-3 fatty acid in a human pilot trial as an alternative treatment for joint management.
Methods and design
First, antioxidant activity of the supplement was determined through an oxygen radical absorbance capacity (ORAC) assay. In a randomized, double-blinded placebo-controlled trial, 45 subjects with pain discomfort received the nutritional supplement or placebo for 9 weeks. Western Ontario MacMaster (WOMAC) and Lequesne's questionnaires, which are disease-specific measurements validated to measure joint dysfunction and pain, were administered and evaluated once per week in the placebo and intervention groups.
Outcome measures
Pain and stiffness symptoms, and joint function were determined once per week through recording their respective WOMAC and Lequesne's scores in the placebo and intervention groups. Statistically significant differences were determined at every measurement point between the two groups.
Results
Lemon verbena extract showed strong antioxidant properties as measured by the ORAC assay. The nutritional supplement containing standardized lemon verbena extract (14% verbascoside, w/w) and fish oil omega-3 fatty acid reduced symptoms of pain and stiffness significantly, and improved physical function as shown by WOMAC and Lequesne's scores after 9 weeks of treatment. WOMAC and Lequesne's total scores decreased 53% and 78%, respectively, at the end of the study compared to initial conditions. Onset of the effect was observed at the third and fourth weeks, when statistically significant differences were detected, compared to placebo.
Conclusions
This pilot study reveals that supplementation with lemon verbena combined with omega-3 fatty acids may be considered for further investigation as a complementary and alternative treatment for improving joint status in subjects with joint discomfort.
Introduction
Joint diseases, such as osteoarthritis (OA) and rheumatoid arthritis (RA), have quite different origins, but both are closely related to inflammation and oxidative stress.1 OA is a common inflammatory degenerative condition of the joints associated with aging, leading to pain and decreased mobility, while RA is an autoimmune chronic inflammatory condition.
Oxidants play a significant role in the pathogenesis of a large number of inflammatory-related disorders. Chondrocytes, cells that maintain and restore cartilage components, are also sources of reactive oxygen species (ROS), which may damage cartilage.2–5 Reactive oxygen and nitrogen species (RONS) are responsible for damage on cartilage, synovial fluid structures, and mitochondria, which may also contribute to the age-related loss of chondrocyte function.2,4,6–9 Strong evidence suggests that RONS oxidative damage is present in aging and osteoarthritic cartilage,10 which is correlated with the extent of cartilage damage.11 Patients with chondral or meniscal lesions also have significantly increased levels of ROS in their synovial fluid.12
Physical exercise may have either beneficial or injurious effects on the joints. Harmful effects of intensive physical exercise on articular cartilage are well-documented.13 Increased risk of OA in the knee has been found among weight lifters14 and soccer players.15 Increased oxidative stress biomarkers following strenuous exercise, which correlate to reductions in muscle force as well as augmented joint and muscle pain, have been reported. Different forms of exercise and different lengths of overtraining can possibly lead to varying degrees of oxidative stress and impaired physical performance.16
Medicinal plants are common sources of antioxidant or anti-inflammatory/immunomodulatory herbal agents that might help minimize the muscular and joint injuries related to oxidative stress and inflammation. Plant phytochemicals with antioxidant activity have also shown significant protective effects against inflammation-related chronic diseases.1,17 Lemon verbena (Aloysia triphylla, Lippia citriodora) is widely used as a food spice and as a medicinal plant in South America and Southern Europe. This plant has traditionally been used in infusions for treating asthma, colds, fevers, flatulence, colic, diarrhea, and indigestion.18 Flavones, iridoids, and phenylpropanoids are the main compounds of this plant. Verbascoside is the most abundant compound19 and it has strong antioxidant19–21 and anti-inflammatory properties.22–25
Fish oils contain the long chain omega-3 polyunsaturated fatty acids (ω-3 PUFAs; i.e., eicosapentaenoic acid [EPA: C20:5n-3] and docosahexanoic acid [DHA: C22:6n-3]), which are found in abundance in cold-water fish, such as salmon, trout, mackerel, and tuna. Evidence from animal and human studies shows that ω-3 PUFAs have immunomodulatory effects.26 Other studies have shown that fish-oil consumption reduces production of proinflammatory eicosanoids in RA.27,28 This has prompted researchers to investigate the usefulness of ω-3 PUFAs for treating inflammatory conditions, such as RA, inflammatory bowel disease, systemic lupus erythematosus, psoriasis, asthma, etc.1,29,30
The aim of the present study was to evaluate the ability of a nutritional supplement based on a combination of a lemon verbena extract and fish oil omega-3 fatty acid to improve the general condition of joints (pain, stiffness and function) in subjects who have joint discomfort. The evaluation was performed with disease-specific measurements that have been validated to measure joint dysfunction. In addition, the antioxidant activity of the lemon verbena extract used in the study and the final supplement was evaluated in vitro.
Materials and Methods
Reagents
2,2′-Azobis (2-methyl-propionamine) dihydrochloride (AAPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox; Hoffman-La Roche), disodium fluorescein (3,6-dihydroxy-spiro[isobenzofuran-1[3H],9,[9H]-xanthen]-3-one) were purchased from the Sigma–Aldrich Corp. (Dorset, UK). Verbascoside was purified through semipreparative high-performance liquid chromatography (HPLC).31 Double-distilled deionized water was used throughout this work. All other compounds were of analytical, spectroscopic, or chromatographic reagent grade and were obtained from Merck KGaA (Darmstadt, Germany).
Analysis of verbascoside content of the lemon verbena extract
Lemon verbena extract was kindly provided by Monteloeder, Ltd. (Elche, Spain). A solution of the extract was prepared in water at 5 mg/mL, and analyzed by HPLC with a diode array-detection coupled to electrospray ion trap mass spectrometry technique (HPLC-DAD-ESI-MS/MS). HPLC system chromatographic procedures, identification, and verbascoside quantitation were identical to those previously described in the literature.19
Determination of antioxidant capacity via oxygen radical absorbance capacity assay
To assay the capacity of the extract and supplement to scavenge peroxyl radicals, a validated oxygen radical absorbance capacity (ORAC) method,32 which uses fluorescein (FL) as a fluorescent probe (ORACFL), was utilized with minor modifications.33 ORAC values were expressed as μmole Trolox equivalents (TE) per g of antioxidant substance.
Design and methodology for the human intervention study
This was a double-blinded, placebo-controlled, randomized short-time study in participants with joint discomfort. Most participants agreed to maintain their regular physical activities. Each participant was treated with a placebo or the supplement for 9 weeks. A washout period of 2 weeks was included to discontinue any medication, physiotherapy, or dietary supplements until the end of the study.
Inclusion and exclusion criteria
The inclusion criteria were as follows: diagnosed joint discomfort/pain (knee, hip, elbow, hands, or shoulder) by a physician for more than 3 months; and age over 18. Both males and females were included. The exclusion criteria were: medication (NSAR, corticoids, antibiotics) use, except for cholesterol-lowering agents, antihypertonics, and antiarhythmics; and participants reporting allergies to plant products, vitamins, minerals, or any phytotherapy supplementation.
Randomization
Participants fulfilling the study criteria were randomized to receive placebo or the supplement, using a 12×12 randomization table. Capsules used in both groups were entirely indistinguishable from each other and the manufacturer (Laboratorium Medisan BV., Zevenbergen, The Netherlands) supplied the two types of capsules (placebo and supplement) in a codified form.
Study drug and administration
After giving written informed consent, 45 participants were enrolled in the study. Twenty-two (22) participants were assigned to placebo and 23 were assigned to the supplement. Each hard gelatin capsule of supplement (0.6 g) contained 370 mg of fish-oil powder EPA/DHA (10/8) and 230 mg of the standardized lemon verbena extract (14% w/w verbascoside). Each group, placebo (n=22) or supplement (n=23), consumed 6 capsules per day (before every meal) from weeks 1 to 5. Then, starting at week 6 until the end of the study (week 9), the dose was halved and participants consumed 3 capsules per day. The content of verbascoside for 6 or 3 capsules (daily dosages) was 193 and 97 mg, respectively.
Primary endpoint measurements
Pain/stiffness and function were recorded according to the corresponding scoring obtained from the Western Ontario MacMaster (WOMAC) and Lequesne's questionnaires at the beginning of the study and weekly during the study. The participants completed the questionnaires a minimum of once per week (see Appendix 1 for details). These two questionnaires (Lequesne Index and WOMAC Osteoarthritis Index) are disease-specific measurements that have validated to measure dysfunction and pain in participants with knee or hip OA.34–36 The WOMAC scale is a disease-specific, self-report multidimensional questionnaire for assessing pain, stiffness, and physical functional disability in OA. An adaptation of the WOMAC questionnaire was used in this study (see supplemental data). The Lequesne Index is used to evaluate three components: pain/discomfort; maximum distance walked; and activities of daily living. Lower scores indicate a lesser degree of pain, stiffness, or physical dysfunction. In this study, pain+stiffness (PS) WOMAC score was calculated by the addition of their individual scores.
Statistical analysis
Statistical significance and comparison between the placebo and treatment groups at every week point were performed using a Wilcoxon two-sample test. A Wilcoxon matched-pairs signed-ranks test was used to compare baseline findings with those after every week of treatment in each group separately. All the data were expressed as mean±standard deviation (SD) unless indicated otherwise. Statistical significance was set at p<0.05.
Results and Discussion
Evaluation of the composition and antioxidant capacity of the supplement and its components
The character of the main active compounds in the lemon verbena extract by HPLC-DAD-ESI-MS/MS analysis has been reported.19,37 Phenylpropanoids were the major compounds in the extract utilized in the present study, with verbascoside, also known as acteoside, being the most abundant one, quantitated as 14.75%±0.85 (w/w). As has been previously reported, other phenylpropanoids and diglucuronidated flavones were also present in the lemon verbena extract.19,37,38
The antioxidant capacity of verbascoside and the lemon verbena extract used in the supplement was quantified by ORAC assay, which is an antioxidant measurement based on a hydrogen atom transfer (HAT) reaction.32,39 Whereas pure verbascoside (97% w/w) showed an ORAC value of 7307±66 μmol TE/mmol (11710±106 μmol TE/g), lemon verbena extract (14%) had an ORAC value of 5183±300 TE/g. It was noted that the theoretical ORAC value of the extract obtained based only on a verbascoside contribution would have been 1639 μmol TE/g dw; however the value obtained was more than three times this value. It was then postulated that other minor components of the extract, such as flavones, could be contributing to enhance the antioxidant capacity of the extract in an additive manner.
An ORACFL value of 4075±234 TE/g dw for a lemon verbena extract containing 20% verbascoside19 has been reported. Despite the lower verbascoside content of the extract used here, a higher ORAC value was obtained instead, which may have been the result of the higher flavone content of this extract. This additive behavior has been also previously observed for lemon verbena extract and verbascoside by using the Trolox equivalent antioxidant capacity (TEAC) assay.19 The experimentally determined ORAC value of the complete supplement (lemon verbena+omega 3 fatty acid) yielded 1065±122 μmol TE/g. Given that one g of the supplement contained 383 mg of lemon verbena extract, a theoretical ORAC value of 1985 μmol TE/g would result. This difference could have been the result of inhibition of the ORAC assay by some excipients or omega 3 fatty-acid carriers present in the supplement.
An average person consumes ∼1200–1700 ORAC units per day (see: www.ars.usda.gov/is/AR/archive/feb99/aging0299.htm). Researchers estimate a daily intake of 3000–5000 ORAC units to have a significant impact on plasma and tissue antioxidant capacity. Daily intake of lemon verbena in the human study presented here varied from 0.7 to 1.4 g. Thus, the supplement would have provided 3500–7000 ORAC units per day, satisfying the established requirements.
Dropout rate of the study
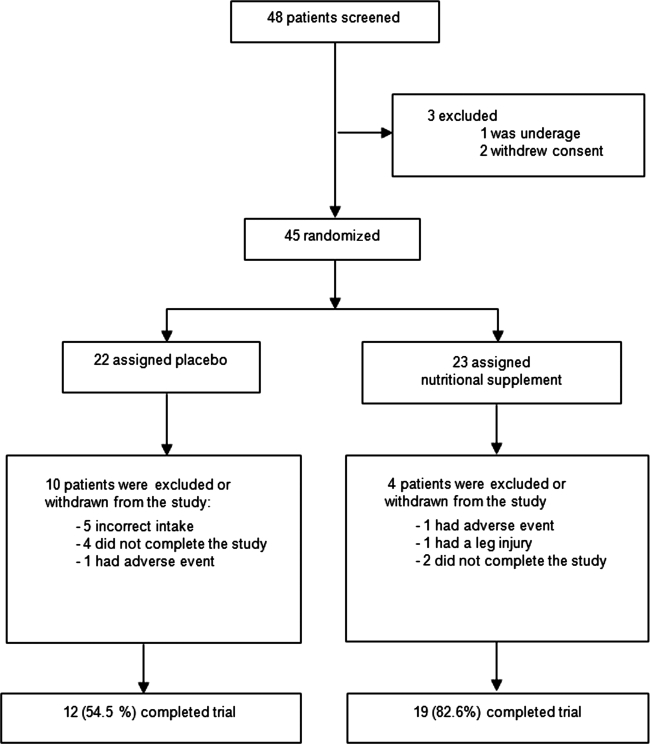
Forty-eight (48) participants were enrolled for screening evaluation (Fig. 1) and after 3 exclusions, 45 participants were randomly assigned either to placebo or nutritional supplement groups, n=22 and n=23, respectively. Of these, 14 participants were withdrawn during the study for different reasons; there were 10 dropouts in the placebo group and 4 dropouts in the supplement group (treatment refusal, irregular treatment, starting on medication, or occurrence of an adverse event [AE]). Finally, 31 participants completed the study (12 in the placebo and 19 in the supplement group; Fig. 1).
FIG. 1.
Flow chart showing the dropout rate at different timepoints in the study.
Subjects' characteristics
No significant differences between the treatment group and the placebo group were detected with respect to average age, gender, body–mass index (BMI), or other physical characteristics (Table 1). Most participants maintained regular physical activity. Both the placebo and intervention groups had similar sociodemographic characteristics (Table 1), with a mean age of ∼39, a height of 171 cm, a weight of 76 kg, and a BMI of 26 (Table 1).
Table 1.
Baseline Demographic Characteristics of Participants Who Completed the Study
| Placebo group (n=12) | Intervention group (n=19) | |
|---|---|---|
| Characteristics | ||
| Gender (m:f) | 8/4 | 12/7 |
| Age (years)a | 39.91±12.41 | 39.15±11.77 |
| Height (cm)a | 171.58±9.43 | 171.15±8.51 |
| Weight (kg)a | 76.66±11.75 | 76.55±13.65 |
| Body–mass indexa | 25.96±2.98 | 26.15±2.86 |
| Previous treatment, activity, and history | ||
| Treatment | ||
| Anti-inflammatory drugs (ibuprofen, paracetamol, etc.) | 6 (50 %) | 12 (63 %) |
| Glizolan (osteoarthritis medication) | – | 1 (5.2 %) |
| Robaxin (muscle-relaxing agent) | 1 (8.33 %) | – |
| Homeopathy, nutritional supplements (chondroitin, glucosamine, etc.) | 4 (33.3 %) | 5 (26.31%) |
| Others (massages, ultrasounds, physiotherapy, other treatments) | 3 (25 %) | 2 (10.52%) |
| Physical activity | ||
| Cardiovascular exercise (elliptical bicycle, walking, swimming) | 7 (58.33%) | 13 (68.42%) |
| Body building | 4 (33.33%) | 6 (31.57%) |
| Other sports (soccer, tennis, t'ai chi) | 1 (8.33 %) | 2 (10.52%) |
| Medical history | ||
| Joint pain/stiffness: (knee, shoulder, elbow, hip, etc.) | 6 (50%) | 14 (73.68%) |
| Rheumatoid arthritis | 1 (8.33%) | 2 (10.52%) |
| Degenerative osteoarthritis (knees) | 1 (8.33 %) | 1 (5.26 %) |
| Back pain (herniated disc, lumbago) | 2 (16.67 %) | 3 (15.78%) |
| Tendonitis (shoulder, elbow, wrist) | 3 (25 %) | 3 (15.78 %) |
Data are expressed as median±standard deviation of number of participants, except for gender distribution of male:female (m:f) that is expressed as a ratio.
Adverse effects
Only 1 patient reported an AE (i.e., a heartburn sensation). The subject, who was in the placebo group, stopped the treatment immediately and was excluded from the study (Table 1). No major complications were reported by this subject.
Efficacy in joint discomfort measured by WOMAC and Lequesne's indexes
First, the WOMAC multidimensional questionnaire was used to assess pain, stiffness, and physical function of the joints throughout the study. Pain+Stiffness PS WOMAC parameter, Function (F) WOMAC parameter, WOMAC Total Score and Lequesne's Functional Index were determined for both groups prior to the intervention to establish baseline scores (Table 2). No statistical differences were found at baseline WOMAC and Lequesne's different scores between the two groups (Table 2). Therefore, both groups could be considered to be very well-matched. A decrease in any of the absolute baseline scores for WOMAC or Lequesne's values must be interpreted as an improvement in the joint status.
Table 2.
Baseline Scores for Placebo and Intervention Groups
| Placebo group | Intervention group | Wilcoxon two-sample test (p) | |
|---|---|---|---|
| WOMAC—Pain+Stiffness | 8.9±5.14 | 11.46±6.21 | 0.544 |
| WOMAC—Function | 18.28±10.76 | 26.51±13.56 | 0.375 |
| WOMAC—Total Score | 27.43±15.55 | 38.03±18.75 | 0.421 |
| Lequesne Functional Index | 12.04±5.01 | 8.64±3.9 | 0.125 |
Results of pretesting on the comparison of the placebo group (n=12) with the. nutritional supplement group (n=19). All data are expressed as a median with a 95% confidence ratio.
WOMAC, Western Ontario McMaster index.
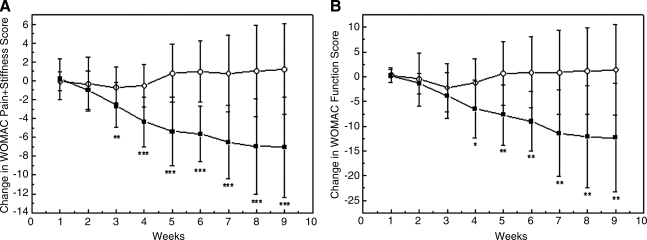
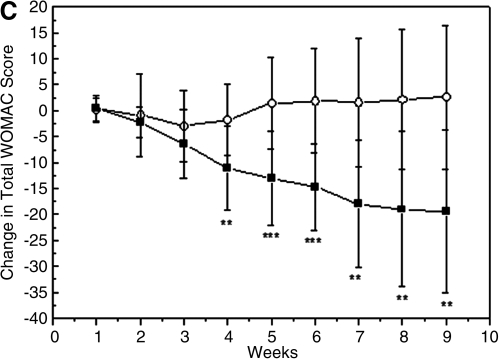
The evolution of symptoms was determined by studying the weekly evolution in mean changes of (PS) WOMAC parameter (Fig. 2A), F WOMAC parameter (Fig. 2B), WOMAC Total Score (Fig. 2C), and Lequesne's Functional Index for both groups (Fig. 3). The comparison between baseline and endpoint of the study (week 9) for both WOMAC parameters, revealed a continuous decrease in the group having the nutritional supplement, indicating improvement of joint status and pain relief. Moreover, decreases of PS WOMAC (Fig. 2A) and F WOMAC (Fig. 2B) were significant starting at the third and fourth weeks, respectively (p<0.01 at the third week and p<0.05 at the fourth week), compared to placebo, and continued to decrease until the end of the study. No significant changes in the PS and F WOMAC subscales were observed for the placebo group throughout the study. The total WOMAC index decreased approximately 20 points after 9 weeks in the group receiving the supplement (Fig. 2C), meaning that there was a 53% decrease, compared to initial WOMAC value. After 21 days, significant differences (p<0.01 at the fourth week) were already observed when the supplement group was compared to the placebo group. In contrast, no changes were observed in the total WOMAC index for the placebo group either (Fig. 2C).
FIG. 2.
Western Ontario MacMaster (WOMAC) index results. Change from baseline (start of treatment) to endpoint in Pain+Stiffness (PS) (A), function (F) (B) and total (C) WOMAC scores, during the weeks of treatment in participants having the supplement (filled squares) or placebo (empty circles). Data are expressed as mean±standard deviation. *p≤0.05; **p≤0.01; and ***p≤0.001 between groups.
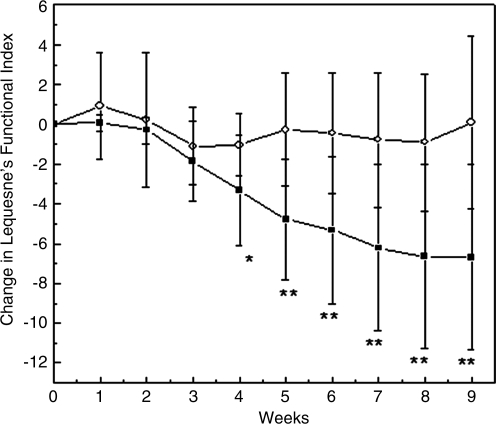
FIG. 3.
Lequesne's Functional Index results. Change from baseline (start of treatment) to endpoint in Lequesne's Functional Index during the weeks of treatment in participants having the supplement (filled squares) or placebo (empty circles). Data are expressed as mean±standard deviation. *p≤0.05; **p≤0.01; and (not shown) ***p≤0.001 between groups.
The Lequesne's questionnaire was also used as a complementary measurement to test the efficacy of the supplement. During the first 3 weeks, both placebo and supplement group had similar Lequesne's Functional Index scores (Fig. 3). However, starting at the fourth week and, until the end of the study, significant differences (p<0.05 at the fourth week and p<0.01 at the fifth week) were obtained in the supplement group, compared to the placebo group. The Lequesne Functional Index scores decreased continously in the supplement group until the end of the study; the scores dropped 78%, compared to the initial values. The placebo group had similar Lequesne's Functional Index scores throughout the study.
To the current authors' knowledge, this is the first intervention study to show, in subjects who had joint discomfort, improvement of the general condition of their joints, after consuming a nutritional supplement based on an herbal extract combined with omega-3 fatty acids. Recently, the use of different dietary supplements based on antioxidant/anti-inflammatory herbal ingredients, vitamins or omega-3 fatty acid, has been reviewed for their ability to manage joint problems.1,40 These studies concluded that only intervention studies using cat's claw (Uncaria tomentosa), ginger (Zingiber officinale),41 or omega-3 fatty acid seem to be reasonably supported. Moreover, weak evidence of efficacy was concluded by researchers who conducted a review of the use of vitamins and selenium for the same purpose.42 Finally, meta-analysis studies of the use of chondroitin and glucosamine for joint diseases show that that symptomatic benefits of these supplements are minimal or nonexistent.43,44 In any event, most review studies agree that there is a clear need for well-designed, placebo-controlled double-blinded studies to determine the potential benefits of herbs for treating ROS-mediated inflammatory disorders.
In addition, physical exercise associated with a low intake of antioxidant nutrients may cause greater vulnerability to oxidative stress. Thus, intake of an antioxidant-enriched diet is still the most cautious recommendation for minimizing the deleterious actions of free radicals that result from exercise.45 Accordingly, several studies have revealed that moderate doses of dietary polyphenols diminish this exercise-induced oxidative stress and decrease inflammatory markers.46–48 The supplement used in the current study could also lower the negative impact of physical exercise, because lemon verbena extract has shown significant free-radical scavenging properties, both in vitro31 and in animal models.19
Conclusions
In conclusion, this pilot study indicates that a standardized lemon verbena extract with antioxidant and anti-inflammatory properties may be used in combination with fish-oil omega-3 fatty acid as an alternative treatment for joint management. Use of the supplement for 9 weeks in subjects who had joint discomfort revealed a significant improvement of joint status and pain relief, compared to placebo. These results show that a combination of lemon verbena with omega-3 fatty acid may be considered for further investigation, either as an alternative treatment or to reduce the use of anti-inflammatory drugs in joint management.
Appendix 1. Evaluation of Womac and Lequesne's Scores
Acknowledgments
This investigation has been supported by Grants Torres-Quevedo (PTQ04-3-0778) to N. Caturla, and AGL2007—60778, both from MEC, and Grant ACOMP/2010/107 from Generalitat Valenciana. We also thank Monteloeder, S.L. and New Developments in Nutraceuticals, SL for providing us with the nutritional supplement.
Disclosure Statement
No competing financial interests exist.
References
- 1.Rosenbaum CC. O'Mathuna DP. Chavez M. Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16:32–40. [PubMed] [Google Scholar]
- 2.Henrotin Y. Deby-Dupont G. Deby C, et al. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32:562–567. doi: 10.1093/rheumatology/32.7.562. [DOI] [PubMed] [Google Scholar]
- 3.Henrotin Y. Deby-Dupont G. Deby C, et al. Active oxygen species, articular inflammation and cartilage damage. EXS. 1992;62:308–322. doi: 10.1007/978-3-0348-7460-1_31. [DOI] [PubMed] [Google Scholar]
- 4.Rathakrishnan C. Tiku K. Raghavan A. Tiku ML. Release of oxygen radicals by articular chondrocytes: A study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J Bone Miner Res. 1992;7:1139–1148. doi: 10.1002/jbmr.5650071005. [DOI] [PubMed] [Google Scholar]
- 5.Tiku ML. Allison GT. Naik K. Karry SK. Malondialdehyde oxidation of cartilage collagen by chondrocytes. Osteoarthr Cartilage. 2003;11:159–166. doi: 10.1016/s1063-4584(02)00348-5. [DOI] [PubMed] [Google Scholar]
- 6.Martin JA. Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257–264. doi: 10.1023/a:1020185404126. [DOI] [PubMed] [Google Scholar]
- 7.McCord JM. Free radicals and inflammation: Protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald RA. Moy WW. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979;22:251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- 9.Soltes L. Mendichi R. Kogan G, et al. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 10.Loeser RF. Carlson CS. Del Carlo M. Cole A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 11.Yudoh K. Nguyen T. Nakamura H, et al. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–R391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haklar U. Yuksel M. Velioglu A, et al. Oxygen radicals and nitric oxide levels in chondral or meniscal lesions or both. Clin Orthop Relat Res. 2002;403:135–142. [PubMed] [Google Scholar]
- 13.Manninen P. Riihimaki H. Heliovaara M. Suomalainen O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxf) 2001;40:432–437. doi: 10.1093/rheumatology/40.4.432. [DOI] [PubMed] [Google Scholar]
- 14.Kujala UM. Kettunen J. Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38:539–546. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 15.Roos H. Lindberg H. Gardsell P, et al. The prevalence of gonarthrosis and its relation to meniscectomy in former soccer players. Am J Sports Med. 1994;22:219–222. doi: 10.1177/036354659402200211. [DOI] [PubMed] [Google Scholar]
- 16.Bloomer RJ. Effect of exercise on oxidative stress biomarkers. Adv Clin Chem. 2008;46:1–50. doi: 10.1016/s0065-2423(08)00401-0. [DOI] [PubMed] [Google Scholar]
- 17.Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70(suppl):491S–499S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 18.Newall CA. Anderson LA. Phillipson JD. Herbal Medicines–A Guide for Health Care Professionals. London: The Pharmaceutical Press; 1996. p. 179. [Google Scholar]
- 19.Funes L. Fernández-Arroyo S. Laporta O, et al. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009;117:589–598. [Google Scholar]
- 20.Valentao P. Fernandes E. Carvalho F, et al. Studies on the antioxidant activity of Lippia citriodora infusion: Scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull. 2002;25:1324–1327. doi: 10.1248/bpb.25.1324. [DOI] [PubMed] [Google Scholar]
- 21.Liu MJ. Li JX. Guo HZ, et al. The effects of verbascoside on plasma lipid peroxidation level and erythrocyte membrane fluidity during immobilization in rabbits: A time course study. Life Sci. 2003;73:883–892. doi: 10.1016/s0024-3205(03)00354-0. [DOI] [PubMed] [Google Scholar]
- 22.Korkina LG. Mikhal'chik EV. Suprun MV, et al. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell Mol Biol. 2007;53:84–91. [PubMed] [Google Scholar]
- 23.Hausmann M. Obermeier F. Paper DH, et al. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2007;148:373–381. doi: 10.1111/j.1365-2249.2007.03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JY. Woo ER. Kang KW. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J Ethnopharmacol. 2005;97:561–566. doi: 10.1016/j.jep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Díaz AM. Abad MJ. Fernández L, et al. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004;74:2515–2526. doi: 10.1016/j.lfs.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DS. Hubbard NE. Erickson KL. Regulation of human immune and inflammatory responses by dietary fatty acids. Adv Food Nutr Res. 2005;50:101–138. doi: 10.1016/S1043-4526(05)50004-4. [DOI] [PubMed] [Google Scholar]
- 27.James MJ. Cleland LG. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum. 1997;27:85–97. doi: 10.1016/s0049-0172(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 28.Cleland LG. French JK. Betts WH, et al. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1998;15:1471–1475. [PubMed] [Google Scholar]
- 29.Goldberg RJ. Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Ruxton CHS. Reed SC. Simpson MJA. Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J Human Nutr Dietetics. 2004;17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 31.Funes L. Laporta O. Cerdán-Calero M. Micol V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem Phys Lipids. 2010;163:190–199. doi: 10.1016/j.chemphyslip.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Ou B. Hampsch-Woodill M. Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 33.Laporta O. Perez-Fons L. Mallavia R, et al. Isolation, characterization and antioxidant capacity assessment of the bioactive compounds derived from Hypoxis rooperi corm extract (African potato) Food Chem. 2007;101:1442–1454. [Google Scholar]
- 34.Bellamy NBW. Goldsmith CH. Campbell J. Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1999;15:1833–1840. [PubMed] [Google Scholar]
- 35.Faucher MPS. Lefevre-Colau MM. Rannou F, et al. Algo-functional assessment of knee osteoarthritis: Comparison of the test–retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarth Cartil. 2002;10:602–610. doi: 10.1053/joca.2002.0533. [DOI] [PubMed] [Google Scholar]
- 36.Lequesne M. Informational indices: Validation of criteria and tests. Scand J Rheumatol. 1989;80:17–27. doi: 10.3109/03009748909103708. [DOI] [PubMed] [Google Scholar]
- 37.Quirantes-Piné R. Funes L. Micol V, et al. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J Chromatogr A. 2009;1216:5391–5397. doi: 10.1016/j.chroma.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 38.Bilia AR. Giomi M. Innocenti M, et al. HPLC-DAD-ESI-MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J Pharm Biomedical Anal. 2008;46:463–470. doi: 10.1016/j.jpba.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Huang D. Ou B. Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan M. Mutlu EA. Benson M, et al. Use of herbal preparations in the treatment of oxidant-mediated inflammatory disorders. Complement Ther Med. 2007;15:207–216. doi: 10.1016/j.ctim.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Altman R. Marcussen K. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44:2531–2538. doi: 10.1002/1529-0131(200111)44:11<2531::aid-art433>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 42.Canter PH. Wider B. Ernst E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: A systematic review of randomized clinical trials. Rheumatology (Oxf) 2007;46:1223–1233. doi: 10.1093/rheumatology/kem116. [DOI] [PubMed] [Google Scholar]
- 43.Reichenbach S. Sterchi R. Scherer M, et al. Meta-analysis: Chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. 2007;146:580–590. doi: 10.7326/0003-4819-146-8-200704170-00009. [DOI] [PubMed] [Google Scholar]
- 44.Towheed TE. Maxwell L. Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;2:CD002946. doi: 10.1002/14651858.CD002946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urso ML. Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 46.Panza VSP. Wazlawik E. Ricardo Schutz G, et al. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition. 2008;24:433–442. doi: 10.1016/j.nut.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Nieman DC. Henson DA. Davis JM, et al. Quercetin's influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007;103:1728–1735. doi: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- 48.Morillas-Ruiz JM. Villegas García JA. López FJ, et al. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr. 2006;25:444–453. doi: 10.1016/j.clnu.2005.11.007. [DOI] [PubMed] [Google Scholar]