Abstract
The antibacterial activity of methanol, ethanol, chloroform, and hexane extracts of the leaves of Himalayan gymnospermous plant Ginkgo biloba L. was assessed against five animal and plant pathogenic strains (Agrobacterium tumefaciens, Bacillus subtilis, Escherichia coli, Erwinia chrysanthemi, and Xanthomonas phaseoli) employing disc-diffusion and broth-dilution assays. The methanol extract showed the highest activity (zone of inhibition of 15–21 mm) followed by ethanol (14–19 mm), chloroform (15–20 mm), and hexane (14–19 mm) extracts at 250 μg/mL. A minimum inhibitory concentration (MIC) of 7.8 μg/mL was found for the methanol extract against most of the pathogens tested.
Keywords: Ginkgo biloba, antimicrobial activity, antibacterial, gymnosperms
1. INTRODUCTION
Ginkgo biloba L. is considered a living fossil due to its survival over millions of years [1, 2]. One of the greatest properties of Ginkgo is its resistance to serious plant diseases. Ginkgo contains a number of biologically active compounds for its defence against insects, bacteria, and fungi [3]. An extract from the leaves of G. biloba, used in the treatment of cerebral insufficiency, was developed in the 1960s [4] and designated EGb 761. This preparation is an acetone-water (60 : 40) extract from the dried leaves [5]. A number of secondary metabolites, like terpenoids, polyphenols, organic acids, and amino acids have been isolated from the plant. However, the main bioactive constituents are terpene trilactones and flavonoid glycosides which are considered responsible for the pharmacological activities of the standardized leaf extract [6]. Ginkgo leaves have attracted much attention as agents for improving cerebral circulation and they possess antitumor, antiparasitic, and antiviral activities [7–9]. The role of the extract in the treatment of diseases involving free radicals and oxidative damage has also been suggested [10, 11].
However, little is known about the antimicrobial activity of gymnospermous plants including G. biloba [12]. Therefore, the antibacterial potential of various crude organic extracts of leaves of G. biloba collected at high altitude in Kumaun Himalaya, India was evaluated against a wide range of pathogenic bacterial strains.
2. MATERIALS AND METHODS
2.1. Collection of Plant Material
The tree of Ginkgo biloba (Ginkgoaceae) or maidenhair tree (Figure 1(a)) is characterized by fan-shaped leaves and fleshy, yellow, foul-smelling seeds, enclosing a silvery, edible inner kernel. The green leaves of G. biloba (Ginkgoaceae) were collected in the month of June 2010 from Nainital, Kumaun Himalaya, India and authenticated by the Department of Botany, Kumaun University, Nainital. A voucher specimen was deposited in the herbarium of the department (KU 203).
Figure 1.
(a) Ginkgo biloba; (b) leaves powder.
2.2. Extraction Procedure
Leaves of the plant were thoroughly washed and dried at room temperature. The dried material was powdered in an electric grinder (Figure 1(b)). To prepare a stock solution, 50 g of this powder was added to 200 mL of solvents (w/v, 50 g/200 mL). Solvents used for extraction were methanol, ethanol, chloroform, hexane, and water. Each extract was shaken for at least 6 h and after that each extract was passed through Whatman filter paper no.1. The final filtrate was concentrated on a rotary evaporator under vacuum at 20°C and was utilized for the experiments.
2.3. Microorganisms Used
Five (Gram +ve and −ve) bacteria (Bacillus subtilis MTCC no.121, Escherichia coli MTCC no.40, Agrobacterium tumefaciens MTCC no.609, borrowed from Institute of Microbial Technology, Chandigarh, India and Xanthomonas phaseoli and Erwinia chrysanthemi were obtained from Plant Pathology Department, G. B. Pant University, Pantnagar, India) were used.
2.4. Screening of Antibacterial Activity
Antibacterial tests of selected microorganisms were carried out using a disc-diffusion method [13]. A small sterile cotton swab was dipped into the 24-hour-old culture of bacteria and was inoculated by streaking the swab over the entire agar surface. After inoculation the plates were allowed to dry at room temperature in laminar chamber. The filter paper discs (5 mm) loaded with 40 μL of extract were placed on the surface of the agar plates. After 5 min the plates were incubated at 37°C for 24 h. Gentamycin (30 mg), erythromycin (15 mg), and ampicillin (10 mg; Central Drug House Ltd., New Delhi) were used as positive controls and the respective solvent as negative control. After 24 h of incubation, the diameter was observed for zone of inhibition, ZOI (measured in mm including disc size). All tests were performed in triplicate and observed values of ZOI are expressed as mean value with standard error of means (SEM).
2.5. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
All the fractions were tested for the minimum inhibitory concentration and minimum bactericidal concentration. MIC was performed at five concentrations of extracts (125, 62.5, 31.25, 15.62, 7.81 μg/mL) following serial dilution technique. All the wells showing no visible growth of microorganisms were subcultured and incubated at 37°C overnight. The highest dilution showing 100% inhibition was recorded as MBC.
3. RESULTS AND DISCUSSION
3.1. Results
The antibacterial activity of G. biloba methanol, ethanol, chloroform, and hexane extracts against microorganisms were examined in the present study and their potency were quantitatively assessed by ZOI (Table 1), MIC, and MBC values (Table 2). The results indicated that the methanol extract showed the highest activity among all the tested extracts and inhibited the growth of all the bacterial strains (Table 1). MIC and MBC values for the microorganisms sensitive to the methanol extract were in the range of 7.8–15.62 μg/mL and 31.25–62.5 μg/mL, respectively (Table 2). The ethanol and chloroform extracts showed MIC and MBC values in the same range. For the hexane extract, the MIC values were in the range of 15.62–31.25 μg/mL and MBC were 62.5–125 μg/mL. It was interesting to note that all the fractions were found more active than the used standard antibiotics (Figure 2).
Table 1.
Zone of inhibition of different extracts of Ginkgo biloba.
| Microorganisms | Diameter of inhibition zone (mm)* | ||||||
|---|---|---|---|---|---|---|---|
| H | C | E | M | E | A | G | |
| A. tumefaciens | 16 ± 0.8 | 19 ± 0.6 | 14 ± 1.0 | 15 ± 0 | na | na | 20 ± 0.6 |
| B. subtilis | 20 ± 1.7 | 18 ± 0 | 19 ± 0.6 | 19 ± 0.6 | na | na | 20 ± 0.4 |
| E. coli | 15 ± 0 | 14 ± 0.3 | 19 ± 1.0 | 19 ± 0.6 | na | na | 21 ± 1.2 |
| E. chrysanthemi | 16 ± 0.5 | 17 ± 0.8 | 16 ± 0.6 | 17 ± 0.3 | na | na | 21 ± 1.2 |
| X. phaseoli | 16 ± 0.8 | 16 ± 0.6 | 19 ± 1.0 | 21 ± 0.2 | na | na | 21 ± 0.7 |
*All the values are mean ± SEM of three determinations. H, C, E, M: hexane, chloroform, ethanol, methanol; E, A, G: erythromycin, ampicillin, gentamycin; na: not active.
Table 2.
Minimum inhibitory/bactericidal concentration (μg/mL).
| Microorganisms | MIC and MBC values (μg/mL) | |||
|---|---|---|---|---|
| H | C | E | M | |
| A. tumefaciens | 31.25 | 15.62 | 31.25 | 7.81 |
| *125 | *31.25 | *62.25 | *31.25 | |
| B. subtilis | 15.62 | 7.81 | 7.81 | 7.81 |
| *62.5 | *31.25 | *15.25 | *31.25 | |
| E. coli | 15.62 | 31.25 | 31.25 | 15.62 |
| *125 | *62.5 | *62.5 | *31.25 | |
| E. chrysanthemi | 15.62 | 15.62 | 31.25 | 15.62 |
| *62.5 | *62.5 | *62.25 | *62.5 | |
| X. phaseoli | 31.25 | 15.62 | 15.62 | 7.81 |
| *62.5 | *31.25 | *62.25 | *62.5 | |
*MBC values.
Figure 2.
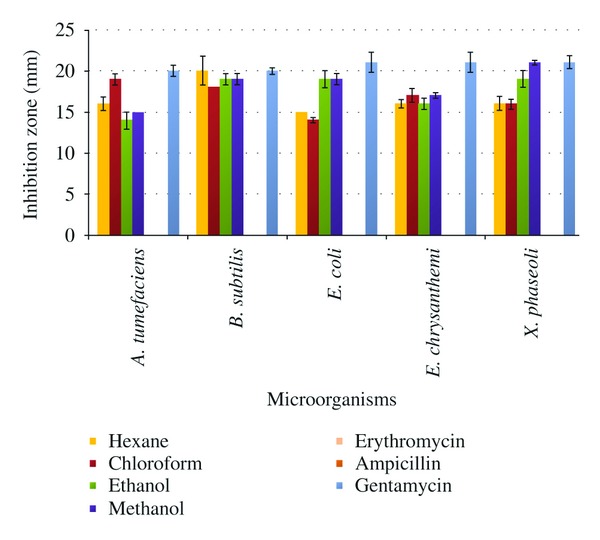
Inhibition zones (in mm) observed in methanol, ethanol, chloroform, and hexane extracts as compared to ampicillin, erythromycin, and gentamycin. Bar represents standard errors and mean value of inhibition zone.
3.2. Discussion
G. biloba, a high altitude grown Kumaun Himalayan plant has not been studied for its antibacterial activity. Therefore, the present study highlights for the first time the in vitro antibacterial activities of different solvent fractions of this plant.
The present data show that there is no uniform response between bacterial strains studied in terms of susceptibility in all these crude extract of G. biloba (Table 1). Our findings show that small amounts of crude extracts of Ginkgo leaves (7.8 μg/mL) possess inhibitory activity against Gram-positive and Gram-negative bacteria. The present investigation supports the ethnobotanical uses of G. biloba. Relying upon [6] terpene trilactones and flavonoid glycosides, the active compounds of Ginkgo biloba might be responsible for its antimicrobial potential.
This study also provides new leads as there are no previous records on the antibacterial activity of G. biloba extracts against the plant pathogenic strains X. phaseoli, A. tumefaciens, and E. chrysanthemi.
This is the first report to demonstrate the antibacterial potential of ethanol, chloroform, and hexane extracts of G. biloba. These results also confirm the evidences in previous studies that methanol is a better solvent to extract antimicrobial substances from medicinal plants [14–16]. This study provides a strong scientific basis for the ethnomedical use of G. biloba against bacterial diseases of animals and plants.
ACKNOWLEDGMENTS
The paper was supported by DRS (SAP)UGC, New Delhi. The authors wish to thank the Department of Plant Pathology, G. B. Pant University of Agriculture and Technology (GBPUA & T), Pantnagar and Microbial Type Culture Collection (MTCC) for providing bacterial strains, and Department of Botany, DSB Campus, Kumaun University, Nainital for providing necessary facilities to carry out the present investigation.
References
- 1.Braquet P. The ginkgolides from Chinese pharmacopeia to a new class of pharmacological agents: the antagonists of platelet activating factor. In: Braquet P, editor. Ginkgolides—Chemistry, Biology, Pharmacologyand Chemical Perspectives. Vol. 1. Barcelona, Spain: Prous Science; 1988. pp. 15–34. [Google Scholar]
- 2.Boonkaew T, Camper ND. Biological activities of Ginkgo extracts. Phytomedicine. 2005;12(4):318–323. doi: 10.1016/j.phymed.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Bombardelli E, Cristoni A, Morazzoni P. Cosmetical uses of Ginkgo extracts and constituents. In: van Beek TA, editor. Ginkgo Biloba. Singapore: Harwood Academic Publishers; 2000. pp. 475–489. [Google Scholar]
- 4.Maurer K, Ihl R, Dierks T, Frölich L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. Journal of Psychiatric Research. 1997;31(6):645–655. doi: 10.1016/s0022-3956(97)00022-8. [DOI] [PubMed] [Google Scholar]
- 5.Drieu K. Preparation et definition de l’extract de Ginkgo biloba. Presse Medicale. 1986;15(31):1455–1457. [PubMed] [Google Scholar]
- 6.Singh B, Kaur P, Gopichand, Singh RD, Ahuja PS. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79(6):401–418. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Atzori C, Bruno A, Chichino G, Bombardelli E, Scaglia M, Ghione M. Activity of bilobalide, a sesquiterpene from Ginkgo biloba, on Pneumocystis carinii. Antimicrobial Agents and Chemotherapy. 1993;37(7):1492–1496. doi: 10.1128/aac.37.7.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Archives of Neurology. 1998;55(11):1409–1415. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 9.Wadsworth TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced release of nitric oxide. Chemico-Biological Interactions. 2001;137(1):43–58. doi: 10.1016/s0009-2797(01)00208-3. [DOI] [PubMed] [Google Scholar]
- 10.Bridi R, Crossetti FP, Steffen VM, Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytotherapy Research. 2001;15(5):449–451. doi: 10.1002/ptr.814. [DOI] [PubMed] [Google Scholar]
- 11.Pitchumoni SS, Doraiswamy PM. Current status of antioxidant therapy for Alzheimer’s disease. Journal of the American Geriatrics Society. 1998;46(12):1566–1572. doi: 10.1111/j.1532-5415.1998.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 12.Sati SC, Joshi S. Antibacterial potential of leaf extracts of Juniperus communis L. from Kumaun Himalaya. African Journal of Microbiology Research. 2010;4(12):1291–1294. [Google Scholar]
- 13.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 14.Karaman I, Şahin F, Güllüce M, Öğüçü H, Şengül M, Adigüzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. Journal of Ethnopharmacology. 2003;85(2-3):231–235. doi: 10.1016/s0378-8741(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. Journal of Ethnopharmacology. 1998;62(2):183–193. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 16.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? Journal of Ethnopharmacology. 1998;60(1):1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]


