Abstract
Background: The association between antidiabetic medications and the prognosis of human prostate cancer has not been explored. This study examined the impact of these drugs on the outcomes of diabetic patients with prostate cancer to provide a basis for diabetes management strategy in these patients.
Patients and methods: Records of consecutive prostate cancer patients with coexisting diabetes mellitus type 2 who were treated at the study institution between 15 July 1999 and 31 December 2008 were reviewed. The survival, cancer pathological grade, stage at the time of diagnosis, and antidiabetic pharmacotherapy of the patients were analyzed.
Results: A total of 233 consecutive cases were analyzed. In Kaplan–Meier analysis, thiazolidinedione (log-rank, P = 0.005) and metformin (log-rank, P = 0.035) usage were significant predictors of improved overall survival, while insulin and insulin secretagogue usage were not significant predictors. Multivariate Cox regression analysis showed that thiazolidinedione {hazard ratio [HR] = 0.454 [95% confidence interval (CI) 0.213–0.965], P = 0.040} and metformin [HR = 0.550 (95% CI 0.315–0.960), P = 0.035] usage remained as significant predictors of favorable survival after controlling for variables including age, race, Gleason grade, and stage.
Conclusions: Thiazolidinediones and metformin appear to be associated with improved overall survival of diabetic prostate cancer patients. The choice of antidiabetic pharmacotherapy may influence overall survival of these patients.
Keywords: metformin, overall survival, prostate cancer, thiazolidinediones
introduction
Extensive epidemiological data suggest important roles of type 2 diabetes mellitus (DM2) in carcinogenesis [1–4]. However, an exception to this general trend is seen in prostate cancer [5–14]. Prostate cancer is the most common cancer in men and the second leading cause of cancer death in men. The incidence of this very important malignancy in males is inversely associated with DM2 [5–14]. Genetic susceptibility to DM2 may be associated with reduced prostate cancer risk [15–18].
Being distinct from cancer risk, which is related to carcinogenesis, cancer prognosis is related to the biological processes contributing to cancer progression after the malignancy has formed. Hyperglycemia [19] and hyperinsulinemia [20] are predictive risk factors for increased mortality in prostate cancer patients, and men with DM2 are more likely to present with high-grade prostate cancer [21]. A meta-analysis suggests that overall mortality is increased in prostate cancer patients with preexisting DM2 compared with those without DM2 [22]. There is also evidence from animal models that DM2 can promote migration, proliferation, and other pathological processes that facilitate cancer progression [23].
Current medications for DM2 include thiazolidinediones (e.g. pioglitazone and rosiglitazone), biguanides (e.g. metformin), sulfonylureas, meglitinides, α-glucosidase inhibitors, amylin analogues, dipeptidyl peptidase-4 inhibitors, incretin mimetics (e.g. glucagon-like peptide-1 analogues), bioengineered insulin analogues, and insulin preparations. Although all these different classes of medications can lower blood glucose, the mechanisms of actions of these agents are different and they have different impacts on the circulating insulin levels. Since hyperinsulinemia is a prospective risk factor for prostate cancer mortality [20], one would expect an increase in prostate cancer risk in patients on insulin and insulin secretagogues compared with those on agents that improve insulin sensitivity. Yet evidence for increase in prostate cancer risk by insulin and insulin secretagogues is still lacking. Metformin appears to reduce the incidence of pancreatic cancer [24, 25], colon cancer [25], and perhaps prostate cancer [26]. In contrast, thiazolidinediones appear to reduce the incidence of lung cancer [27] but may be neutral toward the risk for prostate cancer [24, 27]. Larger studies or meta-analyses remain to be carried out to conclusively address the impact on prostate cancer risk by these different classes of antidiabetic medications.
In addition to the impact on circulating insulin levels, some antidiabetic drugs may have direct antitumor effects. For example, thiazolidinediones [agonists of peroxisome proliferator-activated receptor-γ (PPARγ)] have been shown to suppress various types of cancer cells in cell culture and in animal models [28–30], and so has metformin [31–33], which activates AMP-activated protein kinase (AMPK) and decreases signaling through mammalian target of rapamycin (mTOR) [34]. In a population-based cohort study [35], diabetic patients treated with sulfonylureas and/or insulin were more likely to die of cancer than patients treated with metformin. It is not clear whether metformin has a beneficial effect, or sulfonylureas and/or insulin have a detrimental effect, or a combination of both possibilities. Amylin analogues, dipeptidyl peptidase-4 inhibitors, and incretin mimetics are relatively new antidiabetic agents, and cancer survival data for patients with long-term usage of these agents are not yet available. Nevertheless, this study [35] has highlighted the differential impact of antidiabetic medications on cancer prognosis in diabetic patients. However, there remains a gap in knowledge about the impact of specific classes of antidiabetic medications on the prognosis of specific types of cancer (including prostate cancer).
Metformin [36], the AMP analog 5-aminoimidazole-4-carboxamide riboside (AICAR) [37], or rosiglitazone inhibit prostate cancer cell lines [37]. Troglitazone stabilized prostate-specific antigen (PSA) in a phase II study of advanced prostate cancer patients [38], while rosiglitazone did not increase PSA doubling time or prolong the time to disease progression more than placebo in men with a rising PSA level after radical prostatectomy and/or radiation therapy and without radiological evidence of metastatic disease [39]. To examine the impact of specific classes of antidiabetic medications on the prognosis of prostate cancer, we have conducted a retrospective analysis of diabetic prostate cancer patients treated at our institution. In this report, we present the first clinical evidence that thiazolidinediones may improve the overall survival of diabetic prostate cancer patients, which is similar to metformin but is in sharp contrast with the lack of impact of insulins and insulin secretagogues.
patients and methods
study population
The study was approved by the University of Texas M. D. Anderson Cancer Center (MDACC) Institutional Review Board in accordance with an assurance filed with and approved by the Department of Health and Human Services. The study population consists of consecutive cases of prostate cancer with coexisting or preexisting DM2 treated by the Genitourinary Medical Oncology Department in MDACC between 15 July 1999 and 31 December 2008 (a time period after the introduction of thiazolidinediones in the US market). In total, we reviewed 250 cases.
data collection
Trained personnel reviewed online patient records to collect information on demographics and known or suspected risk factors for prostate cancer prognosis. Since the black race is an adverse prognostic factor for prostate cancer [40], the race of this patient cohort was categorized as black versus non-black. The pathological diagnosis and Gleason grade of the primary tumor were recorded. The TNM (tumour–node–metastasis) stage of the prostate cancer at the time of diagnosis was also ascertained.
Mean body mass index (BMI) was calculated using the recorded height and body weight during clinic visits. According to the World Health Organization standard, BMIs of 18.5–24.9, 25–29.9, and ≥30 kg/m2 were defined as normal body weight, overweight, and obesity, respectively.
Diabetes was defined by the self-reported medical history and was also confirmed from their medical records. Diabetic patients on diet only and not on any form of pharmacotherapy were excluded from the analysis. Patients with type 1 diabetes mellitus were also excluded. Antidiabetic pharmacotherapy was recorded in detail.
statistical analysis
Out of 250 cases reviewed, 17 cases had diet-controlled diabetes and were not on antidiabetic pharmacotherapy; these 17 cases were not included in further statistical analysis. The association of prostate cancer patient survival with each class of antidiabetic pharmacotherapy was evaluated by Kaplan–Meier analysis. Risk factors (e.g. age, race, Gleason grade, stage at the time of diagnosis, and BMI) were analyzed using multivariate Cox regression analysis. Age and race were included in all models. Antidiabetic pharmacotherapy was classified as: (i) insulin or insulin analogues, (ii) insulin secretagogues (e.g. sulfonylureas and meglitinides), (iii) biguanides, (iv) thiazolidinediones, and (v) others, including α-glucosidase inhibitors, dipeptidyl peptidase-4 inhibitors, amylin analogues, and glucagon-like peptide 1 analogues. Because many patients used combination therapy, the drugs or combinations changed over time, and the number of patients in each monotherapy group was small, the final analysis used the categorical variables of ever-use versus never-use of insulins, insulin secretagogues, metformin, or thiazolidinediones. The demographics and risk factors of prostate cancer prognosis were compared between thiazolidinedione- ever-users and never-users by χ2 test, Student’s t-test, or Mann–Whitney rank sum test where appropriate. All statistical analyses were carried out using SPSS version 17.0 (SPSS, Cary, NC) and SigmaPlot version 11.0 (Systat, Chicago, IL) software with two-sided tests, with a P value of ≤0.05 considered statistically significant.
results
association of thiazolidinedione therapy with improved survival of diabetic prostate cancer patients
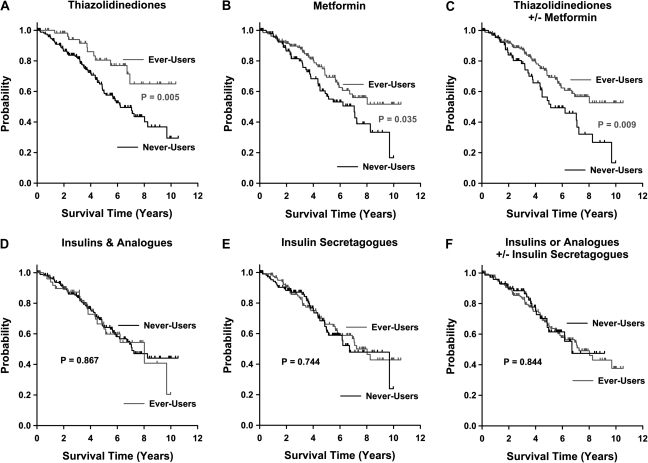
A total of 233 consecutive cases were analyzed. The characteristics of the study population are summarized in Table 1. In Kaplan–Meier analysis, thiazolidinedione usage was a significant (log-rank test, P = 0.005, Figure 1A) predictor of improved survival. The 75th percentile survival of the thiazolidinedione ever-use group was 6.710 years and that of the thiazolidinedione never-use group was 3.627 years; the median survival of thiazolidinedione- never-users was 6.211 years. Metformin usage was also a significant (log-rank test, P = 0.035, Figure 1B) predictor of improved survival. The 75th percentile survival of the metformin ever-use group was 4.660 years and that of the metformin never-use group was 3.444 years; the median survival of metformin never-users was 7.052 years. Likewise, usage of metformin and/or thiazolidinediones was also a significant (log-rank test, P = 0.009, Figure 1C) predictor of improved survival. The 75th percentile survival of the metformin ± thiazolidinedione ever-use group was 4.660 years and that of the metformin ± thiazolidinedione never-use group was 3.216 years; the median survival of metformin ± thiazolidinedione never-users was 5.137 years. In contrast, insulin and insulin secretagogue usage were not significant predictors of survival (Figure 1D–F). When ever-users were compared with never-users for thiazolidinediones, metformin, and metformin ± thiazolidinediones, the mean age at prostate cancer diagnosis of never-users were older than the metformin users by 5.1 years (P <0.001, Table 2) but not for thiazolidinediones and metformin ± thiazolidinediones, and there were no other significant differences in race, Gleason grade, TNM stage, PSA at the time of diagnosis, and BMI between the respective ever-user and never-user groups (Table 2).
Table 1.
Characteristics of the study population
| Number of patients | Missing data | Mean | Median | Maximum | Minimum | |
| Race | 233 | 0 | ||||
| Black | 41 | |||||
| Hispanic | 22 | |||||
| Asian | 4 | |||||
| White | 166 | |||||
| Age of diagnosis (years) | 233 | 0 | 63.48 | 63 | 84 | 42 |
| ≤65 | 137 | |||||
| >65 | 96 | |||||
| Gleason grade | 231 | 2 | ||||
| ≤6 | 28 | |||||
| 7–8 | 111 | |||||
| ≥9 | 92 | |||||
| TNM stage | 216 | 17 | ||||
| ≤2 | 97 | |||||
| ≥3 | 119 | |||||
| PSA at diagnosis | 227 | 6 | 76.56 | 12.7 | 3602 | 1 |
| BMI, kg/m2 | 226 | 7 | 31 | 30.30 | 55.61 | 18.80 |
| <30 | 105 | |||||
| 30–35 | 64 | |||||
| >35 | 57 |
TNM, tumour–node–metastasis; PSA, prostate-specific antigen; BMI, body mass index.
Figure 1.
Thiazolidinediones and metformin are associated with improved survival of diabetic prostate cancer patients. Kaplan–Meier survival curves comparing ever-users and never-users are shown for thiazolidinediones (A), metformin (B), thiazolidinediones and/or metformin (C), insulins and insulin analogues (D), insulin secretagogues (E), and insulins or analogues and/or insulin secretagogues (F). The P values of the log-rank tests are as labeled.
Table 2.
Comparison of the user groups versus the never-user groups
| Thiazolidinedione ever-users | Thiazolidinedione never-users | Statistical test | P | |
| Black race | 11/61 (18.0%) | 30/172 (17.4%) | χ2 | 0.927 |
| Age of diagnosis (years) | Mean = 61.69 | Mean = 64.12 | t | 0.060 |
| Gleason grade | Median = 8 | Median = 8 | Mann–Whitney rank sum | 0.236 |
| TNM stage ≥3 | 26/56 (46.43%) | 93/160 (58.1%) | χ2 | 0.174 |
| PSA at diagnosis | Median = 9.3 | Median = 15.7 | Mann–Whitney rank sum | 0.147 |
| BMI | Median = 30.58 | Median = 30.27 | Mann–Whitney rank sum | 0.525 |
| Metformin ever-users | Metformin never-users | Statistical test | P | |
| Black race | 26/132 (19.7%) | 15/101 (14.8%) | χ2 | 0.430 |
| Age of diagnosis (years) | Mean = 61.29 | Mean = 66.39 | t | <0.001 |
| Gleason grade | Median = 8 | Median = 8 | Mann–Whitney rank sum | 0.502 |
| TNM stage ≥3 | 48/93 (51.6%) | 70/122 (57.4%) | χ2 | 0.482 |
| PSA at diagnosis | Median = 15.9 | Median = 11.2 | Mann–Whitney rank sum | 0.112 |
| BMI | Median = 30.33 | Median = 29.48 | Mann–Whitney rank sum | 0.364 |
| Metformin ± thiazolidinediones ever-users | Metformin ± thiazolidinediones never-users | Statistical test | P | |
| Black race | 24/160 (15%) | 17/73 (23.3%) | χ2 | 0.182 |
| Age of diagnosis (years) | Mean = 64.14 | Mean = 62.03 | t | 0.122 |
| Gleason grade | Median = 8 | Median = 8 | Mann–Whitney rank sum | 0.147 |
| TNM stage ≥3 | 80/93 (53.7%) | 38/67 (56.7%) | χ2 | 0.190 |
| PSA at diagnosis | Median = 11.7 | Median = 16.42 | Mann–Whitney rank sum | 0.935 |
| BMI | Median = 29.74 | Median = 31.27 | Mann–Whitney rank sum | 0.208 |
TNM, tumour–node–metastasis; PSA, prostate-specific antigen; BMI, body mass index.
Cox regression analysis was carried out using a model consisting of the categorical covariates: black race, Gleason grade ≥8, TNM stage ≥3, obesity (BMI >30), insulin usage, insulin secretagogue usage, thiazolidinedione usage and metformin usage, and continuous covariates: age at diagnosis and PSA at diagnosis (Table 3). As expected, this multivariate analysis showed that Gleason grade was a significant (P < 0.001) predictor of survival of these diabetic prostate cancer patients. Thiazolidinedione usage was a significant (P = 0.040) predictor of favorable survival [hazard ratio (HR) = 0.454, 95% confidence interval (CI) 0.213–0.965] and so was metformin usage (P = 0.035, HR = 0.550, 95% CI 0.315–0.960).
Table 3.
Multivariate Cox regression analysis of survival
| Covariate | Coefficient | Hazard ratio | Wald chi-square | P |
| Black race | −0.141 (−0.818 to 0.537) | 0.869 (0.441–1.710) | 0.165 | 0.684 |
| Age at diagnosis | 0.0235 (−0.00627 to 0.053) | 1.024 (0.994–1.055) | 2.394 | 0.122 |
| Gleason grade ≥8 | 0.469 (0.192–0.745) | 1.598 (1.212–2.107) | 11.027 | <0.001 |
| Stage ≥3 | 0.533 (−0.0462 to 1.113) | 1.705 (0.955–3.044) | 3.253 | 0.071 |
| PSA at diagnosis | −0.000226 (−0.00112 to 0.000674) | 1.000 (0.999–1.001) | 0.242 | 0.623 |
| BMI >30 | −0.0586 (−0.587 to 0.469) | 0.943 (0.556–1.599) | 0.0473 | 0.828 |
| Insulin | −0.165 (−0.812 to 0.483) | 0.848 (0.444–1.620) | 0.249 | 0.618 |
| Insulin secretagogues | −0.0973 (−0.677 to 0.482) | 0.907 (0.508–1.620) | 0.108 | 0.742 |
| Thiazolidinediones | −0.790 (−1.545 to 0.0353) | 0.454 (0.213–0.965) | 4.209 | 0.040 |
| Metformin | −0.598 (−1.155 to −0.0407) | 0.550 (0.315–0.960) | 4.424 | 0.035 |
PSA, prostate-specific antigen; BMI, body mass index. The 95% confidence intervals are presented in parentheses. The P values that are <0.05 are highlighted in bold fonts.
discussion
Most epidemiological studies involving antidiabetic medications and prostate cancer have focuses the impact of the medications on the risk of having prostate cancer [26, 27, 41–43]. Despite basic scientific evidence that metformin and thiazolidinediones may have antineoplastic effects against prostate cancer [36–38, 44], epidemiological investigation on the impact of antidiabetic medications on prostate cancer patient survival is lacking. This study addresses the gap in knowledge about the impact of specific classes of antidiabetic medications on the prognosis of prostate cancer in DM2 patients. Different classes of antidiabetic pharmacotherapy have differential impact on the progression of cancer cells [45] and on the survival of pancreatic cancer patients [24]. In contrast to our findings in the pancreatic cancer study [24], thiazolidinediones usage is associated with improved survival of diabetic prostate cancer patients as well as metformin. This difference in impact of different classes of antidiabetic pharmacotherapy on cancers originating in different tissues/organs is likely due to fundamental differences in the tumor biology of malignancies originating from different tissues.
Metformin [36] and the AMP analogue, AICAR [37] inhibit prostate cancer cell lines through the impact of AMPK on the protein kinase B (AKT)/mTOR signaling pathway. Thiazolidinediones inhibit prostate cancer cells and down-regulate PSA [38, 44]. Up-regulation of phosphatase and tensin homolog gene expression by PPARγ can regulate cell proliferation and apoptosis through interaction with the insulin-like growth factor-I receptor/AKT/mTOR signaling pathway at multiple points [46]. Our results provided the first clinical evidence that both metformin usage and thiazolidinediones usage were associated with improved overall survival of diabetic prostate cancer patients. However, our patient cohort has too few patients that were taking both metformin and thiazolidinediones to adequately assess the impact of this drug combination on prostate cancer. Other limitations of our retrospective study included: (i) that close to one-third of the patients resided outside our city and received most of the diabetes care from their local physicians and (ii) that the majority of patients died while under the care of physicians outside our institution. Therefore, reliable records of long-term diabetes control (i.e. HbA1c) and reliable determination of causes of death were not available in enough number of cases for further analyses. Cardiovascular disease and cancer are the two leading causes of death for this age group. Since rosiglitazone may increase the risk of cardiovascular events and mortality [47], the survival benefit of thiazolidinediones in diabetic prostate cancer patients is likely to be due to a beneficial impact on prostate cancer-specific mortality. Future randomized prospective clinical trials are necessary to confirm the survival benefits of metformin and thiazolidinediones, alone or in combination, for prostate cancer.
Thus far, early phase clinical trials with thiazolidinediones in prostate cancer have mixed results: troglitazone stabilized PSA in advanced prostate cancer patients [38] versus no impact by rosiglitazone on the PSA doubling time or time to disease progression in early-stage prostate cancer with a rising PSA but no radiological evidence of metastasis [39]. These seemingly discordant results may be explained by the difference in the stage of disease. Bone metastases are almost inevitable in advanced prostate cancer. Prostate cancer cells influence bone homeostasis by paracrine factors (e.g. platelet-derived growth factor, bone morphogenic proteins, transforming growth factor-α, etc.) that regulate osteoblasts and by secreted factors that modify growth factors in the bone microenvironment (e.g. PSA) [48]. The canonical Wnt–β-catenin pathway inhibits transcription of PPARγ, whereas the non-canonical pathway activates a histone methyltransferase to methylate PPARγ target genes to repress PPARγ transactivation. Osteoblast differentiation is inhibited by PPARγ, and thiazolidinediones are PPARγ agonists that favor differentiation of mesenchymal stem cells into adipocytes instead of osteoblasts [49]. It is highly probable that the survival benefit of thiazolidinediones in diabetic prostate cancer patients is at least in part mediated by disrupting the interaction between metastatic prostate cancer cells and stromal cells in the bone microenvironment. It is very important that future interventional trials will recruit the patient population that will most likely benefit from these agents.
Our study has an important implication for the clinical management of DM2 in prostate cancer patients. In the absence of contraindications for metformin and thiazolidinediones, these two classes of antidiabetic pharmacotherapy may be preferred over other classes. Moreover, many prostate cancer patients who are receiving short-term [50] or long-term androgen-deprivation therapy [51] are at risk for developing insulin resistance and hyperglycemia (i.e. DM2 [52]), and thiazolidinediones may be useful in the treatment of DM2 precipitated by androgen-deprivation therapy with leuprolide in prostate cancer patients [53]. More research will be necessary to confirm our findings and hopefully will change the standard of clinical management of DM2 for diabetic prostate cancer patients.
funding
This work was supported by an RO1 grant from the US National Institute of Health (NIHRO1CA 089266) to M.-H.L., Department of Defense, Breast Cancer Research Program of the Office of the Congressionally Directed Medical Research Programs (CDMRP). CDMRP Synergistic Idea Development Award BC062166 to S.-C.Y. and M.-H.L., and Cancer Center Core Grant to the University of Texas MD Anderson Cancer Center (CA16672).
disclosure
The authors declare no conflict of interest.
References
- 1.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84:417–422. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verlato G, Zoppini G, Bonora E, Muggeo M. Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care. 2003;26:1047–1051. doi: 10.2337/diacare.26.4.1047. [DOI] [PubMed] [Google Scholar]
- 3.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm EB, Stampfer MJ, et al. Diabetes mellitus and risk of prostate cancer (United States) Cancer Causes Control. 1998;9:3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg DJ, Neugut AI, Ahsan H, Shea S. Diabetes mellitus and the risk of prostate cancer. Cancer Invest. 2002;20:157–165. doi: 10.1081/cnv-120001141. [DOI] [PubMed] [Google Scholar]
- 7.Zhu K, Lee IM, Sesso HD, et al. History of diabetes mellitus and risk of prostate cancer in physicians. Am J Epidemiol. 2004;159:978–982. doi: 10.1093/aje/kwh139. [DOI] [PubMed] [Google Scholar]
- 8.Coker AL, Sanderson M, Zheng W, Fadden MK. Diabetes mellitus and prostate cancer risk among older men: population-based case-control study. Br J Cancer. 2004;90:2171–2175. doi: 10.1038/sj.bjc.6601857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47:1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez C, Patel AV, Mondul AM, et al. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol. 2005;161:147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Perez A, Garcia Rodriguez LA. Prostate cancer risk among men with diabetes mellitus (Spain) Cancer Causes Control. 2005;16:1055–1058. doi: 10.1007/s10552-005-4705-5. [DOI] [PubMed] [Google Scholar]
- 12.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 13.Waters KM, Henderson BE, Stram DO, et al. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169:937–945. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124:1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudmundsson J, Sulem P, Steinthorsdottir V, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 16.Meyer TE, Boerwinkle E, Morrison AC, et al. Diabetes genes and prostate cancer in the Atherosclerosis Risk in Communities study. Cancer Epidemiol Biomarkers Prev. 2010;19:558–565. doi: 10.1158/1055-9965.EPI-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierce BL, Ahsan H. Genetic susceptibility to type 2 diabetes is associated with reduced prostate cancer risk. Hum Hered. 2010;69:193–201. doi: 10.1159/000289594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens VL, Ahn J, Sun J, et al. HNF1B and JAZF1 genes, diabetes, and prostate cancer risk. Prostate. 2010;70:601–607. doi: 10.1002/pros.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gapstur SM, Gann PH, Colangelo LA, et al. Postload plasma glucose concentration and 27-year prostate cancer mortality (United States) Cancer Causes Control. 2001;12:763–772. doi: 10.1023/a:1011279907108. [DOI] [PubMed] [Google Scholar]
- 20.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41:2887–2895. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico AV, Braccioforte MH, Moran BJ, Chen MH. Causes of death in men with prevalent diabetes and newly diagnosed high- versus favorable-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:1329–1337. doi: 10.1016/j.ijrobp.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 22.Snyder CF, Stein KB, Barone BB, et al. Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis. 2010;13:58–64. doi: 10.1038/pcan.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro DL, Taboga SR, Goes RM. Diabetes induces stromal remodelling and increase in chondroitin sulphate proteoglycans of the rat ventral prostate. Int J Exp Pathol. 2009;90:400–411. doi: 10.1111/j.1365-2613.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 26.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 28.Galli A, Mello T, Ceni E, et al. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin Investig Drugs. 2006;15:1039–1049. doi: 10.1517/13543784.15.9.1039. [DOI] [PubMed] [Google Scholar]
- 29.Blanquicett C, Roman J, Hart CM. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008;6:25–34. [PMC free article] [PubMed] [Google Scholar]
- 30.Wei S, Yang J, Lee SL, et al. PPARgamma-independent antitumor effects of thiazolidinediones. Cancer Lett. 2009;276:119–124. doi: 10.1016/j.canlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efferth T, Sauerbrey A, Steinbach D, et al. Analysis of single nucleotide polymorphism C3435T of the multidrug resistance gene MDR1 in acute lymphoblastic leukemia. Int J Oncol. 2003;23:509–517. [PubMed] [Google Scholar]
- 32.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67:1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 35.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 36.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 37.Xiang X, Saha AK, Wen R, et al. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 38.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci U S A. 2000;97:10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MR, Manola J, Kaufman DS, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569–1574. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 40.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol. 1996;155:1667–1673. [PubMed] [Google Scholar]
- 41.Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf. 2007;16:485–492. doi: 10.1002/pds.1352. [DOI] [PubMed] [Google Scholar]
- 42.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008;168:925–931. doi: 10.1093/aje/kwn190. [DOI] [PubMed] [Google Scholar]
- 43.Velicer CM, Dublin S, White E. Diabetes and the risk of prostate cancer: the role of diabetes treatment and complications. Prostate Cancer Prostatic Dis. 2007;10:46–51. doi: 10.1038/sj.pcan.4500914. [DOI] [PubMed] [Google Scholar]
- 44.Shiau CW, Yang CC, Kulp SK, et al. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res. 2005;65:1561–1569. doi: 10.1158/0008-5472.CAN-04-1677. [DOI] [PubMed] [Google Scholar]
- 45.Feng YH, Velazquez-Torres G, Gully C, et al. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med. doi: 10.1111/j.1582-4934.2010.01083.x. 2010 May 3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao H, Dupont J, Yakar S, et al. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene. 2004;23:786–794. doi: 10.1038/sj.onc.1207162. [DOI] [PubMed] [Google Scholar]
- 47.Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363:1489–1491. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 48.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310:71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 50.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 51.Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 52.Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inaba M, Otani Y, Nishimura K, et al. Marked hyperglycemia after androgen-deprivation therapy for prostate cancer and usefulness of pioglitazone for its treatment. Metabolism. 2005;54:55–59. doi: 10.1016/j.metabol.2004.07.010. [DOI] [PubMed] [Google Scholar]