Abstract
Vestibular tissues (cristae ampullares, macular otolithic organs, and Scarpa’s ganglia) in chinchilla, rat, and guinea pig were examined for immunoreactivity to the α9 nicotinic acetylcholine receptor (nAChR) subunit. The α9 antibody was generated against a conserved peptide present in the intracellular loop of the predicted protein sequence of the guinea pig α9 nAChR subunit. In the vestibular periphery, staining was observed in calyces around type I hair cells, at the synaptic pole of type II hair cells, and in varying levels in Scarpa’s ganglion cells. Ganglion cells were also triply labeled to detect α9, calretinin, and peripherin. Calretinin labels calyx-only afferents. Peripherin labels bouton-only afferents. Dimorphic afferents, which have both calyx and bouton endings, are not labeled by calretinin or peripherin. In these experiments, α9 was expressed in both calyx and dimorphic afferents. A subpopulation of small ganglion cells did not contain the α9 nAChR but did stain for peripherin. We surmise that these are bouton-only afferents. Bouton (regularly discharging) afferents also show efferent responses, although they are qualitatively different from those in irregularly discharging (calyx and dimorphic) afferents, much slower and longer lasting. Thus, regular afferents are probably more affected via a muscarinic cholinergic or a peptidergic mechanism, with a much smaller superimposed fast nicotinic-type response. This latter response could be due to one of the other nicotinic receptors that have been described in studies from other laboratories.
Indexing terms: efferent, hair cells, crista ampullaris, otolith, macular organ, vestibular ganglion
Acetylcholine (ACh) is well-established as a transmitter in auditory and vestibular efferents (Bobbin and Konishi, 1971, 1974; Klinke and Galley, 1974; Guth et al., 1998). It has long been known that the cholinergic response of hair cells and afferents has an unusual pharmacology (Desmedt and Monaco, 1960). Until cloning efforts in the 1990s, the inner ear cholinergic response was not attributable to any previously described cholinergic receptors. In mammals, electrophysiological stimulation of auditory efferents inhibits sensory transmission (Wiederhold and Kiang, 1970), whereas stimulation of vestibular efferents in mammals excites vestibular afferents (Goldberg and Fernández, 1980; McCue and Guinan, 1994; Plotnik et al., 2002; Marlinski et al., 2004). Both cochlear and vestibular systems exhibit choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) staining in their respective efferent nuclei (Gacek and Lyon, 1974; Warr, 1975; Goldberg and Fernández; 1980; Schwarz et al., 1986; Perachio and Kevetter, 1989), which supports the pharmacological evidence that these systems are primarily cholinergic (for recent reviews on vestibular efferents see Goldberg et al., 2000; Lysakowski and Goldberg, 2004). The efferent innervation patterns of the cochlea have long been adequately described (Spoendlin, 1970; Warr and Guinan, 1979), but the regional pattern of peripheral vestibular efferent innervation has only recently begun to be mapped (Purcell and Perachio, 1997; Lysakowski and Goldberg, 1997; Maksoud and Lysakowski, 2004).
Receptors for ACh are of two types: 1) muscarinic, G-protein-linked metabotropic receptors and 2) nicotinic, ligand-gated ion channel receptors. This paper concerns nicotinic receptors. Several subtypes have been cloned. The nicotinic acetylcholine receptor (nAChR; Noda et al., 1983a,b), found in skeletal muscle and fish electric organs, is composed of five pentameric subunits: 2α, β, δ, and γ, with a sixth subunit, ε, found during embryonic development. There have so far been shown to be at least 10 α subunits, 4 β subunits, and 1 each of the δ, γ, and ε. The classic “neuromuscular junction-type” α subunit, α1, binds α-bungarotoxin irreversibly. Neuronal nicotinic receptors consist of two sets of isoforms: 1) α2–6, which form heteromers and do not bind α-bungarotoxin, and 2) α7–10, which can form homomers or heteromers and bind α-bungarotoxin reversibly. The nAChR most commonly found in neural tissue comprises subunits with the stoichiometry (α4)2(β2)3 (Lindstrom, 1996).
Previous studies have examined the distribution of nAChR subunits in vestibular end organs by using various nucleic acid and ligand-binding methods (Ohno et al., 1993; Anderson et al., 1997; Hiel et al. 1996, 2000; Lustig et al. 1999; Holt et al., 2001; Ishiyama et al., 1995; Wackym et al., 1995). Localization of the receptor protein in mammalian vestibular tissue by means of immunohistochemistry is more precise but has not previously been done. Park et al. (1997) have used antibodies raised against a portion of the α9 subunit to localize it to the base of inner hair cells and outer hair cells in the guinea pig organ of Corti. Holt and colleagues (2001) used the same antibodies with similar results on hair cells in the frog saccule, a seismic (Lewis et al., 1985) and acoustic (Lewis, 1992) sensory organ.
Pharmacological studies have shown that classic cholinergic compounds have potent effects in the inner ear, but strychnine, a glycine antagonist, has proved to be one of the most potent antagonists (Bobbin and Konishi, 1974; Fuchs and Murrow, 1992a; Shigemoto and Ohmori, 1990, 1991; Erostegui et al., 1994; Sridhar et al., 1997; Rothlin et al., 1999). More recent studies have implicated the α9 and α10 nAChR subunits functionally. When expressed in Xenopus oocytes, the pharmacological signatures of α9 (Elgoyhen et al., 1994) and the recently cloned α10 nAChR subunits (Elgoyhen et al., 2001; Sgard et al., 2002) are unique in comparison with other nAChRs. Their response to ACh is antagonized by nicotine; blocked by atropine, curare, strychnine, bicuculline; and reversibly blocked by α-bungarotoxin.
Studies in the last decade or so have clarified the morphophysiological organization of afferents innervating the vestibular labyrinth in the chinchilla (Baird et al., 1988, Fernández et al., 1988, 1990; Goldberg et al., 1990a,b), squirrel monkey (Fernández et al., 1995; Lysakowski et al., 1995), reptiles (Schessel et al., 1991; Brichta and Goldberg, 2000a,b), pigeon (Si et al., 2003; Zakir et al., 2003), frog (Honrubia et al., 1984, 1989; Myers and Lewis, 1990, 1991), and toadfish (Boyle et al., 1991; Boyle and Highstein, 1990). There are three morphological types of vestibular afferents in mammals, birds, and reptiles, each with its own distinct physiology: calyx, dimorphic, and bouton afferents (Baird et al., 1988; Lysakowski et al., 1995; Brichta and Goldberg, 2000a,b). These three types are also distributed in varying proportions in the sensory epithelium in mammalian crista and macular organs (Fernández et al., 1988, 1990, 1995). It is fortuitous that calyx afferents stain with calretinin antibodies (Desmadryl and Dechesne, 1992; Desai et al., 2005a,b) and that bouton afferents stain with antibodies to peripherin (Lysakowski et al., 1999), because we can use these immunological markers to correlate the presence of receptor antibodies with specific vestibular afferent classes. So far, no specific marker has been described for dimorphic afferents, but we have used the absence of staining for either of the other markers as an indicator of dimorphic afferents.
From physiological studies on vestibular efferents, it is known that they have distinct effects on afferents in squirrel monkeys (Goldberg and Fernández, 1980), cats (McCue and Guinan, 1994), and chinchillas (Plotnik et al., 2002; Marlinski et al., 2004). Although different afferent classes were differentially distributed within the chinchilla (Fernández et al., 1988, 1990) and monkey vestibular sensory epithelia (Fernández et al., 1995; Lysakowski et al., 1995), efferent boutons were evenly distributed within these same sensory epithelia (Lysakowski and Goldberg, 1993, 1997). Efferent boutons make synapses with afferent processes, including calyces, boutons, and afferent dendrites, and with type II hair cells (Engstrom et al., 1965; Smith and Rasmussen, 1968; Wersäll, 1968; Iurato et al., 1972; Lysakowski and Goldberg, 1997). Those contacting afferent processes are considered postsynaptic to the hair cell-afferent ribbon synapse and are marked by asymmetric membrane thickenings or postsynaptic densities. On the other hand, efferent boutons directly contacting type II hair cells are marked by subsynaptic cisterns and are described as presynaptic to the hair cell synapse (Engstrom et al., 1965; Lysakowski and Goldberg, 1997). We wanted to determine whether efferent synapses in mammals that involved α9 nAChRs contacted a particular class of vestibular afferent or participated in one or the other type of efferent synapse, which could then be compared with the physiological effects of efferent activation in the vestibular system. Preliminary reports of this work have been presented previously (Maroni et al., 1998; Guth et al., 1999; Ouyang et al., 2003).
MATERIALS AND METHODS
All procedures described below involving animals conform to NIH guidelines and have been approved by the Institutional Animal Care and Use Committees at the University of Miami and the University of Illinois at Chicago.
α9 nAChR antibody generation
An antibody (MU43) was generated in rabbits (Covance, Inc., Princeton, NJ) against a synthetic peptide (SKPKTARNKDL) conjugated to keyhole limpet hemocyanin (Luebke, 1996; Luebke and Foster, 2002). This peptide is part of the proposed intracellular loop (between TM III and TM IV) of the guinea pig α9 nAChR. The MU43 antibody was tested by both ELISA and Western analysis and is specific for the α9 nAChR in heterologous expression studies using α9-injected Xenopus oocytes. This peptide sequence is not present in any other neuronal nAChR subunits (α2–8, α10, β2–4, δ, γ, or ε) discovered to date.
Western blot and protein assay
For each Western blot, inner ear tissues and pituitary glands were harvested from at least three to five adult rats, chinchillas, and guinea pigs within 5 minutes of death. One of the few locations initially described for α9 was in the pituitary gland (Elgoyhen et al., 1994), so the pituitary gland was an important positive control for our antibody. All animals were anesthetized (Nembutal, 80 mg/kg) and quickly decapitated. All tissues (cristae ampullares, utricular and saccular maculae, cochleae, vestibular and cochlear ganglia, and pituitary glands) were dissected in ice-cold phosphate-buffered saline (PBS). Each tissue was homogenized separately in Laemmli buffer with a disposable pestle in a sterile Eppendorf tube for 30 seconds. Samples of whole chinchilla brain and skeletal muscle were used for controls and treated similarly. The tubes were sealed, and samples were boiled at 95°C for 5 minutes and then frozen in crushed dry ice. Then samples were thawed at 37°C for 30 seconds to 1 minute and kept on ice. Stop dye was added, and samples were again boiled at 97°C for 5 minutes, separated by SDS-PAGE on a 4–15% gradient acrylamide gel, and transferred to an Immobilon P membrane (Amersham ECL detection kit). Prestained “kaleidoscope” molecular weight markers (Bio-Rad, Hercules, CA) were run in a parallel lane to allow size estimations of the protein bands. Protein assays were performed three times with the DC protein assay kit (Bio-Rad), which is a modified form of the high-sensitivity Lowry protein assay. Values given are an average of the three replications.
For detection of proteins in tissue by Western blot analysis, membranes were soaked in primary antibody (rabbit anti-guinea pig α9 antibody, MU43, 1:500), diluted in a 1% nonfat milk blocking solution and incubated in a sealed bag for 1 hour. Membranes were rinsed twice (3 minutes each) and incubated in goat anti-rabbit secondary antibody conjugated to horseradish peroxidase for 30 minutes, blotted dry, rinsed twice again for 3 minutes each, reacted with Pierce illuminator (Pierce, Rockford, IL) and peroxidase solutions for 5 minutes, and finally exposed to Kodak LS film. All incubations were performed on a shaker at RT. Films were scanned into Adobe Photoshop and labels added. The analysis was repeated three times for each tissue in each species.
Fixation
Twenty chinchillas were transcardially perfused, first with 100 ml of a heparinized vascular rinse (1,000 IU heparin per 100 cc of a buffered aqueous rinse solution, containing 8.5 g NaCl, 0.25 g KCl, and 0.2 g NaHCO3, pH 7.4) for about 1 minute. This was followed by 500 ml of a mixed aldehyde fixative (3% paraformaldehyde, 2% acrolein, 0–0.02% glutaraldehyde, 0.1% picric acid, 1% 0.1M CaCl2, and 5% sucrose) in 0.1 M phosphate buffer (PB) for 10 minutes. After the perfusion, animals were decapitated, and the calvaria were opened. Temporal bones were postfixed in 3% paraformaldehyde-30% sucrose buffer for 20 minutes. The vestibular end organs and pituitary glands were dissected and placed in 0.1 M PB. Some tissue was embedded in gelatin, allowed to harden at 4°C, placed in 3% paraformaldehyde-30% sucrose buffer for 2 hours, and rinsed in PB overnight before sectioning.
Immunochemistry
Vestibular end organs and pituitary glands were sectioned in several ways, each requiring slightly different preparation and treatment. Otolithic organs were put into 100% Cal-Ex (Fisher Scientific, Pittsburgh, PA) for 5 minutes and rinsed in 0.01 M phosphate-buffered saline (PBS) prior to sectioning. Material cut with a vibratome was freeze-thawed with isopentane (2 × 30 seconds) chilled to −20°C. Gelatin-embedded vestibular tissue was sectioned with either a vibratome or a freezing sliding microtome at 20–30 µm and placed in 0.1 M PB. Unembedded pituitaries were serially sectioned with a cryostat at 12 µm. Frozen vestibular tissue and cryostat pituitary tissue sections were then digested with Triton (2% and 0.1% respectively) for 30 minutes and rinsed five times with PBS. All material was blocked with 10% normal goat serum in PBS (containing 0.2% Triton for frozen sections). Sections were incubated in the rabbit anti-α9 MU43 antibody at 1:3,000 in fresh blocking solution overnight at 4°C, rinsed, then treated with goat anti-rabbit IgG at 1:8,000 in PBS at 25°C for 4 hours. Preabsorption and preimmune serum controls were done simultaneously. Tissue was then incubated in a peroxidase antiperoxidase (PAP) soluble complex (Sigma, St. Louis, MO) for 2 hours at 1:200 dilution and rinsed. This was followed by a slightly modified Graham and Karnovsky (1966) diaminobenzidine (DAB) reaction, consisting of a 15-minute preincubation in a solution of 0.5% 3,3′-diaminobenzidine tetrahydrochloride (Sigma) in 0.1 M PB, pH 7.4 (made by first dissolving the DAB in distilled water, then diluting with double strength buffer). The preincubation was followed by incubation in the same DAB solution with 0.003% hydrogen peroxide added. The reaction was monitored under a microscope until completed (generally 5–20 minutes for sections or whole organs). In earlier experiments, we used ABC kits (Vector, Burlingame, CA) but found that we obtained unacceptably high background over the hair cell nuclei. This background was eliminated by using the PAP method (Sternberger and Sternberger, 1986). Sections were mounted on gelatinized slides, dehydrated in a series of ethanols, and coverslipped with DPX, a nonfluorescent mounting medium (BDH Chemicals, Poole, England). Some vibratome sections were also serially dehydrated in ethanols, embedded in Araldite resin, and resectioned at 2–4 µm.
Ganglion cell counts
Counts were performed using the disector method (Sterio, 1984). Briefly, counts of cells at each level of staining density (unstained, light, moderate, and dark) were made in three vestibular ganglia from two chinchillas. Vestibular ganglia were embedded in gelatin, sectioned on a cryostat at 12 µm, and reacted for α9 immunohistochemistry as described in the paragraph above for pituitary tissue. Pairs of adjacent sections spaced every 60 µm were used, with the first of the pair serving as a look-up section and the second as a reference section. Cells were counted first in one direction, then the other, and averaged. No significant differences in the percentages of neurons with different staining density were noted in different portions of the ganglion.
Confocal microscopy
We prepared some vestibular ganglia and otolithic organs for confocal microscopy, using triple-label immunofluorescence methods. Whole chinchilla vestibular ganglia and end organs, obtained as described above, were placed into 4% Triton in 0.01 M PBS for 4 hours, then rinsed in 0.01 M PBS. The otolithic organs were placed into 100% Cal-Ex, as described above for sectioned material. Then, all organs and ganglia were placed into 1% sodium borohydride in aqueous solution for 10 minutes and rinsed in 0.01 M PBS. After incubation in a blocking solution of 1% bovine serum albumin in 0.01 M PBS for 1 hour, the tissue was rinsed with 0.01 M PBS and incubated with the primary antibody cocktail, consisting of goat anticalretinin at 1:700 (Chemicon, Temecula, CA), rabbit anti-α9 (MU-43) at 1:1,000, and mouse antiperipherin (Chemicon) at 1:300, all in 0.01 M PBS, for 48 hours at 4°C. Tissue was rinsed and incubated with a secondary antibody cocktail consisting of AMCA-conjugated donkey anti-goat (Chemicon), fluorescein-conjugated donkey anti-rabbit (Chemicon), and rhodamine-conjugated donkey anti-mouse (Chemicon), all at 1:150 in 0.01 M PBS, for 48 hours at 4°C. The whole end organs and ganglia were mounted with Vectashield (Vector) on slides with spacers inserted, and coverslips were sealed with nail polish. Slides were stored horizontally at 4°C in the dark.
Whole end organs and vestibular ganglia were imaged with a Zeiss LSM 510 confocal laser scanning microscope. Three lasers of the microscope were utilized corresponding to the excitation wavelengths for the three secondary antibody labels: AMCA at 364 nm set at 15.3% power; fluorescein at 488 nm at 30.4% power; rhodamine at 568 nm at 30.1% power. Each channel’s image was optimized individually, leading to a composite picture of the three channels. Computer settings were recorded, and the no-primary-antibody controls were viewed under the same computer settings. Images were labeled in Adobe Photoshop 7.0.
RESULTS
Western blot
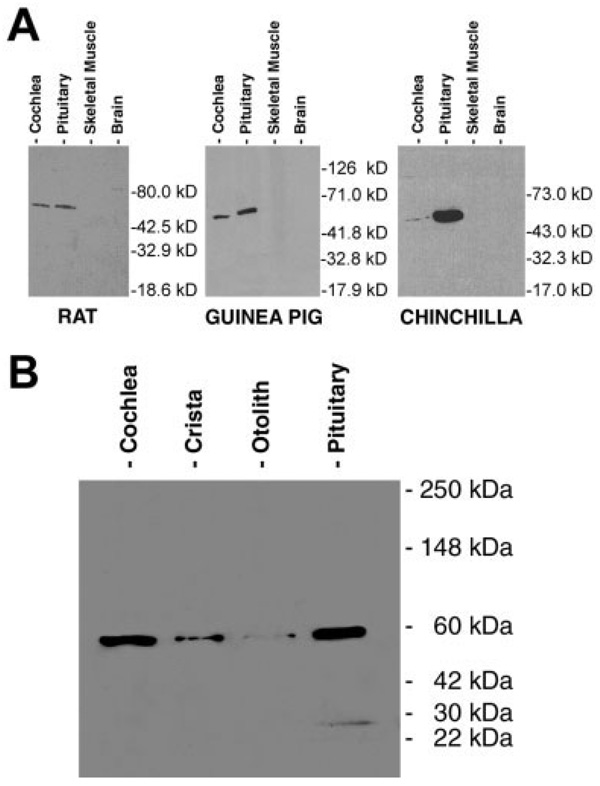
The effectiveness of the anti-α9 antibody (MU43) for use in the chinchilla was determined by comparing Western blots of rat and guinea pig (Fig. 1A, left and center) inner ear and control (pituitary) samples to similar samples in chinchilla (Fig. 1A, right). One of the few locations initially described for α9 nAChR was the pituitary gland (Elgoyhen, 1994), so pituitary tissue was used as a positive control in Western analysis.
Fig. 1.
A: Western blots of rat, guinea pig, and chinchilla immunoreactivity in cochlea, pituitary, skeletal muscle, and whole-brain homogenates. Thirty micrograms of tissue protein was loaded for each lane. Cochlear samples were not microdissected, but rather tissue was homogenized with some surrounding bone present. B: Western blot demonstrating the variation in levels of reactivity among two cochleas, six cristae, four otolith organs, and a pituitary from one chinchilla. Vestibular and cochlear end organs were microdissected in this case, thus minimizing the amount of bone present. In order not to overload the lanes, 22 µg of cochlear protein (one-tenth of one cochlea), 72 µg protein from six microdissected cristae (all the cristae present in one animal), 60 µg of four microdissected otolith organs (one animal’s otolith organs), and 30 µg of pituitary protein (one-fifth of one pituitary) were used.
The molecular weights of the bands shown in Figure 1 match the estimated weight of one α9 subunit in the range of ~55 kDa. Western blotting using the MU43 antibody on cochlea and pituitary homogenate showed immunoreactivity, whereas skeletal muscle and whole brain homogenate (without pituitary gland) did not (Fig. 1A). The pituitary contains more reactive α9 material than the cochlea, which is particularly noticeable in the first chinchilla blot (Fig. 1A, right) in which equal amounts of protein (30 µg) were loaded in each lane. In a second series of Western blots, chinchilla vestibular end organs and pituitary were loaded into separate lanes (Fig. 1B), with amounts loaded determined from our protein assay results (Table 1).
TABLE 1.
Protein Assays Using Chinchilla End Organs and Pituitary Glands1
| Tissue | Amount of protein (µg/organ) |
|---|---|
| Crista | 11.91 ± 0.60 |
| Otolith organ2 | 14.98 ± 1.03 |
| Cochlea | 218.75 ± 37.123 |
| Pituitary | 156.17 ± 15.86 |
Values are mean ± SD. N = 3 animals in each case except cochlea, for which N = 2.
Otolith organ refers to either the utricular or saccular macula.
Cochlear tissue includes some bone, which adds to the total protein content.
Immunochemistry of pituitary tissue
α9 Immunoreactivity was noted in all peripheral vestibular tissue and in the pituitary gland. The chinchilla pituitary stained darkly and was used as a gauge of fixation quality and interindividual differences (data not shown). Such interanimal differences in α9 protein levels are related to the size of the physiological effect that efferents have in the cochlea (Luebke and Foster, 2002) and possibly also in the vestibular periphery, although the latter hypothesis has not been tested. If the pituitary showed strong reactivity, results in the vestibular periphery were generally better. The adenohypophysis (anterior lobe), pars tuberalis of the median eminence, and pituitary stalk exhibited a high density of reaction product (data not shown).
Immunochemistry of vestibular periphery
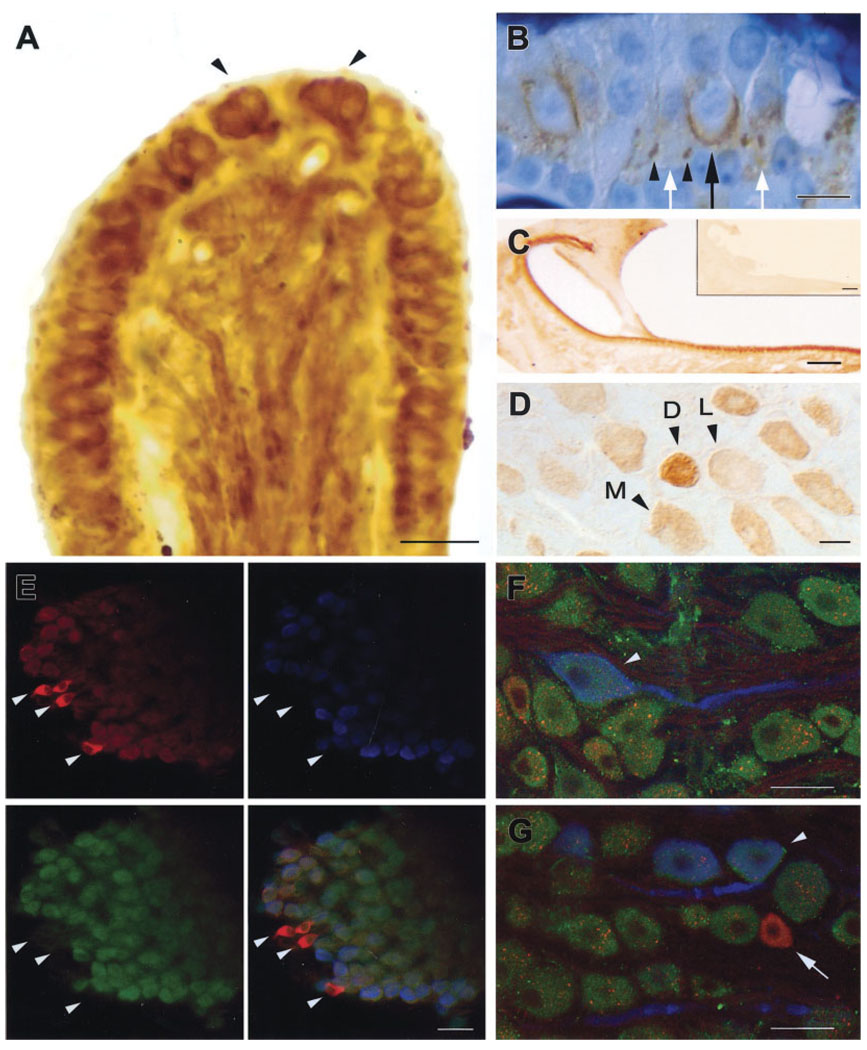
Immunoreaction to the α9 antibody was particularly noted in the crista ampullaris (Fig. 2A). The entire sensory epithelium, both central and peripheral zones, showed some level of reactivity. Transitional epithelium and dark cell epithelium (not shown) showed less staining. Calyces, in particular, exhibited dense immunostaining. Fiber staining in the stroma was observed, and the morphology of their peripheral terminal endings suggests that they are afferent terminals (Fernández et al., 1988, 1990). Semithin (2 µm) plastic-embedded sections (Fig. 2B) provided better localization of the reaction product than frozen sections (Fig. 2A). Calyces around several type I hair cells throughout the crista, boutons at the bases of type II hair cells, and basal poles of type II hair cells themselves were all immunoreactive, although the latter were less intensely stained (Fig. 2B). Preabsorption and preimmune serum controls were negative (data not shown).
Fig. 2.
A: A 30-µm frozen section cut transversely through the center of the chinchilla crista ampullaris. α9 immunoreactivity is observed in both the peripheral and the central zones in this section, but not in the transitional epithelium or the dark cell epithelium (not shown). The calyx endings of calyx and dimorphic afferents (arrowheads) can be seen to be densely α9-immunoreactive. B: A 2-µm plastic-embedded transverse section through the middle of the crista ampullaris shows reaction product in a calyx (black arrow) surrounding a type I hair cell, in bouton endings (arrowheads), presumably from dimorphic afferents, in that these are located in the central zone, and in local areas of density near the bases of type II hair cells (white arrows). C: A 30-µm transverse section of the chinchilla utricular macula immunostained with α9 primary antibody and reacted with DAB. Inset: An adjacent section of the utricular macula incubated identically to C, but with no primary antibody. D: Photomicrograph of the vestibular ganglion in which the variation in staining density can be observed. Cells are unstained (not shown), lightly stained (L), moderately stained (M), or darkly stained (D). E: Split-screen confocal image of whole-mount triple-labeled vestibular ganglion with peripherin (top left, red TRITC label), calretinin (top right, blue AMCA label), and α9 (bottom left, green FITC label) primary antibodies. The combined image is at bottom right. Calretinin and peripherin label calyx and bouton afferent ganglion cells, respectively, whereas ganglion cells unlabeled with either of these are presumed to belong to dimorphic afferents. In the combined image, it can be seen that α9 is found in calyx and dimorphic afferents, but not in bouton afferents (arrowheads). F: A calyx afferent ganglion cell (arrowhead) doubly labeled with anticalretinin (blue AMCA label) and α9 (green FITC label) in a field of FITC-labeled, presumably dimorphic afferent ganglion cells. Lipofuchsin, which typically accumulates in neurons such as these ganglion cells, is present and is seen as orange autofluorescent granules in this and the next panel. G: A calyx afferent ganglion cell (arrowhead) doubly labeled with anticalretinin (blue AMCA label) and α9 (green FITC label) and a bouton afferent ganglion cell, labeled with peripherin (arrow, red TRITC label) but not labeled with α9. Scale bars = 25 µm in A,D,F,G; 10 µm in B; 100 µm in C, inset; 50 µm in E.
It was difficult to obtain good staining in the utricular (Fig. 2C) or saccular macula. The reasons for this were unclear. Immunohistochemical staining in otolithic organs might have been affected by the decalcification procedure, which the cristae did not undergo and which could have interfered with antigen immunoreactivity, although a similar procedure is used successfully in our laboratory for other antisera. A subset of cells in the vestibular (Scarpa’s) ganglion also demonstrated reactivity to the α9 antibody. This staining was variable in density. Under higher magnification, the differences became clearer. Figure 2D shows a 30-µm frozen section of the vestibular ganglion. The different levels of staining density in ganglion cells were distributed uniformly throughout the ganglion. Percentages of ganglion cells from three vestibular ganglia with four levels of staining (unstained, light staining, moderate staining, and dark staining) are presented in Table 2.
TABLE 2.
α9 nAChR Immunoreactivity in Vestibular Ganglion Cells
| Level of staining | No. of cells | Cells counted/ganglion (mean ± SD) | Percentage stained |
|---|---|---|---|
| Unstained cells | 2,066 | 689 ± 14 | 23 ± 9 |
| Lightly stained cells | 4,210 | 1,403 ± 19 | 45 ± 2 |
| Moderately stained cells | 2,556 | 852 ± 25 | 28 ± 9 |
| Darkly stained cells | 409 | 136 ± 7 | 4 ± 3 |
| Total | 9,241 | 3,080 ± 40 | 100 |
To determine which class of afferents (calyx, dimorphic, or bouton) contained α9 nAChR, triple-labeling immunohistochemical studies were performed (Figs. 2E–G). Calyx-only afferents were labeled with calretinin (blue, AMCA label), the bouton-only afferents were labeled with peripherin (red, TRITC label), and the α9-containing afferents were labeled with anti-α9 nAChR antibody MU43 (green, FITC label). There was no specific label for dimorphic afferents, but their identification was determined by a lack of staining for either calretinin or peripherin. In Figure 2E–G, it can be observed that the α9-containing afferents were either of the calyx or of the dimorphic types. We did not observe peripherin-positive (bouton only) ganglion cells containing the α9 nAChR protein.
DISCUSSION
The vestibular efferent system has long been known to be a primarily cholinergic system (Gacek and Lyon, 1975; Warr, 1975; Schwarz et al., 1986; Perachio and Kevetter, 1989). Several other neurotransmitters may also be involved, including calcitonin gene-related peptide (CGRP; Perachio and Kevetter, 1989; Tanaka et al., 1989; Wackym et al., 1990; Lysakowski 1999; Popper et al., 2002), met-enkephalin (Perachio and Kevetter, 1989; Ryan et al., 1991; Popper et al., 2004), nitric oxide (Lyon et al., 1994; Lysakowski and Singer, 2000; Chen and Eatock, 2000), ATP (Rossi et al., 1994), and norepinephrine (Hozawa and Kimura, 1989; Hozawa and Takasaka, 1993). Pharmacological studies, however, show that classic cholinergic or anticholinergic compounds have the greatest effects (Bernard et al., 1985; Fuchs and Murrow, 1992a; Shigemoto and Ohmori, 1990, 1991; Yamaguchi and Ohmori, 1993).
α9 was cloned and shown by earlier in situ hybridization and PCR studies in the rat to have a limited distribution in the CNS, pituitary, nasal epithelium, and inner ear (Elgoyen et al., 1994). More recent studies have demonstrated the presence of the α9 nAChR in lymphocytes (Peng et al., 2004), dorsal root ganglion cells (Lips et al., 2002), and epidermal basal cell and follicular central cell layers in skin (Kurzen et al., 2004). Previous studies have used in situ hybridization in the cochlea (Morley et al., 1998; Luo et al., 1998; Simmons and Morley, 1998; Hiel et al., 2000) to demonstrate the presence of α9 nAChR. α9 was also shown to be present in vestibular sensory epithelium, in in situ hybridization and RT-PCR studies (Hiel et al., 1996, 2000; Anderson et al., 1997; Luo et al., 1998; Simmons and Morley, 1998; Lustig et al., 1999; Cameron et al., 2004; Drescher et al., 2004) as well as in studies utilizing green fluorescent protein (GFP) coexpressed with α9 mRNA (Zuo et al., 1999). Efferent modulation of afferent responses in the cochlea can now be partially explained by the unique physiology and pharmacology of this receptor subunit and its localization to outer hair cells (Fuchs and Murrow, 1992a,b; Elgoyhen et al., 1994; Glowatzki et al., 1995; Blanchet et al., 1996; Rothlin et al., 1999; Verbitsky et al., 2000; Holt et al., 2001). However, the desensitization kinetics and the Ca2+ sensitivity of the ACh response in outer hair cells are most closely matched when α9 is coexpressed with the α10 nAChR subunit (Katz et al., 2000; Elgoyhen et al., 2001; Sgard et al., 2002). α9 Homomers also respond to strychnine, typically regarded as a glycine antagonist (Elgoyen et al., 1994), and bicuculline, a γ-aminobutyric acid (GABA) antagonist (Erostegui et al., 1994; Sridhar et al., 1995; for review see Housley and Ryan, 1997), which had earlier been shown to inhibit crista efferents (Norris et al., 1988).
Previous studies have used rather indirect methods to determine the distribution of nAChR subunits. Nevertheless, several possible candidates have been identified. Ohno et al. (1993) used in situ hybridization to show that rat vestibular ganglion cells express both α4 and β2 subunit mRNAs. Histological studies using labeled α-bungarotoxin, a toxin specific for neuromuscular (α1) and some neuronal nicotinic (α7–10) receptor subtypes, showed reversible binding to calyces surrounding type I hair cells and the bases of type II hair cells (Ishiyama et al., 1995; Wackym et al., 1995; Dailey et al., 2000). Hiel et al. (1996) also used in situ hybridization to localize α4–7 and β2–3 in Scarpa’s ganglion cells and α9 alone in the end organs of the rat. Ganglion cells showed varying degrees of nAChR subunit expression, with only α6 present in all ganglion cells. Anderson et al. (1997) used RT-PCR to show α2–7 and β2–3 mRNA in Scarpa’s ganglion and α3, α5–7, α9, and β2–4 mRNA in the vestibular end organs of the rat. With chick vestibular sensory organs and ganglia, Lustig et al. (1999) used RT-PCR, single-cell RT-PCR, and in situ hybridization. They found evidence for α9 nAChR in both hair cells and ganglion cells of the chick vestibular end organs, whereas Holt et al. (2001), using mRNA profiling, found α9 nAChR mRNA present in frog saccule hair cells. Qualitatively, α4 and β2 subunits appear to be the predominant subunits in the ganglion, whereas α9 was expressed most strongly in the vestibular periphery in these studies. Anderson and colleagues (1997) suggest that some of the differences among studies using different techniques might be due to the higher sensitivity of the RT-PCR technique compared with in situ hybridization.
The presence of α9 mRNA in type I hair cells in in situ hybridization studies (Hiel et al., 1996; Lustig et al., 1999), even though efferent boutons do not directly contact type I hair cells in the adult, has been a bit of a mystery. One explanation for its presence in type I hair cells was that in situ hybridization detects mRNA and that this mRNA might still be present as a small number of copies in type I hair cells in adult tissue. The notion was that mRNA may be derived from a developmental stage before calyces surround type I hair cells at about postnatal day 4 in mouse (Favre and Sans, 1979; Rüsch et al., 1998). Thus α9 mRNA expression without subsequent protein expression could be due to posttranscriptional regulation of the α9 receptor subunit. In support of this notion, the rat cochlea has inner hair cells and outer hair cells, which both contain α9 and α10 mRNA during development (Simmons and Morley, 1998; Simmons and Morley, 2001; Katz et al., 2004). ACh currents can be recorded in neonatal inner hair cells, yet, by adulthood, α10 mRNA is no longer expressed in these cells (Elgoyhen et al., 2001; Lustig et al, 2001; Simmons and Morley, 2001; Katz et al., 2004), although α9 mRNA is still present, along with the AChR currents (Glowatzki and Fuchs, 2000). α10 mRNA expression persists in outer hair cells, however, and in vestibular hair cells in the adult (Elgoyhen et al., 2001; Simmons and Morley, 2001). This finding suggests that functional nAChR expression may be controlled by posttranscriptional events.
Although antibodies against the α9 receptor have been used in the guinea pig cochlea (Park et al., 1997; Luebke and Foster, 2002) and in the fish saccule, another auditory organ (Drescher et al., 2004), direct localization of individual ACh receptor subunits using immunohistochemistry in the mammalian vestibular periphery has not been previously demonstrated. The peptide recognized by the polyclonal MU43 antibody is not present in α10 or in any other nicotinic AChR subunit known to date. We used this antibody to determine the location of this receptor subunit in the vestibular periphery. The antibody shows affinity for certain cells in Scarpa’s ganglion with a variable staining pattern. Although the ganglion cell is not the site of receptor activity, receptor manufacturing occurs in endoplasmic reticulum located in the cell’s soma and dendrites (Hille, 2001).
Our clearest data on the microstructure of α9 distribution were derived from semithin sections of the crista ampullaris. Reaction product is found in calyx terminals surrounding type I hair cells, in bouton terminals (derived from dimorphic afferents), at the basal poles of type II hair cells in the sensory epithelium, and also, though labeling is not as strong, in type II hair cells (Fig. 2B). The mRNA for α9 nAChR is present in type I and type II hair cells during development in the mouse and chick (Elgoyhen et al., 1994; Simmons and Morley, 1998; Hiel et al., 2000), but, in the adult (present study), α9 nAChR protein was detected only in the calyces surrounding type I hair cells and not in the type I hair cells themselves. Data supporting this finding are those from a gene microarray study on individual type I and type II hair cells (Cristobal et al., 2005). In this study, the authors used laser-captured hair cells and, thus, were able to identify type I vs. type II hair cells histologically. In this manner, they could determine genes expressed in each cell type and show that type I hair cells do not express the α9 receptor gene, whereas type II hair cells do. Perhaps α9 nAChR mRNA found in developing type I hair cells is present because these cells are initially contacted by efferent fibers before the calyx has developed (Favre and Sans, 1979; Rüsch et al., 1998), but an answer to this question may await a gene expression microarray study or a proteomic study in the developing inner ear.
Vestibular ganglion cells also showed specific reactivity, and they appeared to have variable levels of α9 nAChR production. Triple labeling for various afferent classes showed that the α9 nAChR is present in calyx and dimorphic afferents and is not found in the bouton-only afferents. Both regularly discharging and irregularly discharging afferents have fast and slow efferent-mediated responses. These fast and slow responses are much larger in irregular afferents. Calyx and central dimorphic afferents are both irregularly discharging afferents and are found in the central zone. Our results indicate that they both express α9 nAChR. When the α9 nAChR is expressed with α10 in Xenopus ooctyes, it exhibits rapid kinetics (Oliver, et al., 2000), similar to the large, fast effect of efferent stimulation on irregularly discharging, centrally located afferents. Bouton and peripheral dimorphic afferents are both regularly discharging afferents and are found in the peripheral zone. Bouton afferents, as identified by peripherin labeling in vestibular ganglion cells, do not appear to express α9 nAChR. We do not know whether peripheral dimorphic afferents express α9 nAChR, although we did observe staining in the peripheral zone, so this staining may be the peripheral dimorphic afferents. We do not know yet about differences in responsiveness to efferent-mediated stimulation between bouton and peripheral dimorphic afferents. We do know that efferent-mediated responses are smaller in regular afferents and that efferent responses are proportional to encoder sensitivity (Marlinski et al., 2004). Perhaps the cholinergic receptor mediating the slow response is of the muscarinic type or involves other nicotinic α or β subunits. The complete efferent transmitter receptor story lies beyond the scope of this investigation, but it is a topic of ongoing investigation in our laboratory.
Immunohistochemical studies with muscarinic receptor subtypes have not yet been published, but mRNA studies have indicated that mRNA for muscarinic receptor subtypes is present (Wackym et al., 1996). Additional candidates to be considered for the slow efferent response are peptide neurotransmitters or neuromodulators. The results of a preliminary report (Lysakowski, 1999) show that CGRP is present in a majority (~90%) of peripheral efferent boutons, compared with only 25% of central efferent boutons. These results were partially confirmed (no regional variation was discussed) in a study in which CGRP immunoreactivity was found in human crista ampullaris (Popper et al., 2002). Opiate peptides are another strong candidate. Popper et al. (2004) found mu opioid receptors in vestibular afferents, both in the ganglion and in the end organs, in all afferent types, using afferent markers in a manner similar to the present study. They did not find opiate receptors in hair cells. Thus, it is possible that CGRP and opiate signaling play a more important role in the slow efferent response, whereas α9 nAChR signaling is more effective in the fast response.
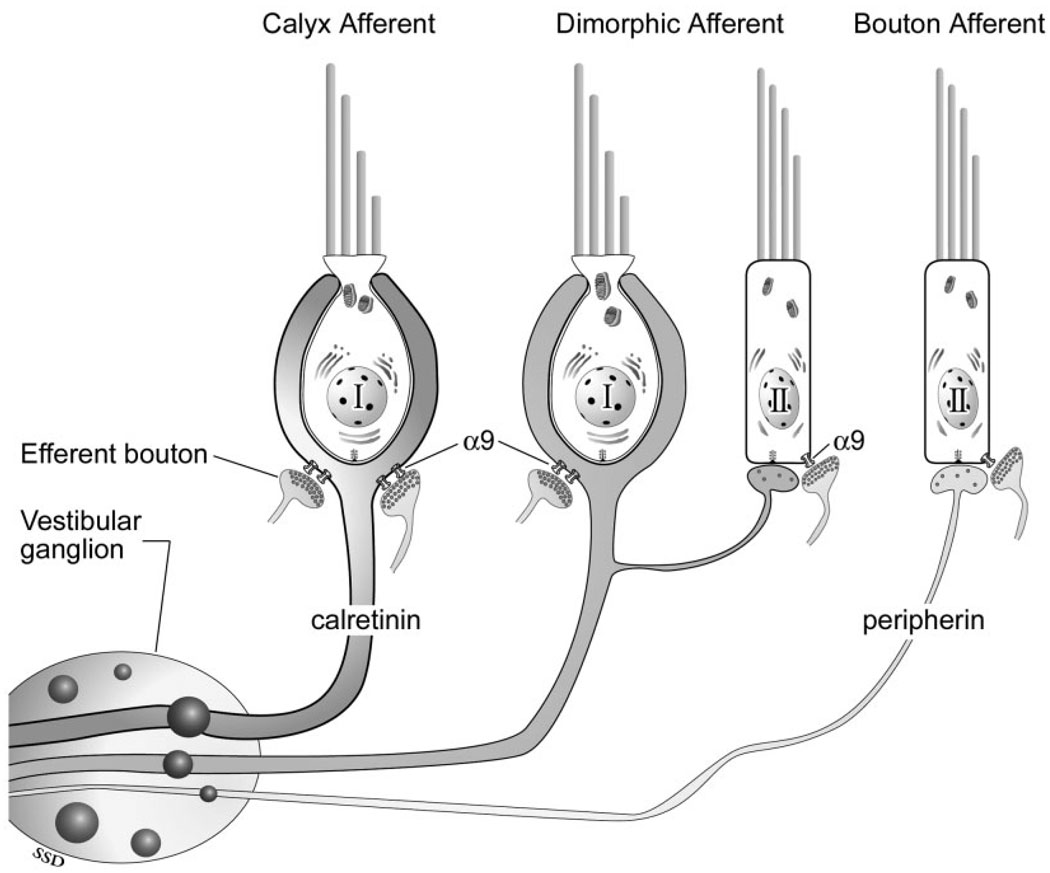
A diagram of our α9 immunohistochemical findings with respect to the three afferent classes (calyx, dimorphic, and bouton) is shown in Figure 3. Further studies should be performed to determine the identity and regional expression of other efferent transmitter receptor subtypes.
Fig. 3.
Diagram of vestibular afferent classes and α9 nAChR reactivity. There are three morphological classes of vestibular afferents (Fernández et al., 1988): calyx (left), dimorphic (center), and bouton (right). Our data indicate that α9 receptors are found on two of these classes, the calyx and dimorphic afferents, but not on bouton afferents.
ACKNOWLEDGMENTS
Grant sponsor: National Institutes of Health; Grant number: R01 DC02521 (to A.L.); Grant number: R01 DC03086 (to A.E.L.).
We gratefully acknowledge the expert technical and photographic assistance of Mr. Steven Price and Ms. Amanda Lowrey, and we thank Mr. Sapan Desai for the illustration in Figure 3 and for his computer graphics expertise. Drs. Joseph C. Holt and Jay M. Goldberg commented on a previous version of the article. We also thank Dr. Mei-Ling Chen of the University of Illinois College Research Resources Center for her confocal imaging expertise.
LITERATURE CITED
- Anderson AD, Troyanovskaya M, Wackym PA. Differential expression of α2–7, α9 and β2–4 nicotinic acetylcholine receptor subunit mRNA in the vestibular endorgans and Scarpa’s ganglia of the rat. Brain Res. 1997;778:409–413. doi: 10.1016/s0006-8993(97)01121-9. [DOI] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Bernard C, Cochran SL, Precht W. Presynaptic actions of cholinergic agents upon the hair cell-afferent fiber synapses in the vestibular labyrinth of the frog. Brain Res. 1985;338:225–236. doi: 10.1016/0006-8993(85)90151-9. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbin RP, Konishi T. Acetylcholine mimics crossed olivocochlear bundle stimulation. Nat New Biol. 1971;231:222–223. doi: 10.1038/newbio231222a0. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, Konishi T. Action of cholinergic and anticholinergic drugs at the crossed olivocochlear bundle-hair cell junction. Acta Otolaryngol. 1974 Jan–Feb;77(1):56–65. doi: 10.3109/00016487409124598. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein SM. Resting discharge and response dynamics of horizontal semicircular canal afferents in the toadfish, Opsanus tau. J Neurosci. 1990;10:1557–1569. doi: 10.1523/JNEUROSCI.10-05-01557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Carey JP, Highstein SM. Morphological correlates of response dynamics and efferent stimulation in horizontal semicircular canal afferents. J Neurophysiol. 1991;66:1504–1521. doi: 10.1152/jn.1991.66.5.1504. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Morphological identification of physiologically characterized afferents innervating the turtle posterior crista. J Neurophysiol. 2000 Mar;83(3):1202–1223. doi: 10.1152/jn.2000.83.3.1202. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Responses to efferent activation and excitatory response-intensity relations of turtle posterior-crista afferents. J Neurophysiol. 2000 Mar;83(3):1224–1242. doi: 10.1152/jn.2000.83.3.1224. [DOI] [PubMed] [Google Scholar]
- Cameron P, Gould R, Holt JC, Goldberg JM, Lysakowski A. Sequencing of the turtle alpha-9 nicotinic acetylcholine receptor subunit gene. Abstr Mol Biol Deafness Hearing Mtg. 2004;2004(Oct.):160. [Google Scholar]
- Chen JW, Eatock RA. Major potassium conductance in type I hair cells from rat semicircular canals: characterization and modulation by nitric oxide. J Neurophysiol. 2000;84:139–151. doi: 10.1152/jn.2000.84.1.139. [DOI] [PubMed] [Google Scholar]
- Cristobal R, Wackym PA, Cioffi JA, Erbe CB, Roche JP, Popper P. Assessment of differential gene expression in vestibular epithelial cell types using microarray analysis. Brain Res Mol Brain Res. 2005;133:19–36. doi: 10.1016/j.molbrainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Dailey SH, Wackym PA, Brichta AM, Gannon PJ, Popper P. Topographic distribution of nicotinic acetylcholine receptors in the cristae of a turtle. Hear Res. 2000;141:51–56. doi: 10.1016/s0378-5955(99)00208-7. [DOI] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005 Jan;93(1):251–266. doi: 10.1152/jn.00746.2003. Epub 2004 Jul 7. [DOI] [PubMed] [Google Scholar]
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol. 2005 Jan;93(1):267–280. doi: 10.1152/jn.00747.2003. Epub 2004 Jul 7. [DOI] [PubMed] [Google Scholar]
- Desmadryl G, Dechesne CJ. Calretinin immunoreactivity in chinchilla and guinea pig vestibular end organs characterizes the calyx unit subpopulation. Exp Brain Res. 1992;89:105–108. doi: 10.1007/BF00229006. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Monaco P. Suppression par la strychnine de l’effet inhibiteur centrifuge exercé par le faisceau olivo-cochléaire. Arch Int Pharmacodyn. 1960;129:244–248. [PubMed] [Google Scholar]
- Drescher DG, Ramakrishnan NA, Drescher MJ, Chun W, Wang X, Myers SF, Green GE, Sadrazodi K, Karadaghy AA, Poopat N, Karpenko AN, Khan KM, Hatfield JS. Cloning and characterization of alpha9 subunits of the nicotinic acetylcholine receptor expressed by saccular hair cells of the rainbow trout (Oncorhynchus mykiss) Neuroscience. 2004;127:737–752. doi: 10.1016/j.neuroscience.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacologic properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. α10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström H, Ades HW, Hawkins JE., Jr . The vestibular sensory cells and their innervation. In: Szentágothai J, editor. Modern trends in neuromorphology. Symp Biol Hung. vol 5. Budapest: Akadémiai Kiadó; 1965. pp. 21–41. [Google Scholar]
- Erostegui C, Norris CH, Bobbin RP. In vitro pharmacologic characterization of a cholinergic receptor on outer hair cells. Hear Res. 1994 Apr;74(1–2):135–147. doi: 10.1016/0378-5955(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Favre D, Sans A. Morphological changes in afferent vestibular hair cell synapses during the postnatal development of the cat. J Neurocytol. 1979;8:765–775. doi: 10.1007/BF01206675. [DOI] [PubMed] [Google Scholar]
- Fernández C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla: I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol. 1988;60:167–181. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla: III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:767–780. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- Fernández C, Lysakowski A, Goldberg JM. Hair-cell counts and afferent innervation patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J Neurophysiol. 1995;73:1253–1281. doi: 10.1152/jn.1995.73.3.1253. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells in the chick’s cochlea. J Neurosci. 1992 Mar;12(3):800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc Biol Sci. 1992 Apr 22;248(1321):35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol. 1974;77:92–101. doi: 10.3109/00016487409124603. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Wild K, Brändle U, Fakler G, Fakler B, Zenner HP, Ruppersberg JP. Cell-specific expression of the alpha 9 n-ACh receptor subunit in auditory hair cells revealed by single-cell RT-PCR. Proc Biol Sci. 1995 Nov 22;262(1364):141–147. doi: 10.1098/rspb.1995.0188. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol. 1980;43:986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol. 1990 Apr;63(4):781–790. doi: 10.1152/jn.1990.63.4.781. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990 Apr;63(4):791–804. doi: 10.1152/jn.1990.63.4.791. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brichta AM, Wackym PA. Efferent vestibular system: anatomy, physiology, and neurochemistry. In: Beitz AJ, Anderson JH, editors. Neurochemistry of the vestibular system. Boca Raton, FL: CRC Press; 2000. pp. 61–94. [Google Scholar]
- Graham RC, Jr, Karnovsky MJ. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Guth PS, Perin P, Norris CH, Valli P. The vestibular hair cells: posttransductional signal processing. Prog Neurobiol. 1998;54:193–247. doi: 10.1016/s0301-0082(97)00068-3. [DOI] [PubMed] [Google Scholar]
- Guth SM, Price SD, Luebke A, Lysakowski A. Alpha-9 nicotinic acetylcholine receptor is not found on the peripherin-labelled (bouton) class of afferents in the vestibular ganglion of the chinchilla. ARO Abstr. 1999;22:186–187. [Google Scholar]
- Hiel H, Elgoyhen AB, Drescher DG, Morley B. Expression of nicotinic acetylcholine receptor mRNA in the adult rat peripheral vestibular system. Brain Res. 1996;738:347–352. doi: 10.1016/s0006-8993(96)01046-3. [DOI] [PubMed] [Google Scholar]
- Hiel H, Luebke AE, Fuchs PA. Cloning and expression of α9 nicotinic acetylcholine receptor subunit in cochlear hair cells of the chick. Brain Res. 2000;858:215–225. doi: 10.1016/s0006-8993(00)01947-8. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3rd ed. Sunderland, MA: Sinauer Assoc; 2001. Chap. 21. [Google Scholar]
- Holt JC, Lioudyno M, Athas G, Garcia MM, Perin P, Guth PS. The effect of proteolytic enzymes on the alpha9-nicotinic receptor-mediated response in isolated frog vestibular hair cells. Hear Res. 2001;152:25–42. doi: 10.1016/s0378-5955(00)00225-2. [DOI] [PubMed] [Google Scholar]
- Honrubia V, Sitko S, Lee R, Kuruvilla A, Schwartz I. Anatomical characteristics of the anterior vestibular nerve of the bullfrog. Laryngoscope. 1984;94:464–474. doi: 10.1288/00005537-198404000-00004. [DOI] [PubMed] [Google Scholar]
- Honrubia V, Hoffman LF, Sitko S, Schwartz IR. Anatomic and physiological correlates in bullfrog vestibular nerve. J Neurophysiol. 1989;61:688–701. doi: 10.1152/jn.1989.61.4.688. [DOI] [PubMed] [Google Scholar]
- Housley GD, Ryan AF. Cholinergic and purinergic neurohumoral signalling in the inner ear: a molecular physiological analysis. Audiol Neurootol. 1997;2:92–110. doi: 10.1159/000259233. [DOI] [PubMed] [Google Scholar]
- Hozawa K, Kimura RS. Vestibular sympathetic nervous system in guinea pig. Acta Otolaryngol. 1989;107:171–181. doi: 10.3109/00016488909127496. [DOI] [PubMed] [Google Scholar]
- Hozawa K, Takasaka T. Catecholaminergic innervation in the vestibular labyrinth and vestibular nucleus of guinea pigs. Acta Otolaryngol Suppl. 1993;503:111–113. doi: 10.3109/00016489309128089. [DOI] [PubMed] [Google Scholar]
- Ishiyama A, Lopez I, Wackym PA. Distribution of efferent cholinergic terminals and α-bungarotoxin binding to putative nicotinic acetylcholine receptors in the human vestibular endorgans. Laryngoscope. 1995;105:1167–1172. doi: 10.1288/00005537-199511000-00005. [DOI] [PubMed] [Google Scholar]
- Iurato S, Luciano L, Pannese E, Reale E. Efferent vestibular fibers in mammals: morphological and histochemical aspects. Prog Brain Res. 1972;37:429–443. doi: 10.1016/s0079-6123(08)63917-5. [DOI] [PubMed] [Google Scholar]
- Katz E, Verbitsky M, Rothlin CV, Vetter DE, Heinemann SF, Elgoyhen AB. High calcium permeability and calcium block of the alpha9 nicotinic acetylcholine receptor. Hear Res. 2000;141:117–128. doi: 10.1016/s0378-5955(99)00214-2. [DOI] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci. 2004;24:7814–7820. doi: 10.1523/JNEUROSCI.2102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke R, Galley N. Efferent innervation of vestibular and auditory receptors. Physiol Rev. 1974;54:316–357. doi: 10.1152/physrev.1974.54.2.316. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Berger H, Jager C, Hartschuh W, Naher H, Gratchev A, Goerdt S, Deichmann M. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol. 2004;123:937–949. doi: 10.1111/j.0022-202X.2004.23425.x. [DOI] [PubMed] [Google Scholar]
- Lewis ER. Convergence of design in vertebrate acoustic sensors. In: Webster DB, Fay RR, Popper AN, editors. The evolutionary biology of hearing. New York: Springer-Verlag; 1992. pp. 163–184. [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. Boca Raton, FL: CRC Press; 1985. [Google Scholar]
- Lindstrom J. Neuronal nicotinic acetylcholine receptors. Ion Channels. 1996;4:377–450. doi: 10.1007/978-1-4899-1775-1_10. [DOI] [PubMed] [Google Scholar]
- Lips KS, Pfeil U, Kummer W. Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;115:1–5. doi: 10.1016/s0306-4522(02)00274-9. [DOI] [PubMed] [Google Scholar]
- Luebke AE. Isolation of the α9 receptor from the guinea pig organ of Corti. ARO Abstr. 1996;19:187. [Google Scholar]
- Luebke AE, Foster PK. Variation in inter-animal susceptibility to noise damage is associated with alpha 9 acetylcholine receptor subunit expression level. J Neurosci. 2002;22:4241–4247. doi: 10.1523/JNEUROSCI.22-10-04241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Bennett T, Jung HH, Ryan AF. Developmental expression of alpha 9 acetylcholine receptor mRNA in the rat cochlea and vestibular inner ear. J Comp Neurol. 1998;393:320–331. [PubMed] [Google Scholar]
- Lustig LR, Hiel H, Fuchs PA. Vestibular hair cells of the chick express the nicotinic acetylcholine receptor subunit alpha 9. J Vestib Res. 1999;9:359–367. [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10) Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- Lyon MJ, Godin D, Mayer B. Localization of nitric oxide synthase immunoreactivity and nicotinamide adenine dinucleotide phosphate diaphorase in the rat inner ear. Soc Neurosci Abstr. 1994;20:969. [Google Scholar]
- Lysakowski A. CGRP shows regional variation in efferent innervation of chinchilla vestibular periphery. Soc Neurosci Abstr. 1999;25:1670. [Google Scholar]
- Lysakowski A, Goldberg JM. Regional variations in the synaptic organization of the squirrel monkey cristae. Soc Neurosci Abstr. 1993;19:1578. [Google Scholar]
- Lysakowski A, Goldberg JM. Regional variations in the cellular and synaptic architecture of the chinchilla cristae. J Comp Neurol. 1997;389:419–443. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Morphophysiology of the vestibular sensory periphery. In: Highstein SM, Fay RR, Popper AN, editors. The vestibular system. New York: Springer-Verlag; 2004. pp. 57–152. [Google Scholar]
- Lysakowski A, Singer M. Nitric oxide synthase localized in a sub-population of vestibular efferents with NADPH diaphorase histochemistry and nitric oxide synthase immunohistochemistry. J Comp Neurol. 2000;427:508–521. doi: 10.1002/1096-9861(20001127)427:4<508::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernández C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol. 1995;73:1270–1281. doi: 10.1152/jn.1995.73.3.1270. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Alonto A, Jacobson L. Peripherin immunoreactivity labels small diameter vestibular “bouton” afferents in rodents. Hear Res. 1999;133:149–154. doi: 10.1016/s0378-5955(99)00065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud Y, Lysakowski A. Regional distribution of efferent innervation to the chinchilla vestibular periphery. Program No. 530.11. Soc Neurosci Abstr Online. 2004 [Google Scholar]
- Marlinski V, Plotnik M, Goldberg JM. Efferent actions in the chinchilla vestibular labyrinth. J Assoc Res Otolaryngol. 2004;5:126–143. doi: 10.1007/s10162-003-4029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni PD, Lysakowski A, Luebke A. Alpha-9 nicotinic receptor subunit immunoreactivity in the rodent inner ear. ARO Abstr. 1998;21:65. [Google Scholar]
- McCue MP, Guinan JJ., Jr Influence of efferent stimulation on acoustically responsive vestibular afferents in the cat. J Neurosci. 1994;14:6071–6083. doi: 10.1523/JNEUROSCI.14-10-06071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley BJ, Li HS, Hiel H, Drescher DG, Elgoyhen AB. Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT-PCR and in situ hybridization. Brain Res Mol Brain Res. 1998;53:78–87. doi: 10.1016/s0169-328x(97)00272-6. [DOI] [PubMed] [Google Scholar]
- Myers SF, Lewis ER. Hair cell tufts and afferent innervation of the bullfrog crista ampullaris. Brain Res. 1990;534:15–24. doi: 10.1016/0006-8993(90)90107-m. [DOI] [PubMed] [Google Scholar]
- Myers SF, Lewis ER. Vestibular afferent responses to microrotational stimuli. Brain Res. 1991;543:36–44. doi: 10.1016/0006-8993(91)91045-3. [DOI] [PubMed] [Google Scholar]
- Noda M, Furutani Y, Takahashi H, Toyosato M, Tanabe T, Shimizu S, Kikyotani S, Kayano T, Hirose T, Inayama S, et al. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. Nature. 1983 Oct 27–Nov 2;305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Noda M, Takahashi H, Tanabe T, Toyosato M, Kikyotani S, Furutani Y, Hirose T, Takashima H, Inayama S, Miyata T, Numa S. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Norris CH, Housley GD, Williams WH, Guth SL, Guth PS. The acetylcholine receptors of the semicircular canal in the frog (Rana pipiens) Hear Res. 1988;32:197–206. doi: 10.1016/0378-5955(88)90092-5. [DOI] [PubMed] [Google Scholar]
- Ohno K, Takeda N, Kiyama H, Kato H, Fujita S, Matsunaga T, Tohyama M. Synaptic contact between vestibular afferent nerve and cholinergic efferent terminal: its putative mediation by nicotinic receptors. Brain Res Mol Brain Res. 1993;18:343–346. doi: 10.1016/0169-328x(93)90100-4. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Lysakowski A, Luebke A. Immunohistochemical localization of alpha 9 and alpha 10 nicotinic acetylcholine receptor subunits in the rodent inner ear. ARO Abst. 2003;26:159. [Google Scholar]
- Park H-J, Niedzielski AS, Wenthold RJ. Expression of the nicotinic acetylcholine receptor subunit, α9, in the guinea pig cochlea. Hear Res. 1997;112:95–105. doi: 10.1016/s0378-5955(97)00111-1. [DOI] [PubMed] [Google Scholar]
- Peng H, Ferris RL, Matthews T, Hiel H, Lopez-Albaitero A, Lustig LR. Characterization of the human nicotinic acetylcholine receptor subunit alpha 9 (CHRNA9) and alpha 10 (CHRNA10) in lymphocytes. Life Sci. 2004;76:263–280. doi: 10.1016/j.lfs.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Perachio AA, Kevetter GA. Identification of vestibular efferent neurons in the gerbil: histochemical and retrograde labelling. Exp Brain Res. 1989;78:315–326. doi: 10.1007/BF00228903. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Marlinski V, Goldberg JM. Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol. 2002;88:1234–1244. doi: 10.1152/jn.2002.88.3.1234. [DOI] [PubMed] [Google Scholar]
- Popper P, Ishiyama A, Lopez I, Wackym PA. Calcitonin gene-related peptide and choline acetyltransferase colocalization in the human vestibular periphery. Audiol Neurootol. 2002;7:298–302. doi: 10.1159/000064445. [DOI] [PubMed] [Google Scholar]
- Popper P, Cristobal R, Wackym PA. Expression and distribution of mu opioid receptors in the inner ear of the rat. Neuroscience. 2004;129:225–233. doi: 10.1016/j.neuroscience.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol. 1997;78:3234–3248. doi: 10.1152/jn.1997.78.6.3234. [DOI] [PubMed] [Google Scholar]
- Rossi ML, Martini M, Pelucchi B, Fesce R. Quantal nature of synaptic transmission at the cytoneural junction in the frog labyrinthq. J Physiol. 1994;478:17–35. doi: 10.1113/jphysiol.1994.sp020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Katz E, Verbitsky M, Elgoyhen AB. The alpha9 nicotinic acetylcholine receptor shares pharmacological properties with type A gamma-aminobutyric acid, glycine, and type 3 serotonin receptors. Mol Pharmacol. 1999;55:248–254. doi: 10.1124/mol.55.2.248. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci. 1998;18:7487–7501. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Simmons DM, Watts AG, Swanson LW. Enkephalin mRNA production by cochlear and vestibular efferent neurons in the gerbil brainstem. Exp Brain Res. 1991;87:259–267. doi: 10.1007/BF00231843. [DOI] [PubMed] [Google Scholar]
- Schessel DA, Ginzberg R, Highstein SM. Morphophysiology of synaptic transmission between type I hair cells and vestibular primary afferents. An intracellular study employing horseradish peroxidase in the lizard, Calotes versicolor. Brain Res. 1991;544:1–16. doi: 10.1016/0006-8993(91)90879-z. [DOI] [PubMed] [Google Scholar]
- Schwarz DWF, Satoh K, Schwarz IE, Hu K, Fibiger HC. Cholinergic innervation of the rat’s labyrinth. Exp Brain Res. 1986;64:19–26. doi: 10.1007/BF00238197. [DOI] [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol. 2002;61:150–159. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- Shigemoto T, Ohmori H. Muscarinic agonists and ATP increase the intracellular Ca2+ concentration in chick cochlear hair cells. J Physiol. 1990;442:127–148. doi: 10.1113/jphysiol.1990.sp017904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto T, Ohmori H. Muscarinic receptor hyperpolarizes cochlear hair cells of chick by activating Ca2+-activated K+ channels. J Physiol. 1991;442:669–690. doi: 10.1113/jphysiol.1991.sp018814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Zakir MM, Dickman JD. Afferent innervation of the utricular macula in pigeons. J Neurophysiol. 2003;89:1660–1677. doi: 10.1152/jn.00690.2002. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Morley BJ. Differential expression of the alpha 9 nicotinic acetylcholine receptor subunit in neonatal and adult cochlear hair cells. Brain Res Mol Brain Res. 1998;56:287–292. doi: 10.1016/s0169-328x(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Morley BJ. Developmental expression of the α10 nicotinic acetylcholine receptor subunit in the rat inner ear. ARO Abstr. 2001;24:98. [Google Scholar]
- Smith CA, Rasmussen GL. Nerve endings in the maculae and cristae of the chinchilla vestibule, with a special reference to the efferents. Washington, DC: U.S. Govt. Printing Office (NASA SP-152); 1968. Third symposium on the role of the vestibular organs in space exploration; pp. 183–201. [Google Scholar]
- Spoendlin HH. Structural basis for peripheral frequency analysis. In: Plomp R, Smoorenberg GF, editors. Frequency analysis and periodicity detection in hearing. Leiden: A.W Sijthoff; 1970. pp. 2–40. [Google Scholar]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J Neurosci. 1995;15:3667–3678. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC, Sewell WF. Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci. 1997;17:428–437. doi: 10.1523/JNEUROSCI.17-01-00428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. The unlabeled antibody method: comparison of peroxidase-antiperoxidase with avidin-biotin complex by a new method of quantification. J Histochem Cytochem. 1986 May;34(5):599–605. doi: 10.1177/34.5.3517144. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takeda N, Senba E, Tohyama M, Kubo T, Matsunaga T. Localization, origin and fine structure of calcitonin gene-related peptide-containing fibers in the vestibular endorgans of the rat. Brain Res. 1989;504:31–35. doi: 10.1016/0006-8993(89)91593-x. [DOI] [PubMed] [Google Scholar]
- Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the α9 nicotinic receptor. Neuropharmacology. 2000;39:2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- Wackym PA, Popper P, Ward PH, Micevych PE. In-situ hybridization for the study of gene expression in neuro-otologic research. Otolaryngol Head Neck Surg. 1990;103:519–526. doi: 10.1177/019459989010300402. [DOI] [PubMed] [Google Scholar]
- Wackym PA, Popper P, Lopez I, Ishiyama A, Micevych PE. Expression of α4 and β2 nicotinic acetylcholine receptor subunit mRNA and the localization of α-bungarotoxin binding proteins in the rat vestibular periphery. Cell Biol Int. 1995;19:291–300. doi: 10.1006/cbir.1995.1071. [DOI] [PubMed] [Google Scholar]
- Wackym PA, Chen CT, Ishiyama A, Pettis RM, Lopez I, Hoffman L. Muscarinic acetylcholine receptor subtype mRNAs in the human and rat vestibular periphery. Cell Biol Int. 1996;20:187–192. doi: 10.1006/cbir.1996.0023. [DOI] [PubMed] [Google Scholar]
- Warr WB. Olivocochlear and vestibular efferent neurons of the feline brain stem: their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J Comp Neurol. 1975;161:159–182. doi: 10.1002/cne.901610203. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ., Jr Efferent innervation of the organ of Corti: two separate systems. Brain Res. 1979;173:152–155. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Wersäll J. Efferent innervation of the inner ear. In: von Euler C, Skoglund C, Söderberg U, editors. Structure and function of inhibitory neuronal mechanisms. Oxford: Pergamon; 1968. pp. 123–139. [Google Scholar]
- Wiederhold ML, Kiang NYS. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Ohmori H. Suppression of the slow K+ current by choline agonists in cultured chick cochlear ganglion cells. J Physiol. 1993;464:213–228. doi: 10.1113/jphysiol.1993.sp019631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakir M, Huss D, Dickman JD. Afferent innervation patterns of the saccule in pigeons. J Neurophysiol. 2003;89:534–550. doi: 10.1152/jn.00817.2001. [DOI] [PubMed] [Google Scholar]
- Zuo J, Treadaway J, Buckner TW, Fritzsch B. Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci U S A. 1999;96:14100–14105. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]