Abstract
Purpose
To assess the feasibility of adding dose-intensive topotecan and cyclophosphamide to induction therapy for newly diagnosed high-risk neuroblastoma (HRNB).
Patients and Methods
Enrolled patients received two cycles of topotecan (approximately 1.2 mg/m2/d) and cyclophosphamide (400 mg/m2/d) for 5 days followed by four cycles of multiagent chemotherapy (Memorial Sloan-Kettering Cancer Center [MSKCC] regimen). Pharmacokinetically guided topotecan dosing (target systemic exposure with area under the curve of 50 to 70 ng/mL/hr) was performed. Peripheral-blood stem cell (PBSC) harvest and surgical resection of residual primary tumor occurred after cycles 2 and 5, respectively. Patients achieving at least a partial response received myeloablative chemotherapy with PBSC rescue and radiation to the presurgical primary tumor volume. Oral 13-cis-retinoic acid maintenance therapy was administered twice daily for 14 days in six 28-day cycles.
Results
Thirty-one patients were enrolled onto the study. No deaths related to toxicity or dose-limiting toxicities occurred during induction. Mucositis rarely occurred after topotecan cycles (9.7%) in contrast to 30% after MSKCC cycles. Thirty patients underwent PBSC collection with median 31.1 × 106 CD34+ cells/kg (range, 1.8 to 541.8 × 106 CD34+ cells/kg), all negative for tumor contamination by immunocytochemical analysis. Targeted topotecan systemic exposure was achieved in 26 (84%) of 31 patients. At the end of induction, 26 patients (84%) had tumor response and one patient had progressive disease. In the overall cohort, 3-year event-free and overall survival were 37.8% ± 9.4% and 57.1% ± 9.4%, respectively.
Conclusion
This pilot induction regimen was well tolerated with expected and reversible toxicities. These data support investigation of efficacy in a phase III clinical trial for newly diagnosed HRNB.
INTRODUCTION
Outcome for patients with advanced-stage neuroblastoma or those whose tumors exhibit unfavorable biologic characteristics such as MYCN gene amplification is poor, with survival of only 20% to 35%.1–6 Achievement of complete tumor response following induction therapy is associated with improved survival, and escalation in chemotherapy dose intensity improves initial tumor response.7–10 However, recent multicenter trials of dose-intensive chemotherapy induction regimens reveal response rates of only 52% to 75%.11–14 An alternative strategy is to incorporate new agents with non–cross-resistant mechanisms of cytotoxicity into induction therapy. This led to our testing the feasibility of adding cyclophosphamide and topotecan to a backbone dose-intensive induction regimen.10 Topotecan, an inhibitor of topoisomerase I, has activity against neuroblastoma,15–18 potentiates the effects of other DNA damaging agents,19,20 and demonstrates a non–cross-resistant mechanism of action.21,22 Topotecan has a toxicity profile most notable for dose-limiting myelosuppression23–25 and can be safely combined with cyclophosphamide.26–29 A randomized study of topotecan alone versus topotecan-cyclophosphamide in patients with recurrent neuroblastoma demonstrated a trend toward improved response rate (19% v 32%, respectively), decreased rate of grades 3 or 4 infectious complications, and increased time to tumor progression following topotecan-cyclophosphamide therapy.30
Early clinical trials estimated a topotecan maximum-tolerated dose of 0.75 mg/m2/d daily for 5 days when combined with cyclophosphamide.26 Xenograft models predict that a higher topotecan systemic exposure (52 to 88 ng/mL/hr), achieved by topotecan dosages of 1.0 to 1.6 mg/m2/d will improve antineuroblastoma tumor activity31,32 and support further topotecan dose intensification. We report the feasibility of incorporating dose-intensive topotecan plus cyclophosphamide (TopoCy) into a conventional chemotherapy backbone as a novel induction regimen for newly diagnosed patients with high-risk neuroblastoma. Pharmacokinetically guided topotecan dosing33,34 was used to achieve the optimal topotecan systemic exposure suggested by preclinical models.31,32 The feasibility of peripheral-blood stem cell (PBSC) mobilization, in vivo PBSC tumor purging following TopoCy, tumor response, and survival rates were determined.
PATIENTS AND METHODS
Patient Selection
Patients had a diagnosis of neuroblastoma or ganglioneuroblastoma verified by histology or demonstration of clumps of tumor cells in bone marrow with increased urinary catecholamine metabolites. Diagnosis, staging, and response assessments were performed according to International Neuroblastoma Staging System (INSS) criteria.35 Centralized analysis of MYCN gene amplification (defined as more than 10 copies) by fluorescent in situ hybridization and Shimada histology classification was performed.36,37
Patients ≤ 30 years of age at initial diagnosis and without prior systemic therapy were enrolled at one of five participating Children's Oncology Group (COG) institutions. High-risk neuroblastoma included patients with INSS stage 3, age ≥ 365 days with MYCN amplification and/or unfavorable histology; INSS stage 3, 4, or 4S and age less than 365 days old with MYCN amplification; INSS stage 4 and age ≥ 547 days old; INSS stage 4, and age 365 to 547 days old with MYCN amplification, unfavorable histology, and/or diploidy; and age ≥ 365 days initially with INSS stage 1, 2, or 4S that progressed to stage 4 without interval chemotherapy.
The study protocol and consent forms were approved by each participating hospital's institutional review board, and signed informed consent was obtained from the patient or parents or guardian, as appropriate, after neuroblastoma diagnosis was confirmed. The protocol was activated on March 29, 2004, and was closed to accrual on November 30, 2005.
Treatment
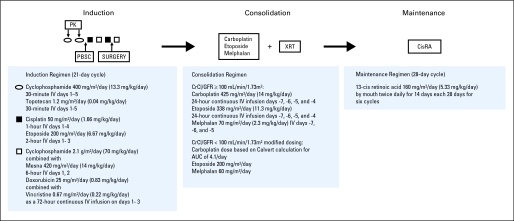
Protocol therapy consisted of three phases: induction, consolidation, and maintenance (Fig 1). Induction included six cycles of chemotherapy administered every 21 days or thereafter when absolute neutrophil count (ANC) was ≥ 750/μL and platelet count was ≥ 75,000/μL. Granulocyte colony-stimulating factor 5 μg/kg/d was administered 24 hours after completion of chemotherapy until ANC was more than 1,500/μL. Doses were based on body-surface area for those who weighed more than 12 kg or on weight (kg) for those who weighed ≤ 12 kg. Topotecan dose was adjusted to achieve a topotecan lactone systemic exposure (ie, area under the curve [AUC]) of 50 to 70 ng/mL/hr. PBSC harvest occurred after cycle 2 or later, if clinically indicated. Surgical resection of residual primary tumor or sites of regional dissemination (nodal disease) occurred after cycle 5.
Fig 1.
Study schema. AUC, area under the curve; CisRA, 13 cis-retinoic acid; CrCl, creatinine clearance; GFR, glomerular filtration rate; IV, intravenous; PBSC, peripheral-blood stem cell; PK, pharmacokinetics; XRT, external beam radiation therapy.
Patients with at least partial response (PR) at the end of induction, PBSC product of ≥ 1.5 × 106 CD34+ cells/kg without evidence of tumor contamination as documented by immunocytochemical assay,38 a cardiac shortening fraction of ≥ 28% or ejection fraction ≥ 55%, a creatinine clearance or glomerular filtration rate ≥ 60 mL/min/1.73 m2, and serum creatinine less than 1.5 mg/dL proceeded to consolidation chemotherapy (carboplatin, etoposide, and melphalan).39 On day 0, PBSCs were infused, and daily granulocyte colony-stimulating factor (5 μg/kg/d) was administered until engraftment (ANC > 2,000 × 3 days). External beam radiotherapy (21.6 Gy in 1.8 Gy fractions) beginning ≥ 28 days following PBSC infusion was administered to the presurgical gross tumor volume and sites of residual disease on the basis of computed tomography scan, magnetic resonance imaging, and/or metaiodobenzylquanadine (MIBG) scans performed at the end of induction. Maintenance isotretinoin began after day 66 after PBSC infusion and after radiation therapy for 6 months duration.40
Adverse events were assessed by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v3.0. The treating institutions assessed tumor response by using the INSS response criteria35 for chest, abdomen, or pelvic computed tomography scan, magnetic resonance imaging evaluation, iodine-123 [123I]-MIBG scintigraphy, urinary catecholamine levels, and bone marrow aspirate and biopsy obtained from bilateral iliac crests. Response assessments occurred after induction cycle 2 and at end of induction, consolidation, and maintenance. Patients were followed until they met the criteria for being off study (death, loss to follow-up, entry into another COG therapeutic study, withdrawal of consent for further data submission, or secondary malignancy).
Pharmacokinetically Guided Topotecan Dosing
Topotecan pharmacokinetics samples were obtained at all institutions, shipped overnight on dry ice, and analyzed at St. Jude Children's Research Hospital (SJCRH; North American patients) or on site at The Children's Hospital at Westmead (Australian patients) by using a method cross-validated with the SJCRH lab. Final dosage recommendations were calculated by the SJCRH laboratory and implemented on the next day after sample analysis.
Pharmacokinetic analysis was performed on day 1 of cycles 1 and 2. If pharmacokinetic analysis confirmed achievement of target (AUC 50 to 70 ng/mL/hr), no additional pharmacokinetic studies were performed. If the patient was outside the target range, topotecan dosage was adjusted for day 3, and the pharmacokinetic analysis was repeated. Dose adjustment was repeated on day 5 if the patient remained outside the target range, and pharmacokinetic studies were performed again on day 5. The initial dosage for cycle 2 was determined on the basis of the patient-specific pharmacokinetic data collected during cycle 1. Pharmacokinetic study times and results from the cycle 2 pharmacokinetic analyses were applied in the same manner as for cycle 1.
Sample Collection and Analysis
Samples (2.5 mL) were collected by using a limited sampling model: preinfusion, and 5 minutes, 2 hours, and 3 hours after the end of infusion. Whole blood was collected into a heparinized tube from a site contralateral to the site of topotecan infusion, plasma was processed, and topotecan lactone concentrations were measured. The lower limit of quantification for this high-performance liquid chromatography method was 0.25 ng/mL.34
Pharmacokinetic Analysis
A two-compartment model was fit to the topotecan lactone plasma concentrations by using a maximum a posteriori Bayesian algorithm as implemented in ADAPT II.41 Estimated model parameters included the volume of the central compartment, elimination rate constant, and the intercompartment rate constants. Clearance and volume of distribution at steady state were calculated by using standard equations and the parameter estimates. A plasma concentration time profile was simulated from model parameters for each patient and the AUC0-∞ was calculated by using a log-linear trapezoidal method. Because of previously published data suggesting a linear disposition,42,43 topotecan dosage adjustments were made by using the following equation: adjusted dosage = (current dosage/current AUC) × target AUC.
Statistical Analysis
The pilot regimen was deemed feasible (primary end point) if induction rules were met: ≥ 25 patients received 75% or more of the intended cycles 3 to 6 chemotherapy doses, no more than one death related to toxicity during induction, eight or fewer patients experiencing dose-limiting toxicities (DLTs) during cycles 1 and 2, and no more than two patients with PBSC tumor contamination by immunocytochemical analysis or having less than 1.5 × 106 CD34+ cells/kg collected. DLT was defined as inability to achieve ANC of more than 750/μL or platelet count of more than 75,000/μL by day 35 from the start of the cycle (unless there was documented tumor involvement of marrow); any grade 3 or higher nonhematopoietic, nonmucosal (mucositis/stomatitis) toxicity that did not resolve to grade ≤ 2 by day 35 of the cycle (excluding grade 3 anorexia or nausea); any grade 4 nonhematopoietic/nonmucosal toxicity (excluding grade 4 febrile neutropenia, anorexia, dehydration, vomiting) not attributable to infectious complication; or grade 4 mucositis requiring intubation.
Descriptive statistical analyses were performed to assess patient characteristics, toxicities, and secondary end points of topotecan pharmacokinetics, response, and survival. Event-free survival (EFS) and overall survival (OS) were calculated by using the Kaplan-Meier method44 with SEs per Peto et al.45 For EFS, time to event was calculated from the time of enrollment onto the study until the first occurrence of relapse, progressive disease, secondary malignancy, or death, or until the time of last contact if no event occurred. For OS, time to event was calculated from the time of enrollment onto the study until the time of death or until the time of last contact if the patient did not die. EFS and OS were compared with a log-rank test, and Kaplan-Meier curves were plotted. P values less than .05 were considered statistically significant.
RESULTS
Patients' Characteristics
Thirty-one patients were enrolled onto the study and all met eligibility requirements (Table 1). The majority of patients were male (81%) and age ≥ 18 months (84%), with median age at diagnosis of 30 months (range, 10 months to 9 years). One patient progressed to INSS stage 4 from stage 1 following only surgical resection, three patients had stage 3, and 27 patients had stage 4 neuroblastoma.
Table 1.
Patient Characteristics With 3-Year EFS and OS
| Characteristic | No. | % | 3-Year EFS ± SE (%) | P | 3-Year OS ± SE (%) | P |
|---|---|---|---|---|---|---|
| Stratum | ||||||
| Newly diagnosed | 30 | 97 | 39.1 ± 9.7 | N/A | 59.1 ± 9.5 | N/A |
| Relapsed | 1 | 3 | 1 event | 1 death | ||
| Age, months | ||||||
| < 18 | 5 | 16 | 40.0 ± 31.0 | .5608 | 40.0 ± 31.0 | .2056 |
| ≥ 18 | 26 | 84 | 38.5 ± 10.1 | 61.5 ± 9.9 | ||
| Race/ethnicity | ||||||
| White | 23 | 88 | 39.1 ± 10.8 | 60.9 ± 10.6 | ||
| Black | 2 | 8 | 1 event | N/A | 1 death | N/A |
| Other | 1 | 4 | 1 event | 1 death | ||
| Sex | ||||||
| Male | 25 | 81 | 32.0 ± 10.0 | .2740 | 48.0 ± 10.4 | .1873 |
| Female | 6 | 19 | 62.5 ± 22.1 | 100.0 ± 0 | ||
| INSS stage | ||||||
| Not stage 4 | 4 | 13 | 25.0 ± 21.7 | .4786 | 25.0 ± 21.7 | .1522 |
| Stage 4 | 27 | 87 | 39.7 ± 10.3 | 62.0 ± 9.9 | ||
| MYCN status | ||||||
| Not amplified | 15 | 60 | 26.7 ± 13.2 | .2386 | 53.3 ± 13.8 | .9987 |
| Amplified | 10 | 40 | 48.0 ± 17.3 | 46.7 ± 17.0 | ||
| Ploidy | ||||||
| Hyperdiploid | 9 | 50 | 33.3 ± 19.2 | .6092 | 25.9 ± 15.8 | .0652 |
| Diploid | 9 | 50 | 44.4 ± 16.6 | 77.8 ± 13.9 | ||
| Shimada histology | ||||||
| Favorable | 1 | 5 | 0 events | N/A | 0 deaths | N/A |
| Unfavorable | 21 | 95 | 47.1 ± 12.1 | 50.6 ± 11.9 | ||
| INPC MKI | ||||||
| Low/intermediate | 8 | 62 | 37.5 ± 21.0 | .2795 | 37.5 ± 21.0 | .3702 |
| High | 5 | 38 | 60.0 ± 21.9 | 60.0 ± 21.9 | ||
| INPC grade | ||||||
| Undifferentiated/poorly differentiated | 11 | 85 | 54.5 ± 16.4 | .1698 | 54.5 ± 16.4 | .1698 |
| Differentiating | 2 | 15 | 2 events | 2 deaths |
Abbreviations: EFS, event-free survival; INPC, International Neuroblastoma Pathology Classification; INSS, International Neuroblastoma Staging System; MKI, mitosis karyorrhexis index; N/A, not applicable; OS, overall survival.
Topotecan Pharmacokinetic Analyses and Dose Adjustments in Cycles 1 and 2
The population's average topotecan lactone clearance was 26.4 L/h/m2 (range, 10.2 to 60.9 L/h/m2). The inter- and intrapatient variabilities in topotecan systemic clearance assessed by using a mixed effects model were 23% and 30%, respectively.
A total of 56 pharmacokinetic studies were performed in 31 patients during cycle 1, and 54 studies were performed in 31 patients during cycle 2 (Table 2). After cycle 1 fixed topotecan dose, 16 (52%) of 31 analyses were within the target range (dose success). Cycle 2 topotecan dose was based on the cycle 1 dose that successfully achieved the target AUC or was calculated on the basis of the final pharmacokinetic study; 12 (39%) of 31 analyses were dose successes. Fourteen patients required a dose adjustment during cycles 1 and 2. Pharmacokinetically guided topotecan dosing achieved the target AUC in 84% of patients in either cycle 1 or cycle 2. Only one patient did not achieve the target AUC during either cycle 1 or cycle 2. Twelve patients (39%) and 18 patients (58%) required an increase in topotecan dose during cycle 1 and cycle 2, respectively. The median topotecan dosages required to achieve the target AUC in cycles 1 and 2 were 1.2 mg/m2/d (range, 0.75 to 2.1 mg/m2/d) and 1.3 mg/m2/d (range, 1.2 to 2.9 mg/m2/d), respectively.
Table 2.
Topotecan Pharmacokinetic Analyses
| Variable | No. of Patients |
|||
|---|---|---|---|---|
| Cycle 1 |
Cycle 2 |
|||
| No. | % | No. | % | |
| Pharmacokinetic studies | 31 | 31 | ||
| Fixed-dose topotecan* | 31 | 31† | ||
| Dosing success | 14 | 12 | ||
| Dosing failure | 17 | 19 | ||
| Pharmacokinetic guided dosing‡ | 17 | 19 | ||
| Targeting success | 12 | 14 | ||
| Targeting failure | 5 | 5 | ||
| Total successes | 26 | 84 | 26 | 84 |
| Dosage to achieve target, mg/m2 | ||||
| Median | 1.2 | 1.3 | ||
| Range | 0.75-2.1 | 1.2-2.9 | ||
| Dosage to achieve target, mg/kg | ||||
| Median | 0.06 | 0.11 | ||
| Range | 0.04-0.1 | 0.04-0.13 | ||
Success if target area under the curve of 50 to 70 ng/mL/hr was achieved after protocol-defined dose (cycle 1 or cycle 2) or cycle 1–based pharmacokinetic analysis (cycle 2).
Fixed dosage per protocol (n = 11) or dosage based on cycle 1 pharmacokinetic analyses.
Success if target area under the curve of 50 to 70 ng/mL/hr was achieved by pharmacokinetic-guided dosing.
Stem-Cell Harvest
PBSCs were successfully collected in 30 patients (> 1.5 × 106 CD34+ cells/kg). One patient had disease progression before PBSC collection and went off therapy. Median PBSC collection was 31.1 × 106 CD34+ cells/kg (range, 1.8 to 541.8 × 106 CD34+ cells/kg) collected over a median of 1 day (range, 1 to 3 days), with 27 collections occurring before cycle 3. Three patients had less than 4 × 106 CD34+ cells/kg collected; one patient had CD34+ cells/kg collected following cycle 5 of induction at the physician's discretion. No tumor contamination was detected in the PBSC products by immunocytochemical analysis performed centrally (n = 29) or at a local institution (n = 1).
Toxicities
No DLTs or deaths related to toxicity occurred during induction. Only one patient required a dose reduction of more than 25% of the intended chemotherapy doses (in cycle 6) because of prolonged myelosuppression. Table 3 lists reported grade 3 or higher key toxicities during induction cycles 1 to 2 and 3 to 6. The majority of patients experienced grade 3 or 4 hematopoietic toxicity and febrile neutropenia throughout induction. Mucositis involving the oropharnyx or lower intestinal tract and documented infections were more common during cycles 3 to 6 (30% and 46.7%, respectively) compared with cycles 1 and 2 (9.7% and 9.7%, respectively).
Table 3.
Observed Induction and Consolidation Toxicity
| Toxicity | Cycle |
|||
|---|---|---|---|---|
| 1 and 2(n = 31) |
3 Through 6(n = 30) |
|||
| Count | Incidence (%) | Count | Incidence (%) | |
| Induction grade 3 or 4 toxicity* | ||||
| Hemoglobin | 23 | 74.2 | 22 | 73.3 |
| Neutrophils/granulocytes (ANC/AGC) | 25 | 80.6 | 25 | 83.3 |
| Platelets | 24 | 77.4 | 25 | 83.3 |
| Mucositis/stomatitis/colitis/enteritis/diarrhea | 3 | 9.7 | 9 | 30.0 |
| Febrile neutropenia (fever of unknown origin without infection, ANC < 1 × 109; fever ≥ 38.5°C) | 22 | 71.0 | 20 | 66.7 |
| Infection (clinical or microbiologic diagnosis) with grades 3 to 4 neutrophils, ANC < 1.0 × 109 | 3 | 9.7 | 9 | 30.0 |
| Other infection | 0 | 0.0 | 5 | 16.7 |
| Consolidation grade 3, 4, or 5 toxicity (n = 24) * | ||||
| Mucositis/stomatitis/colitis/enteritis/diarrhea | 7 | 29.2 | ||
| Febrile neutropenia (fever of unknown origin without infection, ANC < 1 × 109; fever ≥ 38.5°C) | 8 | 33.3 | ||
| Infection (clinical or microbiologic diagnosis) with grade 3 to 4 neutrophils, ANC < 1.0 × 109 | 11 | 45.8 | ||
| ALT | 13 | 54.2 | ||
| AST | 13 | 54.2 | ||
| Bilirubin (hyperbilirubinemia) | 1 | 4.2 | ||
| Hypoxia | 3 | 12.5 | ||
| Pleural effusion (nonmalignant) | 1 | 4.2 | ||
| Renal failure | 1 | 4.2 | ||
Abbreviations: AGC, absolute granulocyte count; ANC, absolute neutrophil count.
Only the maximum grade of toxicity per patient per type of toxicity is counted within the given time period.
Table 3 also summarizes reported grade 3 or higher nonhematopoietic toxicities experienced during consolidation. Thirteen patients (54%) developed increases in liver transaminase levels, three in the setting of systemic infection. One patient died after consolidation with myeloablative chemotherapy because of sinusoidal obstructive syndrome and another died from complications of renal failure.
Feasiblity
In summary of the primary end point, all of the induction feasibility rules were met: delivery of intended cycles, no deaths related to toxicity or DLTs, and sufficient PBSC collection with no detectable tumor contamination.
Responses
Table 4 lists the number of overall responses for induction and consolidation therapy. A best overall response of complete response (CR), very good partial response (VGPR), or partial response (PR) was achieved in 12 (40%) of 30 evaluated patients following two cycles of induction, 26 (83.8%) of 31 at end of induction, and 21 (87.5%) of 24 at completion of consolidation. Twenty-four patients proceeded to consolidation. The reasons for removing patients from protocol therapy before consolidation were progression (n = 1), physician discretion (n = 2), less than PR to induction (n = 2), prolonged delay in beginning consolidation due to viral infection (n = 1), and parental refusal (n = 1).
Table 4.
Response Assessment
| Reporting Period | CR | VGPR | PR | MR | NR | PD | Not Evaluable* | Not Evaluated |
|---|---|---|---|---|---|---|---|---|
| Cycles 1 and 2 (n = 31) | 2 | 1 | 9 | 9 | 8 | 1 | 0 | 1 |
| End induction (n = 31) | 7 | 8 | 11 | 2 | 1 | 1 | 1 | 0 |
| End consolidation (n = 24)* | 8 | 8 | 5 | 0 | 0 | 1 | 2 | 0 |
Abbreviations: CR, complete response; MR, mixed response; NR, no response; PD, progressive disease; PR, partial response; VGPR, very good partial response.
One patient removed from protocol therapy prior to end of induction per physician discretion; two patients with death related to toxicity during consolidation.
Survival Analysis
Fourteen of the 31 patients were alive at last follow-up. Sixteen patients relapsed or progressed, and thirteen of those subsequently died. Four of the 17 deaths on this study were first events. Causes of death included disease (n = 15) and stem-cell transplantation–related complications (n = 2).
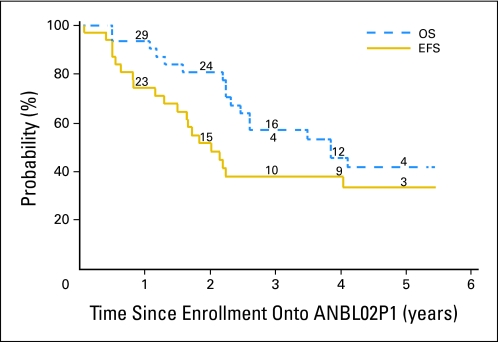
The median follow-up time for the 11 patients who did not experience an event was 4.5 years (range, 2.0 to 5.4 years). The overall 3-year EFS and OS rates were 37.8% ± 9.4% and 57.1% ± 9.4%, respectively (Fig 2). No statistically significant difference in outcome was found by patient characteristics (Table 1).
Fig 2.
Event-free survival (EFS) and overall survival (OS) for all patients (N = 31). The numbers at risk at the start of years 1 to 5 are given along the curves.
DISCUSSION
The induction regimen from the COG ANBL02P1 study demonstrates tolerability and feasibility of delivering a dose-intensive TopoCy regimen to newly diagnosed patients with high-risk neuroblastoma. The regimen uses a dose-intensive chemotherapy backbone previously described by Kushner et al10 (Memorial Sloan-Kettering Cancer Center [MSKCC] regimen) and used in the completed COG A3973 study.13 The MSKCC regimen's initial induction cycles—high-dose cyclophosphamide plus doxorubicin and vincristine—cause significant mucosal and infectious toxicities10 similar to those observed during ANBL02P1 cycles 3 to 6. In comparison, dose-intensive TopoCy rarely caused severe mucositis and documented infection and did not limit the ability to deliver the subsequent intended dose-intensive chemotherapy.
The use of myeloablative consolidation chemotherapy and autologous bone marrow infusion improves EFS of high-risk neuroblastoma.2,46,47 PBSCs carry less burden of collection48 and are less likely to contain tumor cells49 than bone marrow although the optimal timing of PBSC collection remains controversial. Our study confirmed prior results from Bensihom et al50 that sufficient PBSC cells without tumor contamination can be harvested after two cycles of chemotherapy. Sufficient numbers of stem cells were harvested for a minimum of two PBSC infusions in all but one patient, which was essential when planning the current COG tandem transplantation study. The occurrence of secondary malignancy following intensive neuroblastoma therapy51 may be related to the mutagenic potential of induction alkylator or topoisomerase 1 inhibitor chemotherapy, further providing a rationale for earlier PBSC harvest.
Kretchmar et al18 reported no improvement in outcome when adding cyclophosphamide and topotecan to neuroblastoma induction therapy. However, topotecan doses were significantly lower than those used in ANBL02P1 (0.75 mg/m2/d) and may not provide maximal antitumor activity.31,32 Indeed, the response rate observed following two cycles of ANBL02P1 TopoCy are similar to the response rate in recurrent disease following lower-dose topotecan.30 Within the context of the entire ANBL02P1 induction regimen, patients achieved an overall response rate of 84% and 15 (48%) of 31 patients achieved CR or VGPR. These induction response rates are similar to metastatic response of CR in 17 of 44 patients observed in a multicenter trial52 and the 47% CR or VGPR response rate observed with the European Neuroblastoma Study Group 5 (ENSG5) cooperative trial.12 These similarities highlight the need for a larger phase III clinical trial to more accurately assess the efficacy of the ANBL02P1 induction regimen.
ANBL02P1 and previous pediatric topotecan trials demonstrate marked interpatient variability in clearance suggesting that pharmacokinetically guided dosing may be of clinical benefit.33,53 We were able to achieve the target exposure in 84% of patients. The median topotecan dose required to achieve the target AUC (cycle 1, 1.2 mg/m2/d; cycle 2, 1.3 mg/m2/d), achieved by either a dose increase or decrease, supports the use of 1.2 mg/m2/d dosing administered daily for 5 days in future trials. The ANBL02P1 trial was not intended to assess the maximum tolerated topotecan dose that could be combined into induction therapy. Higher topotecan (6 to 8 mg/m2 per cycle) and cyclophosphamide dosages (approximately 4,200 mg/m2 per cycle) have been successfully administered to patients with recurrent neuroblastoma.28,29 Future trials incorporating a higher topotecan dosage into induction therapy may be warranted given ANBL02P1 results, although feasibility studies may be needed given the risk for increased mucositis24 and infection associated with higher topotecan dosages.30
ANBL02P1 confirmed the feasibility of incorporating pharmacokinetically guided dosing in an international multi-institutional clinical trial among institutions equipped with personnel and resources to perform real-time sampling and processing. However, in this patient population, more intrapatient variability in topotecan clearance was observed than in previous studies. Changing clinical factors (eg, renal function, extent of disease, concomitant medications) may account for some of this variability,54 but since this was, to the best of our knowledge, the first COG study of its kind, unfamiliarity with logistic aspects of the study also may have contributed to the greater intrapatient variability observed. Regardless, our study shows that although feasible, current real-time sampling techniques are time and labor intensive and are not likely to be feasible in a COG group-wide study because most sites do not have the appropriate infrastructure. Ultimately, methods must be developed to individualize therapy without burdening the health care system, either by minimization of the number of topotecan samples required or determination of a population model to predict topotecan exposure.
The feasibility of PBSC harvest and tolerability of the ANBL02P1 induction regimen support further investigation of its efficacy in a phase III clinical trial. However, suboptimal response still occurs in a subset of patients with neuroblastoma. Future trials need to translate our improved understanding of the neuroblastoma genetic signature by introducing novel molecularly targeted therapy. The tolerability of the topotecan-containing induction regimen provides a backbone regimen on which to add such agents.
Footnotes
See accompanying editorial on page 4345
Supported by GlaxoSmithKline Beecham Grant No. NCI 1 K23 CA87058-01A2 (to the National Childhood Cancer Foundation) and National Institutes of Health Grants No. U10 CA98413 (Children's Oncology Group [COG] Statistics and Data Center grant) and U10 CA98543 (COG Chair's grant).
Presented as an oral presentation at Advances in Neuroblastoma Research 2006, Los Angeles, CA, May 17-20, 2006, and the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, GA, June 2-6, 2006.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Clinton F. Stewart, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Julie R. Park, Clinton F. Stewart, Wendy B. London, Victor M. Santana, Susan L. Cohn, Katherine K. Matthay
Administrative support: Wendy B. London
Provision of study materials or patients: Julie R. Park, Victor M. Santana, Peter J. Shaw, Susan L. Cohn, Katherine K. Matthay
Collection and assembly of data: Julie R. Park, Jeffrey R. Scott, Wendy B. London, Peter J. Shaw, Susan L. Cohn
Data analysis and interpretation: Julie R. Park, Jeffrey R. Scott, Wendy B. London, Arlene Naranjo, Katherine K. Matthay
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Park JR, Eggert A, Caron H. Neuroblastoma: Biology, prognosis, and treatment. Pediatr Clin North Am. 2008;55:97–120. doi: 10.1016/j.pcl.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children's Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canete A, Gerrard M, Rubie H, et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: The International Society of Paediatric Oncology European Neuroblastoma Experience. J Clin Oncol. 2009;27:1014–1019. doi: 10.1200/JCO.2007.14.5839. [DOI] [PubMed] [Google Scholar]
- 4.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladenstein R, Philip T, Lasset C, et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: A report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–965. doi: 10.1200/JCO.1998.16.3.953. [DOI] [PubMed] [Google Scholar]
- 6.London WB, Boni L, Simon T, et al. The role of age in neuroblastoma risk stratification: The German, Italian, and Children's Oncology Group perspectives. Cancer Lett. 2005;228:257–266. doi: 10.1016/j.canlet.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 8.Pinkerton CR, Zucker JM, Hartmann O, et al. Short duration, high dose, alternating chemotherapy in metastatic neuroblastoma (ENSG 3C induction regimen): The European Neuroblastoma Study Group. Br J Cancer. 1990;62:319–323. doi: 10.1038/bjc.1990.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castleberry RP, Cantor AB, Green AA, et al. Phase II investigational window using carboplatin, iproplatin, ifosfamide, and epirubicin in children with untreated disseminated neuroblastoma: A Pediatric Oncology Group study. J Clin Oncol. 1994;12:1616–1620. doi: 10.1200/JCO.1994.12.8.1616. [DOI] [PubMed] [Google Scholar]
- 10.Kushner BH, LaQuaglia MP, Bonilla MA, et al. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. J Clin Oncol. 1994;12:2607–2613. doi: 10.1200/JCO.1994.12.12.2607. [DOI] [PubMed] [Google Scholar]
- 11.Valteau-Couanet D, Michon J, Boneu A, et al. Results of induction chemotherapy in children older than 1 year with a stage 4 neuroblastoma treated with the NB 97 French Society of Pediatric Oncology (SFOP) protocol. J Clin Oncol. 2005;23:532–540. doi: 10.1200/JCO.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Pearson AD, Pinkerton CR, Lewis IJ, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 13.Kreissman SG, Villablanca JG, Seeger RC, et al. A randomized phase III trial of myeloablative autologous peripheral blood stem cell (PBSC) transplant (ASCT) for high-risk neuroblastoma (HR-NB) employing immunomagnetic purged (P) versus unpurged (UP) PBSC: A Children's Oncology Group study. J Clin Oncol. 2008;26(suppl):541s. abstr 10011. [Google Scholar]
- 14.Ladenstein R, Valteau-Couanet D, Brock P, et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28:3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 15.Potmesil M. Camptothecins: From bench research to hospital wards. Cancer Res. 1994;54:1431–1439. [PubMed] [Google Scholar]
- 16.Nitschke R, Parkhurst J, Sullivan J, et al. Topotecan in pediatric patients with recurrent and progressive solid tumors: A Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 1998;20:315–318. doi: 10.1097/00043426-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Tubergen D, Pratt C, Stewart C, et al. Phase I study of topotecan in children with refractory solid tumors: A Pediatric Oncology Group Study. Proc Am Soc Clin Oncol. 1994;13:463. [Google Scholar]
- 18.Kretschmar CS, Kletzel M, Murray K, et al. Response to paclitaxel, topotecan, and topotecan-cyclophosphamide in children with untreated disseminated neuroblastoma treated in an upfront phase II investigational window: A Pediatric Oncology Group study. J Clin Oncol. 2004;22:4119–4126. doi: 10.1200/JCO.2004.08.174. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann SH, Peereboom D, Buckwalter CA, et al. Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst. 1996;88:734–741. doi: 10.1093/jnci/88.11.734. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Motzer RJ, Tong Y, et al. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: A rational approach to clinical protocol design. J Natl Cancer Inst. 1994;86:1517–1524. doi: 10.1093/jnci/86.20.1517. [DOI] [PubMed] [Google Scholar]
- 21.Keshelava N, Groshen S, Reynolds CP. Cross-resistance of topoisomerase I and II inhibitors in neuroblastoma cell lines. Cancer Chemother Pharmacol. 2000;45:1–8. doi: 10.1007/PL00006736. [DOI] [PubMed] [Google Scholar]
- 22.Mattern MR, Hofmann GA, Polsky RM, et al. In vitro and in vivo effects of clinically important camptothecin analogues on multidrug-resistant cells. Oncol Res. 1993;5:467–474. [PubMed] [Google Scholar]
- 23.Pratt CB, Stewart C, Santana VM, et al. Phase I study of topotecan for pediatric patients with malignant solid tumors. J Clin Oncol. 1994;12:539–543. doi: 10.1200/JCO.1994.12.3.539. [DOI] [PubMed] [Google Scholar]
- 24.Rowinsky EK, Adjei A, Donehower RC, et al. Phase I and pharmacodynamic study of the topoisomerase I-inhibitor topotecan in patients with refractory acute leukemia. J Clin Oncol. 1994;12:2193–2203. doi: 10.1200/JCO.1994.12.10.2193. [DOI] [PubMed] [Google Scholar]
- 25.Saltz L, Sirott M, Young C, et al. Phase I clinical and pharmacology study of topotecan given daily for 5 consecutive days to patients with advanced solid tumors, with attempt at dose intensification using recombinant granulocyte colony-stimulating factor. J Natl Cancer Inst. 1993;85:1499–1507. doi: 10.1093/jnci/85.18.1499. [DOI] [PubMed] [Google Scholar]
- 26.Saylors RL, 3rd, Stewart CF, Zamboni WC, et al. Phase I study of topotecan in combination with cyclophosphamide in pediatric patients with malignant solid tumors: A Pediatric Oncology Group Study. J Clin Oncol. 1998;16:945–952. doi: 10.1200/JCO.1998.16.3.945. [DOI] [PubMed] [Google Scholar]
- 27.Saylors RL, 3rd, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: A Pediatric Oncology Group phase II study. J Clin Oncol. 2001;19:3463–3469. doi: 10.1200/JCO.2001.19.15.3463. [DOI] [PubMed] [Google Scholar]
- 28.Kushner BH, Kramer K, Meyers PA, et al. Pilot study of topotecan and high-dose cyclophosphamide for resistant pediatric solid tumors. Med Pediatr Oncol. 2000;35:468–474. doi: 10.1002/1096-911x(20001101)35:5<468::aid-mpo5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Kushner BH, Kramer K, Modak S, et al. Differential impact of high-dose cyclophosphamide, topotecan, and vincristine in clinical subsets of patients with chemoresistant neuroblastoma. Cancer. 2010;116:3054–3060. doi: 10.1002/cncr.25232. [DOI] [PubMed] [Google Scholar]
- 30.London WB, Frantz CN, Campbell LA, et al. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: A Children's Oncology Group study. J Clin Oncol. 2010;28:3808–3815. doi: 10.1200/JCO.2009.27.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tubergen DG, Stewart CF, Pratt CB, et al. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1996;18:352–361. doi: 10.1097/00043426-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Zamboni WC, Stewart CF, Thompson J, et al. Relationship between topotecan systemic exposure and tumor response in human neuroblastoma xenografts. J Natl Cancer Inst. 1998;90:505–511. doi: 10.1093/jnci/90.7.505. [DOI] [PubMed] [Google Scholar]
- 33.Santana VM, Furman WL, Billups CA, et al. Improved response in high-risk neuroblastoma with protracted topotecan administration using a pharmacokinetically guided dosing approach. J Clin Oncol. 2005;23:4039–4047. doi: 10.1200/JCO.2005.02.097. [DOI] [PubMed] [Google Scholar]
- 34.Santana VM, Zamboni WC, Kirstein MN, et al. A pilot study of protracted topotecan dosing using a pharmacokinetically guided dosing approach in children with solid tumors. Clin Cancer Res. 2003;9:633–640. [PubMed] [Google Scholar]
- 35.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 36.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 37.Shapiro DN, Valentine MB, Rowe ST, et al. Detection of N-myc gene amplification by fluorescence in situ hybridization: Diagnostic utility for neuroblastoma. Am J Pathol. 1993;142:1339–1346. [PMC free article] [PubMed] [Google Scholar]
- 38.Seeger RC, Reynolds CP, Gallego R, et al. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: A Children's Cancer Group Study. J Clin Oncol. 2000;18:4067–4076. doi: 10.1200/JCO.2000.18.24.4067. [DOI] [PubMed] [Google Scholar]
- 39.Villablanca JG, Matthay KK, Swift P, et al. Phase I trial of carboplatin, etoposide, melphalan, and local irradiation (CEM-LI) with purged autologous bone marrow transplantation (ABMT) for high risk neuroblastoma. Proc Intl Soc Pediatr Oncol. 1999:168. abstr O-114. [Google Scholar]
- 40.Matthay KK. Intensification of therapy using hematopoietic stem-cell support for high-risk neuroblastoma. Pediatr Transplant. 1999;3(suppl 1):72–77. doi: 10.1034/j.1399-3046.1999.00070.x. [DOI] [PubMed] [Google Scholar]
- 41.d'Argenio DZ, Schumitzky A. ADAPT II User's Guide. Los Angeles, CA: Biomedical Simulations Resource, University of Southern California; 1990. [Google Scholar]
- 42.van Warmerdam LJ, Verweij J, Schellens JH, et al. Pharmacokinetics and pharmacodynamics of topotecan administered daily for 5 days every 3 weeks. Cancer Chemother Pharmacol. 1995;35:237–245. doi: 10.1007/BF00686554. [DOI] [PubMed] [Google Scholar]
- 43.Zamboni WC, Bowman LC, Tan M, et al. Interpatient variability in bioavailability of the intravenous formulation of topotecan given orally to children with recurrent solid tumors. Cancer Chemother Pharmacol. 1999;43:454–460. doi: 10.1007/s002800050923. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 45.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc A. 1972;135:185–198. [Google Scholar]
- 46.Pritchard J, Cotterill SJ, Germond SM, et al. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer. 2005;44:348–357. doi: 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 47.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 48.To LB, Roberts MM, Haylock DN, et al. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 1992;9:277–284. [PubMed] [Google Scholar]
- 49.Dominici C, Deb G, Angioni A, et al. Peripheral blood stem cells in children with solid tumors: Part II. Immunocytologic detection of tumor cells in bone marrow and peripheral blood stem cell harvests. Anticancer Res. 1993;13:2573–2575. [PubMed] [Google Scholar]
- 50.Bensimhon P, Villablanca JG, Sender LS, et al. Peripheral blood stem cell support for multiple cycles of dose intensive induction therapy is feasible with little risk of tumor contamination in advanced stage neuroblastoma: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2010;54:596–602. doi: 10.1002/pbc.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kushner BH, Kramer K, Modak S, et al. Reduced risk of secondary leukemia with fewer cycles of dose-intensive induction chemotherapy in patients with neuroblastoma. Pediatr Blood Cancer. 2009;53:17–22. doi: 10.1002/pbc.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valteau-Couanet D. Event-free survival of patients with high-risk neuroblastoma treated with an N6-like induction treatment. J Clin Oncol. 2005;23:6262–6263. doi: 10.1200/JCO.2005.01.2062. author reply 6263. [DOI] [PubMed] [Google Scholar]
- 53.Panetta JC, Schaiquevich P, Santana VM, et al. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res. 2008;14:318–325. doi: 10.1158/1078-0432.CCR-07-1243. [DOI] [PubMed] [Google Scholar]
- 54.O'Reilly S, Rowinsky EK, Slichenmyer W, et al. Phase I and pharmacologic study of topotecan in patients with impaired renal function. J Clin Oncol. 1996;14:3062–3073. doi: 10.1200/JCO.1996.14.12.3062. [DOI] [PubMed] [Google Scholar]