Abstract
For shotgun cloning into M13 vectors, a double-stranded cassette of synthetic oligonucleotides containing a SmaI site within the two halves of an EcoK site, has been introduced into the vector M13mp8. Cloning of blunt end DNA into the SmaI site destroys the EcoK site, and recombinants are therefore preferentially selected on transfection into a K strain of E.coli. For deletion mutagenesis using synthetic oligonucleotides, an M13 vector with four copies of the EcoK cassette has been made to facilitate the joining of lacZ or a Factor Xa cleavage site to any protein reading frame.
Full text
PDF


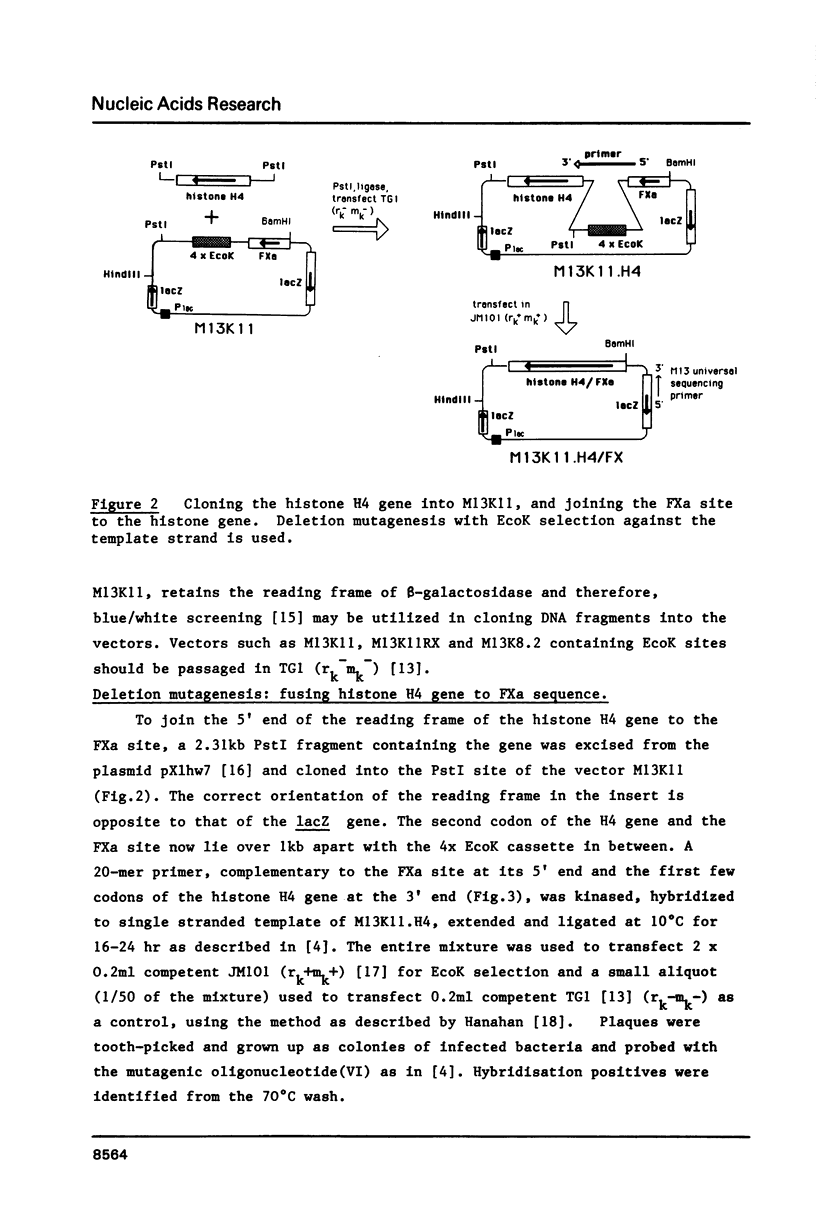

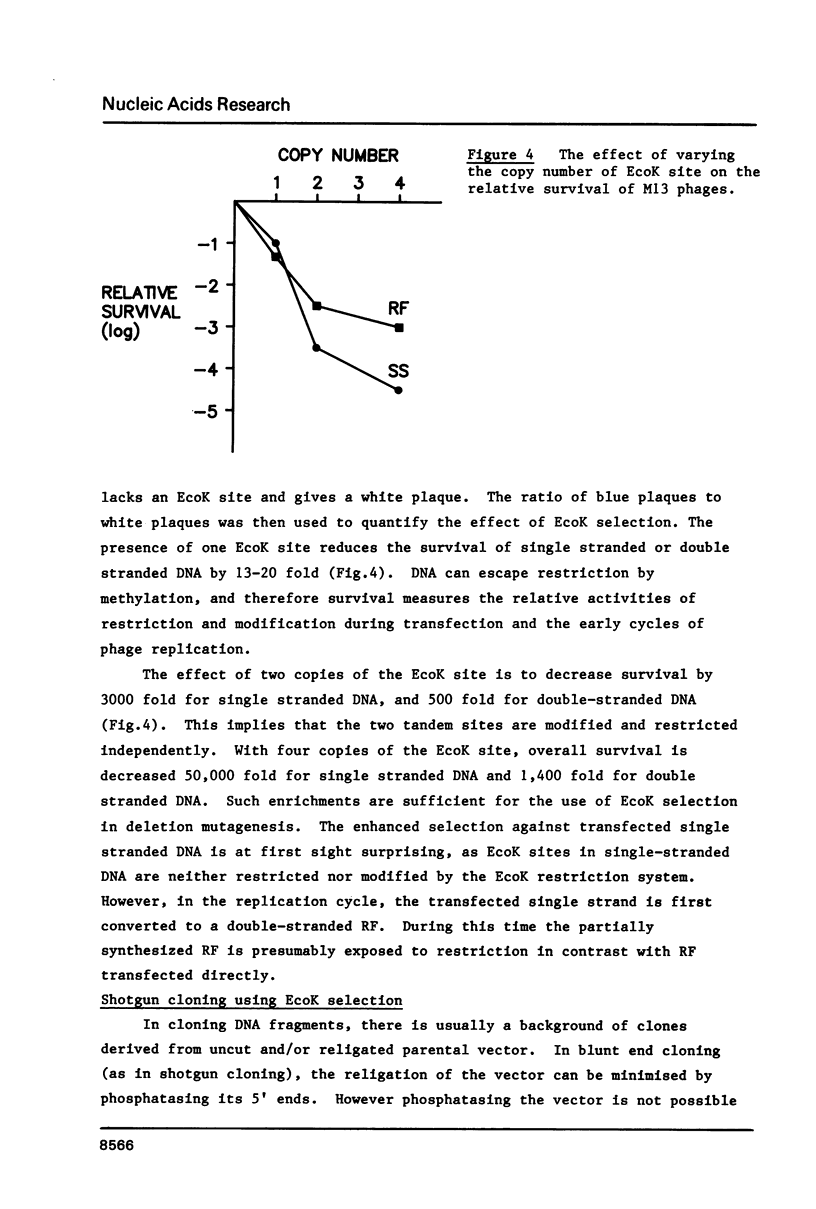
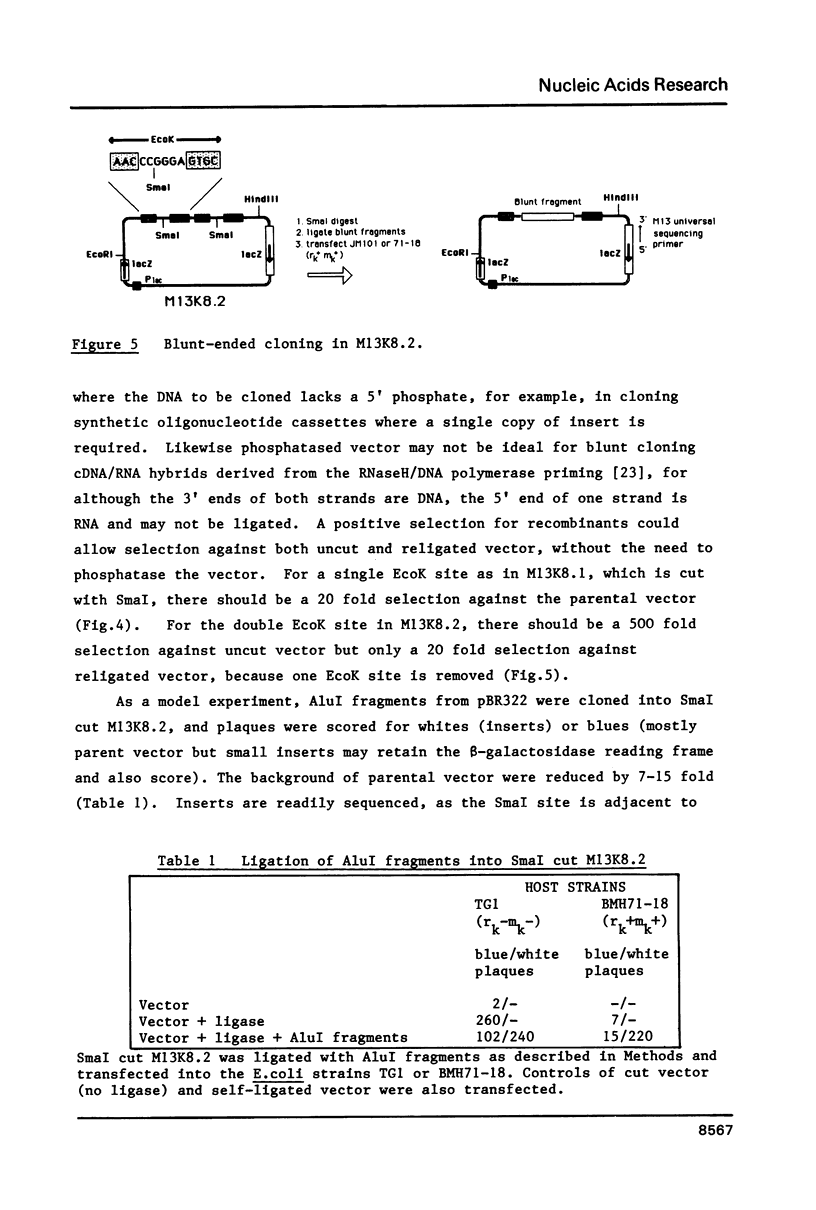




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Hayflick J. S., Vasser M., Seeburg P. H. In vitro deletional mutagenesis for bacterial production of the 20,000-dalton form of human pituitary growth hormone. DNA. 1983;2(3):183–193. doi: 10.1089/dna.1983.2.183. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. Positive selection vectors: a small plasmid vector useful for the direct selection of Sau3A-generated overlapping DNA fragments. Gene. 1984 Mar;27(3):323–325. doi: 10.1016/0378-1119(84)90077-5. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. L., Smith M. In vitro generation of specific deletions in DNA cloned in M13 vectors using synthetic oligodeoxyribonucleotides: mutants in the 5'-flanking region of the yeast alcohol dehydrogenase II gene. Nucleic Acids Res. 1984 Mar 12;12(5):2407–2419. doi: 10.1093/nar/12.5.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Matthes H. W., Singh M., Sproat B. S., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides. VII. Solid phase synthesis of oligodeoxyribonucleotides by a continuous flow phosphotriester method on a kieselguhr-polyamide support. Nucleic Acids Res. 1982 Oct 25;10(20):6243–6254. doi: 10.1093/nar/10.20.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil G., Faust J. R., Chin D. J., Goldstein J. L., Brown M. S. Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell. 1985 May;41(1):249–258. doi: 10.1016/0092-8674(85)90078-9. [DOI] [PubMed] [Google Scholar]
- Gronenborn B. Overproduction of phage lambda repressor under control of the lac promotor of Escherichia coli. Mol Gen Genet. 1976 Nov 17;148(3):243–250. doi: 10.1007/BF00332898. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Lautenberger J. A., Edgell M. H., Hutchison C. A., 3rd The nucleotide sequence recognized by the Escherichia coli K12 restriction and modification enzymes. J Mol Biol. 1979 May 15;130(2):191–209. doi: 10.1016/0022-2836(79)90426-1. [DOI] [PubMed] [Google Scholar]
- Mariuzza R. A., Janković D. L., Boulot G., Amit A. G., Saludjian P., Le Guern A., Mazié J. C., Poljak R. J. Preliminary crystallographic study of the complex between the Fab fragment of a monoclonal anti-lysozyme antibody and its antigen. J Mol Biol. 1983 Nov 15;170(4):1055–1058. doi: 10.1016/s0022-2836(83)80206-x. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miyada C. G., Soberón X., Itakura K., Wilcox G. The use of synthetic oligodeoxyribonucleotides to produce specific deletions in the araBAD promoter of Escherichia coli B/r. Gene. 1982 Feb;17(2):167–177. doi: 10.1016/0378-1119(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Modrich P. Structures and mechanisms of DNA restriction and modification enzymes. Q Rev Biophys. 1979 Aug;12(3):315–369. doi: 10.1017/s0033583500005461. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Old R. W., Woodland H. R., Ballantine J. E., Aldridge T. C., Newton C. A., Bains W. A., Turner P. C. Organization and expression of cloned histone gene clusters from Xenopus laevis and X. borealis. Nucleic Acids Res. 1982 Dec 11;10(23):7561–7580. doi: 10.1093/nar/10.23.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Van der Bliek A. M., Van der Horst G., Groot Koerkamp M. J., Tabak H. F., Veeneman G. H., Van Boom J. H. In vitro site-directed mutagenesis with synthetic DNA oligonucleotides yields unexpected deletions and insertions at high frequency. Nucleic Acids Res. 1983 Dec 20;11(24):8595–8608. doi: 10.1093/nar/11.24.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Stiles J. I., Bahl C. P., Wu R. Specific binding of a synthetic oligodeoxyribonucleotide to yeast cytochrome c mRNA. Nature. 1977 Jan 6;265(5589):61–63. doi: 10.1038/265061a0. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Van Rompuy L., Jou W. M., Huylebroeck D., Fiers W. Complete nucleotide sequence of the influenza B/Singapore/222/79 virus hemagglutinin gene and comparison with the B/Lee/40 hemagglutinin. Nucleic Acids Res. 1983 Jul 25;11(14):4703–4712. doi: 10.1093/nar/11.14.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R. Structure and mechanism of multifunctional restriction endonucleases. Annu Rev Biochem. 1981;50:285–319. doi: 10.1146/annurev.bi.50.070181.001441. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]




