Abstract
Purpose
The 21-gene breast cancer assay recurrence score (RS) is widely used for assessing recurrence risk and predicting chemotherapy benefit in patients with estrogen receptor (ER) –positive breast cancer. Pathologic and clinical factors such as tumor size, grade, and patient age also provide independent prognostic utility. We developed a formal integration of these measures and evaluated its prognostic and predictive value.
Patients and Methods
From the National Surgical Adjuvant Breast and Bowel (NSABP) B-14 and translational research cohort of the Arimidex, Tamoxifen Alone or in Combination (TransATAC) studies, we included patients who received hormonal monotherapy, had ER-positive tumors, and RS and traditional clinicopathologic factors assessed (647 and 1,088, respectively). Individual patient risk assessments from separate Cox models were combined using meta-analysis to form an RS-pathology-clinical (RSPC) assessment of distant recurrence risk. Risk assessments by RS and RSPC were compared in node-negative (N0) patients. RSPC was compared with RS for predicting chemotherapy benefit in NSABP B-20.
Results
RSPC had significantly more prognostic value for distant recurrence than did RS (P < .001) and showed better separation of risk in the study population. RSPC classified fewer patients as intermediate risk (17.8% v 26.7%, P < .001) and more patients as lower risk (63.8% v 54.2%, P < .001) than did RS among 1,444 N0 ER-positive patients. In B-20, the interaction of RSPC with chemotherapy was not statistically significant (P = .10), in contrast to the previously reported significant interaction of RS with chemotherapy (P = .037).
Conclusion
RSPC refines the assessment of distant recurrence risk and reduces the number of patients classified as intermediate risk. Adding clinicopathologic measures did not seem to enhance the value of RS alone nor the individual biology RS identifies in predicting chemotherapy benefit.
INTRODUCTION
Validated prognostic and predictive factors currently play an important role in treatment planning for patients with early-stage breast cancer.1 Important traditional clinicopathologic measures such as nodal status, tumor size, tumor grade, and patient age have been used for many years in clinical practice.1–3
Recently, multiple studies in more than 4,000 patients have led to the development and validation of the 21-gene reverse transcriptase polymerase chain reaction assay, Oncotype DX (Genomic Health, Redwood City, CA). The Oncotype DX recurrence score (RS) has been shown to independently predict risk of distant recurrence and also the magnitude of chemotherapy benefit in patients with estrogen receptor (ER) –positive, tamoxifen-treated breast cancer.4–9
The RS assay was initially validated as a predictor of risk of distant recurrence in ER-positive hormonal therapy–treated early-stage breast cancer in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 study and, more recently, in the translational research cohort within the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial (TransATAC) study.4,9 Weak or moderate correlations between RS and traditional measures have been reported in multiple studies.5–7,9–11 RS cannot be predicted from traditional measures, and vice versa. Both RS and traditional measures provide independent prognostic information not contained in each other.7,9,10
RS was shown to be predictive of chemotherapy benefit in 651 patients with available tumor blocks from the NSABP B-20 study, which randomly assigned patients with N0, ER-positive breast cancer to therapy with tamoxifen alone; methotrexate, fluorouracil, and tamoxifen; or cyclophosphamide, methotrexate, fluorouracil, and tamoxifen.5 However, in NSABP B-20 and other studies, tumor size and tumor grade have not been shown to be predictive of chemotherapy benefit.5,12
The published American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines include both traditional measures and RS in treatment planning.1,2 In clinical practice, physicians currently combine RS with pathology and clinical measures subjectively, on the basis of individual experience. As a next logical step, we developed a formal integration of RS and traditional pathology and clinical measures and evaluated its prognostic and predictive value.
PATIENTS AND METHODS
The criteria for patient inclusion and the statistical methods were specified in advance. The inclusion criteria and methodology for the NSABP B-14 and TransATAC validation studies of RS have been described previously.4,9 Patients from these validation studies were included in the meta-analysis calculations if they also had ER-positive disease by the Oncotype DX assay (ER expression ≥ 6.5) and the tumor grade, tumor size, and age of the patient were known. TransATAC included the tamoxifen and anastrozole monotherapy arms of ATAC, but not the combination arm.9 The TransATAC patients treated with anastrozole and those with node-positive disease were included because the relation of RS, tumor grade, tumor size, and patient age to the risk of distant recurrence did not depend on hormonal therapy or nodal status.9 The study databases were located at the NSABP Biostatistical Center at the University of Pittsburgh, Pittsburgh, PA, and the TransATAC data center at Queen Mary University of London and remained separate throughout the study.
Predictive Variables and Primary End Point
RS, assessed on a scale from 0 to 100, and patient age at the time of surgery were used as continuous variables. Histologic tumor grade was used as assessed by a single central laboratory breast cancer pathologist in each study. Tumor size was used as a continuous variable and was prespecified to be truncated at 0.5 cm and 5 cm to avoid undue influence on the model fit from exceptionally large or small tumors. The prespecified end point was time to first distant recurrence.
Statistical Analyses
Multivariate Cox proportional hazards models were fit separately to the data sets from NSABP B-14 and TransATAC.13 RS was fit using a natural cubic spline with 2 df with knots at RS = 5, 18, and 50 (Data Supplement).14 Tumor size and patient age were fit as linear covariates. Besides RS, tumor grade, tumor size, patient age, nodal status (N0, one to three positive nodes, four or more positive nodes), and hormonal treatment were included in the model for TransATAC after statistical tests verified that they did not interact with the other covariates.
The baseline cumulative hazard for distant recurrence at 10 years was estimated in NSABP B-14 by Breslow's method.15 Because patient follow-up in TransATAC was limited after 9 years, the 10-year baseline cumulative hazard was extrapolated from the 9-year Breslow estimate by assuming that the average hazard 9 to 10 years after surgery equaled that from 8 to 9 years.
To assess whether clinicopathologic factors jointly add prognostic value to RS, a meta-analysis likelihood ratio (LR) test was formed by summing across studies the LR test statistic, Δχ2, from comparing the full (RS-pathology-clinical [RSPC]) model, which includes RS, age, tumor size, and grade, with the reduced (RS alone) model. Nodal status and hormonal treatment were retained in the model for TransATAC to adjust for these variables.
To assess the 10-year risk of distant recurrence for a given patient with N0, ER-positive disease with specified RS, tumor grade, tumor size, and age, the log cumulative hazard for distant recurrence at 10 years was first estimated using the multivariate Cox model for each study. These two estimates were then averaged, weighting by their (patient-specific) inverse variances, and transformed to obtain a risk estimate and CI. This estimate is the RSPC risk assessment.
In practice, patients with RS less than 18 are classified as low risk, those with RS ≥ 31 as high risk, and those with RS 18 to 30 as intermediate risk.4 On the basis of the NSABP B-14 validation study, the estimated 10-year risk of distant recurrence for a patient with N0, ER-positive, tamoxifen-treated breast cancer is approximately 12% when RS is 18 and 20% when RS is 30.4 Comparable risk groups for RSPC were predefined using these boundary risks: patients with RSPC risk assessment less than 12% were classified as low risk, those with more than 20% as high risk, and those with 12% to 20% as intermediate risk. A sensitivity analysis was conducted using the exact RS risk estimates of 11.40% at RS = 18 and 20.01% at RS = 30. The patients with N0, ER-positive disease in the two studies were cross-classified by RSPC and RS risk group. Kaplan-Meier estimates of the 10-year distant recurrence incidence were combined across studies using meta-analysis methods (Data Supplement). Predictiveness curves, showing the RS and RSPC meta-analysis estimates of 10-year distant recurrence risk plotted against their population percentiles, were used to compare the ability of RS and RSPC to characterize recurrence risk among N0, ER-positive patients.16,17
To assess the prediction of chemotherapy benefit using NSABP B-20, the RSPC risk assessment was transformed to the same log cumulative hazard scale as RS when it was developed. The resulting RSPC risk score and RS were each fit to the data using a Cox model with factors for risk score, chemotherapy treatment, and the interaction of risk score with treatment.
All analyses were performed using SAS versions 9.1 and 9.2 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the Study Populations
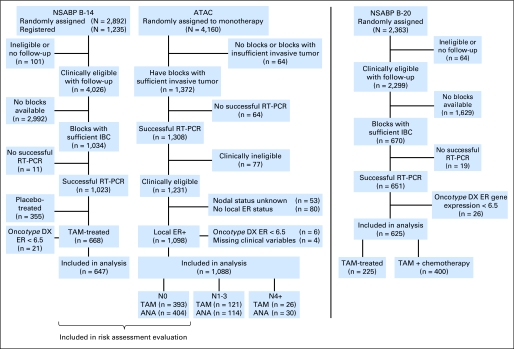
The numbers of patients from the two studies (NSABP B-14 and TransATAC) available for the meta-analysis are summarized in Figure 1. In all, 1,735 patients contributed data to the meta-analysis (647 from NSABP B-14 and 1,088 from TransATAC). B-14 patients were enrolled between January 1982 and October 1988; TransATAC patients were enrolled between July 1996 and March 2000.
Fig 1.
CONSORT diagram. ANA, anastrozole; ATAC, Arimidex, Tamoxifen Alone or in Combination study; ER, estrogen receptor; IBC, invasive breast cancer; NSABP B-14, National Surgical Adjuvant Breast and Bowel study B-14; RT-PCR, reverse transcription polymerase chain reaction; TAM, tamoxifen.
Descriptive statistics for the covariates used in the meta-analysis are shown in Table 1. RS was similarly distributed in these two studies. More tumors were assessed as moderately differentiated in TransATAC than in NSABP B-14. Tumors tended to be slightly smaller in TransATAC than in NSABP B-14. TransATAC enrolled only postmenopausal women; 28% of the B-14 patients were younger than 50 years.
Table 1.
Covariate Distributions in NSABP B-14 and TransATAC Meta-Analysis Evaluable Patients (n = 1,735)
| Covariate | NSABP B-14 (n = 647) |
TransATAC (n = 1,088) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| RS | ||||
| Median | 17 | 16 | ||
| 1st-3rd quartile | 11-31 | 11-24 | ||
| Tumor grade | ||||
| Well differentiated | 224 | 35 | 242 | 22 |
| Moderately differentiated | 293 | 45 | 655 | 60 |
| Poorly differentiated | 130 | 20 | 191 | 18 |
| Tumor size, cm | ||||
| Median | 2.0 | 1.8 | ||
| 1st-3rd quartile | 1.3-2.5 | 1.2-2.3 | ||
| Age at surgery, years | ||||
| Median | 58 | 63 | ||
| 1st-3rd quartile | 48-65 | 57-70 | ||
| Hormonal therapy | ||||
| Tamoxifen alone | 647 | 100 | 540 | 50 |
| Anastrozole alone | — | 548 | 50 | |
| Nodal status | ||||
| No positive nodes (N0) | 647 | 100 | 797 | 73 |
| 1-3 positive nodes (N1-3) | — | 235 | 22 | |
| 4+ positive nodes (N4+) | — | 56 | 5 | |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; RS, Recurrence Score; TransATAC, translational research cohort of Arimidex, Tamoxifen Alone or in Combination trial.
Multivariate Cox Regression Analyses
Hazard ratios and Wald test P values from the multivariate Cox models of the two studies are presented in Table 2. The directions of association of each covariate with risk of distant recurrence are the same in the two studies, and the magnitudes of association are broadly consistent.
Table 2.
Results of Multivariate Cox Proportional Hazards Regression Analysis of NSABP B-14 and TransATAC: Meta-Analysis of Evaluable Patients (n = 1,735)
| Covariate | NSABP B-14 (n = 647) |
TransATAC (n = 1,088) |
||
|---|---|---|---|---|
| Hazard Ratio | Wald Test P | Hazard Ratio | Wald Test P | |
| RS linear component | 5.344* | < .001 | 2.766* | .02 |
| RS nonlinear component | .004 | .37 | ||
| Tumor poorly differentiated | 2.845 | .008† | 2.477 | .012† |
| Tumor moderately differentiated | 1.223 | .50† | 1.625 | .14† |
| Tumor size | 1.266‡ | .006 | 1.72‡ | < .001 |
| Age at surgery | 0.892§ | .22 | 0.933§ | .53 |
| Treatment (anastrozole v tamoxifen) | — | — | 0.886 | .48 |
| 1-3 positive nodes (N1-3) | — | — | 1.429 | .083 |
| 4+ positive nodes (N4+) | — | — | 4.548 | < .001 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; RS, recurrence score; TransATAC, translational research cohort of Arimidex, Tamoxifen Alone or in Combination trial.
Hazard ratio for RS is the ratio of the hazards at 3rd and 1st quartiles of RS, estimated using both the linear and nonlinear components. Overall test for RS P < .001 for NSABP B-14 and for TransATAC.
Trend test for tumor grade P = .008 for NSABP B-14 and P = .023 for TransATAC. Overall test for tumor grade P < .001 for NSABP B-14 and P = .019 for TransATAC.
The hazard ratio for tumor size is for a 1-cm increase.
The hazard ratio for age is for a 10-year increase.
The RSPC model had significantly improved prognostic value compared with a model using RS alone (P < .001) and compared with a model using tumor grade, tumor size, and patient age (P < .001; Table 3).
Table 3.
Meta-Analysis for Likelihood Ratio Tests for Improved Prognostic Value: Meta-Analysis of Evaluable Patients (n = 1,735)
| Comparison | Likelihood Ratio | df | P |
|---|---|---|---|
| RSPC v RS alone | 76.9 | 8 | < .001 |
| RSPC v tumor grade, tumor size, and age | 45.4 | 4 | < .001 |
Abbreviations: RS, recurrence score; RSPC, recurrence score– pathology–clinical.
There was no evidence of interaction between the RSPC covariates and hormonal treatment (LR test, P = .27) or nodal status (P = .60) in the TransATAC study. This supports the use of the data from both treatment groups and the inclusion of N0, N1-3, and N4+ patients in the assessment of risk for N0 tamoxifen-treated patients. The baseline cumulative hazard estimates for the two studies (Data Supplement) support the extrapolation of the cumulative hazard estimate from 9 to 10 years in the TransATAC study. Log-cumulative hazard estimates for the two studies were similar, supporting the meta-analysis approach.
Comparative Evaluation of Distant Recurrence Risk Assessment by RSPC and RS in Patients With Node-Negative Disease
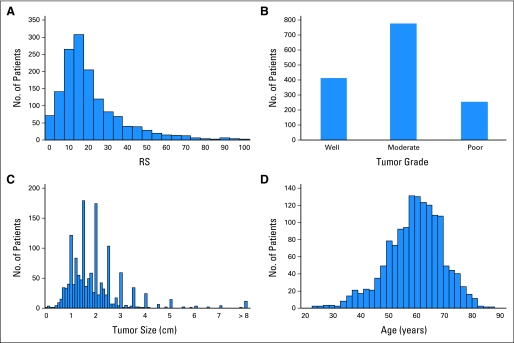
The distant recurrence risk assessments by RSPC and RS were compared using the large population of 1,444 patients with N0, ER-positive disease taking either tamoxifen or anastrozole (647 from NSABP B-14 and 797 from TransATAC). The distributions of RS, tumor grade, tumor size, and age of these patients are shown in Figure 2.
Fig 2.
Distribution of covariates in the 1,444 patients with N0, estrogen receptor–positive breast cancer. (A) Recurrence score (RS); (B) tumor grade; (C) tumor size; (D) age.
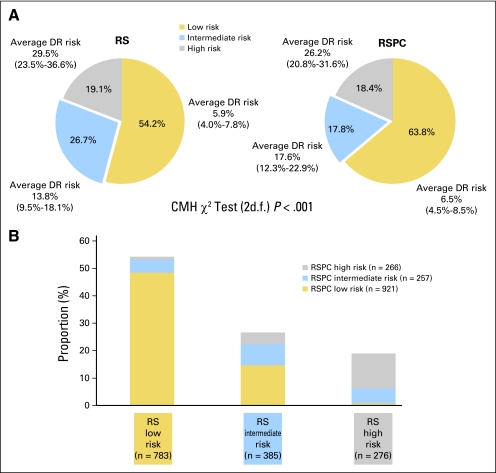
The proportions of patients classified as low, intermediate, and high risk by RSPC and by RS and the meta-analysis Kaplan-Meier estimates of the 10-year incidence of distant recurrence are shown in Figure 3. Fewer patients were classified as having intermediate risk by RSPC (17.8%) than by RS (26.7%). More were classified as low risk by RSPC than by RS; the proportion of patients classified as high risk was similar. The difference between RSPC and RS was significant (P < .001) using the Cochran-Mantel-Haenszel test (2 df) stratifying by patient. The RSPC and RS classifications had similar average 10-year risk within each risk group.
Fig 3.
(A) Proportion of patients with N0, estrogen receptor–positive breast cancer classified as low, intermediate, and high risk by recurrence score (RS) and RS-pathology-clinical assessment (RSPC), and (B) cross-classification of patients (N = 1,444). Cochran-Mantel-Haenszel (CMH) test compares RS with RSPC for the proportions of patients in the three risk classes. Average risk for each risk group is the meta-analysis weighted average estimate (with 95% CI) of the 10-year incidence of distant recurrence (DR). Average risks are not significantly different between RS and RSPC (P = .68, .27, .42 for low-, intermediate-, and high-risk groups, respectively, z-test); average risk significantly increases with increasing risk group (P < .001 for both RS and RSPC, z-test for trend).
The improved separation of risk by RSPC compared with RS in this patient population is also shown by the corresponding predictiveness curves (Data Supplement). A sensitivity analysis using the exact RS risk estimates of 11.40% at RS = 18 and 20.01% at RS = 30 gave similar results (61.6%, 19.9%, and 18.4% of patients classified as low, intermediate, and high risk, respectively, with RSPC).
In the cross-classification of patients by RSPC and RS risk group, many (71.9%) of the 272 patients classified as intermediate risk by RS were upstaged to high risk (16.9%) or downstaged to low risk (55.1%) in the RSPC classification. A few (10.9%) of the 783 patients classified as low risk by RS were upstaged to intermediate (8.9%) or high risk (1.9%) by RSPC. The very small percentage of patients with RS less than 18 who were classified by RSPC as high risk typically had very large tumors (median 4.8 cm). Some patients classified as RS low risk (8.9%) or high risk (28.6%) were classified as intermediate risk by RSPC. It was rare for RS high-risk patients to be classified by RSPC as low risk; such patients had well or moderately differentiated tumors of median size 1 cm.
Comparison of RSPC and RS Risk Assessments for Individual Patients
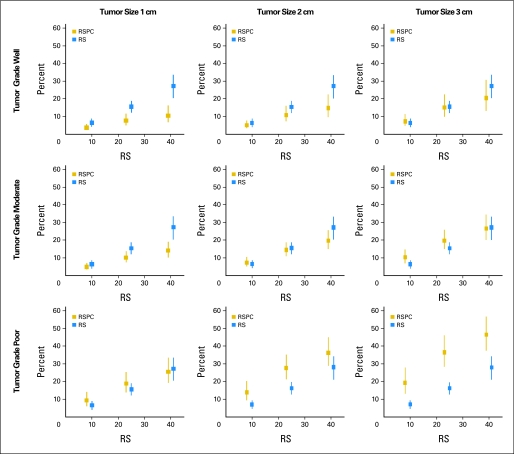
To assess the practical clinical utility of RSPC relative to RS, the risk assessments and CIs produced by RSPC were compared with those produced by RS.4 Figure 4 shows example RSPC and RS risk assessments for patients of age 50 years with various specific tumor grades and sizes and RS values. The effect of changes in the covariates is evident in the risk estimates. For example, the risk is higher when tumor grade is poorly differentiated or with increasing tumor size.
Fig 4.
Example of recurrence score (RS) and RS-pathology-clinical (RSPC) risk assessments with 95% CIs for patients of age 50 years with specific tumor grades and sizes.
For a representative sample of actual patients, 10 patients were randomly selected from NSABP B-14 and 10 from TransATAC, and their 10-year risk of distant recurrence was assessed with 95% CIs using RSPC and RS (Data Supplement). Among all 1,444 patients with N0, ER-positive disease, 68% had an RSPC risk estimate within 5% (absolute value) of the RS risk estimate.
Assessment of Prediction of Chemotherapy Benefit
Of the 651 NSABP B-20 patients with available tumor blocks, 625 had Oncotype DX ER expression ≥ 6.5 and complete information on tumor grade, tumor size, and patient age. Among these 625 patients, 60 patients experienced a distant recurrence. Both RS and the RSPC risk score showed a statistically significant association with the time to distant recurrence (standardized hazard ratios [HR] of 2.22, Wald test P < .001 for RS, and 2.43, P < .001 for the RSPC risk score). RS also showed a significant interaction with chemotherapy treatment (P = .037), as reported previously,5 with a standardized HR of 0.66 (95% CI, 0.44 to 0.97). The interaction of the RSPC risk score with treatment was not significant (P = .10), although the trend was in the same direction as with RS (standardized HR, 0.65; 95% CI, 0.39 to 1.09).
DISCUSSION
The RSPC assessment of prognosis, integrating RS with pathology and clinical measures, is a refinement of the quantitative risk assessment provided by RS. RSPC can provide greater accuracy in the assessment of distant recurrence risk when RS and pathology and clinical measures are discordant. RSPC CIs tend to be narrower when the predictive covariates are internally concordant (have the same risk direction) and wider when the predictive covariates are internally discordant. The RSPC classifier yielded a 33% relative reduction in the number of patients with intermediate risk and an 18% relative increase in the number of patients with low risk compared with RS alone.
RS alone was shown to be predictive of chemotherapy benefit in patients with N0 and N+ ER-positive breast cancer in previous studies in which neither tumor grade, tumor size, nor patient age showed a significant interaction with chemotherapy treatment.5,8 Therefore, it is not surprising that the RSPC risk assessment, which combines RS with these variables, does not improve over RS alone for the prediction of chemotherapy benefit. On the basis of currently available information, RS used alone, and the individual biology it identifies, remains the best predictive factor for chemotherapy benefit in patients with N0, ER-positive breast cancer.
We did not include the tamoxifen-alone arm of NSABP B-20 in our meta-analysis because the 31 distant recurrence events in this arm of the study were insufficient to support the six-term multivariate analysis. However, the 60 distant recurrences in both treatment arms were sufficient for the three-term interaction models to assess prediction of chemotherapy benefit. Other validation studies of RS were excluded from the meta-analysis because of one or more of the following reasons: (1) distant recurrence was not assessed, (2) node-negative patients were not eligible, or (3) all patients received chemotherapy.6–8 Having only two studies in the meta-analysis prevented the use of methodology that estimates between-study variation in the risk estimates.18,19 Hence the CIs for the meta-analysis risk estimates, derived assuming no variation between studies, may be narrower than if between-study variation could have been reliably estimated.
Treatment planning is optimally based on consideration of the absolute benefits and the absolute risks in the context of the individual patient's disease and informed preferences. The results of this study highlight the importance of RS in revealing the underlying biology relevant to both prognosis, which defines residual risk after standard hormonal treatment, and prediction, which defines relative risk reduction for the proposed addition of chemotherapy, in estimating the absolute benefit of added treatment. For patients with breast cancer with low RS or high RS disease, the practical clinical importance of using RSPC in most cases is not likely to be great. For patients with low RS disease, there is little, if any, relative risk reduction with the addition of chemotherapy. Most patients with low RS disease remain low risk by the RSPC classification (89.1%), attesting to the strength of RS itself in affirming the low-risk status of the vast majority of patients with RS less than 18. For the small number of patients who are high risk by RSPC yet predict low sensitivity to chemotherapy on the basis of low RS biology, one could still opt to use chemotherapy until further evidence becomes available; however, current data do not suggest a significant clinical benefit. For patients with high RS disease, there is substantial benefit of chemotherapy, regardless of the other pathology and clinical risk factors for distant recurrence.5,8 RSPC will likely have the greatest clinical utility in patients with intermediate RS. There may be small relative risk reduction with chemotherapy for patients with intermediate RS. A patient with intermediate RS can be expected to have lower absolute benefit from chemotherapy if the risk of distant recurrence by RSPC is lower and higher absolute benefit if the RSPC risk assessment is higher.
Although RSPC will have its greatest utility in the subset of patients with intermediate RS, it is not the case that RS is of value in only a subset of patients. As reported in numerous previous studies, many patients who might seem to be low risk by pathology and clinical factors have high RS disease, and many patients who might seem to be at average or high risk by pathology and clinical factors have low RS disease.4,5,7,9,20
The main ATAC study result shows an overall benefit of anastrozole over tamoxifen, and the test for treatment effect (P = .48) in Table 2 does not contradict that conclusion. Our multivariate analysis used the TransATAC subset of ATAC, which, because of the smaller sample size, inevitably increased the P value for the treatment test despite the treatment HR in our analysis and ATAC (0.83 for the end point disease-free survival) being similar. RSPC assessment of distant recurrence risk with aromatase inhibitor treatment would be useful. The RSPC methodology might also be used with an analysis of competing risks, as described by Ravdin et al.3
In summary, RSPC risk assessment integrating RS with traditional pathology and clinical measures adds significant prognostic information to RS. RSPC can aid in making chemotherapy decisions by refining assessments of recurrence risk where RS and traditional measures are discordant, especially with intermediate RS, by reducing the number of patients classified as intermediate risk and enhancing confidence in the integration of RS with traditional measures. The addition of clinicopathologic measures did not seem to enhance the value of RS alone in predicting relative chemotherapy benefit. As with other meta-analyses, this analysis reflects all of the currently available evidence and can be updated and refined as new data sets become available. The RSPC risk assessment tool will be made available online.
Supplementary Material
Acknowledgment
We thank Barbara C. Good, PhD, Wendy L. Rea, and Christine L. Rudock of the National Surgical Adjuvant Breast and Bowel Project Operations Center for invaluable editorial assistance and help on the plots during the preparation of this manuscript.
Footnotes
See accompanying editorial on page 4347
Supported in part by Public Health Service Grants No. U10CA-12027, U10CA-69974, U10CA-37377, and U10CA-69651 from the National Cancer Institute, Department of Health and Human Services, with additional support from AstraZeneca, Breakthrough Breast Cancer, and the Royal Marsden National Institute for Health Research Biomedical Research Centre. Analysis of the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial was partially funded by the Cancer Research UK Programme Grant No. C569-A10404.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: PDQ: NSABP-B-14.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Michael Crager, Genomic Health (C); Steven Shak, Genomic Health (C) Consultant or Advisory Role: Jack Cuzick, AstraZeneca (C); Mitch Dowsett, AstraZeneca (C); Eleftherios P. Mamounas, Genomic Health (C) Stock Ownership: Steven Shak, Genomic Health Honoraria: Jack Cuzick, AstraZeneca; Mitch Dowsett, AstraZeneca; John F. Forbes, AstraZeneca; Eleftherios P. Mamounas, Genomic Health Research Funding: Jack Cuzick, AstraZeneca; Mitch Dowsett, AstraZeneca Expert Testimony: Mitch Dowsett, AstraZeneca (C) Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Gong Tang, Jack Cuzick, Mitch Dowsett, John F. Forbes, Michael Crager, Eleftherios P. Mamounas, Steven Shak, Norman Wolmark
Financial support: Steven Shak
Administrative support: Joseph P. Costantino, Norman Wolmark
Provision of study materials or patients: John F. Forbes, Steven Shak
Collection and assembly of data: Gong Tang, Joseph P. Costantino, Mitch Dowsett, Eleftherios P. Mamounas, Steven Shak
Data analysis and interpretation: Gong Tang, Jack Cuzick, Mitch Dowsett, Michael Crager, Eleftherios P. Mamounas, Steven Shak
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer (version 2.2008) http://www.nccn.org. [DOI] [PubMed]
- 3.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 6.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 10.Bryant J. Toward a more rational selection of tailored adjuvant therapy. Data from the National Surgical Adjuvant Breast and Bowel Project; Ninth International St. Gallen Breast Cancer Conference; January 26-29, 2005; St. Gallen, Switzerland. [Google Scholar]
- 11.Paik S, Shak S, Tang G, et al. Risk classification of breast cancer patients by the Recurrence Score assay: Comparison to guidelines based on patient age, tumor size, and tumor grade. Twenty-Seventh Annual San Antonio Breast Cancer Symposium; December 8-11 2004; San Antonio, TX. abstr 104. [Google Scholar]
- 12.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 13.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 14.Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modeling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 15.Breslow NE. Contribution to the discussion on the paper by DR Cox, Regression and life tables. J R Stat Soc B. 1972;34:216–217. [Google Scholar]
- 16.Bura E, Gastwirth JL. The binary regression quantile plot: Assessing the importance of predictors in binary regression visually. Biom J. 2001;43:5–21. [Google Scholar]
- 17.Huang Y, Pepe MS, Feng Z. Evaluating the predictiveness of a continuous marker. Biometrics. 2007;63:1181–1188. doi: 10.1111/j.1541-0420.2007.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand. 1982;87:377–385. doi: 10.6028/jres.087.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: Results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127:133–142. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.