Abstract
Purpose
Recent studies have reported increased mortality for right-sided colon cancers but had limited adjustment for patient characteristics and conflicting results by stage. We examined the relationship between colon cancer location (right- v left-side) and 5-year mortality by stage.
Patients and Methods
We identified Medicare beneficiaries from 1992 to 2005 with American Joint Commission on Cancer stages I to III primary adenocarcinoma of the colon who underwent surgery for curative intent through Surveillance, Epidemiology, and End Results (SEER) –Medicare data. Adjusted hazard ratios (HRs) and 95% CIs for predictors of all-cause 5-year mortality were obtained by using Cox proportional hazards regression.
Results
Of 53,801 patients, 67% had right-sided colon cancer. Patients with right-sided cancer were more likely to be older, to be women, to be diagnosed with a more advanced stage, and to have more poorly differentiated tumors. Adjusted Cox regression showed no significant difference in mortality between right- and left-sided cancers for all stages combined (HR, 1.01; 95% CI, 0.98 to 1.04; P = .598) or for stage I cancers (HR, 0.95; 95% CI, 0.88 to 1.03; P = .211). Stage II right-sided cancers had lower mortality than left-sided cancers (HR, 0.92; 95% CI, 0.87 to 0.97; P = .001), and stage III right-sided cancers had higher mortality (HR, 1.12; 95% CI, 1.06 to 1.18; P < .001).
Conclusion
When analysis was adjusted for multiple patient, disease, comorbidity, and treatment variables, no overall difference in 5-year mortality was seen between right- and left-sided colon cancers. However, within stage II disease, right-sided cancers had lower mortality; within stage III, right-sided cancers had higher mortality.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths for both men and women in the United States, with 147,000 new occurrences and 50,000 deaths in 2009.1 Notably, over the past 2 decades, there has been a slow but steady decline in overall CRC mortality rates. Deaths from CRC have decreased by 3.9% per year from 2002 to 2006 for men and by 3.4% per year from 2001 to 2006 for women.2 However, this trend is not consistently present across all subgroups, such as ethnicity, age, and anatomic location of tumors. Multiple studies have shown an increase in the percentage of right-sided colon cancers over this same time frame in the United States,3,4 which has led to a focus on the potential etiologies for variation by anatomic sites of colon tumors. Differences have been noted in the following characteristics: right-sided colon cancers are more likely to be exophytic, to be diploid, and to have mucinous histology, high microsatellite instability, CpG island methylation, whereas left-sided colon cancers are often infiltrating lesions, present with obstructive symptoms, have chromosomal instability, and are more often aneuploid.5–7 Analysis of tumor specimens also has shown a difference in gene expressions between tumors in the right and left colon.8–10 However, it is unclear whether these biologic differences translate into meaningful differences in mortality.
There has been conflicting information regarding the relationship between cancer location and mortality. In 2008, Meguid et al11 reported a 4% increase in mortality for right-sided compared with left-sided colon cancers by using the Surveillance, Epidemiology, and End Results Program (SEER) database. More recently, Benedix et al12 queried the database created by the German multicentered observational study, Colon/Rectal Carcinoma (Primary Tumor), and found an even larger increase (12%) in mortality for right-sided compared with left-sided colon cancers. However, when the results were separated by tumor stage, conflicting results emerged from both studies. Both studies were limited in their ability to adjust for a wide range of patient characteristics. We used the linked SEER-Medicare data set to re-examine the relationship between tumor site (right- v left-side) and 5-year mortality, and more specifically, to determine if this relationship is consistent across tumor stage.
PATIENTS AND METHODS
This study was approved by the University of Wisconsin, Madison, Health Sciences institutional review board. The study was given a waiver of consent.
Data Sources
We examined data from the linked SEER-Medicare database for patients diagnosed with colon cancer between 1992 and 2005. The SEER cancer registries include information on patient demographics, tumor characteristics, first course of treatment, and survival for persons newly diagnosed with cancer. For individuals who are eligible for Medicare services, the SEER-Medicare database includes claims for covered health care services, including hospital, physician, outpatient, home health, and hospice bills. The SEER-Medicare data set has successfully linked 93% of individuals older than age 65 years at diagnosis to their Medicare records.13,14 SEER registries from 1992 to 2005 contain incident cancer diagnoses in the following cities, states, and regions: Los Angeles; San Francisco, Oakland; San Jose, Monterey; Greater California; Connecticut; Detroit; Atlanta; rural Georgia; Hawaii; Iowa; Kentucky; Louisiana; New Jersey; New Mexico; Seattle, Puget Sound; and Utah. In 2000, SEER regions included approximately 26% of the US population.14
Patient Selection
All Medicare-enrolled patients age 66 years and older who were diagnosed with primary adenocarcinoma of the colon in a SEER area from 1992 to 2005 were included in the study if they had a diagnosis of American Joint Committee on Cancer (AJCC) stage I to III colon (SEER cancer site codes 18.0 to 18.9 and 19.9) adenocarcinoma (SEER histology codes 8140 to 8147, 8210 to 8211, 8220 to 8221, 8260 to 8263, 8480 to 8481, and 8490). Patients with rectal cancer (SEER cancer site code 20.9) were excluded, because treatment is different from that of colon cancer, and patients with mucinous cystadenocarcinoma (SEER histology code 8470) were excluded, because the natural history of this disease (which occurs in the appendix and is associated with pseudomyxoma peritonei) is different from other histologic subtypes of colon adenocarcinoma.15 We additionally selected those patients who underwent primary tumor resection with likely curative intent within 6 months of diagnosis by using the following International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) procedure codes: 45.7x (partial excision of large intestine) and 45.8x (total intra-abdominal colectomy). Patients also were required to have continuous enrollment in Medicare Part A and Part B from 12 months before their diagnosis to 5 years after date of discharge, death, or December 31, 2005 (whichever came first) to facilitate ascertainment of comorbidities, postoperative chemotherapy administration, and survival. Patients were excluded if they were also enrolled in a Health Maintenance Organization during the same time period. Patients also were excluded if they were diagnosed with another malignancy 1 year before or after the date of colon cancer diagnosis or if their first diagnosis of colon cancer was made after death (ie, on autopsy). From an initial cohort of 61,846 patients, the following sequential exclusions were carried out. First, tumors with an unknown location and appendiceal site were identified with SEER cancer site codes 18.8 (overlapping lesion of colon), 18.9 (colon not otherwise specified), and 18.1 (appendix) and were excluded from this study (n = 1,143). Subsequent exclusions were for missing tumor grade (n = 2,391), initial cancer treatment other than surgery (eg, preoperative radiation or chemotherapy; n = 222), missing nodal assessment (n = 1,499), and death within 30 days of surgery or before discharge from the surgical hospitalization (n = 2,790). The final sample size was 53,801 patients.
Outcome Variable
The primary outcome measure for this study was all-cause 5-year mortality, defined as death within 5 years of primary surgery for colon cancer on the basis of dates of death recorded in the SEER Patient Entitlement and Diagnosis Summary File.
Main Explanatory Variables
The primary explanatory variable was tumor location. The approach for dividing tumor location into right- and left-side follows the previously mentioned studies.11,12 Right-sided colon cancers were identified with the following SEER cancer site codes: 18.0 (cecum), 18.2 (ascending colon), 18.3 (hepatic flexure of colon), and 18.4 (transverse colon). Left-sided colon cancers were defined with codes 18.5 (splenic flexure of colon), 18.6 (descending colon), 18.7 (sigmoid colon), and 19.9 (rectosigmoid). In addition to tumor location, AJCC stages I to III were identified from the SEER data and were used to stratify all analyses.
Control Variables
Basic patient-related variables included date of birth, sex, race/ethnicity, marital status, SEER registry region, rural/urban county of residence, and census tract median level of household income and median level of education (used as proxies for socioeconomic status). An advantage of the SEER-Medicare linked data set is the ability to use Medicare claims data for risk adjustment and treatment measures. A risk score was assigned to each patient through the use of Centers for Medicare and Medicaid Services Hierarchical Condition Categories (HCCs)16 on the basis of outpatient and inpatient diagnoses from the 12 months before colon cancer diagnosis. We also extracted hospitalization in the year before diagnosis, oncologist visit within 30 days of surgical discharge, and number of months of chemotherapy.
In addition to the patient-related, risk adjustment, and treatment variables described in the Patients and Methods section, we measured a variety of disease-related variables. Disease-related variables included poor prognostic features and year of diagnosis, which was divided into periods of colonoscopy coverage by Medicare previously described by Fenton et al.17 Poor prognostic features included diagnosis in the setting of intestinal obstruction or perforation, emergent admission for surgery, T4 stage, poor/undifferentiated tumor histology, and fewer than 12 lymph nodes examined at surgery.18
Statistical Analysis
We compared the frequency of all patient-related, disease-related, risk adjustment, and treatment variables by tumor location (right- v left-sided colon cancer) by using χ2 tests for categoric variables and two-way analysis of variance tests for continuous variables. Univariate Kaplan-Meier survival analysis was performed. Comparison of overall 5-year survival between right- and left-sided colon cancers within each stage (ie, I to III) and for all stages combined was done with a Cox regression–based test for equality of survival curves. Cox proportional hazards regression analysis was conducted to obtain adjusted hazard ratios (HRs) and 95% CIs for different predictors of overall 5-year mortality and for 5-year mortality controlled for patient-related, disease-related, risk adjustment, and treatment covariates. Models included tumor location, stage, a stage-location interaction term, and all covariates. The interaction between stage and tumor location was significant (likelihood-ratio χ2, 37.86; P < .001), and all models subsequently were stratified by stage. Analyses were carried out with Stata 11.0 software (StataCorp, College Station, TX) and SAS 8.02 software (SAS Institute, Cary, NC). All tests of significance used two-sided P values at the P < .05 level, and robust estimates of the standard error were used in all regression analyses.
RESULTS
Sample Characteristics
Of 53,801 patients, two thirds (66%) were age 75 years and older, 59% were women, 86% were white, and most of them were married and lived in a major metropolitan area (Table 1). Frequency of the disease-related characteristics showed that the majority of patients were diagnosed with stage II disease (44%), followed by stage III disease (32%). Only 18% required emergent admission at diagnosis; and 70% of the tumors were moderately differentiated; 21% were poorly differentiated or undifferentiated.
Table 1.
Demographics and Clinical Characteristics of Medicare Beneficiaries Undergoing Resection for Stages I to III Colon Cancer
| Characteristic | Patients (%) |
P | ||
|---|---|---|---|---|
| All (N = 53,801) | Right-Sided Cancer (n = 36,066) | Left-Sided Cancer (n = 17,735) | ||
| Demographic | ||||
| Age, years | < .001 | |||
| 65-69 | 12.5 | 11.1 | 15.4 | |
| 70-74 | 21.3 | 19.9 | 24.0 | |
| 75-79 | 25.2 | 25.0 | 25.5 | |
| 80-84 | 22.1 | 23.0 | 20.3 | |
| ≥ 85 | 19.0 | 21.1 | 14.8 | |
| Male sex | 41.4 | 38.3 | 47.8 | < .001 |
| Ethnicity | < .001 | |||
| White | 85.9 | 86.9 | 83.7 | |
| Black | 6.2 | 6.0 | 6.7 | |
| Asian or Pacific Islander | 4.2 | 3.5 | 5.8 | |
| Hispanic | 3.4 | 3.3 | 3.5 | |
| Other | 0.4 | 0.4 | 0.3 | |
| Marital status | < .001 | |||
| Married | 50.7 | 49.1 | 53.8 | |
| Widowed | 34.7 | 36.2 | 31.5 | |
| Single, separated, or divorced | 11.6 | 11.6 | 11.7 | |
| Unknown | 3.1 | 3.1 | 3.0 | |
| SEER registry* | < .001 | |||
| California | 28.8 | 28.8 | 28.8 | |
| Connecticut | 10.4 | 10.2 | 10.8 | |
| Detroit | 10.3 | 10.2 | 10.4 | |
| Hawaii | 1.8 | 1.5 | 2.4 | |
| Iowa | 13.0 | 13.2 | 12.5 | |
| New Mexico | 2.5 | 2.3 | 2.9 | |
| Seattle | 7.3 | 7.6 | 6.7 | |
| Utah | 3.1 | 3.0 | 3.1 | |
| Atlanta and rural Georgia | 3.9 | 3.9 | 3.9 | |
| Kentucky | 4.9 | 4.9 | 4.8 | |
| Louisiana | 4.1 | 3.9 | 4.6 | |
| New Jersey | 10.0 | 10.4 | 9.1 | |
| Residence location | .12 | |||
| Major metropolitan | 55.8 | 56.1 | 55.2 | |
| Metropolitan or urban | 34.0 | 33.7 | 34.6 | |
| Less urban or rural | 10.3 | 10.3 | 10.3 | |
| Median household income (census tract), $ in thousands† | < .001 | |||
| Mean | 45.2 | 45.6 | 44.3 | |
| SD | 22.1 | 22.3 | 21.8 | |
| Less than 12 years education (census tract), %† | < .001 | |||
| Mean | 18.9 | 18.6 | 19.6 | |
| SD | 12.5 | 12.3 | 12.8 | |
| Year of diagnosis by Medicare colonoscopy coverage, % | < .001 | |||
| 1992-1997 | 31.8 | 30.1 | 35.0 | |
| 1998-June 2001 | 24.7 | 24.9 | 24.1 | |
| July 2001-2005 | 43.6 | 44.9 | 40.9 | |
| AJCC stage | < .001 | |||
| I | 24.3 | 23.3 | 26.3 | |
| II | 43.8 | 44.3 | 42.8 | |
| III | 31.9 | 32.3 | 31.0 | |
| Poor prognostic feature | ||||
| Emergent admission | 17.5 | 17.7 | 17.3 | .29 |
| Intestinal obstruction on admission | 4.0 | 3.0 | 5.9 | < .001 |
| Intestinal perforation on admission | 1.6 | 1.3 | 2.3 | < .001 |
| T stage | < .001 | |||
| Tis, T0, T1, or T2 | 27.7 | 26.6 | 29.9 | |
| T3 | 60.1 | 60.9 | 58.4 | |
| T4 | 12.3 | 12.5 | 11.7 | |
| No. of nodes resected | < .001 | |||
| 0-11 | 55.7 | 49.6 | 68.2 | |
| ≥ 12 | 44.3 | 50.4 | 31.8 | |
| Tumor grade | < .001 | |||
| Well differentiated | 9.3 | 8.7 | 10.6 | |
| Moderately differentiated | 69.6 | 66.5 | 75.8 | |
| Poorly or undifferentiated | 21.1 | 24.9 | 13.6 | |
| Comorbidity and treatment measure | ||||
| HCC risk score† | < .001 | |||
| Mean | 2.11 | 2.13 | 2.06 | |
| SD | 1.38 | 1.36 | 1.41 | |
| Any hospitalization in the previous year | 26.6 | 27.8 | 24.2 | < .001 |
| Rehospitalization within 30 days of discharge | 11.1 | 11.1 | 11.2 | .93 |
| Any in-hospital surgical complication | 3.4 | 2.8 | 4.7 | < .001 |
| Oncologist visit within 30 days of surgery | 30.4 | 30.7 | 29.7 | .02 |
| Postoperative adjuvant chemotherapy | < .001 | |||
| None | 72.4 | 73.0 | 71.1 | |
| Incomplete: 1-4 months | 8.9 | 9.0 | 8.6 | |
| Complete: ≥ 5 months | 18.7 | 18.0 | 20.2 | |
Abbreviations: AJCC, American Joint Committee on Cancer; HCC, Hierarchical Condition Categories; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results.
Three SEER registries (ie, Kentucky, Louisiana, and New Jersey) were added in 2000.
N = 2,856 individuals with missing data not included in these means.
Two thirds of patients (n = 36,066) had right-sided colon cancer, and one third (n = 17,735) had left-sided colon cancer. Between right- and left-sided colon cancers, there were significant differences in age distribution, sex, ethnicity distribution, stage groups, tumor grade, and completion of postoperative adjuvant chemotherapy (P < .001). Patients with right-sided cancers, compared with those who had left-sided cancers, were significantly older (69% v 61% ≥ 75 years old), were more likely to be women (62% v 52%), had more poorly differentiated tumors (25% v 14%), and had a higher percentage of stages II and III disease (76% v 74%). A higher percentage of patients with left-sided cancers than with right-sided cancers completed a course of adjuvant chemotherapy (20% v 18%). Sample characteristics by stage and location are listed in Table 2.
Table 2.
Demographics and Clinical Characteristics of Medicare Beneficiaries Undergoing Resection for Stages I to III Colon Cancer Stratified by Stage and Location
| Variable | Patients (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage I (n = 13,075) |
Stage II (n = 23,578) |
Stage III (n = 17,148) |
|||||||
| Right-Sided Cancer(n = 8,419) | Left-Sided Cancer(n = 4,656) | P | Right-Sided Cancer(n = 15,992) | Left-Sided Cancer(n = 7,586) | P | Right-Sided Cancer(n = 11,665) | Left-Sided Cancer (n = 5,493) | P | |
| Demographic | |||||||||
| Age, years | < .001 | < .001 | < .001 | ||||||
| 65-69 | 10.6 | 17.3 | 10.3 | 13.7 | 12.4 | 16.0 | |||
| 70-74 | 21.4 | 25.7 | 18.7 | 22.3 | 20.5 | 24.9 | |||
| 75-79 | 26.4 | 27.0 | 24.2 | 24.4 | 25.1 | 25.9 | |||
| 80-84 | 23.1 | 18.8 | 23.5 | 21.5 | 22.2 | 20.0 | |||
| ≥ 85 | 18.5 | 11.2 | 23.3 | 18.1 | 19.8 | 13.3 | |||
| Male sex | 40.5 | 49.0 | < .001 | 37.7 | 46.1 | < .001 | 37.5 | 49.1 | < .001 |
| Ethnicity | < .001 | < .001 | < .001 | ||||||
| White | 87.7 | 85.5 | 87.6 | 84.4 | 85.4 | 81.1 | |||
| Black | 5.7 | 5.8 | 5.7 | 6.4 | 6.6 | 7.8 | |||
| Asian or Pacific Islander | 3.2 | 5.7 | 3.1 | 5.1 | 4.1 | 7.1 | |||
| Hispanic | 2.9 | 2.9 | 3.3 | 3.8 | 3.6 | 3.8 | |||
| Other | 0.4 | 0.2 | 0.4 | 0.4 | 0.4 | 0.3 | |||
| Marital status, % | < .001 | < .001 | < .001 | ||||||
| Married | 51.8 | 57.2 | 47.5 | 51.2 | 49.4 | 54.6 | |||
| Widowed | 33.7 | 28.9 | 37.9 | 33.9 | 35.7 | 30.4 | |||
| Single, separated, or divorced | 11.3 | 10.7 | 11.5 | 11.9 | 11.9 | 12.2 | |||
| Unknown | 3.2 | 3.2 | 3.1 | 3.1 | 3.0 | 2.8 | |||
| SEER registry | < .001 | < .001 | < .001 | ||||||
| California | 28.3 | 28.7 | 29.8 | 28.5 | 27.8 | 29.3 | |||
| Connecticut | 9.3 | 11.0 | 10.7 | 10.8 | 10.1 | 10.8 | |||
| Detroit | 9.8 | 10.3 | 10.1 | 10.5 | 10.8 | 10.3 | |||
| Hawaii | 1.7 | 2.5 | 1.3 | 1.9 | 1.7 | 2.8 | |||
| Iowa | 12.9 | 14.1 | 13.8 | 12.5 | 12.6 | 11.1 | |||
| New Mexico | 2.1 | 2.6 | 2.2 | 3.0 | 2.7 | 3.0 | |||
| Seattle | 7.7 | 6.3 | 7.7 | 7.3 | 7.4 | 6.3 | |||
| Utah | 3.4 | 2.7 | 2.7 | 3.1 | 3.3 | 3.6 | |||
| Atlanta and rural Georgia | 4.2 | 3.5 | 3.8 | 4.2 | 3.9 | 4.0 | |||
| Kentucky | 5.6 | 5.1 | 4.6 | 4.5 | 4.9 | 4.8 | |||
| Louisiana | 4.2 | 4.5 | 3.7 | 4.6 | 4.0 | 4.6 | |||
| New Jersey | 11.0 | 8.9 | 9.7 | 9.0 | 10.9 | 9.5 | |||
| Residence location | .02 | .88 | .25 | ||||||
| Major metropolitan | 55.4 | 52.8 | 55.9 | 56.2 | 56.7 | 55.7 | |||
| Metropolitan or urban | 34.4 | 36.6 | 33.9 | 33.6 | 33.0 | 34.3 | |||
| Less urban or rural | 10.2 | 10.6 | 10.2 | 10.3 | 10.3 | 10.0 | |||
| Median household income (census tract), $ in thousands* | < .001 | < .001 | < .001 | ||||||
| Mean | 47.0 | 44.9 | 45.2 | 44.1 | 45.4 | 44.1 | |||
| SD | 23.1 | 21.4 | 22.1 | 22.2 | 22.0 | 21.5 | |||
| Less than 12 years of education (census tract)* | < .001 | < .001 | < .001 | ||||||
| Mean | 17.9 | 19.2 | 18.7 | 19.7 | 18.8 | 19.8 | |||
| SD | 12.0 | 12.4 | 12.3 | 12.9 | 12.6 | 12.9 | |||
| Year of diagnosis by Medicare colonoscopy coverage | < .001 | < .001 | < .001 | ||||||
| 1992-1997 | 25.0 | 32.9 | 32.7 | 37.5 | 30.4 | 33.4 | |||
| 1998-June 2001 | 25.4 | 24.4 | 24.9 | 24.3 | 24.7 | 23.6 | |||
| July 2001-2005 | 49.7 | 42.7 | 42.4 | 38.2 | 44.9 | 43.0 | |||
| Poor prognostic feature | |||||||||
| Emergent admission | 11.6 | 9.2 | < .001 | 18.4 | 20.0 | .002 | 21.0 | 20.4 | .33 |
| Intestinal obstruction on admission | 0.6 | 1.3 | < .001 | 3.3 | 7.5 | < .001 | 4.4 | 7.3 | < .001 |
| Intestinal perforation on admission | 0.3 | 0.5 | .09 | 1.6 | 3.4 | < .001 | 1.8 | 2.3 | .04 |
| T stage | < .001 | .07 | < .001 | ||||||
| Tis, T0, T1, or T2 | 100.0 | 100.0 | 0.0 | 0.0 | 10.0 | 11.8 | |||
| T3 | 0.0 | 0.0 | 86.1 | 85.2 | 70.3 | 70.9 | |||
| T4 | 0.0 | 0.0 | 13.9 | 14.8 | 19.7 | 17.3 | |||
| No. of nodes resected | < .001 | < .001 | < .001 | ||||||
| 0-11 | 58.7 | 81.0 | 48.8 | 65.8 | 44.1 | 60.7 | |||
| ≥ 12 | 41.3 | 19.1 | 51.2 | 34.2 | 56.0 | 39.3 | |||
| Tumor grade | < .001 | < .001 | < .001 | ||||||
| Well differentiated | 17.4 | 19.7 | 7.1 | 8.6 | 4.5 | 5.7 | |||
| Moderately differentiated | 71.1 | 72.9 | 69.0 | 79.5 | 59.6 | 73.4 | |||
| Poorly or undifferentiated | 11.5 | 7.5 | 23.8 | 12.0 | 35.9 | 21.0 | |||
| Comorbidity and treatment measure | |||||||||
| HCC risk score* | < .001 | .02 | .03 | ||||||
| Mean | 1.81 | 1.58 | 1.95 | 2.00 | 2.60 | 2.55 | |||
| SD | 1.26 | 1.24 | 1.28 | 1.35 | 1.42 | 1.48 | |||
| Any hospitalization in the previous year | 27.8 | 24.3 | < .001 | 28.1 | 24.6 | < .001 | 27.4 | 23.5 | < .001 |
| Rehospitalization within 30 days of discharge | 10.3 | 10.1 | .71 | 11.0 | 11.6 | .19 | 11.8 | 11.4 | .44 |
| Any in-hospital surgical complication | 2.4 | 3.3 | .002 | 2.9 | 5.4 | < .001 | 3.1 | 4.8 | < .001 |
| Oncologist visit within 30 days of surgery | 15.9 | 13.8 | .00 | 28.0 | 29.5 | .02 | 45.2 | 43.7 | .07 |
| Postoperative adjuvant chemotherapy | .17 | < .001 | < .001 | ||||||
| None | 96.3 | 96.9 | 81.6 | 77.7 | 44.3 | 40.2 | |||
| Incomplete: 1-4 months | 2.4 | 2.0 | 6.8 | 7.5 | 16.9 | 15.9 | |||
| Complete: ≥ 5 months | 1.3 | 1.2 | 11.6 | 14.8 | 38.8 | 43.9 | |||
NOTE. Total No. of patients = 53,801.
Abbreviations: HCC, Hierarchical Condition Categories; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results.
Individuals with missing data (n = 2,856) are not included in these means.
Survival Analysis by Tumor Location and Stage
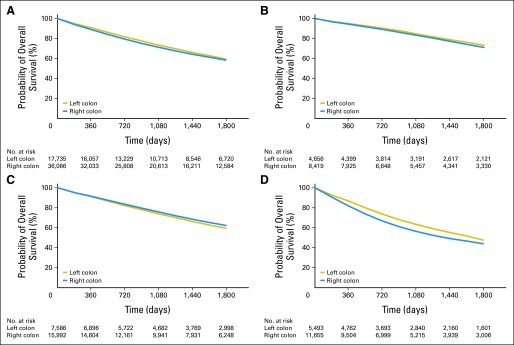
Unadjusted survival curves demonstrated a significant difference in 5-year survival between right- and left-sided cancers for all stages. Right-sided cancers had a worse survival for all stages except for stage II (Fig 1). Unadjusted survival models with tumor location as the explanatory variable showed increased risk of 5-year mortality for right-sided compared with left-sided cancers for all stages combined (HR, 1.06; 95% CI, 1.03 to 1.1), stage I (HR, 1.10; 95% CI, 1.02 to 1.18), and stage III (HR, 1.18; 95% CI, 1.13 to 1.24). For stage II cancers, patients with right-sided tumors had a decreased risk of mortality (HR, 0.92; 95% CI, 0.88 to 0.96). After adjustment, no significant difference in mortality was found between right- and left-sided cancers for all stages combined (HR, 1.01; 95% CI, 0.98 to 1.04; P = .598) or for stage I cancers (HR, 0.95; 95% CI, 0.88 to 1.03; P = .211). Stage II right-sided cancers had lower mortality than left-sided cancers (HR 0.92; 95% CI, 0.87-0.97, P = .001), while stage III right-sided cancers had higher mortality (HR, 1.12; 95% CI, 1.06 to 1.18; P < .001; Table 3). Additional analyses were carried out with models that adjusted for only patient- and comorbidity-related covariates as well as with a model that adjusted for all covariates with the exception of tumor grade, and the findings, when stratified by stage and location, remained the same.
Fig 1.
Kaplan-Meier survival estimates for patients with right-sided (n = 36,066) and left-sided (n = 17,735) colon cancer. (A) All stages combined; (B) stage I disease; (C) stage II disease; and (D) stage III disease.
Table 3.
Adjusted HRs and 95% CIs for 5-Year Mortality by Stage
| Analysis Type | All Stages Combined(N = 53,801) |
Stage I (n = 13,075) |
Stage II (n = 23,578) |
Stage III (n = 17,148) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Unadjusted | ||||||||||||
| Left sided | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Right sided | 1.06 | 1.03 to 1.1 | < .001 | 1.10 | 1.02 to 1.18 | .01 | 0.92 | 0.88 to 0.96 | < .001 | 1.18 | 1.13 to 1.24 | < .001 |
| Adjusted for all covariates* | ||||||||||||
| Left sided | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Right sided | 1.01 | 0.98 to 1.04 | .60 | 0.95 | 0.88 to 1.03 | .21 | 0.92 | 0.87 to 0.97 | .001 | 1.12 | 1.06 to 1.18 | < .001 |
Abbreviation: HR, hazard ratio.
Cox regression model controlling for age; sex; ethnicity; marital status; residence; Surveillance, Epidemiology, and End Results registry; year of diagnosis by Medicare colonoscopy coverage; census-tract income and education; Hierarchical Condition Categories score; hospitalizations per year; in-hospital complications; chemotherapy; and poor tumor prognostic features.
Predictors of Overall Mortality
Significant patient-related predictors of increased mortality were increasing age, male sex, and being widowed or single, separated, or divorced rather than currently married (Table 4). Patients with Asian or Pacific Islander ethnicity had a lower risk of mortality than whites, whereas all other groups did not show a significant difference compared with whites. We also observed regional variation in 5-year mortality. Compared with California (the SEER region that contributed the most patients to our sample), patients from Connecticut, Kentucky, and Louisiana had higher risks of mortality.
Table 4.
Adjusted HRs and 95% CIs of Independent Variables for Overall5-Year Mortality by Cox Proportional Hazards Regression
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Demographic or clinical characteristic | |||
| Tumor location | |||
| Left-sided cancer | 1.00 | ||
| Right-sided cancer | 1.01 | 0.98 to 1.04 | .58 |
| Tumor stage | |||
| I | 1.00 | ||
| II | 0.82 | 0.75 to 0.91 | < .001 |
| III | 1.48 | 1.35 to 1.63 | < .001 |
| Age, years | |||
| 65-69 | 1.00 | ||
| 70-74 | 1.15 | 1.08 to 1.23 | < .001 |
| 75-79 | 1.33 | 1.25 to 1.41 | < .001 |
| 80-84 | 1.69 | 1.59 to 1.80 | < .001 |
| ≥ 85 | 2.24 | 2.10 to 2.38 | < .001 |
| Sex | |||
| Female | 1.00 | ||
| Male | 1.26 | 1.22 to 1.30 | < .001 |
| Ethnicity | |||
| White | 1.00 | ||
| Black | 1.05 | 0.99 to 1.12 | .11 |
| Asian or Pacific Islander | 0.79 | 0.72 to 0.86 | < .001 |
| Hispanic | 0.95 | 0.87 to 1.03 | .22 |
| Other | 0.83 | 0.64 to 1.09 | .18 |
| Marital status | |||
| Married | 1.00 | ||
| Widowed | 1.14 | 1.10 to 1.18 | < .001 |
| Single, separated, or divorced | 1.16 | 1.11 to 1.22 | < .001 |
| Unknown | 1.07 | 0.98 to 1.16 | .13 |
| SEER registry | |||
| California | 1.00 | ||
| Connecticut | 1.06 | 1.00 to 1.12 | .04 |
| Detroit | 0.95 | 0.90 to 1.00 | .04 |
| Hawaii | 1.05 | 0.92 to 1.20 | .50 |
| Iowa | 1.00 | 0.94 to 1.07 | 1.00 |
| New Mexico | 1.07 | 0.97 to 1.18 | .20 |
| Seattle | 1.04 | 0.98 to 1.10 | .24 |
| Utah | 1.02 | 0.93 to 1.12 | .67 |
| Atlanta and rural Georgia | 1.07 | 0.99 to 1.16 | .07 |
| Kentucky | 1.18 | 1.09 to 1.28 | < .001 |
| Louisiana | 1.10 | 1.02 to 1.20 | .02 |
| New Jersey | 1.04 | 0.98 to 1.10 | .15 |
| Residence location | |||
| Major metropolitan | 1.00 | ||
| Metropolitan or urban | 0.97 | 0.93 to 1.01 | .11 |
| Less urban or rural | 0.97 | 0.91 to 1.04 | .42 |
| Median household income (census tract), $ in thousands | 1.00 | 1.00 to 1.00 | .18 |
| Less than 12 years education (census tract), % | 1.00 | 1.00 to 1.00 | .02 |
| Missing census tract data | 1.11 | 1.01 to 1.22 | .02 |
| Year of diagnosis by Medicare colonoscopy coverage | |||
| 1992-1997 | 1.00 | ||
| 1998-June 2001 | 0.95 | 0.92 to 0.99 | .01 |
| July 2001-2005 | 0.89 | 0.85 to 0.92 | < .001 |
| Poor prognostic feature | |||
| Emergent admission | |||
| No | 1.00 | ||
| Yes | 1.29 | 1.25 to 1.34 | < .001 |
| Intestinal obstruction on admission | |||
| No | 1.00 | ||
| Yes | 1.15 | 1.08 to 1.22 | < .001 |
| Intestinal perforation on admission | |||
| No | 1.00 | ||
| Yes | 1.33 | 1.22 to 1.46 | < .001 |
| T stage | |||
| Tis, T0, T1, or T2 | 1.00 | ||
| T3 | 1.58 | 1.45 to 1.72 | < .001 |
| T4 | 2.36 | 2.16 to 2.59 | < .001 |
| No. of nodes resected | |||
| 0-11 | 1.00 | ||
| ≥ 12 | 0.83 | 0.80 to 0.85 | < .001 |
| Tumor grade | |||
| Well differentiated | 1.00 | ||
| Moderately differentiated | 1.05 | 0.99 to 1.10 | .10 |
| Poorly or undifferentiated | 1.26 | 1.19 to 1.34 | < .001 |
| Comorbidity and treatment measures | |||
| HCC risk score | 1.26 | 1.25 to 1.27 | < .001 |
| Any hospitalization in the previous year | |||
| No | 1.00 | ||
| Yes | 1.18 | 1.15 to 1.22 | < .001 |
| Any in-hospital surgical complication | |||
| No | 1.00 | ||
| Yes | 1.19 | 1.11 to 1.28 | < .001 |
| Postoperative adjuvant chemotherapy | |||
| None | 1.00 | ||
| Incomplete: 1-4 months | 1.20 | 1.14 to 1.26 | < .001 |
| Complete: ≥ 5 months | 0.59 | 0.56 to 0.62 | < .001 |
NOTE. Cox regression model controlling for age; sex; ethnicity; marital status; residence; Surveillance, Epidemiology, and End Results registry; year of diagnosis by Medicare colonoscopy coverage; census-tract income and eduction; Hierarchical Condition Categories score; hospitalizations per year; in-hospital complications; chemotherapy; and poor tumor prognostic features.
Abbreviations: HCC, Hierarchical Condition Categories; HR, hazard ratio; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results.
Several disease- and treatment-related variables were also predictive of increased 5-year mortality. As expected, stage was an independent predictor of mortality. However, when compared with the reference group of stage I, patients with stage II disease had a lower risk of mortality, and patients with stage III disease had a higher risk. All of the previously identified poor prognostic features significantly predicted an increased risk of mortality as well as greater patient risk quantified by the HCC risk score. Patients with any hospitalization in the previous year and any in-hospital complication had a higher mortality. With respect to treatment, patients who completed adjuvant chemotherapy had a decreased risk of mortality, and patients with incomplete courses of adjuvant chemotherapy had an increased risk. Year of diagnosis by Medicare colonoscopy coverage period was associated with mortality, for which the risk decreased as coverage increased.
DISCUSSION
Differences in clinical presentation, patient demographics, and tumor biology between right- and left-sided colon cancers have long been reported in the literature,3–10 but it is unclear whether these differences translate into clinically meaningful prognostic differences. We found that, after controlling for multiple patient, disease, risk adjustment, and treatment variables, there was no overall difference in 5-year mortality between right- and left-sided colon cancers. More important, this relationship is not consistent across tumor stage. Stage II right-sided cancers had lower mortality, and stage III right-sided cancers had higher mortality.
Our results across all stages combined differ substantially from two recent studies by Meguid et al11 and Benedix et al.12 With SEER data, Meguid et al11 reported that right-sided cancers had a higher risk of mortality than left-sided cancers across all stages (HR, 1.04; 95% CI, 1.02 to 1.07) when analysis was controlled for age, sex, ethnicity, tumor characteristics (ie, stage, size, grade), number of lymph nodes examined, and year of diagnosis. However, when their adjusted results were stratified by stage, they reported no difference in mortality between right- and left-sided cancers for stage I (HR, 1.003; P = .93) and lower mortality for right-sided stage II cancers (HR, 0.91; P < .001), which was more similar to our results. Notably, they were not able to risk adjust or control for chemotherapy administration with the data set, and their inclusion and exclusion criteria differed. They included all patients in the SEER database from 1988 to 2003 who underwent surgical resection for a primary diagnosis of invasive colon adenocarcinoma for all AJCC stages I to IV, and they excluded patients who died within 60 days of surgery. We limited our sample to AJCC stages I to III and excluded patients who died within 30 days of surgery. Benedix et al12 showed an even higher risk of mortality for right-sided cancers (odds ratio, 1.12; P = .02) compared with left-sided cancers than Meguid et al,11 but again showed conflicting results when stratified by stage. Their unadjusted analysis showed significantly shorter 5-year survival for right-sided cancer for stage I (78% v 84%; P = .01) and stage III (55% v 60%; P < .01) but not for stage II (74% v 72%). This study included approximately 17,000 German patients with colon cancer, and analysis was controlled for multiple patient- and disease-related variables, including comorbidity (but not including chemotherapy administration). Inclusion and exclusion criteria also differed from our study. Similar to the study by Meguid et al,11 Benedix et al12 included all four stages of colon cancer and did not limit patients to age 66 or older.
The reasons for the inconsistent relationship between mortality and tumor location by stage is not clear, but it is most likely related to tumor biology. One specific aspect of tumor biology that lends particular credence to our findings is microsatellite instability (MSI). Multiple studies have found that patients with MSI-positive tumors have a better overall prognosis and that MSI status is an independent favorable predictor of survival.19–23 MSI is predominantly seen in right-sided colon cancers,10 and less than 5% of left-sided cancers show MSI.7 MSI-positive tumors also have a more favorable stage profile. Jernvall et al24 estimate that 20% to 25% of stage II right-sided cancers are MSI positive and that fewer than 15% of stage III right-sided tumors have this same attribute, with even fewer in stage IV colon cancers. Additional evidence that stage III right-sided cancers may be more biologically distinct from stage II right-sided cancers is that MSI-positive status has also been associated with a significantly decreased risk of lymph node (odds ratio, 0.31; 95% CI, 0.17 to 0.56) and distant organ (odds ratio, 0.13; 95% CI 0.05 to 0.33) metastases.22
This study has several limitations. First, we examine only Medicare beneficiaries age 66 years and older at the time of diagnosis, which may limit the applicability of our findings to younger patients with colon cancer. However, the risk of colon cancer increases with age, and the average age of diagnosis of patients with nonfamilial colon cancer is older than 65 years.25 Second, unmeasured factors, such as patient preferences or provider practice patterns, may play a role in patient outcome. If these unmeasured factors are also associated with tumor location, our results could be subject to unmeasured confounding. However, we included a wide variety of clinically relevant patient, disease, risk adjustment, and treatment variables. Finally, MSI tumor status was not available in this data set, which prohibits direct testing of our hypothesis that MSI is a major contributor to the decreased mortality seen in stage II, right-sided colon cancers.
Despite these limitations, our findings have important implications different from previous studies. Prior research has reported an increase in mortality for right-sided colon cancers compared with left-sided colon cancers. Conversely, this study shows no significant difference in mortality after more extensive adjustment and limiting of the sample to a more homogeneous group of patients who have a more narrow age distribution (limited to age 66 years and older as a result of Medicare linkage) and patients considered for surgery with a curative intent (by excluding AJCC stage IV and patients undergoing palliative procedures). We also highlight that the relationship between mortality and tumor location in colon cancer is not straightforward. Specifically, this relationship is stage dependent; stage II right-sided cancers show decreased mortality than left-sided tumors, and stage III right-sided tumors show increased mortality. We hypothesize that a major reason for this inconsistent relationship between mortality and tumor location by stage is due to tumor biology and, more specifically, MSI status. Additional research needs to be done to confirm this hypothesis, which, if confirmed, may significantly impact decisions regarding treatment of patients with right-sided colon cancer. More specifically, these results can help inform the treatment decision process for patients with stage II colon cancers for whom use of adjuvant chemotherapy is controversial.
Acknowledgment
We thank Carol Weidel, SAS programmer, for her assistance in preparing the data for analysis, and Colleen Brown, research specialist, for her assistance in formatting and proofing the manuscript. We also thank the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for efforts in the creation of the SEER-Medicare database.
Footnotes
Supported by Research Grant No. T32HS000083 from the Agency for Healthcare Research and Quality National Research Award; by Grant No. 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health (to the Health Innovation Program and the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research); by Grant No. P30CA014520-34 from the National Cancer Institute, National Institutes of Health (to the University of Wisconsin Carbone Cancer Center); and by the University of Wisconsin School of Medicine and Public Health from the Wisconsin Partnership Program.
Presented at Digestive Disease Week, May 7-11, 2011, Chicago, IL.
The ideas and opinions expressed herein are those of the authors; endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended and should not be inferred.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Noelle LoConte, sanofi-aventis, Bayer Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer M. Weiss, Patrick R. Pfau,Maureen A. Smith
Financial support: Maureen A. Smith
Administrative support: Maureen A. Smith
Collection and assembly of data: Jennifer M. Weiss, Jonathan King, Maureen A. Smith
Data analysis and interpretation: Jennifer M. Weiss, Patrick R. Pfau, Erin S. O'Connor, Noelle LoConte, Gregory Kennedy, Maureen A. Smith
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 4.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: A study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41:173–177. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 5.Lanza G, Jr, Maestri I, Ballotta MR, et al. Relationship of nuclear DNA content to clinicopathologic features in colorectal cancer. Mod Pathol. 1994;7:161–165. [PubMed] [Google Scholar]
- 6.Bufill JA. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 7.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 8.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755–762. [PubMed] [Google Scholar]
- 9.Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374–384. doi: 10.1136/gut.2003.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervaz P, Bucher P, Morel P. Two colons-two cancers: Paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–266. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 11.Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–2394. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: Differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 14.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 15.Lo NS, Sarr MG. Mucinous cystadenocarcinoma of the appendix: The controversy persists—A review. Hepatogastroenterology. 2003;50:432–437. [PubMed] [Google Scholar]
- 16.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton JJ, Cai Y, Green P, et al. Trends in colorectal cancer testing among Medicare subpopulations. Am J Prev Med. 2008;35:194–202. doi: 10.1016/j.amepre.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 19.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 21.Hemminki A, Mecklin JP, Järvinen H, et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 22.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831–3839. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 23.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–923. [PubMed] [Google Scholar]
- 24.Jernvall P, Makinen MJ, Karttunen TJ, et al. Microsatellite instability: Impact on cancer progression in proximal and distal colorectal cancers. Eur J Cancer. 1999;35:197–201. doi: 10.1016/s0959-8049(98)00306-2. [DOI] [PubMed] [Google Scholar]
- 25.Patel SA, Zenilman ME. Outcomes in older people undergoing operative intervention for colorectal cancer. J Am Geriatr Soc. 2001;49:1561–1564. doi: 10.1046/j.1532-5415.2001.4911254.x. [DOI] [PubMed] [Google Scholar]