Abstract
Purpose
Outcome in acute myeloid leukemia (AML) worsens with age, at least in part because of higher treatment-related mortality (TRM) in older patients. Eligibility for intensive AML treatment protocols is therefore typically based on age as the implied principal predictor of TRM, although other health- and disease-related factors modulate this age effect.
Patients and Methods
We empirically defined TRM using estimated weekly hazard rates in 3,365 adults of all ages administered intensive chemotherapy for newly diagnosed AML. We used the area under the receiver operator characteristic curve (AUC) to quantify the relative effects of age and other covariates on TRM in a subset of 2,238 patients. In this approach, an AUC of 1.0 denotes perfect prediction, whereas an AUC of 0.5 is analogous to a coin flip.
Results
Regardless of age, risk of death declined once 4 weeks had elapsed from treatment start, suggesting that patients who die during this time comprise a qualitatively distinct group. Performance status (PS) and age were the most important individual predictors of TRM (AUCs of 0.75 and 0.65, respectively). However, multicomponent models were significantly more accurate in predicting TRM (AUC of 0.83) than PS or age alone. Elimination of age from such multicomponent models only minimally affected their predictive accuracy (AUC of 0.82).
Conclusion
These data suggest that age is primarily a surrogate for other covariates, which themselves add significantly to predictive accuracy, thus challenging the wisdom of using age as primary or sole basis for assignment of intensive, curative intent treatment in AML.
INTRODUCTION
Outcome in acute myeloid leukemia (AML) worsens with age; current 5-year survival rates are approximately 60% for patients younger than 15 years of age but only approximately 5% and 2% for patients age older than 65 and older than 75 years, respectively.1,2 Recognizing this relationship between age and outcome, contemporary AML treatment protocols for adults are typically divided into those for younger and older patients, with an arbitrary cutoff of 55 to 60 years commonly used to distinguish these two groups. Implicit in this practice is the assumption that age itself is the principal predictor of outcome of AML therapy.
Yet previous studies indicate that this age effect is related, in part, to changes in the biology of the disease that occur with age and, in part, to the tendency of older patients to present with significant comorbidities or poor performance status (PS)3; the latter increase the risk of treatment-related mortality (TRM) when intensive therapy is administered with curative intent.3 Besides age and PS, other quantifiable factors independently affecting TRM after such therapies include bilirubin, neutrophil count, fibrinogen, albumin, hemoglobin, and creatinine3,4 and probably, as composite measure, the hematopoietic cell transplantation comorbidity index.5 These data indicate that age as sole or primary criterion for allocation to intensive treatment protocols may be suboptimal and suggest that the ability to predict outcome might be improved by inclusion of additional covariates. This prompted us to quantify the value of incorporating various prognostic factors, rather than using age alone, to predict TRM after intensive chemotherapy for AML other than acute promyelocytic leukemia.
PATIENTS AND METHODS
Study Population
Our analyses included 1,127 adults treated in 10 Southwest Oncology Group (SWOG) trials from 1986 to 2009 and 2,238 adults treated on various protocols at MD Anderson Cancer Center (MDA) from 1995 to 2008. Institutional review boards of participating institutions approved all protocols, and patients were treated according to the Declaration of Helsinki.
Definitions of Outcomes
TRM was empirically defined as death within 28 days after initiation of therapy (described in Results). Designation of complete remission (CR) required achievement of a morphologic leukemia-free state (bone marrow blasts < 5%, absence of extramedullary disease) and recovery of peripheral blood counts (absolute neutrophil count > 1,000/μL, platelet count > 100,000/μL).6,7
Statistical Analysis
Overall survival, defined as time from initiation of therapy to death with observations censored at date of last contact, was estimated using the Kaplan-Meier method. Weekly hazard rates (rate of death in specific week for patients alive at beginning of week) were estimated using the life-table method8 for SWOG and MDA patient cohorts overall and by age category. Analyses for prediction models for TRM followed published methodologies.9,10 Ridge estimators for logistic regression models were calculated, with the ridge parameter selected using the effective Akaike information criterion. More parsimonious regression models were found using backward selection. We used the area under the receiver operator characteristic curve (AUC) to quantify the predictive ability of various covariates (such as age) and the regression models containing these covariates. An AUC of 1.0 indicates that a model (or covariate) is perfect at prediction, whereas an AUC of 0.5 indicates no prediction (ie, model is no better than a coin flip). The following pretreatment covariates were included in regression modeling: age at diagnosis, sex, race/ethnicity (black, Hispanic, white, other), PS, serum creatinine, bilirubin, albumin, lactate dehydrogenase (LDH), WBC count, platelet count, peripheral blood blast percentage, peripheral blood neutrophils, bone marrow blast percentage, bone marrow neutrophils, hemoglobin, fibrinogen, secondary AML, and cytogenetic risk (using SWOG/Eastern Cooperative Oncology Group criteria11). When possible, covariates were treated as numerical. Because cytogenetic information may not be available when treatment decisions are made, models were built both including and excluding cytogenetic risk as covariate. The relative importance of predictors in regression models was evaluated by the value of the partial Wald χ2 statistic minus the predictor's degrees of freedom. Bootstrapping, which has been demonstrated to be a more efficient method for model validation than cross validation or splitting data into two groups,9,10 was used to estimate bias-corrected values of AUC, and all reported AUCs are bootstrap-bias corrected. All analyses were performed using R (http://www.r-project.org).
RESULTS
Empiric Definition of TRM
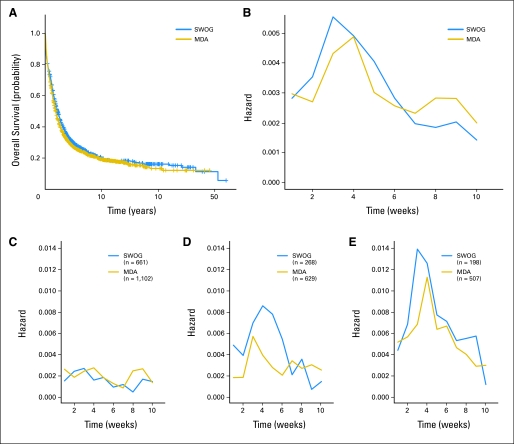
The characteristics of our study cohorts are summarized in Table 1. With the constraint that some variables were not universally collected for SWOG patients, principal differences between the SWOG and MDA cohorts were the slightly younger age and better PS of the SWOG patients and the considerably more frequent use of higher doses of cytarabine during induction therapy at MDA. Relative to SWOG patients, a greater variety of cytarabine-based regimens that contained nonanthracycline drugs (eg, fludarabine, topotecan) were used at MDA (data not shown). Nevertheless, survival in both cohorts was virtually superimposable, with maximum risk of death occurring 3 to 4 weeks after start of treatment (Figs 1A, 1B). Although the likelihood of early death increased with increasing age, this period of maximum risk was similar in patients 60 years of age and younger, 61 to 70 years of age, and 71 years of age and older (Figs 1C through 1E). We therefore defined TRM as death within 28 days of treatment initiation. Using this definition, the probability of TRM in our study population was 10.3% (SWOG, 11.1%; MDA, 9.9%).
Table 1.
Characteristics of Study Population
| Parameter | SWOG |
MDA |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 1,127 | 2,238 | ||
| Age, years* | ||||
| Median | 57 | 61 | ||
| Range | 17-88 | 14-89 | ||
| ≥ 60 | 466 | 41.3 | 1,197 | 53.5 |
| Sex | ||||
| Male | 55 | 56 | ||
| Female | 45 | 44 | ||
| Cytogenetic risk† | ||||
| Favorable | 154 | 13.7 | 179 | 8.0 |
| Intermediate | 622 | 55.2 | 1,437 | 64.2 |
| Unfavorable | 430 | 38.2 | 605 | 27.0 |
| Missing/unknown | 186 | 16.5 | 17 | 0.8 |
| WBC, ×103/μL* | ||||
| Median | NA | 6.2 | ||
| Range | NA | 0.2-433 | ||
| Hemoglobin, g/dL* | ||||
| Median | NA | 8.1 | ||
| Range | NA | 2.0-15.1 | ||
| Platelets, ×103/μL* | ||||
| Median | NA | 49 | ||
| Range | NA | 2-2,292 | ||
| Peripheral blasts, %* | ||||
| Median | NA | 17 | ||
| Range | NA | 0-99 | ||
| Bone marrow blasts, %* | ||||
| Median | NA | 46.4 | ||
| Range | NA | 0-98 | ||
| Total bilirubin, mg/dL* | ||||
| Median | NA | 0.6 | ||
| Range | NA | 0-12.9 | ||
| Creatinine, mg/dL* | ||||
| Median | NA | 0.9 | ||
| Range | NA | 0.3-6.8 | ||
| Fibrinogen, mg/dL* | ||||
| Median | NA | 429 | ||
| Range | NA | 0-1,000 | ||
| Albumin, g/dL* | ||||
| Median | NA | 3.4 | ||
| Range | NA | 0.7-5.3 | ||
| LDH, units/L* | ||||
| Median | NA | 834 | ||
| Range | NA | 15-39,240 | ||
| Performance status* | ||||
| 0 | 326 | 28.9 | 347 | 15.5 |
| 1 | 526 | 46.7 | 1,331 | 59.5 |
| 2 | 141 | 12.5 | 425 | 19.2 |
| > 2 | 55 | 4.9 | 135 | 6.0 |
| ND | 79 | 7.0 | 0 | 0 |
| Patients treated with SD cytarabine | 830 | 73.6 | 319 | 14.2 |
| Patients treated with I/HD cytarabine | 162 | 14.4 | 1,624 | 72.6 |
| Patients treated without cytarabine | 135 | 12.0 | 295 | 13.2 |
| CR with initial therapy | 499 | 39.1 | 1,263 | 56.4 |
| TRM‡ | 125 | 11.1 | 221 | 9.9 |
Abbreviations: CR, complete remission; I/HD, intermediate/high dose; LDH, lactate dehydrogenase; MDA, MD Anderson Cancer Center; NA, data not available for all patients; ND, not determined; SD, standard dose; SWOG, Southwest Oncology Group; TRM, treatment-related mortality.
Determined at time of diagnosis.
SWOG/Eastern Cooperative Oncology Group criteria used to assign cytogenetic risk.
Death within 28 days of therapy initiation.
Fig 1.
Survival analyses. (A) Kaplan-Meier survival analyses of overall survival of 1,127 patients enrolled onto Southwest Oncology Group (SWOG) trials from 1986 to 2009 and 2,238 patients treated at MD Anderson Cancer Center (MDA) from 1995 to 2009 for newly diagnosed acute myeloid leukemia (non–acute promyelocytic leukemia). (B) Plots of probability of death in specific week given that patient was alive at beginning of week (weekly hazard) for patients enrolled onto SWOG trials or treated at MDA. (C-E) Weekly hazard plots for all patients enrolled onto SWOG trials or treated at MDA, stratified by age ([C] ≤ 60, [D] 61-70, [E] > 70 years of age). Slopes of changes in weekly mortality tended to decrease after week 3 in SWOG and week 4 in MDA for patients age 60 years or younger and those older than age 70 years; for patients age 61 to 70 years, slopes tended to decrease after week 4 in SWOG and after week 3 in MDA, respectively.
Composite Models to Predict TRM
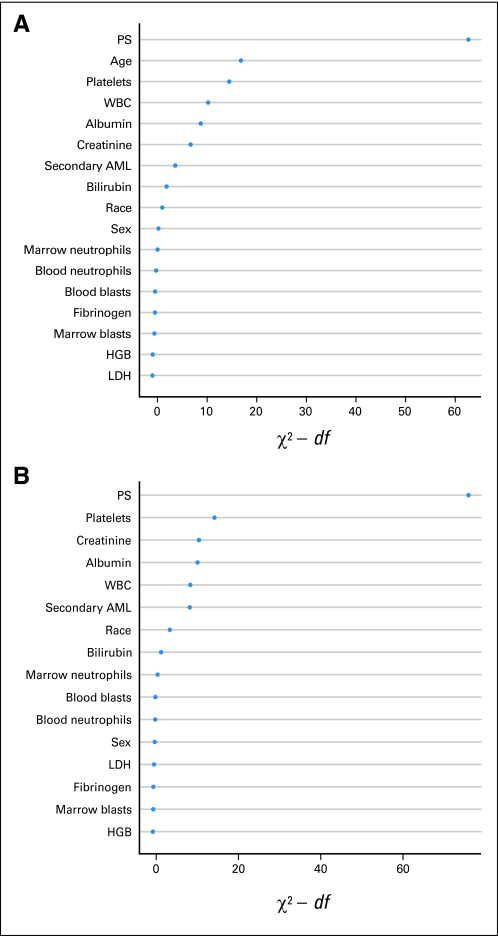
The existence of a discrete cut point after which mortality rates sharply declined after treatment initiation suggested that patients who died early were qualitatively distinct. Consequently, we examined the relative effect of age and other covariates on mortality within the first 28 days of treatment, our criterion for TRM. Because information on many of the covariates was not routinely recorded for SWOG patients, we restricted these analyses to the MDA cohort and used the AUC method9,10 to quantify the effects of individual covariates for the prediction of TRM. PS was the most important single covariate in predicting TRM (AUC for model with PS alone, 0.75; Fig 2A), followed by age (AUC for model with age alone, 0.65). A maximal model comprising all covariates investigated yielded an AUC of 0.83 (Table 2; detailed characteristics of all models provided in Appendix Tables A1, A2, online only). However, our analyses suggested that several covariates were of lesser importance (Fig 2A). Omission of such covariates led to a simplified model that included PS, age, platelet count, serum albumin, secondary AML (yes/no), WBC, peripheral blood blast percentage, and serum creatinine, yielding an AUC of 0.82. Table 2 provides an equation for calculating TRM scores using the covariates contained in this simplified model. Although various treatments were employed at MDA, inclusion of a term for these treatments (without cytarabine, with fludarabine rather than idarubicin, and so on) improved the AUC only marginally (0.83), suggesting that the model was applicable to patients receiving various types of intensive therapy. Importantly, even though age was identified as an important predictor of TRM when covariates were analyzed individually, age was relatively unimportant in the maximal and simplified models; in fact, omission of age from these models only minimally affected the AUC (AUC, 0.82 for maximal model excluding age; AUC, 0.80 for simplified model excluding age; Table 2). Of note, choosing a slightly different cutoff for the definition of TRM did not fundamentally change the predictive ability of our models; for example, choosing a 6-week cutoff, the simplified model yielded an AUC of 0.80 (compared with 0.82 for model using 4-week cutoff).
Fig 2.
Prediction of early death. Importance of individual covariates to predict treatment-related mortality (TRM) using χ2 values with (A) inclusion or (B) exclusion of age. Importance evaluated with Wald χ2 statistic minus predictor's degrees of freedom (df). Covariates with larger χ2 values considered more important in predicting TRM. Covariates listed on y-axis in order of χ2 value, with highest values at top and lowest at bottom. In both panels, performance status (PS) is most important single variable in predicting TRM. Several variables, including hemoglobin (HGB) and fibrinogen, were among least important for both models. AML, acute myeloid leukemia; LDH, lactate dehydrogenase.
Table 2.
Calculation of Maximal and Simplified TRM Scores
| Model | AUC* |
|---|---|
| Maximal† | 0.83 |
| Maximal without age | 0.82 |
| Simplified‡ | 0.82 |
| Simplified without age | 0.80 |
| Basic§ | |
| SWOG | 0.71 |
| MDA | 0.70 |
Abbreviations: AHD, antecedent hematologic disorder; AML, acute myeloid leukemia; AUC, area under receiver operator characteristic curve; LDH, lactate dehydrogenase; MDA, MD Anderson Cancer Center; PS, performance status; SWOG, Southwest Oncology Group; TRM, treatment-related mortality.
All AUCs calculated using MDA data set except for basic TRM model, which was calculated with SWOG and tested in MDA data set.
Includes covariates: age at diagnosis, sex, race/ethnicity (black, Hispanic, white, other), PS, creatinine, bilirubin, albumin, LDH, WBC, platelet count, peripheral blood blast percentage, peripheral blood neutrophils, bone marrow blast percentage, bone marrow neutrophils, hemoglobin, fibrinogen, and secondary AML (defined as documented blood count abnormality for > 1 month before diagnosis of AML [AHD] or AML after cytotoxic therapy).
Includes covariates: PS, age, platelet count, albumin, secondary AML, WBC, peripheral blood blast percentage, and creatinine. This model was used in Tables 3 and 4 with scores calculated according to the formula: 100 / (1 + e[−x]), where x = −4.08 + 0.89 × PS + 0.03 × age − 0.008 × platelets − 0.48 × albumin + 0.47 × (have secondary AML) + 0.007 × WBC − 0.007 × (peripheral blood blast percentage) + 0.34 × creatinine.
Includes covariates: PS, age, and platelet count.
Individual factors can be used to identify subgroups at high risk of experiencing TRM. For example, patients presenting with hyperleukocytosis (WBC > 100,000/μL; n = 105) had a TRM of 30%, whereas patients presenting with a PS of 4 (n = 46) had a TRM of 61%. However, exclusion of such subgroups from our analysis did not significantly change the predictive accuracy of our models (data not shown).
We next investigated the relationship between TRM rates and TRM scores as calculated with the simplified TRM model. As shown in Table 3, 20% of patients had scores between 3.91 and 6.9, and 40% of patients had scores below and 40% had scores above this interval. Although 1% of patients with TRM scores below 3.91 experienced TRM, 20% of patients with scores greater than 6.9 incurred TRM. The highest TRM probability (41%) was seen in the 10% of patients with scores above 22.8. Of particular interest were the comparative effects of age and TRM score in predicting TRM. Given that age was a component of the simplified TRM prediction model, only 20% of patients age older than 60 years had scores of 3.9 or less; conversely, only 21% of patients age older than 60 years had scores greater than 6.9. Nevertheless, in both age groups, the TRM rate was 1% for patients with scores of 3.9 or less (eight of 575 patients age ≤ 60 years and two of 218 patients age > 60 years) but approximately 18% for patients with scores greater than 6.9 (37 of 200 patients age ≤ 60 years and 117 of 662 patients age > 60 years). Qualitatively identical results were obtained when the simplified model without age was used to estimate TRM probabilities (data not shown). These findings emphasize the superiority of the TRM score over age as predictor of TRM, consistent with the higher AUCs of the simplified model or simplified model without age compared with the AUC for age alone (Table 2).
Table 3.
Relationship Between TRM Probability and TRM Score
| TRM Score Interval | All Patients (%) |
Patients Age > 60 Years (%) |
Patients Age ≤ 60 Years (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TRM Score Interval |
TRM Probability |
|||||||||
| Below | Within | Above | Below | Within | Above | Below TRM Score Interval | TRM Probability* | Above TRM Score Interval | TRM Probability† | |
| 0–1.9 | 0 | 20 | 80 | — | 1 | 12 | — | — | 67 | 10 |
| 1.91–3.9 | 20 | 20 | 60 | 1 | 2 | 16 | 8 | 0 | 39 | 15 |
| 3.91–6.9 | 40 | 20 | 40 | 1 | 7 | 20 | 20 | 1 | 21 | 18 |
| 6.91–9.2 | 60 | 10 | 30 | 3 | 7 | 24 | 42 | 2 | 14 | 26 |
| 9.21–13.1 | 70 | 10 | 20 | 4 | 12 | 31 | 55 | 4 | 9 | 35 |
| 13.1–22.8 | 80 | 10 | 10 | 5 | 20 | 41 | 70 | 6 | 5 | 53 |
| 22.81–100 | 90 | 10 | 0 | 6 | 41 | — | 85 | 9 | — | |
NOTE. Calculations based on simplified TRM model.
Abbreviation: TRM, treatment-related mortality.
If below TRM Score Interval.
If above TRM Score Interval.
Development of a Basic TRM Model
Complex, multicomponent models may be cumbersome to use in daily clinical practice. Therefore, we developed a more practical basic model using the SWOG database, which contains information readily available to the great majority of clinicians. Components of the basic model were age, PS, and platelet count (Table 4). Reflecting its simplicity, the AUC of the model was only 0.71. When tested in the MDA data set, its AUC was 0.70, again suggesting that the predictive ability of the model was not influenced by the different treatments used in our cohorts.
Table 4.
TRM Rates According to TRM Score and Age Using Basic Three-Component Prediction Model
| TRM Score | SWOG Patients (years of age)* |
MDA Patients (years of age) |
||||||
|---|---|---|---|---|---|---|---|---|
| > 60 |
≤ 60 |
> 60 |
≤ 60 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| 0–3† | 7 of 68 | 10 | 25 of 558 | 4 | 2 of 96 | 2 | 28 of 879 | 3 |
| 4–6‡ | 27 of 218 | 12 | 11 of 88 | 13 | 63 of 650 | 10 | 44 of 223 | 20 |
| ≥ 7§ | 35 of 113 | 31 | 0 | 0 | 84 of 390 | 22 | 0 | 0 |
NOTE. Calculation of score: 0 × (age < 61 years) + 2 × (age 61 to 70 years) + 4 × (age ≥ 71 years) + 0 × (PS = 0) + 2 × (PS = 1) + 4 × (PS > 1) + 0 × (platelets < 50) + 1 × (platelets ≥ 50).
Abbreviations: MDA, MD Anderson Cancer Center; PS, performance status; SWOG, Southwest Oncology Group; TRM, treatment-related mortality.
Inclusion of only 1,045 SWOG patients in Table (rather than entire SWOG cohort of 1,127 patients, as in Appendix Table A1, online only) reflects 82 patients with missing values for platelet counts. TRM rates were 10% for subset of 1,045 patients and 11% for entire SWOG cohort.
Low risk: score 0-3. Age 61 to 70 years with PS = 0; age ≤ 60 years with PS ≤ 1.
Intermediate risk: score 4-6. Age ≥ 71 years with PS = 0, platelets < 50; age 61 to 70 years with PS = 1; age 61 to 70 years with PS > 1 and platelets < 50; age ≤ 60 years with PS > 1.
High risk: score ≥ 7. Age ≥ 71 years with PS = 1 and platelets ≥ 50; age ≥ 71 years with PS > 1; age 61 to 70 years with PS > 1 and platelets ≥ 50.
DISCUSSION
Many contemporary treatment protocols for newly diagnosed AML are age specific and typically restricted to patients either younger or older an arbitrary age cutoff. Indeed, the recognition that many older adults will not tolerate intensive chemotherapy and die early as a result of excess toxicity3,12 has led to several prognostic models/scoring systems aimed to identify older patients unsuited for intensive therapy,5,12–16 an approach that implies age is the primary predictor of outcome. Although our data confirm that increasing age unequivocally increases risk of TRM, our principal finding is that age is rather an incomplete surrogate for other covariates. Age is incomplete because addition of other covariates to age materially improves predictive ability, and age is a surrogate because its removal from a complex multivariable model has only a minor effect in this regard (compare models with v without inclusion of age in Table 2). Relative contributions of age versus age plus other covariates, as quantified in the TRM scores, can also be appreciated by noting that significant proportions of patients older (younger) than age 60 are relatively unlikely (likely) to incur TRM, although the role of some covariates contributing to TRM scores is not immediately obvious.
Although a widely accepted concept, TRM has been variably defined, most often as death occurring within 28 to 30 days, but not infrequently within 60 days, of initiation of treatment. Our observation that the weekly risk of death declines sharply in adults of all ages 4 weeks after initiation of intensive induction chemotherapy lends empirical support to the 28- to 30-day criterion. The sharp decline also suggests that patients who die within 28 to 30 days comprise a distinct subgroup. Of note, a similar criterion for TRM and similar TRM rates were reported more than two decades ago.4 However, because they were older than the earlier patients, the current patients would have had higher TRM scores and been predicted to be more likely to incur TRM. Thus, the similar TRM rates suggest that changes in therapeutic regimens or supportive care have decreased the risk of TRM over time, although TRM still affects 10% of patients administered intensive therapy.
TRM probabilities could nowadays readily be computed on handheld devices. Because covariates such as those used in the simplified model can be obtained during diagnostic workup in a timely fashion, use of TRM score should be easily implementable in clinical practice; in fact, an ongoing clinical trial (NCT01342887) is employing the TRM score, calculated according to the simplified model, as a major inclusion/exclusion criterion. However, busy physicians may be unlikely to use such programs, prompting development of a basic model that incorporates only age, PS, and platelet count (Table 4). This model, which seems as simple to use as the commonly employed International Prognostic Scoring System for myelodysplasia, places patients into three groups based on estimated TRM risk. Although the basic model is less accurate as a predictive tool than the more complex models (AUC of 0.70 compared with 0.82 and 0.83 in MDA cohort), it too emphasizes the value of examining covariates other than age. For example, the basic model identified 17% of SWOG patients age 60 years or older with a low-risk TRM score (68 of 399; Table 4) and a similar TRM rate (10%) to that seen in SWOG patients younger than age 60 years with low- or intermediate-risk TRM scores (TRM rate of 6%). Similarly, TRM rates were 20% in the 20% of MDA patients age 60 years or younger with high TRM scores but only 6% in the 66% of MDA patients age older than 60 years with low- or intermediate-risk scores. These observations make treatment assignment based on age alone difficult to justify.
Besides death as a result of TRM, patients with AML experience treatment failure because of resistance to therapy, most often manifested as relapse. Conceptually, multicomponent models can be built to predict failure of therapy similar to those predicting TRM. Given that resistance is a more complex outcome, it may be intuitive that such models would be more accurate in forecasting TRM than resistance. Indeed, when defining resistance to therapy as being alive 28 days after initiation of therapy but either not achieving CR or experiencing relapse within 1 year of achieving CR, the single most important individual covariates—namely, cytogenetic risk and age—only yielded AUCs of 0.65 and 0.59, respectively (data not shown). Of note, even multicomponent models only yielded an AUC of 0.72 (ie, predictive ability closer to coin flip than certainty; data not shown). Again, however, removal of age as covariate from such models had no effect on their predictive ability. Although likely to change with the incorporation of additional, recently identified genetic and molecular abnormalities as covariates, this limited ability to predict resistance to therapy based on pretreatment data argues for the importance of post-treatment data (eg, early disease clearance or assessment of minimal residual disease17–20) in assessing risk of resistance. Equally as important, our inability to identify many of the covariates that lead to resistance argues for the importance of randomization.21
Because our study cohorts exclusively consisted of newly diagnosed patients with AML undergoing treatment with curative intent, our results can only be applied to such patients. Furthermore, our models were derived from retrospective data; however, although testing such predictive models in prospective studies is feasible for patients felt to be at low risk of TRM, the prospective validation of such models for patients considered at high risk of TRM may be unethical. Within these constraints, we believe our results have important clinical consequences. For example, a recent randomized phase III trial indicated that daunorubicin at the previously infrequently used dose of 90 mg/m2 improves survival in patients 60 to 65 years of age compared with 45 mg/m2.22 But what if the patient is between 66 or 70 years of age or even older? Although the risk of TRM in many such patients might be too high to warrant use of 90 mg/m2, our results demonstrate that this is not the case in all such patients. Indeed, our results provide a method to estimate these patients' risk of TRM and may allow the identification of subgroups of patients who might be good candidates for intensive therapy, despite advanced age. Similar considerations apply to younger patients as well. For example, trials randomly assigning primarily younger patients to high-dose cytarabine with or without cloretazine have found that the more intense combination therapy was associated with shorter survival consequent to high TRM, despite higher CR rates.23 However, it is plausible that use of models to exclude patients at high risk of TRM despite younger age might have led to improved survival with the combination regimen. Obviously, treatment intensity needs to be weighed against likelihood of therapeutic failure, and a predictably higher risk of TRM might be more acceptable in a patient with favorable-risk cytogenetics or normal karyotype than in a patient of similar age with monosomal karyotype.
The considerations presented herein may seem intuitive, but assignment to intensive AML treatment (whether on protocol or off) primarily based on age is commonplace; indeed, this observation motivated our studies. Although assigning patients to curative intent therapy solely according to age avoids complexity, our data lead us to doubt whether this objective justifies the resultant loss in prognostic information. Instead of assigning treatment based on arbitrary age cutoffs, we believe it is preferable to include all adults and assign them to treatment according to predicted risk of TRM and, possibly, resistance. Regardless of age, patients with low TRM scores might indeed be candidates for intensive standard or investigational therapy; conversely, regardless of age, patients with high TRM scores might be more suitable candidates for lower-intensity regimens. The exact TRM cutoff to be used can be tailored based on an acceptable level of TRM (Table 3). Consistent with the tenets of personalized medicine, age would only be used in the context of the biologic effects of aging (eg, organ dysfunction, unfavorable cytogenetic/molecular abnormalities). More fundamentally, the observation that elimination of age as a covariate has minimal effect on the models in Table 2 suggests that age is largely a surrogate for the other covariates examined in the model. This possibility implicitly underlies the development of various comorbidity indices, which to date have found primary application in patients considered candidates for allogeneic hematopoietic cell transplantation.24 As in other areas of medicine, it seems likely that more such covariates relevant to TRM or resistance will be discovered in the future.
Appendix
Table A1.
Maximal Model
| Covariate | With Age |
Without Age |
||||
|---|---|---|---|---|---|---|
| Coefficient | SE | 95% CI | Coefficient | SE | 95% CI | |
| Intercept | −4.782 | 0.244 | −5.260 to −4.303 | −3.359 | 0.232 | −3.813 to −2.905 |
| Age | 0.031 | 0.002 | 0.028 to 0.034 | |||
| PS | 0.869 | 0.033 | 0.794 to 0.925 | 0.930 | 0.033 | 0.866 to 0.995 |
| Secondary AML | 0.409 | 0.050 | 0.311 to 0.507 | 0.570 | 0.049 | 0.474 to 0.665 |
| Platelets | −0.009 | 3 × 10−4 | −0.009 to −0.008 | −0.008 | 3 × 10−4 | −0.009 to −0.008 |
| Albumin | −0.496 | 0.042 | −0.579 to −0.413 | −0.517 | 0.042 | −0.600 to −0.434 |
| Creatinine | 0.365 | 0.050 | 0.267 to 0.462 | 0.429 | 0.049 | 0.332 to 0.526 |
| Bilirubin | 0.151 | 0.033 | 0.86 to 0.216 | 0.135 | 0.033 | 0.070 to 0.200 |
| WBC | 0.006 | 0.001 | 0.005 to 0.008 | 0.006 | 0.001 | 0.005 to 0.007 |
| LDH | −7 × 10−7 | 1 × 10−5 | −2 × 10−5 to 2 × 10−5 | −3 × 10−5 | 1 × 10−5 | −5 × 10−5 to −6 × 10−6 |
| HGB | −0.009 | 0.014 | −0.037 to 0.018 | 0.023 | 0.014 | −0.004 to 0.050 |
| Sex | ||||||
| Female | Ref | |||||
| Male | −0.202 | 0.049 | −0.298 to −0.106 | −0.145 | 0.049 | −0.241 to −0.049 |
| Peripheral blood blast percentage | −0.003 | 0.001 | −0.005 to −0.001 | −0.004 | 0.001 | −0.006 to −0.002 |
| Peripheral blood neutrophils | 0.004 | 0.001 | 0.001 to 0.007 | 0.004 | 0.001 | 0.001 to 0.007 |
| Bone marrow blast percentage | −0.003 | 0.001 | −0.006 to −5 × 10−4 | −0.003 | 0.001 | −0.005 to 9 × 10−5 |
| Bone marrow neutrophils | 0.013 | 0.004 | 0.006 to 0.021 | 0.015 | 0.004 | 0.007 to 0.022 |
| Fibrinogen | 4 × 10−4 | 1 × 10−4 | 1 × 10−4 to 6 × 10−4 | 3 × 10−4 | 1 × 10−4 | −1 × 10−5 to 6 × 10−4 |
| Race | ||||||
| Hispanic | 0.553 | 0.111 | 0.337 to 0.770 | 0.457 | 0.110 | 0.241 to 0.674 |
| Other | 0.955 | 0.164 | 0.632 to 1.277 | 1.039 | 0.164 | 0.717 to 1.361 |
| White | 0.634 | 0.089 | 0.460 to 0.808 | 0.770 | 0.008 | 0.597 to 0.944 |
| Black | Ref | |||||
NOTE. TRM score calculated as: TRM score = 100 / (1 + e[−x]), where x = intercept + coefficient1 × covariate1 + coefficient2 × covariate2 + coefficient3 × covariate3 + etc.
Abbreviations: AML, acute myeloid leukemia; HGB, hemoglobin; LDH, lactate dehydrogenase; PS, performance status; Ref, reference; TRM, treatment-related mortality.
Table A2.
Simplified Model
| Covariate | With Age |
Without Age |
||||
|---|---|---|---|---|---|---|
| Coefficient | SE | 95% CI | Coefficient | SE | 95% CI | |
| Intercept | −4.08 | 0.053 | −4.185 to −3.976 | −2.625 | 0.052 | −2.727 to −2.523 |
| Age | 0.031 | 5 × 10−5 | 0.030 to 0.032 | |||
| PS | 0.890 | 0.009 | 0.872 to 0.909 | 0.999 | 0.009 | 0.981 to 1.018 |
| Secondary AML | 0.473 | 0.014 | 0.446 to 0.500 | 0.658 | 0.014 | 0.631 to 0.685 |
| Platelets | −0.008 | 9 × 10−5 | −0.009 to −0.008 | −0.008 | 9 × 10−5 | −0.008 to −0.008 |
| Albumin | −0.483 | 0.012 | −0.506 to −0.461 | −0.508 | 0.012 | −0.532 to −0.485 |
| 3Creatinine | 0.341 | 0.013 | 0.315 to 0.367 | |||
| WBC | 0.008 | 2 × 10−4 | 0.006 to 0.007 | 0.007 | 2 × 10−4 | 0.006 to 0.007 |
| Peripheral blood blast percentage | −0.007 | 3 × 10−4 | −0.008 to −0.007 | |||
| Race | ||||||
| Hispanic | 0.440 | 0.032 | 0.377 to 0.503 | |||
| Other | 1.047 | 0.048 | 0.954 to 1.141 | |||
| White | 0.755 | 0.026 | 0.705 to 0.805 | |||
| Black | Ref | |||||
NOTE. TRM score calculated as: TRM score = 100 / (1 + e[−x]), where x = intercept + coefficient1 × covariate1 + coefficient2 × covariate2 + coefficient3 × covariate3 + etc.
Abbreviations: AML, acute myeloid leukemia; PS, performance status; Ref, reference; TRM, treatment-related mortality.
Footnotes
Supported by Grants No. P30-CA15704-35S6 (R.B.W.) and R01-CA090998-09 (M.O.) from the National Cancer Institute/National Institutes of Health.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, December 4-7, 2010, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Roland B. Walter, Elihu H. Estey
Financial support: Roland B. Walter
Provision of study materials or patients: Gautam Borthakur, Farhad Ravandi, Jorge E. Cortes, Frederick R. Appelbaum, Hagop A. Kantarjian, Elihu H. Estey
Collection and assembly of data: Roland B. Walter, Megan Othus, Jorge E. Cortes, Sherry A. Pierce, Elihu H. Estey
Data analysis and interpretation: Roland B. Walter, Megan Othus, Gautam Borthakur, Farhad Ravandi, Jorge E. Cortes, Frederick R. Appelbaum, Hagop A. Kantarjian, Elihu H. Estey
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2010. Childhood Cancer by the ICCC: SEER Cancer Statistics Review, 1975-2007. http://seer.cancer.gov/csr/1975_2007. [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2010. Leukemia: SEER Cancer Statistics Review, 1975-2007. http://seer.cancer.gov/csr/1975_2007. [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estey E, Smith TL, Keating MJ, et al. Prediction of survival during induction therapy in patients with newly diagnosed acute myeloblastic leukemia. Leukemia. 1989;3:257–263. [PubMed] [Google Scholar]
- 5.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 6.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Collett D. ed 2. Boca Raton, FL: Chapman and Hall/CRC; 2003. Modelling Survival Data in Medical Research. [Google Scholar]
- 9.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE, Jr, Margolis PA, Gove S, et al. Development of a clinical prediction model for an ordinal outcome: The World Health Organization multicentre study of clinical signs and etiological agents of pneumonia, sepsis and meningitis in young infants WHO/ARI young infant multicentre study group. Stat Med. 1998;17:909–944. doi: 10.1002/(sici)1097-0258(19980430)17:8<909::aid-sim753>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 14.Malfuson JV, Etienne A, Turlure P, et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93:1806–1813. doi: 10.3324/haematol.13309. [DOI] [PubMed] [Google Scholar]
- 15.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 16.Krug U, Röllig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A web-based application for prediction of outcomes. Lancet. 2010;376:2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 17.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial: United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 18.Haferlach T, Kern W, Schoch C, et al. A new prognostic score for patients with acute myeloid leukemia based on cytogenetics and early blast clearance in trials of the German AML Cooperative Group. Haematologica. 2004;89:408–418. [PubMed] [Google Scholar]
- 19.Elliott MA, Litzow MR, Letendre LL, et al. Early peripheral blood blast clearance during induction chemotherapy for acute myeloid leukemia predicts superior relapse-free survival. Blood. 2007;110:4172–4174. doi: 10.1182/blood-2007-07-104091. [DOI] [PubMed] [Google Scholar]
- 20.Shook D, Coustan-Smith E, Ribeiro RC, et al. Minimal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma. 2009;9(suppl 3):S281–S285. doi: 10.3816/CLM.2009.s.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter RB, Appelbaum FR, Tallman MS, et al. Shortcomings in the clinical evaluation of new drugs: Acute myeloid leukemia as paradigm. Blood. 2010;116:2420–2428. doi: 10.1182/blood-2010-05-285387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 23.Giles F, Vey N, DeAngelo D, et al. Phase 3 randomized, placebo-controlled, double-blind study of high-dose continuous infusion cytarabine alone or with laromustine (VNP40101M) in patients with acute myeloid leukemia in first relapse. Blood. 2009;114:4027–4033. doi: 10.1182/blood-2009-06-229351. [DOI] [PubMed] [Google Scholar]
- 24.Sorror ML. Comorbidities and hematopoietic cell transplantation outcomes. Hematology Am Soc Hematol Educ Program. 2010;2010:237–247. doi: 10.1182/asheducation-2010.1.237. [DOI] [PubMed] [Google Scholar]