Abstract
Although antiestrogen therapies targeting estrogen receptor (ER) α signaling prevent disease recurrence in the majority of patients with hormone-dependent breast cancer, a significant fraction of patients exhibit de novo or acquired resistance. Currently, the only accepted mechanism linked with endocrine resistance is amplification or overexpression of the ERBB2 (human epidermal growth factor receptor 2 [HER2]) proto-oncogene. Experimental and clinical evidence suggests that hyperactivation of the phosphatidylinositol 3-kinase (PI3K) pathway, the most frequently mutated pathway in breast cancer, promotes antiestrogen resistance. PI3K is a major signaling hub downstream of HER2 and other receptor tyrosine kinases. PI3K activates several molecules involved in cell-cycle progression and survival, and in ER-positive breast cancer cells, it promotes estrogen-dependent and -independent ER transcriptional activity. Preclinical tumor models of antiestrogen-resistant breast cancer often remain sensitive to estrogens and PI3K inhibition, suggesting that simultaneous targeting of the PI3K and ER pathways may be most effective. Herein, we review alterations in the PI3K pathway associated with resistance to endocrine therapy, the state of clinical development of PI3K inhibitors, and strategies for the clinical investigation of such drugs in hormone receptor–positive breast cancer.
INTRODUCTION
Approximately 75% of breast cancers express estrogen receptor (ER) α and/or progesterone receptor (PR). Hormone receptor expression typically indicates a degree of estrogen dependence for cancer cell growth. Treatment for these patients inhibits ER function either by antagonizing ligand binding to ER (tamoxifen and other selective ER modulators [SERMs]), downregulating ER (fulvestrant), or blocking estrogen biosynthesis (aromatase inhibitors [AIs]). Although endocrine therapies have changed the natural history of hormone-dependent breast cancer, many tumors exhibit de novo or acquired resistance. For example, more than 30% of patients with early ER-positive breast cancer relapse within 15 years after adjuvant therapy with tamoxifen, and 17% of patients treated with an AI relapse within 9 years.1,2 An accepted mechanism of resistance to endocrine therapy involves overexpression of the ERBB2 (HER2) proto-oncogene.3–5 However, less than 10% of ER-positive breast cancers overexpress HER2, suggesting that for most ER-positive breast cancers, mechanisms of escape from endocrine therapy remain to be elucidated.
Tamoxifen has been a standard treatment for ER-positive breast cancer for more than 30 years. This SERM has dual agonistic/antagonistic effects on ER transcription and breast cancer cell growth.6,7 In contrast, AIs induce estrogen deprivation without agonistic effects on ER. AIs are clinically equivalent if not modestly superior to tamoxifen.2,8 Data on the clinical activity of fulvestrant as first-line therapy in the metastatic setting are limited but suggest it may be superior to AIs despite its inability to completely downregulate ER in patients' tumors.9–11 It is unclear whether mechanisms of antiestrogen resistance are common to SERMs, AIs, and ER downregulators. Studies in human cell lines and xenografts have shown that growth factor receptor signaling pathways, particularly those that converge on phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK/ERK), can mediate resistance to all forms of endocrine therapy. PI3K is the most frequently altered pathway in breast cancer, with mutation and/or amplification of the genes encoding the PI3K catalytic subunits p110α (PIK3CA) and p110β (PIK3CB); the PI3K regulatory subunit p85α (PIK3R1); receptor tyrosine kinases (RTKs) such as HER2 (ERBB2) and fibroblast growth factor receptor 1 (FGFR1); K-Ras; PI3K effectors AKT1, AKT2, and PDK1; and loss of the lipid phosphatases phosphatase and tensin homolog (PTEN) and INPP4B (Table 1).
Table 1.
PI3K Pathway Alterations Associated With Response to Endocrine Therapy
| Gene | Protein | Aberration | Effect on Signaling | Frequency | Patient Prognosis | Reference |
|---|---|---|---|---|---|---|
| ERBB2 | HER2 | Gene amplification or overexpression | Hyperactivation of ErbB2 signaling (PI3K, MEK) | Approximately 10% of ER-positive tumors | Correlates with worse outcome | Ellis et al,3 Arpino et al,4 De Laurentiis et al5 |
| PTEN | Loss-of-function mutation or reduced expression | Hyperactivation of PI3K signaling | 37% to 44% of ER-positive tumors | No consistent correlation | Pérez-Tenorio et al,12 Saal et al,13 Shoman et al14 | |
| PIK3CA | p110α, PI3K | Activating mutation | Hyperactivation of PI3K signaling | 28% to 47% of ER-positive tumors | No correlation or correlation with better outcome | Pérez-Tenorio et al,12 Baselga et al,15 Stemke-Hale et al,16 Ellis et al,17 Campbell et al18 |
| PIK3CB | p110β, PI3K | Amplification | Unknown | 5% of all cases | Unknown | Crowder et al19 |
| IGF1R, INSR | IGF-1R, InsR | Receptor activation | Activates IGF-1R/InsR signaling (PI3K, MEK) | 48% of ER-positive tumors | Unknown | Law et al20 |
| FGFR1 | Amplification | Hyperactivation of FGFR signaling (PI3K, MEK) | 11.6% of ER-positive tumors | Correlates with shorter RFS | Turner et al21 | |
| RPS6K1 | p70S6K | Amplification | Activates mTORC1, protein translation | 8.8% to 12.5% of all tumors | Unknown | Monni et al22 |
| INPP4B | Reduced expression or genomic loss | Hyperactivation of PI3K signaling | 8.4% to 37.7% of ER-positive tumors | Correlates with worse outcome | Gewinner et al,23 Fedele et al24 | |
| PIK3R1 | p85α, PI3K | Inactivating mutation | Derepression of catalytic activity of p110α | 2% of all tumors | Unknown | Jaiswal et al25 |
| AKT1 | Activating mutation | Hyperactivation of AKT signaling | 2.6% to 3.8% of ER-positive tumors | Unknown | Stemke-Hale et al,16 Loi et al,26 Carpten et al27 | |
| AKT2 | Amplification | Hyperactivation of AKT signaling | 2.8% of all tumors | Unknown | Bellacosa et al28 | |
| EGFR | Amplification | Hyperactivation of EGFR signaling (PI3K, MEK) | 0.5% of ER-positive tumors | Unknown | Al-Kuraya et al29 | |
| PDK1 | Amplification or overexpression | Hyperactivation of PDK1 signaling (AKT, mTORC1) | 21% of all tumors | Unknown | Maurer et al30 | |
| KRAS | Activating mutation | Hyperactivation of Ras signaling | 4% to 6% of all tumors | Unknown | Rochlitz et al,31 Di Nicolantonio et al,32 |
Abbreviations: EGFR, epidermal growth factor receptor; ER, estrogen receptor; FGFR, fibroblast growth factor receptor 1; HER2, human epidermal growth factor receptor 2; IGF-1R, insulin-like growth factor-1 receptor; InsR, insulin receptor; mTORC1, mammalian target of rapamycin complex 1; PI3K, phosphatidylinositol 3-kinase; RFS, relapse-free survival.
CROSSTALK BETWEEN ER AND PI3K PATHWAYS
PI3K is activated by growth factor RTKs and G-protein–coupled receptors (GPCRs). PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to produce phosphatidylinositol 3,4,5-trisphosphate (PIP3).33 In turn, PIP3 recruits several pleckstrin homology (PH) domain–containing proteins to the plasma membrane such as PDK1, serine/threonine-protein kinase, and AKT, which on activation drive cell-cycle progression and survival.34,35 Negative regulation of this pathway is conferred by PTEN and INPP4B, which dephosphorylate PIP3 and PIP2, respectively.23,36 AKT activates the mammalian target of rapamycin (mTOR) –containing complex 1 (TORC1),37 which regulates protein synthesis. mTOR is also part of another complex (ie, TORC2), which lies upstream of AKT.38
In addition to its pro-survival and growth-promoting roles, the PI3K pathway interacts with ER directly and indirectly (Fig 1). ER phosphorylation at Ser167 by AKT or p70S6K increases estrogen-induced, tamoxifen-induced, and ligand-independent ER transcriptional activity.39,40 Additionally, PI3K and Ras promote c-Jun phosphorylation.41–45 c-Jun complexes with c-Fos to form the AP-1 complex, which cooperates with ER transcription.46–48 Other oncogenic kinase pathways (eg, MAPK, protein kinase C) also contribute to the modulation of ER and transcription cofactors.49
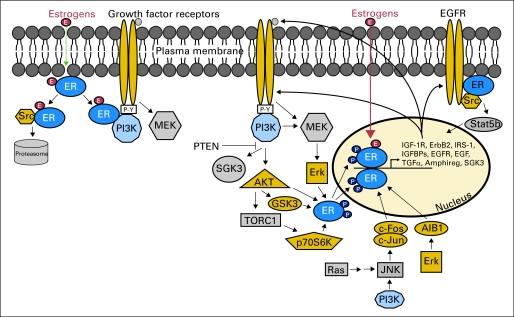
Fig 1.
Reciprocal crosstalk between estrogen receptor (ER) α and growth factor receptor signaling pathways. Receptor tyrosine kinases (RTKs) and G-protein–coupled receptors activate phosphatidylinositol 3-kinase (PI3K; blue) and MEK signaling pathways. These signal transducers can then phosphorylate ER (green) and/or coactivators and corepressors to modulate ER transcriptional activity not necessarily dependent on ER ligands. In turn, ER transcribes genes encoding components of growth factor signaling pathways, thus completing signaling cycle of RTKs to ER to RTKs. ER also complexes with RTKs and Src to rapidly induce nongenomic signaling. ER-interacting proteins shown in color. EGFR, epidermal growth factor receptor; IGF-1R, insulin-like growth factor-1 receptor; IGFBP, insulin-like growth factor binding protein; IRS-1, insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; PTEN, phosphatase and tensin homolog; SGK3, serum/glucocorticoid-regulated kinase 3; TGFα, transforming growth factor alpha; TORC1, target of rapamycin complex 1.
The activation of ER by growth factor RTK signaling is reciprocated in a feed-forward fashion, whereby ER promotes the transcription of genes encoding ligands, RTKs, and signaling adaptors (Fig 1). Clinical evidence also suggests that ER function may sustain PI3K pathway activation in breast cancer cells. Neoadjuvant treatment of patients with ER-positive breast cancer with the AI letrozole reduces P-AKTS473 and P-mTORS2448 tumor levels (markers of PI3K activity), which correlates with clinical response and improved disease outcome.50 Another study detected a reduction in P-S6 levels (marker of TORC1 activity) after neoadjuvant letrozole.15 Hence, estrogen deprivation may suppress ER-positive breast cancer cell growth in part by decreasing PI3K/AKT/mTOR signaling.
Experimentally, PI3K pathway activation has been causally associated with de novo and acquired resistance to endocrine therapy. RNAi-mediated knockdown of PTEN and overexpression of oncogenes that activate PI3K/AKT signaling (eg, HER2, type 1 insulin-like growth factor receptor (IGF1R), activated mutant AKT1) confer resistance to tamoxifen, fulvestrant, and estrogen deprivation in ER-positive breast cancer cells. In most such models, inhibition of PI3K has reversed antiestrogen resistance.7,39,51 In another example, tamoxifen-resistant ER-positive MCF-7 breast cancer xenografts exhibited increased expression of IGF-1R, HER2, and epidermal growth factor receptor (EGFR).52 One model of resistance to estrogen deprivation employs overexpression of aromatase in MCF-7 cells followed by selection with an AI. AI-resistant MCF-7/aromatase cells and xenografts have exhibited higher levels of HER2 and the EGFR ligand amphiregulin along with lower levels of ER.53,54 We reported that four of four long-term estrogen-deprived (LTED) ER-positive breast cancer cell lines exhibited amplification of PI3K/AKT/mTOR signaling, and three of four lines showed hyperactivation of IGF-1R and/or InsR.55 Chronic exposure of MCF-7 cells and xenografts to fulvestrant has been shown to elicit similar changes.56–58
Emerging evidence also implicates nongenomic, estrogen-induced signaling in the activation of PI3K. Estrogen stimulation rapidly initiates intracellular kinase signaling, including activation of IGF-1R/InsR, EGFR, Src, PI3K, and MEK (Fig 1).51,59–61 This implies that a non-nuclear ER binds estrogen and transmits signals to membrane and cytosolic kinases. Several membrane ERs have been identified, including ERα, splice variants of ERα (ER-36, ER-46), ERβ, and GPCR 30.62–64 Although ERα localizes to the plasma membrane in cultured cells, this has not been demonstrated in human tumors.65 Membrane ER might activate oncogenic kinases to promote endocrine resistance; however, these mechanisms remain to be shown clinically.
PI3K pathway activation is required for growth of breast cancer cells resistant to endocrine therapy. Growth of four of four LTED cell lines in the absence of estrogen is inhibited by treatment with the PI3K/mTOR inhibitor BEZ235 or the TORC1 inhibitor everolimus.55 Treatment of mice bearing estrogen-independent MCF-7 xenografts with the pan-PI3K inhibitor BKM120, or bearing letrozole-resistant MCF-7/aromatase xenografts with wortmannin, has been shown to slow tumor growth (unpublished data).66,67 Also, treatment of MCF-7 and MCF-7/LTED cells with the Ras inhibitor farnesylthiosalicylic acid decreases mTOR signaling and hormone-independent growth.68 Interestingly, a recent study showed that exogenous estrogen prevents the apoptotic effects of BEZ235 and RNAi-mediated silencing of PI3K in ER-positive cells.19 Most breast cancers that adapt to antiestrogen therapy retain ER and, presumably, estrogen sensitivity. These data imply that treatment of patients harboring ER-positive breast cancers with a PI3K-targeted therapy alone (without endocrine agent) might be insufficient to maximally inhibit tumor growth.
ASSOCIATION OF PI3K HYPERACTIVATION WITH RESISTANCE TO ENDOCRINE THERAPY
Gain-of-function oncogenes and/or loss of tumor suppressors in breast cancer cells may confer antiestrogen resistance via activation of PI3K. For example, HER2 overexpression predicts weaker response to neoadjuvant AIs or tamoxifen and worse outcome after adjuvant endocrine therapy compared with ER-positive/HER2-negative breast cancers.3–5 Patients with FGFR1-overexpressing ER-positive tumors exhibit a shorter relapse-free survival (RFS) after adjuvant tamoxifen.21 Patients with ER-positive/INPP4B-deficient tumors show worse survival compared with patients with ER-positive/INPP4B-positive tumors.23 Although loss-of-function mutations in PTEN are rare in ER-positive breast cancer, immunohistochemical (IHC) studies have reported a wide range of PTEN loss but found no association of PTEN level with outcome after tamoxifen therapy. Whether other mutations in the PI3K pathway correlate with antiestrogen resistance remains to be determined.
Point mutations in PIK3CA, the gene encoding the p110α catalytic subunit of PI3K, are the most common genetic alterations of this pathway in breast cancer. Up to 80% of PIK3CA mutations occur in hotspots within the helical (E542K and E545K) and kinase (H1047R) domains of p110α. They increase PI3K activity, induce cellular transformation in vitro and tumorigenicity in vivo when overexpressed in human mammary epithelial cells, and induce mammary tumor formation in transgenic mice.69–72 Although such mutations occur in 28% to 47% of ER-positive breast cancers, their clinical significance remains unclear. In retrospective studies, PIK3CA mutations in primary ER-positive tumors have correlated with good long-term outcome.12,15–18,26 In one report, PIK3CA mutations were linked with lower activation of PI3K (assessed by P-AKT) compared with PTEN deficiency in breast tumors.16 In another study, a PIK3CA-mutant gene expression signature was associated with low P-AKT, low TORC1 signaling, and good outcome after adjuvant tamoxifen.26 This association of PIK3CA-mutant status and good prognosis in ER-positive disease does not negate the possibility that combinations of antiestrogens and PI3K pathway inhibitors would be clinically more effective than antiestrogens alone in such tumors. Indeed, a recent analysis of a cohort of 217 patients with multiple solid tumor types enrolled onto phase I trials with PI3K/AKT/mTOR inhibitors showed a higher response rate among patients with PIK3CA-mutant versus PIK3CA–wild-type cancers.73
Understanding the relationship between PIK3CA mutations and endocrine resistance may be confounded by evidence suggesting that these genetic alterations may arise late in tumor development. For example, PIK3CA mutation status is discordant between invasive carcinoma and ductal carcinoma in situ in 33% of patient cases, between primary breast tumors and synchronous lymph node metastases in 13% of patient cases, between primary tumors and asynchronous metastases in 18% to 33% of patient cases, and even within microdissected regions of the same tumor.74–76
In addition to mutational analyses, tumor protein and gene expression profiling of PI3K pathway activation may provide a biomarker to identify patients with antiestrogen-resistant tumors. For example, a gene expression signature of PTEN loss, derived from a comparison of PTEN-positive and -negative tumors by IHC, was predictive of poor RFS after tamoxifen, whereas PTEN IHC status alone was not.13 A gene expression signature of PI3K activation, based on levels of phosphoprotein markers (eg, P-AKT, P-p70S6K) in ER-positive tumors, was enriched in luminal B breast cancers.77 This suggests that luminal B tumors have higher PI3K activity, which may contribute to their lower response to antiestrogens compared with luminal A tumors.78 Similarly, we identified a tumor protein signature of PI3K pathway activation that predicts poor outcome after adjuvant endocrine therapy.55 Therefore, signatures of PI3K activation may complement mutational analyses for the identification of PI3K-driven tumors.
RATIONALE FOR COMBINED USE OF ER AND PI3K INHIBITORS
One of the first PI3K pathway–targeted drugs to be tested in combination with endocrine therapy was the TORC1 inhibitor everolimus. Patients with ER-positive tumors were randomly assigned to neoadjuvant letrozole with or without everolimus for 4 months before surgery. The combination therapy induced a higher clinical response and greater suppression of tumor cell proliferation compared with letrozole alone.15 In another study, patients with advanced disease who had progressed while receiving an AI were randomly assigned to tamoxifen with or without everolimus. Patients receiving tamoxifen plus everolimus showed a significantly improved rate of clinical benefit, time to progression, and disease-free survival compared with women receiving tamoxifen alone.79
Although combinations containing TORC1 inhibitors have shown clinical activity in breast cancer, inhibition of TORC1 relieves negative feedback on activators of PI3K (eg, IGF-1R, IRS-1, HER3), which in turn promote cell survival.80–82 These results suggest that direct inhibitors of PI3K may be more effective. However, inhibition of PI3K or AKT also results in feedback upregulation/activation of several RTKs, which, by providing an upstream input to PI3K, may counteract drug action.83–86 These data suggest that PI3K/AKT/TORC1 inhibitors should be combined with RTK inhibitors to induce an optimal antitumor effect. Consistent with this notion, studies with human xenografts have shown that combinations of inhibitors targeting HER2 and PI3K, HER2 and AKT, HER2 and TORC1, or EGFR and AKT are each superior to single-agent treatments.80,83–85
Additional rationales for combined inhibition of PI3K and ER come from studies in HER2-positive breast cancer. HER2 overexpression, a potent activator of PI3K, confers endocrine resistance.87–90 Preclinical and clinical data suggest that ER-positive breast cancer cells initially inhibited by tamoxifen or estrogen deprivation can upregulate HER2 to bypass ER blockade.88,91 The interdependence of these pathways is highlighted by examples in which HER2 inhibition with trastuzumab or the tyrosine kinase inhibitor lapatinib restores or upregulates ER levels or transcriptional activity in breast cancer cells and patient tumors.92,93 Furthermore, treatment with AIs or downregulation of ER with fulvestrant or RNAi has inhibited the growth of HER2-positive tumors or cells that had progressed with trastuzumab or lapatinib.92,93 These data suggest that combined inhibition of ER and HER2 signaling may provide more effective control of ER-positive/HER2-positive tumors. This inference is supported by results from two clinical trials. In the TAnDEM (Trastuzumab in Dual Human Epidermal Growth Factor Receptor Type 2 [HER2] ER + Metastatic Breast Cancer) study, 207 patients with ER-positive/HER2-positive metastatic breast cancer were randomly assigned to the AI anastrozole with or without trastuzumab. A second study randomly assigned 219 patients with ER-positive/HER2-positive metastatic breast cancer to letrozole with or without lapatinib. In both trials, progression-free survival and clinical benefit were superior in the combination arm compared with the AI-alone arm, suggesting that both HER2 and ER should be simultaneously targeted for maximal therapeutic efficacy.94,95 Whether combined inhibition of PI3K and ER is equally effective against ER-positive/HER2-positive tumors remains to be determined.
PI3K PATHWAY INHIBITORS IN CLINICAL DEVELOPMENT
Several drugs targeting multiple levels of the PI3K network (ie, PI3K, AKT, mTOR) are in clinical development. Class IA PI3K isoforms are heterodimeric lipid kinases that contain a p110 catalytic subunit and p85 regulatory subunit. Three genes (ie, PIK3CA, PIK3CB, and PIK3CD) encode the homologous p110α, p110β, and p110δ isozymes, respectively.96,97 p110δ expression is largely restricted to immune and hematopoietic cells, whereas p110α and p110β are ubiquitously expressed.98 p110α is essential for signaling and growth of tumors driven by PIK3CA mutations, RTKs, and/or mutant Ras, whereas p110β lies downstream of GPCRs and has been shown to mediate tumorigenesis in PTEN-deficient cells.99 A number of ATP-mimetics that bind competitively and reversibly to the ATP-binding pocket of p110 are in early clinical development.100,101 These include the pan-PI3K inhibitors BKM120, XL-147, PX-866, PKI-587, and GDC-0941; p110α-specific inhibitors BYL719, GDC-0032, and INK-1117; p110δ-specific inhibitor CAL-101; and dual PI3K/mTOR inhibitors BEZ235, BGT226, PF-4691502, GDC-0980, and XL-765.
The pan-PI3K and p110α-specific inhibitors are equally potent against oncogenic p110α mutants.102–104 The rationale for the development of isozyme-specific antagonists is to allow higher doses of anti-p110α and anti-p110β drugs to be delivered without incurring adverse effects caused by pan-PI3K inhibitors. Interim results from a phase I trial with the p110δ-specific inhibitor CAL-101 in patients with hematologic malignancies showed that treatment reduced P-AKT levels by more than 90% in peripheral blood lymphocytes and induced objective clinical responses.105 Recently completed phase I trials with BKM120, BEZ235, and XL-147 showed that treatment partially inhibited PI3K as measured by levels of P-S6 and P-AKT in patients' skin or tumors and [18F]fluorodeoxyglucose uptake measured by PET. Main toxicities were rash, hyperglycemia, diarrhea, fatigue, and mood alterations. Few clinical responses were observed in patients with and without detectable PI3K pathway mutations, although screening for genetic lesions was not comprehensive.106–108
Both allosteric and ATP-competitive pan-inhibitors of the three isoforms of AKT are being developed. AZD5363, GDC-0068, GSK2141795, and GSK690693 are ATP-competitive compounds that have shown antitumor activity in preclinical models and recently entered phase I trials.109,110 Allosteric inhibitors such as MK-2206 bind to the AKT PH domain and/or hinge region to promote a conformation incapable of membrane localization.111 MK-2206 inhibits AKT signaling in vivo and suppresses growth of breast cancer xenografts harboring PIK3CA mutations or ERBB2 amplification.112 Phase I data have shown that treatment with MK-2206 decreases levels of P-AKT, P–proline-rich AKT substrate 40, and P-GSK3β in tumor cells, peripheral blood mononuclear cells, and hair follicles.113,114
The mTOR kinase is a component of PI3K-driven oncogenesis that functions within two signaling complexes: TORC1 and TORC2.37,38 The macrolide rapamycin and its analogs complex with FK506-binding protein (FKBP12), which then binds to mTOR and inhibits the kinase activity of TORC1 but not TORC2.38 The formulation problems of rapamycin prompted the development of analogs such as CCI-779 (temsirolimus), RAD001 (everolimus), AP-23573 (deferolimus), and MK-8669 (ridaferolimus). These rapalogs have shown cytostatic activity in preclinical models and clinical trials, particularly in patients with renal cell cancer and in those with mutations in the tuberous sclerosis complex (upstream of TORC1) who harbor renal angiolipomas. Compounds that target the ATP-binding cleft of mTOR (ie, OSI-027, AZD8055, INK-128), and are thus active against both TORC1 and TORC2, are also in phase I trials.115
CLINICAL INVESTIGATION OF PI3K PATHWAY INHIBITORS IN ER-POSITIVE BREAST CANCER
The somatic alterations described (ie, PIK3CA and AKT1 mutations, PTEN and INPP4B loss, PIK3CB and AKT2 amplification, and so on) identify cancers with aberrant activation of and potential dependence on the PI3K pathway. This is an important consideration for the selection of patients into trials with PI3K inhibitors. Like mutant PI3K, other somatic mutations in tumors have revealed molecules critical for cancer survival and progression. Pharmacologic targeting of these mutants has resulted in remarkable clinical responses in patients bearing tumors with such mutant oncogenes. Examples include imatinib and dasatinib in chronic myelogenous leukemia harboring the BCR-ABL oncogene, EGFR TKIs gefitinib and erlotinib in lung cancers with EGFR-activating mutations, HER2 antagonists trastuzumab and lapatinib in breast cancers with HER2 gene amplification, and Raf inhibitors against metastatic melanomas containing B-Raf–activating mutations.116
As with other targeted therapies, only a fraction of patients with tumors containing PI3K pathway mutations will likely benefit from single-agent PI3K inhibitors. There is increasing agreement that initial phase II efficacy studies with PI3K inhibitors in patients with advanced disease should be enriched with, if not limited to, patients harboring mutations in this pathway. However, testing these drugs in single-arm phase II trials in patients with metastatic cancer with PI3K pathway alterations is intrinsically problematic because: first, the difficulty of obtaining biopsies from metastatic sites, and second, the limitations of assessing tumor response as a meaningful clinical end point in the absence of a placebo control arm. We will discuss alternative approaches that may address these issues.
PRESURGICAL AND NEOADJUVANT CLINICAL TRIALS
There are examples of short-term, pharmacodynamic trials providing information that can be later used for patient selection into trials with novel targeted therapies such as PI3K antagonists. In ER-positive breast cancer, data from presurgical studies suggest that such trials can be used to predict longer-term outcome after adjuvant endocrine therapy. Dowsett et al117 reported that patients with ER-positive tumors with high Ki67 scores after 2 weeks of neoadjuvant antiestrogen therapy had shorter RFS compared with those with low Ki67 scores. Ellis et al118 found that Ki67 scores and ER status after 3 to 4 months of neoadjuvant endocrine therapy were predictive of RFS after adjuvant tamoxifen. Furthermore, ER-positive/HER2-negative tumors showed a reduction in Ki67 in response to neoadjuvant letrozole, whereas ER-positive/HER2-positive tumors did not.3 Therefore, presurgical evaluation of molecular markers after short-term treatment may be valuable to predict long-term patient benefit. However, no single biomarker has been shown to accurately predict disease outcome in individual patients. Ki67 score was found to vary between biopsies from the same breast tumor such that a change greater than 36% to 50% in the post-treatment compared with pretreatment biopsy would be required to detect a potentially informative difference.119,120 Furthermore, identifying patients who would benefit from neoadjuvant therapy using Ki67 score is challenging in tumors with rather low baseline cell proliferation.120
Although some PI3K pathway mutations (eg, HER2, FGFR1 amplification) have been linked with antiestrogen resistance, the role of PIK3CA mutations is less clear.3–5,21 Neoadjuvant trials provide a setting to investigate this role. For example, in a neoadjuvant study of letrozole with or without everolimus, PIK3CAexon9 mutations were associated with a statistically lower reduction in cell proliferation in response to letrozole, as measured by a change in the Ki67 IHC score on day 15, compared with PIK3CA–wild-type tumors.15 We have observed similar preliminary data in a presurgical trial of short-term letrozole (10 to 21 days) in patients with ER-positive/HER2-negative breast cancer (NCT00651976). In this study, analysis of the first 21 patients showed that PIK3CA-mutant tumors exhibited a statistically lower reduction in Ki67 score compared with tumors with wild-type PIK3CA (data not shown). However, another study in which tumor cell proliferation was assessed at baseline and after 4 months of antiestrogen therapy found no association between PIK3CA status and drug-induced change in Ki67 score.17 This discrepancy may be the result of the different timing (2 to 3 weeks v 4 months) of the biopsies from which cell proliferation was assessed.
RANDOMIZED TRIALS OF ANTIESTROGENS WITH OR WITHOUT PI3K PATHWAY INHIBITORS
Although PI3K pathway activation may confer antiestrogen resistance, breast tumors that acquire hormone independence may still be stimulated by (host) estradiol. Thus, we speculate that PI3K inhibitors may have limited efficacy against ER-positive breast cancers as single agents. Because antiestrogen therapies are effective against a large fraction of ER-positive tumors as single agents, there is a need for randomized clinical trials of standard ER-targeted therapies versus combinations targeting both the ER and PI3K pathways.
In Table 2, we summarize the randomized trials in which inhibitors of TORC1, HER2, EGFR, IGF-1R, protein kinase C-β/PDK1/p70S6K, and farnesyltransferase have been combined with endocrine therapy. The molecular targets of these drugs rely on PI3K and have been linked to endocrine resistance. Neoadjuvant treatment with letrozole and the TORC1 inhibitor everolimus more effectively suppressed tumor cell proliferation and increased clinical response compared with letrozole alone in patients with early-stage ER-positive breast cancer.15 In the TAMRAD (Tamoxifen and RAD001) trial, 111 patients bearing AI-resistant metastatic breast cancer (resistance defined as primary [relapse during adjuvant AI or < 6 months after starting adjuvant AI in metastatic setting] or secondary [relapse ≥ 6 months on adjuvant AI or prior response to AI and subsequent metastatic progression]) were randomly assigned to tamoxifen with or without everolimus. Patients in the combination arm showed an improved clinical benefit rate (61% v 42%), time to progression (8.6 v 4.5 months), and overall survival compared with patients receiving tamoxifen alone. Patients with secondary but not primary AI resistance who received both drugs showed an increased time to progression compared with patients receiving tamoxifen alone.79 In contrast, results from other studies have not favored the combination arm. For example, addition of the IGF-1R antibody AMG479 to fulvestrant or exemestane did not alter progression-free survival in patients with ER-positive breast cancer compared with endocrine therapy alone.124 The addition of the farnesyltransferase inhibitor tipifarnib to letrozole did not improve response in patients with advanced ER-positive disease compared with letrozole alone.123
Table 2.
Prospective Randomized Trials Testing Combinations of PI3K Pathway Inhibitors and Endocrine Therapies
| Kinase Target | Trial Design | Phase | Reference |
|---|---|---|---|
| mTOR | Letrozole ± everolimus in patients with early-stage ER-positive breast cancer | II | Baselga et al15 |
| Exemestane ± everolimus in ER-positive metastatic breast cancer after progression while receiving another AI | III | NCT00863655 (BOLERO-2; ongoing) | |
| Tamoxifen ± everolimus in ER-positive metastatic breast cancer after progression while receiving AI | II | TAMRAD; Bachelot et al79 | |
| HER2 | Anastrozole ± trastuzumab in patients with ER-positive/HER2-positive metastatic breast cancer | III | Kaufman et al95 |
| HER2/EGFR | Letrozole ± lapatinib in ER-positive metastatic breast cancer | III | Johnston et al94 |
| AI ± lapatinib or AI + fulvestrant ± lapatinib in ER-positive metastatic breast cancer after progression while receiving AI | III | NCT00688194 (ongoing) | |
| AI ± trastuzumab or lapatinib or both in patients with ER-positive/HER2-positive metastatic breast cancer | III | NCT01160211 (not yet open) | |
| EGFR | Anastrozole ± gefitinib in early-stage ER-positive breast cancer | II | Smith et al121 |
| Anastrozole ± gefitinib in metastatic ER-positive patients | II | Cristofanilli et al122 | |
| PKCβ, PDK1, p70S6K | Fulvestrant ± enzastaurin in ER-positive metastatic breast cancer after progression while receiving AI | II | NCT00451555 (ongoing) |
| Farnesyl transferase | Letrozole ± tipifarnib in ER-positive metastatic breast cancer after progression on tamoxifen | II | Johnston et al123 |
| IGF-1R/InsR | BMS-754807 ± letrozole in ER-positive metastatic breast cancer after progression while receiving AI | II | NCT01225172 (ongoing) |
| IGF-1R | IMCA12 ± same antiestrogen (AI, fulvestrant, or tamoxifen) after progression in patients with ER-positive metastatic breast cancer | II | NCT00728949 (ongoing) |
| AI or fulvestrant ± AMG479 in ER-positive advanced or metastatic breast cancer after progression while receiving endocrine Tx | II | Kaufman et al124 |
Abbreviations: AI, aromatase inhibitor; BOLERO, Breast cancer trials of OraL EveROlimus; EGFR, epidermal growth factor receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IGF-1R, insulin-like growth factor-1 receptor; InsR, insulin receptor; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; TAMRAD, Tamoxifen and RAD001.
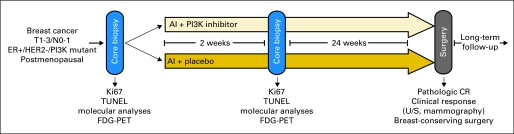
The neoadjuvant trial design depicted in Figure 2 illustrates an approach that can be used to determine whether to pursue combinations of PI3K inhibitors and antiestrogens in patients with ER-positive breast cancer. Such trials would have to be performed after safety of the combinations has been documented in phase I studies. Patients would be randomly assigned to standard endocrine therapy with or without a PI3K pathway inhibitor. A research biopsy could be obtained after 2 weeks to document effects on tumor cell proliferation, apoptosis, and ER/PI3K signaling and for wider exploratory mutational analysis. Incorporation of noninvasive imaging with [18F]fluorodeoxyglucose–positron emission tomography at this time point could identify metabolic changes indicative of a pharmacodynamic effect. Study end points would be clinical and/or pathologic complete response (CR) after 4 to 6 months of therapy. Historically, pathologic CR has had limitations as an end point of neoadjuvant trials in ER-positive breast cancer. However, we cannot rule out that as we optimize targeted approaches such as antiestrogens in combination with PI3K inhibitors in ER-positive/PI3K-mutant tumors, a pathologic CR rate could be used as a primary end point for a trial of this design. This approach potentially addresses the following questions: First, is there an early (at 2 weeks) difference in cellular and molecular response between treatment arms? Second, is clinical and/or pathologic response superior in the arm containing the PI3K pathway inhibitor? A difference in favor of the combination would support further development of PI3K inhibitors and endocrine therapy in patients with advanced disease. Third, is there a tissue and/or imaging pharmacodynamic biomarker in the baseline, 2-week, and/or surgical specimen that correlates with response/resistance to the combination? If so, such a biomarker could be used to select patients with advanced disease who would likely benefit from the combination in phase II trials.
Fig 2.
Diagram of neoadjuvant clinical trial with phosphatidylinositol 3-kinase (PI3K) pathway inhibitor. Patients with estrogen receptor (ER) –positive breast cancer eligible for neoadjuvant therapy randomly assigned to standard treatment (eg, aromatase inhibitor [AI]) with or without PI3K pathway inhibitor. Core biopsies obtained before and after 2 weeks of therapy to document effects on tumor cell proliferation, apoptosis, and pathway inactivation (ie, downregulation of P-AKT, P-S6, P–proline-rich AKT substrate 40 by immunohistochemistry). Incorporation of noninvasive [18F]fluorodeoxyglucose (FDG) –positron emission tomography (PET) at 2 weeks could identify early metabolic changes; however, PI3K pathway inhibitors may induce hyperglycemia,106–108 which could limit utility of FDG-PET. Primary end point of clinical response could be evaluated after approximately 4 to 6 months of therapy by measuring tumor with calipers, ultrasound (U/S), and/or mammography. Absence of tumor in surgical specimen would be scored as pathologic complete response (CR). Rate of breast-conserving surgery could be compared between both arms. Molecular mining of baseline, 2-week, and surgical biopsies could identify biomarkers that could be used for selection of patients enrolled onto subsequent studies of combination. HER2, human epidermal growth factor receptor 2; TUNEL, terminal deoxynucleotidyl nick-end labeling.
DISCUSSION
Alterations in the PI3K pathway are the most common somatic mutations in ER-positive breast cancer. Experimental and clinical evidence implies that such mutations are associated with antiestrogen resistance. Many PI3K pathway inhibitors are in clinical development. Early clinical data suggest that this strategy is feasible and that as single agents, these drugs are well tolerated. Although there is crosstalk between the ER and PI3K/AKT pathways, these networks also signal independently. Because most ER-positive breast cancers that acquire resistance to antiestrogens retain ER and responsiveness to estrogens, we speculate PI3K inhibitors should be used in combination with antiestrogens in patients who progress while receiving the latter. To determine if combined inhibition of PI3K and ER is more active than antiestrogen therapy alone against ER-positive tumors with mutations in the PI3K pathway, randomized clinical trials are required.
Footnotes
Supported by National Institutes of Health Grant No. K99CA142899 (T.W.M.); Breast Cancer Specialized Program of Research Excellence Grant No. P50CA98131; Vanderbilt-Ingram Cancer Center Support Grant No. P30CA68485; a grant from the Breast Cancer Research Foundation (C.L.A.); American Cancer Society Clinical Research Professorship Grant No. CRP-07-234 (C.L.A.); the Lee Jeans Translational Breast Cancer Research Program (C.L.A.); and Stand Up to Cancer/American Association for Cancer Research Dream Team Translational Cancer Research Grant No. SU2C-AACR- DT0209 (C.L.A.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00651976.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Carlos L. Arteaga, Roche (C), Monogram Biosciences (C), AstraZeneca (C), GlaxoSmithKline (C) Stock Ownership: None Honoraria: None Research Funding: Carlos L. Arteaga, U3-Amgen, AstraZeneca, Exelixis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 3.Ellis MJ, Tao Y, Young O, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24:3019–3025. doi: 10.1200/JCO.2005.04.3034. [DOI] [PubMed] [Google Scholar]
- 4.Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A southwest oncology group study. Clin Cancer Res. 2004;10:5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 5.De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 6.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–5187. [PubMed] [Google Scholar]
- 7.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JMS, Brookes CL, van de Velde CJH, et al. Final results of a prospectively planned biomarker analysis: HER1-3 as predictive markers of benefit from early treatment with aromatase inhibitors versus tamoxifen in the TEAM pathology sub-study. Cancer Res. 2010;70 abstr S2-4. [Google Scholar]
- 9.Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: Results from the FIRST study. J Clin Oncol. 2009;27:4530–4535. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 10.Robertson JFR, Dixon JM, Sibbering DM, et al. Tumor biomarker changes following pre-surgical treatment with 500 mg fulvestrant plus anastrozole versus 500 mg fulvestrant alone and 1 mg anastrozole alone. Cancer Res. 2009;69 abstr 24. [Google Scholar]
- 11.Robertson JFR, Lindemann JPO, Llombart-Cussac A, et al. A comparison of fulvestrant 500 mg with anastrozole as first-line treatment for advanced breast cancer: Follow-up analysis from the “FIRST” study. Cancer Res. 2010;70 doi: 10.1007/s10549-012-2192-4. abstr S1-3. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Tenorio G, Alkhori L, Olsson B, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 13.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoman N, Klassen S, McFadden A, et al. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod Pathol. 2005;18:250–259. doi: 10.1038/modpathol.3800296. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 16.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis MJ, Lin L, Crowder R, et al. Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2010;119:379–390. doi: 10.1007/s10549-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell IG, Russell SE, Choong DY, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 19.Crowder RJ, Phommaly C, Tao Y, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955–3962. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 21.Turner N, Pearson A, Sharpe R, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monni O, Barlund M, Mousses S, et al. Comprehensive copy number and gene expression profiling of the 17q23 amplicon in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:5711–5716. doi: 10.1073/pnas.091582298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedele CG, Ooms LM, Ho M, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci U S A. 2010;107:22231–22236. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaiswal BS, Janakiraman V, Kljavin NM, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loi S, Haibe-Kains B, Majjaj S, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci U S A. 2010;107:10208–10213. doi: 10.1073/pnas.0907011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 28.Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 29.Al-Kuraya K, Schraml P, Torhorst J, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64:8534–8540. doi: 10.1158/0008-5472.CAN-04-1945. [DOI] [PubMed] [Google Scholar]
- 30.Maurer M, Su T, Saal LH, et al. 3- phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69:6299–6306. doi: 10.1158/0008-5472.CAN-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochlitz CF, Scott GK, Dodson JM, et al. Incidence of activating ras oncogene mutations associated with primary and metastatic human breast cancer. Cancer Res. 1989;49:357–360. [PubMed] [Google Scholar]
- 32.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitman M, Downes CP, Keeler M, et al. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KE, Coadwell J, Stephens LR, et al. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 35.Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 36.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 37.Gingras AC, Kennedy SG, O'Leary MA, et al. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 40.Yamnik RL, Digilova A, Davis DC, et al. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 41.Coso OA, Chiariello M, Yu JC, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 42.Minden A, Lin A, Claret FX, et al. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 43.Logan SK, Falasca M, Hu P, et al. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hibi M, Lin A, Smeal T, et al. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 45.Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 46.Rauscher FJ, 3rd, Cohen DR, Curran T, et al. Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988;240:1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- 47.DeNardo DG, Cuba VL, Kim H, et al. Estrogen receptor DNA binding is not required for estrogen-induced breast cell growth. Mol Cell Endocrinol. 2007;277:13–25. doi: 10.1016/j.mce.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Petz LN, Ziegler YS, Loven MA, et al. Estrogen receptor alpha and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology. 2002;143:4583–4591. doi: 10.1210/en.2002-220369. [DOI] [PubMed] [Google Scholar]
- 49.Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: New therapeutic opportunities. Clin Cancer Res. 2007;13:1950–1954. doi: 10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 50.Generali D, Fox SB, Brizzi MP, et al. Down-regulation of phosphatidylinositol 3′-kinase/AKT/molecular target of rapamycin metabolic pathway by primary letrozole-based therapy in human breast cancer. Clin Cancer Res. 2008;14:2673–2680. doi: 10.1158/1078-0432.CCR-07-1046. [DOI] [PubMed] [Google Scholar]
- 51.Miller TW, Perez-Torres M, Narasanna A, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 53.Sabnis G, Schayowitz A, Goloubeva O, et al. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–1428. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Masri S, Phung S, et al. The role of amphiregulin in exemestane-resistant breast cancer cells: Evidence of an autocrine loop. Cancer Res. 2008;68:2259–2265. doi: 10.1158/0008-5472.CAN-07-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan M, Yan PS, Hartman-Frey C, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 57.Frogne T, Benjaminsen RV, Sonne-Hansen K, et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009;114:263–275. doi: 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Massarweh S, Osborne CK, Jiang S, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 59.Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song RX, McPherson RA, Adam L, et al. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 61.Song RX, Fan P, Yue W, et al. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocr Relat Cancer. 2006;13(suppl 1):S3–S13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Zhang X, Shen P, et al. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filardo EJ, Quinn JA, Bland KI, et al. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 65.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 66.Voliva CF, Pecchi S, Burger M, et al. Biological characterization of NVP-BKM120, a novel inhibitor of phosphoinosotide 3-kinase in phase I/II clinical trials. Cancer Res. 2010;70 abstr 4498. [Google Scholar]
- 67.Sabnis G, Goloubeva O, Jelovac D, et al. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res. 2007;13:2751–2757. doi: 10.1158/1078-0432.CCR-06-2466. [DOI] [PubMed] [Google Scholar]
- 68.Yue W, Wang J, Li Y, et al. Farnesylthiosalicylic acid blocks mammalian target of rapamycin signaling in breast cancer cells. Int J Cancer. 2005;117:746–754. doi: 10.1002/ijc.21222. [DOI] [PubMed] [Google Scholar]
- 69.Meyer DS, Brinkhaus H, Muller U, et al. Luminal expression of PIK3CA mutant H1047R in the mammary gland induces heterogeneous tumors. Cancer Res. 2011;71:4344–4351. doi: 10.1158/0008-5472.CAN-10-3827. [DOI] [PubMed] [Google Scholar]
- 70.Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci U S A. 2008;105:2652–2657. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 72.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 73.Janku F, Tsimberidou AM, Garrido-Laguna I, et al. PIK3CA Mutations in Patients with Advanced Cancers Treated with PI3K/AKT/mTOR Axis Inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miron A, Varadi M, Carrasco D, et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2011;70:5674–5678. doi: 10.1158/0008-5472.CAN-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dupont Jensen J, Laenkholm AV, Knoop A, et al. PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clin Cancer Res. 2011;17:667–677. doi: 10.1158/1078-0432.CCR-10-1133. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093–1101. doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Creighton CJ, Fu X, Hennessy BT, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bachelot T, Bourgier C, Cropet C, et al. TAMRAD: A GINECO randomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients (pts) with hormone-receptor positive, HER2 negative metastatic breast cancer (MBC) with prior exposure to aromatase inhibitors (AI) Cancer Res. 2010;70 doi: 10.1200/JCO.2011.39.0708. abstr S1-6. [DOI] [PubMed] [Google Scholar]
- 80.Miller TW, Forbes JT, Shah C, et al. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15:7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chakrabarty A, Sánchez V, Kuba MG, et al. Breast cancer special feature: Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1018001108. [epub ahead of print on February 28, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chandarlapaty S, Sawai A, Scaltriti M, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garrett JT, Olivares MG, Rinehart C, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amin DN, Sergina N, Ahuja D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2011;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lipton A, Ali SM, Leitzel K, et al. Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J Clin Oncol. 2003;21:1967–1972. doi: 10.1200/JCO.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 88.Arpino G, Wiechmann L, Osborne CK, et al. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 90.Pietras RJ, Arboleda J, Reese DM, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 91.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 92.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munzone E, Curigliano G, Rocca A, et al. Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res. 2006;8:R4. doi: 10.1186/bcr1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 95.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 96.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 97.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 98.Vanhaesebroeck B, Leevers SJ, Panayotou G, et al. Phosphoinositide 3-kinases: A conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 99.Jia S, Roberts TM, Zhao JJ. Should individual PI3 kinase isoforms be targeted in cancer? Curr Opin Cell Biol. 2009;21:199–208. doi: 10.1016/j.ceb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 101.Maira SM, Voliva C, Garcia-Echeverria C. Class IA phosphatidylinositol 3-kinase: From their biologic implication in human cancers to drug discovery. Expert Opin Ther Targets. 2008;12:223–238. doi: 10.1517/14728222.12.2.223. [DOI] [PubMed] [Google Scholar]
- 102.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 103.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 104.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl- piperazin-1-ylmethyl)-4-morpholin -4-yl-thieno[3,2-d] pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 105.Furman RR, Byrd JC, Flinn IW, et al. Interim results from a phase I study of CAL-101, a selective oral inhibitor of phosphatidylinositol 3-kinase p110d isoform, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2010;28(suppl):241s. abstr 3032. [Google Scholar]
- 106.Baselga J, De Jonge MJ, Rodon J, et al. A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(suppl):233s. doi: 10.1200/JCO.2011.36.1360. abstr 3003. [DOI] [PubMed] [Google Scholar]
- 107.Burris H, Rodon J, Sharma S, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(suppl):234s. abstr 3005. [Google Scholar]
- 108.Edelman G, Bedell C, Shapiro G, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(suppl):234s. abstr 3004. [Google Scholar]
- 109.Rhodes N, Heerding DA, Duckett DR, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 110.Davies TG, Verdonk ML, Graham B, et al. A structural comparison of inhibitor binding to PKB, PKA and PKA-PKB chimera. J Mol Biol. 2007;367:882–894. doi: 10.1016/j.jmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 111.Toral-Barza L, Zhang WG, Huang X, et al. Discovery of lactoquinomycin and related pyranonaphthoquinones as potent and allosteric inhibitors of AKT/PKB: Mechanistic involvement of AKT catalytic activation loop cysteines. Mol Cancer Ther. 2007;6:3028–3038. doi: 10.1158/1535-7163.MCT-07-0211. [DOI] [PubMed] [Google Scholar]
- 112.She QB, Chandarlapaty S, Ye Q, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yap TA, Yan L, Patnaik A, et al. Final results of a translational phase l study assessing a QOD schedule of the potent AKT inhibitor MK-2206 incorporating predictive, pharmacodynamic (PD), and functional imaging biomarkers. J Clin Oncol. 2011;29(suppl):194s. abstr 3001. [Google Scholar]
- 114.Biondo A, Yap TA, Yan L, et al. Phase I clinical trial of an allosteric AKT inhibitor, MK-2206, using a once weekly (QW) dose regimen in patients with advanced solid tumors. J Clin Oncol. 2011;29(suppl):203s. doi: 10.1200/JCO.2011.35.5263. abstr 3037. [DOI] [PubMed] [Google Scholar]
- 115.Fasolo A, Sessa C. MTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1717–1734. doi: 10.1517/13543784.17.11.1717. [DOI] [PubMed] [Google Scholar]
- 116.Stuart D, Sellers WR. Linking somatic genetic alterations in cancer to therapeutics. Curr Opin Cell Biol. 2009;21:304–310. doi: 10.1016/j.ceb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 118.Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ellis PA, Smith IE, Detre S, et al. Reduced apoptosis and proliferation and increased Bcl-2 in residual breast cancer following preoperative chemotherapy. Breast Cancer Res Treat. 1998;48:107–116. doi: 10.1023/a:1005933815809. [DOI] [PubMed] [Google Scholar]
- 120.Assersohn L, Salter J, Powles TJ, et al. Studies of the potential utility of Ki67 as a predictive molecular marker of clinical response in primary breast cancer. Breast Cancer Res Treat. 2003;82:113–123. doi: 10.1023/B:BREA.0000003968.45511.3f. [DOI] [PubMed] [Google Scholar]
- 121.Smith IE, Walsh G, Skene A, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25:3816–3822. doi: 10.1200/JCO.2006.09.6578. [DOI] [PubMed] [Google Scholar]
- 122.Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 123.Johnston SR, Semiglazov VF, Manikhas GM, et al. A phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res Treat. 2008;110:327–335. doi: 10.1007/s10549-007-9726-1. [DOI] [PubMed] [Google Scholar]
- 124.Kaufman PA, Ferrero JM, Bourgeois H, et al. A randomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC) Cancer Res. 2010;70(abstr S1-4) [Google Scholar]