Abstract
Purpose
The response definitions proposed by the European LeukemiaNet (ELN) are defined on the basis of imatinib front-line therapy. It is unknown whether these definitions apply to patients treated with second-generation tyrosine kinase inhibitors (TKIs).
Patients and Methods
One hundred sixty-seven patients with newly diagnosed chronic myelogenous leukemia (CML) in chronic phase were treated with second-generation TKIs in phase II trials (nilotinib, 81; dasatinib, 86). Median follow-up was 33 months. Event-free survival (EFS) was measured from the start of treatment to the date of loss of complete hematologic response, loss of complete or major cytogenetic response, discontinuation of therapy for toxicity or lack of efficacy, progression to accelerated or blastic phases, or death at any time.
Results
Overall, 155 patients (93%) achieved complete cytogenetic response (CCyR), including 146 (87%) with major molecular response (MMR; complete in 46 patients [28%]). According to the ELN definitions, the rates of suboptimal response were 0%, 2%, 1%, and 12% at 3, 6, 12, and 18 months of therapy, respectively. There was no difference in EFS and CCyR duration between patients who achieved CCyR with and without MMR across all the landmark times of 3, 6, 12, and 18 months.
Conclusion
The use of second-generation TKIs as initial therapy in CML induces high rates of CCyR at early time points. The ELN definitions of response proposed for imatinib therapy are not applicable in this setting. We propose that achievement of CCyR and partial cytogenetic response at 3 months should be considered optimal and suboptimal responses, respectively. The achievement of MMR offered no advantage over CCyR in defining long-term outcome in patients with newly diagnosed CML treated with second-generation TKIs.
INTRODUCTION
The European LeukemiaNet (ELN) recommendations were proposed during the era of imatinib front-line therapy,1 a treatment that produced complete cytogenetic responses (CCyRs) in 83% of patients with Philadelphia chromosome (Ph) –positive chronic myelogenous leukemia (CML), with most responses being durable.2–4 Achieving a CCyR correlated with survival.2–4
According to the ELN definitions, the overall response to imatinib can be defined as optimal, suboptimal, and failure. Optimal means that there is no indication that a change of therapy may improve a survival that is currently projected as being close to 100% after 6 to 7 years. Suboptimal response means that the patient may still have a substantial long-term benefit from continuing a specific treatment, but the chances of an optimal outcome are reduced, so that suboptimal responders may be eligible for alternative approaches. Failure means that a favorable outcome is unlikely and that the patient should receive a different treatment whenever available and applicable.
Second-generation tyrosine kinase inhibitors (TKIs), such as dasatinib and nilotinib, are more potent BCR-ABL TKIs with demonstrated efficacy in patients resistant to or intolerant of imatinib.5–7 Dasatinib and nilotinib were first approved for patients resistant to or intolerant of prior imatinib therapy, are active against most BCR-ABL mutations with the exception of T315I, and have well-established safety profiles.2,8 Single-arm phase II studies9–11 first suggested, and phase III randomized trials later confirmed that dasatinib and nilotinib were superior to imatinib, inducing faster and higher rates of CCyRs and molecular responses. Therefore, both drugs were granted approval by the US Food and Drug Administration to be used in patients with newly diagnosed CML in chronic phase (CML-CP).12,13
The application of the ELN definitions—optimal, suboptimal, and failure—in patients receiving front-line therapy with second-generation TKIs may not be relevant since most patients will achieve early CCyRs. To determine the significance of ELN response criteria for patients receiving dasatinib or nilotinib as initial therapy for CML-CP, we conducted an analysis of patients receiving second-generation TKIs in early CML-CP to determine the frequency with which optimal and suboptimal responses occur and the long-term impact of such responses.
PATIENTS AND METHODS
One hundred and sixty-seven patients with newly diagnosed CML-CP were treated with second-generation TKIs in two simultaneous phase II trials (partially reported previously in Cortes et al10 and Rosti et al11): one trial used nilotinib 400 mg twice daily and the other used dasatinib 100 mg once daily. Entry criteria were similar for both trials. CML-CP was defined as the presence in the peripheral blood of less than 15% blasts, less than 20% basophils, less than 30% blasts and promyelocytes, and platelets at more than 100 ×109/L.14 Patients were treated on protocols approved by institutional review boards, and informed consent was obtained in accordance with the Declaration of Helsinki. Response criteria were as previously described.2 A complete hematologic response (CHR) was defined as a WBC count of less than 10 × 109/L, a platelet count of less than 450 ×109/L, no immature cells (blasts, promyelocytes, myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly). This was further categorized by the best cytogenetic response as CCyR (0% Ph-positive metaphases), partial cytogenetic response (PCyR; 1% to 35% Ph-positive), or minor (36% to 95% Ph-positive). A major cytogenetic response (MCyR) included CCyR plus PCyR (ie, < 35% Ph-positive). Cytogenetic response was judged by standard cytogenetic analysis in 20 metaphases performed on bone marrow aspirates. Fluorescent in situ hybridization on peripheral blood was used only when routine cytogenetic analysis was not successful (ie, insufficient metaphases). Major molecular response (MMR) was defined as a BCR-ABL/ABL transcript ratio of ≤ 0.1% (international scale). A complete molecular response (CMR) was defined as undetectable transcripts by using an assay with sensitivity of at least 4.5-log.14
The rates of suboptimal response and failure at each time point were calculated among evaluable patients (those patients still receiving therapy and with an evaluable hematologic, cytogenetic, and/or molecular result, as appropriate) to determine the response at the specified time point to be classified according to the ELN. Differences among variables were evaluated by the χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively. Event-free survival (EFS) was measured from the start of treatment to the date of any of the following events while on therapy: loss of CHR, loss of CCyR or MCyR, discontinuation of therapy for toxicity or lack of efficacy, progression to accelerated or blastic phases, or death from any cause at any time. Survival was measured from the time treatment was started to the date of death from any cause at any time or date of last follow-up. Transformation-free survival was measured from the start of therapy to the date of transformation to accelerated or blastic phases while on therapy or to the date of last follow-up. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test.
RESULTS
A total of 167 patients were treated. The median follow-up time was 33 months (range, 3 to 66 months). Patients' characteristics are provided in Table 1. Eighty-six patients (52%) received dasatinib and 81 (48%) received nilotinib. The median time from diagnosis to therapy was 1 month (range, 0 to 8 months). The majority of patients (76%) were in the Sokal low-risk category.
Table 1.
Patient and Disease Characteristics When Second-Generation TKI Therapy Was Initiated and Overall Outcome
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 46 | |
| Range | 18-85 | |
| Time from diagnosis to treatment, months | ||
| Median | 1 | |
| Range | 0-8 | |
| Platelets ×109/L | ||
| Median | 296 | |
| Range | 73-2,000 | |
| Hemoglobin, g/dL | ||
| Median | 12.2 | |
| Range | 6.7-15.8 | |
| WBC ×109/L | ||
| Median | 31.6 | |
| Range | 0.8-342.5 | |
| Peripheral blood blasts | ||
| Median | 0 | |
| Range | 0-7 | |
| Peripheral blood basophils | ||
| Median | 3 | |
| Range | 0-19 | |
| Marrow blasts | ||
| Median | 2 | |
| Range | 0-8 | |
| Marrow basophils | ||
| Median | 2 | |
| Range | 0-12 | |
| Splenomegaly | 44 | 26 |
| Clonal evolution | 10 | 6 |
| % Patients Ph+ > 90 | 144 | 86 |
| Sokal risk group | ||
| Low | 127 | 76 |
| Intermediate | 31 | 19 |
| High | 9 | 5 |
| Second-generation TKI | ||
| Dasatinib | 86 | 52 |
| Nilotinib | 81 | 48 |
| Response | ||
| CCyR | 155 | 93 |
| MMR | 146 | 87 |
| 4-year outcome | ||
| EFS | 89 | |
| TFS | 97 | |
| OS | 99 | |
Abbreviations: CCyR, complete cytogenetic response; EFS, event-free survival; MMR, major molecular response; OS, overall survival; Ph+, Philadelphia chromosome–positive metaphase; TFS, transformation-free survival; TKI, tyrosine kinase inhibitor.
Overall, 155 patients (93%) achieved a CCyR, including 146 (87%) who achieved an MMR (complete in 46 [28%]). Among the 155 patients who achieved a CCyR at any time, five (3%) had lost such a response at the time of last follow-up, one of whom (20%; one of five) was taken off study because of adverse events. A total of 17 patients (10%) experienced events as defined for EFS, with three (2%) experiencing transformation to accelerated (n = 2) or blastic (n = 1) phase. The 4-year EFS, transformation-free survival, and overall survival (OS) rates for the whole group were 89%, 97%, and 99%, respectively.
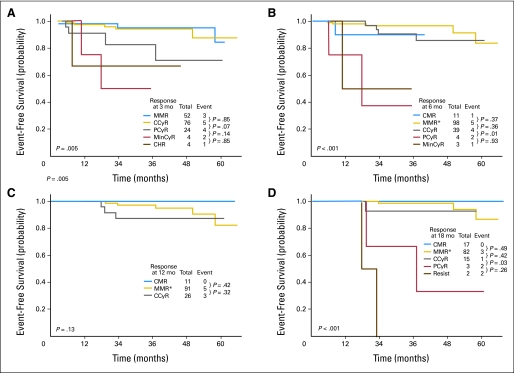
The incidence of optimal and suboptimal responses and failure at 3, 6, 12, and 18 months is provided in Table 2. At 3 months from the initiation of therapy, all patients who had been evaluated for response (n = 160) achieved CHR (ie, optimal response); at 6 months, 152 (98%) of the 155 evaluable patients achieved an optimal response (MCyR), and three patients (2%) met the definition of suboptimal response (ie, less than an MCyR). By 12 months, 128 (99%) of 129 evaluable patients had an optimal response (CCyR), and one (1%) met the definition for suboptimal response (ie, less than CCyR). By 18 months, the percentage of patients with a suboptimal response (ie, less than MMR) increased to 12% (14 of 118 evaluable patients), and 84% achieved an optimal response. Criteria for failure were met by 0%, 0%, 0%, and 4% at 3, 6, 12, and 18 months, respectively. Best responses and events by time on therapy are summarized in Table 3.
Table 2.
Frequency of Suboptimal Response and Failure at 3, 6, 12, and 18 Months of Therapy
| Months of Therapy | Response | Total |
|
|---|---|---|---|
| No. | % | ||
| 3 (n = 160) | Optimal | 160 | 100 |
| Suboptimal | 0 | ||
| Failure | 0 | ||
| 6 (n = 155) | Optimal | 152 | 98 |
| Suboptimal | 3 | 2 | |
| Failure | 0 | ||
| 12 (n = 129) | Optimal | 128 | 99 |
| Suboptimal | 1 | 1 | |
| Failure | 0 | ||
| 18 (n = 119*) | Optimal | 99 | 84 |
| Suboptimal | 14 | 12 | |
| Failure | 5 | 4 | |
One patient achieved a complete cytogenetic response, but no molecular test was performed at that time point; thus, we gave neither optimal nor suboptimal.
Table 3.
Adverse Events According to Cytogenetic and Molecular Response at 3, 6, 12, and 18 Months
| Response | Months to Response |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 (n = 160) |
6 (n = 155) |
12 (n = 129) |
18 (n = 119) |
|||||||||
| No. | % | IRIS/MDACC Events | No. | % | IRIS/MDACC Events | No. | % | IRIS/MDACC Events | No. | % | IRIS/MDACC Events | |
| Molecular | 52 | 32 | 0/3 | 109 | 70 | 0/6 | 102 | 79 | 1/5 | 99 | 83 | 0/3 |
| Complete | 1 | < 1 | 0/0 | 11 | 7 | 0/1 | 11 | 9 | 0/0 | 17 | 14 | 0/0 |
| Major | 51 | 32 | 0/3 | 98 | 63 | 0/5 | 91 | 70 | 1/5 | 82 | 69 | 0/3 |
| Cytogenetic | 104 | 65 | 4/11 | 46 | 30 | 3/7 | 27 | 21 | 2/3 | 18 | 15 | 1/3 |
| Complete | 76 | 48 | 1/5 | 39 | 25 | 2/4 | 26 | 20 | 2/3 | 15 | 13 | 0/1 |
| Partial | 24 | 15 | 2/4 | 4 | 3 | 1/2 | 1 | < 1 | 0/0 | 3 | 2 | 1/2 |
| Minor | 4 | 2 | 2/2 | 3 | 2 | 0/1 | 0 | N/A | 0 | N/A | ||
| Others (CHR) | 4* | 3 | 0/1 | 0 | N/A | 0 | N/A | 2 | 2 | 2/2 | ||
Abbreviations: CHR, complete hematologic response; IRIS, International Randomized Study of Interferon and STI571; MDACC, MD Anderson Cancer Center; N/A, not applicable.
Two patients achieved CHR, but no information on cytogenetic response was available because of insufficient metaphases.
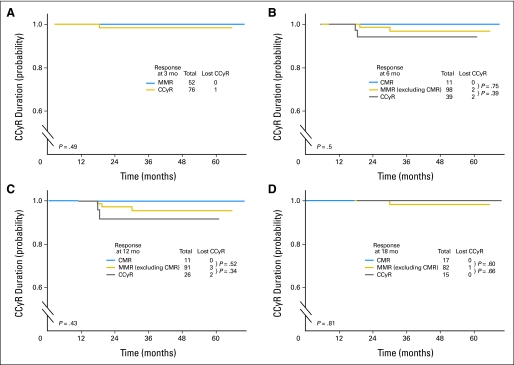
Overall, according to the ELN definitions, the rates of suboptimal response were low and were 2%, 1%, and 12%, at 6, 12, and 18 months of therapy, respectively. No patients met the criteria of suboptimal response at 3 months. The EFS from 3, 6, 12, and 18 months by cytogenetic and molecular response at these time points is shown in Figures 1A to 1D. At 3 months, patients with PCyR had a higher rate of events compared with patients with at least CCyR; patients who did not achieve a CHR had a poor outcome. At 6 months, patients with PCyR had a worse EFS compared with those who achieved a CCyR. At 12 months, all evaluable patients had achieved at least a CCyR, and outcome was similar, regardless of achievement of a molecular response; although not statistically significant, patients with a CMR had no events throughout their follow-up. At 18 months, patients with at least a CCyR (regardless of the molecular response) had a better prognosis than patients with PCyR or worse. Overall, patients with less than CCyR at any time points have a higher rate of events with a worse EFS. Importantly, there was no difference in EFS between patients who achieved a CCyR without molecular response and those who achieved an MMR or a CMR across all landmark time points of 3, 6, 12, and 18 months. In addition, CCyRs were durable, with no statistical difference, whether or not patients had achieved an MMR or a CMR in addition to CCyR at any time points (Figs 2A to 2D).
Fig 1.
The University of Texas MD Anderson Cancer Center event-free by response at (A) 3 months, (B) 6 months, (C) 12 months, and (D)18 months. (*)One patient with partial cytogenetic response (PCyR) had no adverse events and was excluded. CCyR, complete cytogenetic response; CHR, complete hematologic response; CMR, complete molecular response; MinCyR, minor cytogenetic response; Resist, resistant.
Fig 2.
Complete cytogenetic response (CCyR) duration by response at (A) 3 months (mo), (B) 6 months, (C) 12 months, and (D) 18 months. CMR, complete molecular response; MMR, major molecular response.
DISCUSSION
The recommendations from the ELN have been useful in harmonizing the definitions and treatment patterns for patients with CML treated with imatinib. According to the ELN definitions, the overall response to imatinib can be defined as optimal, suboptimal, and failure. These definitions were based on the information available at the time, mostly from the International Randomized Study of Interferon and STI571 (IRIS) study. With the advent of new treatment options with second-generation TKIs for patients with newly diagnosed early CML-CP, we explored whether the prognostic significance of these definitions is applicable for these populations.
Our study of homogeneous patients with early CML-CP treated with second-generation TKIs has confirmed that second-generation TKIs used in the front-line setting are highly efficacious and induced higher rates of CCyRs, with the majority of these responses occurring within 3 months of starting therapy. This is in line with what has been previously reported with the use of second-generation TKIs in the salvage and front-line setting.3,4,9,10 This is in contrast with the results obtained with imatinib therapy in which the CCyR rates peak around 12 to 18 months.15 Thus, with the majority of patients (99%) achieving an optimal response within 3 months, the ELN definitions of response may not be applicable in patients with newly diagnosed CML treated with second-generation TKIs.
At 3 months of therapy, patients with less than a CCyR had a poor outcome, and patients achieving a PCyR had an intermediate outcome. Therefore, it is important to closely monitor, from the earliest time points, all patients treated with second-generation TKIs with a goal of achieving a CCyR at 3 months of therapy since this was associated with the best long-term outcome. Patients with a PCyR, although they cannot be considered to have experienced a treatment failure, have a higher likelihood of adverse events (17%), and therefore should be considered suboptimal. These patients should be reassessed at 6 months of therapy, because by that time, they should have achieved a CCyR; otherwise, they would be considered as having experience treatment failure. Therefore, after 6 months of therapy, patients were either in CCyR or they experienced treatment failure (Fig 1B). Thus a redefinition of response at 3 months is proposed, with an optimal response defined by at least a CCyR, a suboptimal response defined by a PCyR, and treatment failure defined as less than a PCyR. A validation of these responses in independent cohorts is needed, particularly because only a small minority of our patients (5%) were in the Sokal high-risk category compared with a quarter of the patients enrolled in the randomized trials.12,13
The broadly used IRIS definitions of events did not take into consideration intolerance and adverse effects that would lead to switching therapy regardless of efficacy. In our analysis, we used a broader definition of adverse events than what is traditionally used as introduced in the IRIS trial. However, by repeating the analysis with more restricted criteria like those of IRIS (Table 3), the findings were similar: patients achieving less than a CCyR at 3 months did have a worse outcome.
We and others16–20 have shown that the achievement of an early CCyR remains the major surrogate end point for long-term outcome in patients with CML treated with imatinib as well as with second-generation TKIs. The long-term implications of achieving an additional MMR on outcome of patients with CML remains controversial. Our study has failed to show any advantage in EFS and response duration in favor of patients who achieved an MMR compared with those who achieved only a CCyR at all time points. This is in line with our historical experience with imatinib in patients with newly diagnosed CML.19 Similarly, Marin et al20 previously reported that the achievement of an MMR at 12 or 18 months of therapy with imatinib failed to confer any benefit in 5-year progression-free survival or OS. A recent update of the IRIS results has shown no significant impact on EFS or OS, whether a patient has or has not achieved an MMR by 12 months, provided a CCyR was achieved, and a modest but statistically significant benefit in EFS but not OS when assessed by 18 months.21 This observation may have important practical consequences. Recent trends in CML have emphasized the importance of close monitoring of minimal molecular residual disease, advocating molecular studies every 3 months.2 Our analysis suggests that in a patient treated with second-generation TKIs in stable durable CCyR, the depth of molecular response (MMR v less than MMR but with CCyR) may not be a significant prognostic factor for outcome. That said, regular molecular monitoring may allow physicians to identify in less invasive ways those patients achieving a CCyR (ratio below 1% on the international scale)21 and, more importantly, to identify patients achieving a CMR. These last patients may be potential candidates for stopping therapy in the context of a clinical trial.22
In summary, second-generation TKIs used in front-line therapy induce high and fast rates of CCyR that could translate into better outcome. The ELN definitions of response are not applicable in this setting, which should prompt a discussion of what the definitions should be for patients treated with second-generation TKIs. Therefore, we propose that for patients treated with second-generation TKIs as initial therapy for CML, achieving a CCyR by 3 months of therapy should be considered an optimal response, and achieving a PCyR should be considered a suboptimal response. Patients with less than a CCyR at 3 months might be considered for alternative treatment options, provided those options can realistically be expected to offer a better long-term outcome. Finally, the achievement of an MMR offered no advantage over CCyR on midterm outcome in patients with newly diagnosed CML treated with second-generation TKIs; a longer follow-up is needed.
Footnotes
See accompanying article on page 4250
Supported in part by Grant No. P01CA049639 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Hagop M. Kantarjian, Novartis (C); Jorge E. Cortes, Novartis (C), Bristol-Myers Squibb (C), Pfizer (C) Stock Ownership: None Honoraria: Elias Jabbour, Bristol-Myers Squibb, Novartis; Guillermo Garcia-Manero, Novartis Research Funding: Hagop M. Kantarjian, Bristol-Myers Squibb, Novartis, Pfizer; Jorge E. Cortes, Bristol-Myers Squibb, Novartis, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Elias Jabbour, Jorge E. Cortes
Provision of study materials or patients: Elias Jabbour, Hagop M. Kantarjian, Jorge E. Cortes
Collection and assembly of data: Elias Jabbour, Susan O'Brien, Mary Beth Rios, Jorge E. Cortes
Data analysis and interpretation: Elias Jabbour, Hagop M. Kantarjian, Susan O'Brien, Jianqin Shan, Alfonso Quintás-Cardama, Guillermo Garcia-Manero, Jorge E. Cortes
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Deininger M, O'Brien SG, Guilhot F, et al. International randomized study of interferon vs. STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009;114 abstr 1126. [Google Scholar]
- 2.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 4.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 5.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 7.Cortes J, Bruemmendorf T, Kantarjian H, et al. Efficacy and safety of bosutinib (SKI-606) among patients with chronic phase Ph+ chronic myelogenous leukemia (CML) Blood. 2007;110 doi: 10.1182/blood-2011-05-355594. abstr 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 9.Cortes JE, Jones D, O'Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes JE, Jones D, O'Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 12.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, O'Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myeloid leukemia: Need for new response definitions? Cancer. 2008;112:837–845. doi: 10.1002/cncr.23238. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian HM, Talpaz M, O'Brien S, et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha: Follow-up results. Clin Cancer Res. 2002;8:2177–2187. [PubMed] [Google Scholar]
- 17.Jabbour E, Kantarjian H, Shan J, et al. The achievement of an early complete cytogenetic response (CCyR) is a major determinant for outcome in patients (pts) with early chronic phase (CP) chronic myeloid leukemia (CML) treated with tyrosine kinase inhibitors (TKIs) Blood. 2010;116 doi: 10.1182/blood-2011-04-348110. abstr 3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintás-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113:6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 20.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: An analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116:3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]