Abstract
Purpose
Recent increases in incidence and survival of oropharyngeal cancers in the United States have been attributed to human papillomavirus (HPV) infection, but empirical evidence is lacking.
Patients and Methods
HPV status was determined for all 271 oropharyngeal cancers (1984-2004) collected by the three population-based cancer registries in the Surveillance, Epidemiology, and End Results (SEER) Residual Tissue Repositories Program by using polymerase chain reaction and genotyping (Inno-LiPA), HPV16 viral load, and HPV16 mRNA expression. Trends in HPV prevalence across four calendar periods were estimated by using logistic regression. Observed HPV prevalence was reweighted to all oropharyngeal cancers within the cancer registries to account for nonrandom selection and to calculate incidence trends. Survival of HPV-positive and HPV-negative patients was compared by using Kaplan-Meier and multivariable Cox regression analyses.
Results
HPV prevalence in oropharyngeal cancers significantly increased over calendar time regardless of HPV detection assay (P trend < .05). For example, HPV prevalence by Inno-LiPA increased from 16.3% during 1984 to 1989 to 71.7% during 2000 to 2004. Median survival was significantly longer for HPV-positive than for HPV-negative patients (131 v 20 months; log-rank P < .001; adjusted hazard ratio, 0.31; 95% CI, 0.21 to 0.46). Survival significantly increased across calendar periods for HPV-positive (P = .003) but not for HPV-negative patients (P = .18). Population-level incidence of HPV-positive oropharyngeal cancers increased by 225% (95% CI, 208% to 242%) from 1988 to 2004 (from 0.8 per 100,000 to 2.6 per 100,000), and incidence for HPV-negative cancers declined by 50% (95% CI, 47% to 53%; from 2.0 per 100,000 to 1.0 per 100,000). If recent incidence trends continue, the annual number of HPV-positive oropharyngeal cancers is expected to surpass the annual number of cervical cancers by the year 2020.
Conclusion
Increases in the population-level incidence and survival of oropharyngeal cancers in the United States since 1984 are caused by HPV infection.
INTRODUCTION
Human papillomavirus (HPV) causes an epidemiologically and clinically distinct form of oropharyngeal squamous cell carcinoma (OPSCC).1–5 HPV-positive OPSCCs have risk factors related to sexual behavior whereas HPV-negative cancers are strongly associated with tobacco and alcohol use.2–6 In addition, HPV-positive patients have substantially better survival compared with HPV-negative patients.7–9
OPSCC incidence increased from 1973 to 2004 in the United States, particularly among young individuals (< 60 years of age), men, and whites.10,11 Concomitantly, population-level survival for OPSCC has improved.10 The increasing incidence and improving survival are hypothesized to arise from an increasing proportion of OPSCCs caused by HPV over calendar time.10–12 However, empirical evidence is lacking.
The population-level burden of HPV-positive OPSCC is currently unknown and may have important implications for cancer prevention, potentially through HPV vaccination. Therefore, we combined molecular epidemiologic methods that use both sensitive and specific laboratory assays with cancer surveillance methods to investigate whether changes in the population-level epidemiology of OPSCC in the United States are caused by HPV infection and to estimate the historical, current, and future population-level burden of HPV-positive OPSCCs.
PATIENTS AND METHODS
Study Design
We included specimens of OPSCC tissue (N = 271) collected by all three registries (Hawaii, Iowa, and Los Angeles, California [LA]) that participate in the Surveillance, Epidemiology, and End Results (SEER) Program's Residual Tissue Repository (RTR) Program.13,14 Since 2003, these registries have retrospectively obtained tumor specimens from pathology laboratories within their catchment areas.13 The three RTR registries have age and sex distributions similar to those of seven other registries in SEER9, but include a higher proportion of nonwhites (Data Supplement). Eligible specimens included formalin-fixed, paraffin-embedded, pathologically confirmed invasive squamous cell carcinomas of the oropharynx (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] codes C019, C024, C090-099, and C100-109).10 We also included 69 cervical cancers (1986 to 2004) as positive controls (given HPV's role as a necessary cause) and 27 gastric cancers (1984 to 2004) as negative controls.
Laboratory Analysis
After tumor presence was confirmed with hematoxylin and eosin staining, serial tissue sections were produced at the Hawaii RTR by using procedures to minimize interspecimen contamination. DNA was purified from paraffin-embedded tumor tissues as previously described.15 Total RNA was purified by using the Roche High-Pure RNA Paraffin Kit (Roche, Mannheim, Germany). Following DNase treatment, RNA was reverse transcribed to cDNA by using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Carlsbad, CA).
Because DNA and RNA from formalin-fixed, paraffin-embedded tumor specimens are known to degrade over time, laboratory assays were designed to account for this. Specimens were classified as evaluable or unevaluable for DNA amplification by polymerase chain reaction (PCR) by using a real-time TaqMan assay (Applied Biosystems) that amplified a 58 base-pair (bp) region of a control gene, human endogenous retrovirus-3 (ERV-3).15 RNA specimens were similarly classified after reverse transcription to cDNA by using a real-time TaqMan PCR assay that amplified a 73-bp region of a housekeeping gene, human ribosomal protein large P0 (RPLP0). Samples were considered evaluable if ERV-3 or RPLP0 copies were above the lower limit of assay detection (≥ three copies).
The presence of 28 HPV types was evaluated in purified DNA with PCR amplification by using the SPF10 primer system, which amplified a 65-bp region of the conserved L1 viral capsid gene, followed by reverse line blot hybridization for HPV type specification (Inno-LiPA; Innogenetics, Gent, Belgium).
Because HPV16 accounts for a majority (approximately 90% to 95%) of HPV-positive OPSCCs,1 purified tumor DNA (and cDNA) from all tumors was evaluated for the presence of HPV16 E6 DNA (and mRNA) by a real-time TaqMan PCR assay.15 Samples positive for non-HPV16 oncogenic types (eg, 18, 26, 31, 33, 35, 39, 45, 52, and 58) by Inno-LiPA were similarly evaluated by using real-time TaqMan assays targeted to the E6 or E7 gene.16 The number of HPV viral copies was normalized to the number of cells in the PCR reaction (ERV-3 DNA copies) and adjusted for the proportion of tumor in the specimen. Samples with a viral load of at least 1.0 copy per cell were considered positive. Evaluable samples with any detectable HPV E6 or E7 mRNA were considered positive for viral oncogene expression.
All tumor samples were evaluated for HPV16 by using an in situ hybridization–catalyzed signal amplification method for biotinylated probes (Genpoint; Dako, Copenhagen, Denmark).7 An HPV-positive tumor was defined as specific staining of tumor cell nuclei for HPV. Samples were also evaluated by using immunohistochemistry for expression of a surrogate biomarker of HPV E7 oncoprotein function, the cdk inhibitor p16.7 Samples with diffuse nuclear and cytoplasmic staining in ≥ 70% of tumor cells were considered positive (interpreted by one pathologist [R.C.J.]).
Statistical Analyses
HPV assays were analyzed separately. The κ statistic was used to assess agreement across assays. Characteristics of HPV-positive and HPV-negative patients were compared by using a χ2 test. Calendar year of diagnosis was categorized into four periods: 1984 to 1989, 1990 to 1994, 1995 to 1999, and 2000 to 2004. Conclusions remained unchanged in sensitivity analyses when using single-year or 2-year categories. Trends in HPV prevalence across calendar periods were evaluated by using logistic regression, after adjustment for age, sex, race, and registry.
We anticipated loss in assay sensitivity because of the age of specimens beyond the exclusion of unevaluable samples. Therefore, HPV prevalence in 69 cervical cancers was used to estimate sensitivity of each laboratory assay across calendar periods, assuming all cervical cancers were HPV-positive (Data Supplement). These calendar period–specific sensitivities were used to randomly resample HPV-negative tissues and assign them as HPV-positive to correct the observed prevalence (10 imputations). Trends in corrected prevalence were evaluated by using multiple imputation methods.17
Univariable differences in overall survival were evaluated by using the Kaplan-Meier method and the log-rank test. Cox regression was used to evaluate differences in overall survival by HPV status, after adjustment for age, sex, race, registry, calendar period, stage at cancer diagnosis (per SEER classification as localized, regional, or distant14), and primary course of cancer-directed therapy14: radiotherapy, surgery, and chemotherapy. Statistical interactions were evaluated through product terms.
We conducted analyses to account for the nonrandom selection of the 271 tested patients. Given the availability of demographic characteristics of both selected and unselected patients with OPSCC within the three cancer registries, we estimated sampling fractions for inclusion into our study (specific to age group, sex, race, registry, and calendar year [2-year groups]). The inverse of these sampling probabilities were used as weights to reweight the observed HPV prevalence in tested OPSCCs to all patients within Hawaii, Iowa, and LA (1988 to 2004; periods preceding 1988 were excluded because of sparse data). The reweighted, calendar period–specific, population-level HPV prevalence was applied to the total number of OPSCCs to calculate incidence rates separately for HPV-positive and HPV-negative OPSCCs. Bootstrap standard errors around weighted HPV prevalence were used in combination with the delta method to estimate asymptotically normal 95% CIs for percent change in HPV-positive and HPV-negative OPSCC incidence.
The future burden of oropharyngeal, other head and neck, and cervical cancers was projected through 2030 by using observed age-specific incidence trends (1973 to 2007; ages 30 to 84 years) from nine cancer registries within the SEER program in age-period-cohort models (Data Supplement).14,18–20 Potential reductions in cervical cancer incidence from HPV vaccination were not considered to provide a conservative contrast with OPSCC burden. Projected incidence rates were applied to the 2008 US Census population projections21 to estimate the annual number of patients.
All results (HPV prevalence across time and survival comparisons) are based on the 271 tested OPSCC specimens, except incidence rates for HPV-positive and HPV-negative OPSCCs, which were based on reweighted analyses. Results are presented primarily for the Inno-LiPA assay since conclusions were similar across assays. Detailed results for HPV16 viral load, HPV16 oncogene expression, HPV16 in situ hybridization (ISH), and p16 are presented in the DataSupplement.
RESULTS
We included tumors from 271 (4.7%) of 5,755 patients diagnosed with OPSCCs from 1984 to 2004 in Hawaii, Iowa, and LA. Patients included in our study were similar in age and stage compared with those not included but were more likely to be from Hawaii, male, of other races, and be diagnosed during the 1990s (P < .001; Table 1).
Table 1.
Characteristics of Patients With Oropharyngeal Cancer From Hawaii, Iowa, and Los Angeles Included or Not Included in the Study (1984-2004)
| Characteristic | Included in the Study (n = 271) |
Not Included in the Study (n = 5,484) |
χ2P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .18 | ||||
| < 50 | 51 | 18.8 | 880 | 16.0 | |
| 50-59 | 83 | 30.6 | 1,480 | 27.0 | |
| 60-69 | 78 | 28.8 | 1,675 | 3.5 | |
| 70+ | 59 | 21.8 | 1,449 | 26.4 | |
| Sex | .01 | ||||
| Male | 217 | 80.1 | 4,019 | 73.3 | |
| Female | 54 | 19.9 | 1,465 | 26.7 | |
| Race | < .001 | ||||
| White | 171 | 63.1 | 4,541 | 82.8 | |
| Black | 37 | 13.7 | 587 | 1.7 | |
| Other* | 63 | 23.2 | 356 | 6.5 | |
| Calendar years | < .001 | ||||
| 1984-1989 | 52 | 19.2 | 1,440 | 26.3 | |
| 1990-1994 | 78 | 28.8 | 1,198 | 21.8 | |
| 1995-1999 | 95 | 35.0 | 1,236 | 22.5 | |
| 2000-2004 | 46 | 17.0 | 1,610 | 29.4 | |
| Registry | < .001 | ||||
| Hawaii | 117 | 43.2 | 436 | 7.9 | |
| Iowa | 33 | 12.2 | 1,209 | 22.1 | |
| Los Angeles, California | 121 | 44.6 | 3,839 | 7.0 | |
| Stage | .16 | ||||
| Localized | 78 | 29.9 | 1,721 | 33.6 | |
| Regional | 155 | 59.4 | 2,741 | 53.4 | |
| Distant | 28 | 10.7 | 667 | 13.0 | |
NOTE. Oropharyngeal cancers included those arising from the base of tongue (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3]; code C019), lingual and palatine tonsil (C024 and C090-099), and oropharynx (C100-109). Patients were restricted to those with squamous cell histologies (ICD-O-3 codes 8050-8076, 8078, 8083, 8084, and 8094).
Includes all other races and ethnicities (Asian, n = 1; Chinese, n = 8; Filipino, n = 17; Hawaiian, n = 12; Japanese, n = 15; Korean, n = 2; Pacific Islander, n = 4; Vietnamese, n = 1; and other, n = 3).
Of the 271 oropharyngeal specimens, 271 (100%) were evaluable for HPV16 ISH, 263 (97.0%) for Inno-LiPA and viral load, and 216 (80.0%) for viral oncogene expression. The median proportion of tumor in sections was 60% (interquartile range [IQR], 30% to 70%). HPV prevalence in oropharyngeal tumors was 44.1% by Inno-LiPA (38.8% for HPV16), 30.8% by HPV16 viral load, 35.2% by HPV16 oncogene expression, and 28.0% by HPV16 ISH. Type distribution among 116 Inno-LiPA–positive tumors was HPV16 (87.9%; n = 102); HPV35 (3.4%; n = 4); HPV33 and HPV58 (2.6%; n = 3 each); HPV18, HPV26, and HPV52 (1.7%; n = 2 each); and HPV45 (0.9%; n = 1).
Agreement across laboratory assays for HPV16 ranged from good to excellent (Data Supplement). Among 102 Inno-LiPA HPV16-positive specimens, 77.5% were positive by the viral load criterion (median, 22.1 HPV16 copies per cell; IQR, 1.2 to 106.4 copies), and 84.5% were positive by the viral oncogene expression criterion (median, 152.9 transcripts per 1,000 RPLP0 equivalents; IQR, 43.7 to 451.8 transcripts). Of 16 tumors positive for non-HPV16 types by Inno-LiPA, 50.0% and 64.3% were positive by viral load and oncogene expression, respectively. Of 66 evaluable cervical cancers, 59 (89.4%) were Inno-LiPA–positive. Median HPV16 viral load and oncogene transcript levels were similar between oropharyngeal and cervical cancers (Wilcoxon P = .40 and .14, respectively; Data Supplement), supporting the use of cervical cancers for sensitivity corrections. Of 27 gastric cancers, two were positive by Inno-LiPA alone.
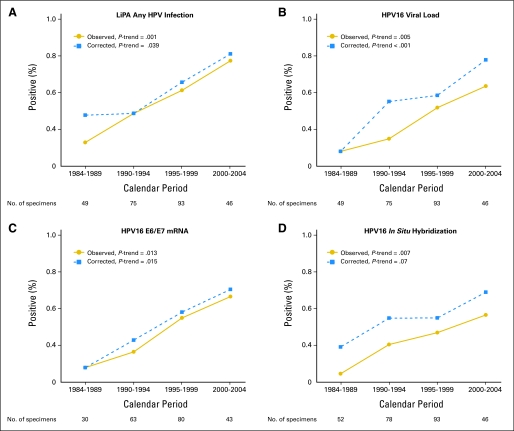
HPV prevalence in oropharyngeal tumors significantly increased across calendar periods for all assays (Fig 1 and Data Supplement; all P trend < .05). For example, HPV prevalence by Inno-LiPA increased more than four-fold from 16.3% during 1984 to 1989 to 72.7% during 2000 to 2004. These increases remained significant even after corrections for potential loss in sensitivity (Fig 1). After such correction, HPV prevalence during 2000 to 2004 was approximately 70% (76.3% by Inno-LiPA [70.9% for HPV16], 72.2% by HPV16 viral load, and 63.0% by HPV16 oncogene expression).
Fig 1.
Prevalence of human papillomavirus (HPV) infection in oropharyngeal cancers across four calendar periods (1984 to 1989, 1990 to 1994, 1995 to 1999, and 2000 to 2004) is shown for any HPV infection as determined by (A) the Inno-LiPA assay, (B) HPV16 viral load, (C) HPV16 E6/E7 oncogene expression, and (D) HPV16 in situ hybridization. Solid lines and circles represent observed prevalence estimates. Dotted lines and squares represent prevalence estimates corrected for potential loss in assay sensitivity because of the age of the specimens. The number of specimens evaluable for each assay is shown below the x-axis.
HPV-positive patients diagnosed from 1984 to 2004 were significantly younger (χ2 P < .001), more likely to be male (P = .03), and more likely to be white or of other races (P = .003; Data Supplement). Increasing HPV prevalence across calendar periods was also most apparent among younger patients, men, and whites and other races (Data Supplement).
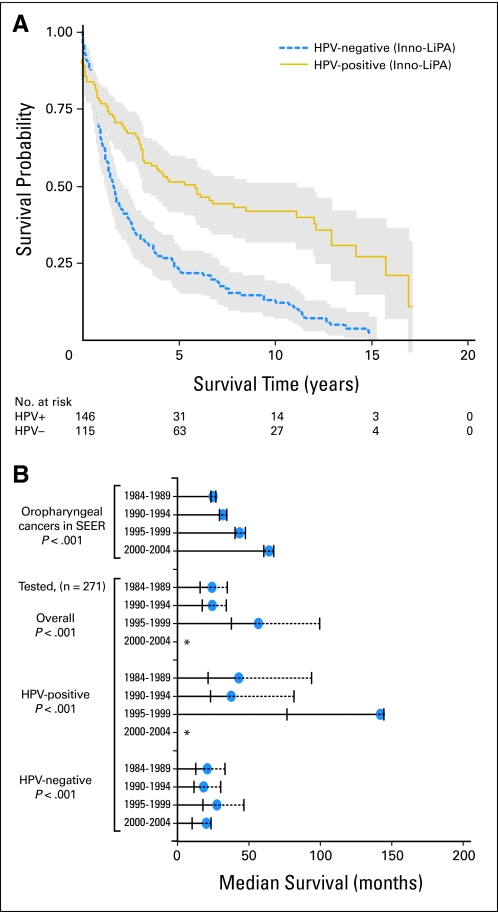
Analogous to the improved survival (2 to 5 years) of HPV-positive patients with OPSCCs observed in clinical trials,7–9,22 in our study, long-term survival (over 15 years after cancer diagnosis) of HPV-positive patients was significantly better than that for HPV-negative patients (62 v 135 deaths; median survival, 131 v 20 months; log-rank P < .001; Fig 2A; Data Supplement). The following were independent predictors of decreased survival: older age, diagnosed during earlier calendar periods, advanced stage, lack of surgery or radiotherapy, and receipt of chemotherapy (Data Supplement). After adjustment for these factors, HPV-positive patients had a 69% reduction in risk of death compared with HPV-negative patients (hazard ratio [HR], 0.31; 95% CI, 0.21 to 0.46; Data Supplement), and risk was similar for HPV16 (HR, 0.31; 95% CI, 0.21 to 0.47) and non-HPV16 Inno-LiPA–positive patients (HR, 0.31; 95% CI, 0.14 to 0.72). The difference in survival between HPV-positive and HPV-negative patients was greater for patients treated by radiation (HR, 0.23; 95% CI, 0.09 to 0.59) than for those not treated by radiation (HR, 0.80; 95% CI, 0.40 to 1.60; P interaction = .002).
Fig 2.
Follow-up for overall survival began on the date of cancer diagnosis and ended at death or the last day of follow-up (November 1, 2009, for Hawaii; December 31, 2007, for Iowa; and May 31, 2010, for Los Angeles). Median follow-up among surviving patients was 112 months (120 for human papillomavirus–negative [HPV−] and 110 for HPV-positive [HPV+] patients). (A) Kaplan-Meier survival estimates and 95% CIs (shaded area) for patients with HPV+ (solid line) and HPV− (dashed line) oropharyngeal cancer, as determined by the Inno-LiPA assay. (B) Median Kaplan-Meier survival estimates in months (circles) and 95% bootstrap CIs (vertical lines) across four calendar periods for oropharyngeal cancers in 17 registries in the Surveillance, Epidemiology, and End Results (SEER) program and for the 271 tested oropharyngeal cancers, including survival for all oropharyngeal cancers, HPV+ oropharyngeal cancers, and HPV− oropharyngeal cancers. (*) Median survival has not yet been reached. Median survival of oropharyngeal cancers in SEER17 was 25 months during 1984 to 1989, 31.9 months during 1990 to 1994, 43.6 months during 1995 to 1999, and 64.1 months during 2000 to 2004. Median survival of tested patients who had oropharyngeal cancer was 24.1 months during 1984 to 1989, 24.4 months during 1990 to 1994, 56.7 months during 1995 to 1999, and was not yet reached during 2000 to 2004. Median survival of patients who had HPV+ oropharyngeal cancer was 43 months during 1984 to 1989, 37.6 months during 1990 to 1994, 142.2 months during 1995 to 1999, and was not yet reached during 2000 to 2004. Median survival of patients who had HPV− oropharyngeal cancer was 20.9 months during 1984 to 1989, 18.3 months during 1990 to 1994, 27.7 months during 1995 to 1999, and 20.2 months during 2000 to 2004.
The survival benefit of HPV-positive OPSCCs manifested at the population-level as increased overall survival for OPSCCs across calendar periods in the SEER data and also in the 271 tested cases (Fig 2B; P < .001). This improvement in overall survival for OPSCCs across calendar periods, in part, arose from increasing median survival for HPV-positive patients across calendar periods (log-rank P < .001), although survival for HPV-negative patients remained unchanged (log-rank P = .67; Fig 2B; P interaction = .03).
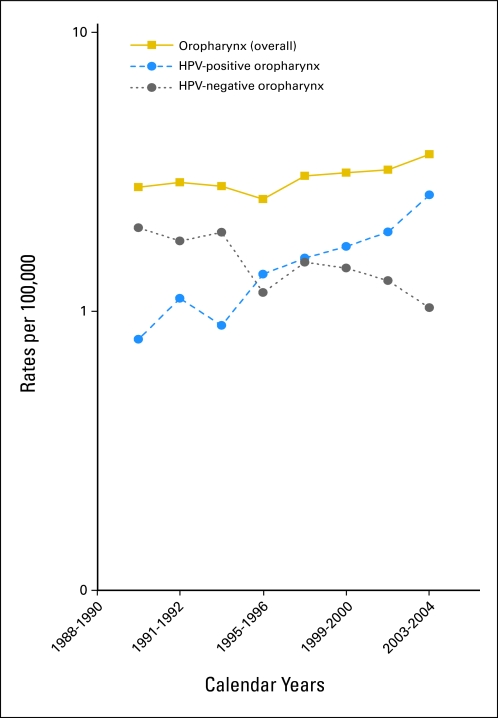
In analyses reweighted to the Hawaii, Iowa, and LA populations, incidence rates for HPV-positive OPSCCs increased by 225% (95% CI, 208% to 242%) during 1988 to 2004, whereas incidence of HPV-negative OPSCCs significantly declined by 50% (95% CI, 47% to 53%; Fig 3). Consequently, overall incidence of OPSCCs increased by 28% during 1988 to 2004. Of note, trends in OPSCC incidence during 1988 to 2004 in these three registries were similar to trends in other registries included in SEER9 (Data Supplement). After reweighting, the prevalence of HPV in all OPSCCs in Hawaii, Iowa, and LA during 2003 to 2004 was approximately 70%, consistent with our measured estimate.
Fig 3.
Incidence rates for overall oropharyngeal cancer, human papillomavirus (HPV)–positive oropharyngeal cancers, and HPV-negative oropharyngeal cancers during 1988 to 2004 in Hawaii, Iowa, and Los Angeles. Incidence rates for HPV-positive oropharyngeal cancers increased from 0.8 per 100,000 during 1988 to 1990 to 2.6 per 100,000 during 2003 to 2004. Incidence rates for HPV-negative oropharyngeal cancers significantly declined from 2.0 per 100,000 during 1988 to 2004 to 1.0 per 100,000 during 2003 to 2004. Overall incidence of oropharyngeal cancers increased from 2.8 per 100,000 during 1988 to 1990 to 3.6 per 100,000 during 2003 to 2004.
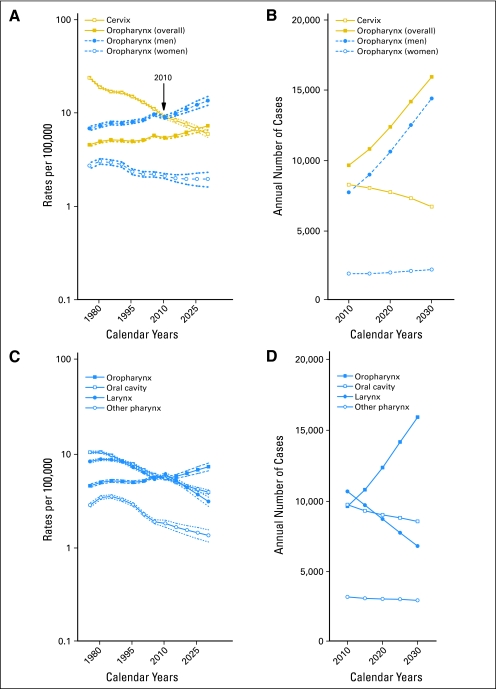
The future burden of OPSCCs through 2030 was estimated by projecting observed incidence rates from nine SEER registries and was compared with the burden of cervical cancer (the current focus of HPV vaccine prevention efforts) and other head and neck cancers. It is estimated that by 2010, OPSCC incidence among men will have surpassed the incidence of cervical cancers (Fig 4A). Likewise, the annual number of OPSCCs among men and women has already surpassed the number of cervical cancers (Fig 4B). By 2020, even under a conservative assumption of 70% of OPSCCs being HPV-positive, the annual number of HPV-positive OPSCCs (approximately 8,700 patients) will surpass the annual number of cervical cancers (approximately 7,700 patients), with a majority occurring among men (approximately 7,400 patients). In addition, OPSCCs will likely constitute a majority (approximately 47%) of all head and neck cancers by 2030 (Figs 4C and 4D).
Fig 4.
(A) Observed and projected incidence rates and bootstrap 95% CIs (ages 30 to 84 years) for oropharyngeal cancers overall (solid squares), oropharyngeal cancers among men (solid circles), oropharyngeal cancers among women (open circles), and cervical cancers (open squares). (B) Projected annual number of patients (ages 30 to 84 years) of oropharyngeal cancers overall, oropharyngeal cancers among men, oropharyngeal cancers among women, and cervical cancers through the year 2030. (C) Observed and projected incidence rates for oropharyngeal (solid squares), oral cavity (open squares), larynx (solid circles), and other pharynx (open circles) cancers. (D) Projected annual number of patients with oropharyngeal, oral cavity, laryngeal, and other pharynx cancers through the year 2030. Observed incidence rates during 1973 to 2007 from nine registries within the Surveillance, Epidemiology, and End Results (SEER) program were used in age-period-cohort models to project expected incidence through the year 2030. Projected incidence rates were applied to the 2008 US population projections to calculate the annual number of patients. Oropharyngeal cancers included base of tongue (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] topography code C019), lingual tonsil (C024), soft palate not otherwise specified (NOS; C051), uvula (C052), tonsil (C090-099), oropharynx (C100-109), and Waldeyer ring (C142). Oral cavity cancers included lip (C000-009), oral tongue (C020-23, C028, and C029), gum (C030-039), floor of mouth (C040-049), hard palate (C051, C058, and C059), and other and unspecified parts of the mouth (C060-069). Laryngeal cancers included glottis (C320), supraglottis (C321), subglottis (C322), laryngeal cartilage (C323), overlapping lesion of larynx (C328), and larynx NOS (C329). Other pharynx cancers included nasopharynx (C110-119), pyriform sinus (C129), postcricoid region (C130), hypopharynx (C130-139), and pharynx NOS (C140 and C148). Oropharyngeal cancers included both HPV-related and HPV-unrelated (soft palate NOS and uvula) anatomic subsites because projections were conducted for all head and neck cancer sites. Oropharyngeal, oral cavity, laryngeal, and other pharynx cancers were restricted to squamous cell histologies (ICD-O-3 codes 8050-8076, 8078, 8083, 8084, and 8094). Cervical cancers (C530-539) included all histologic subtypes.
DISCUSSION
Our study provides strong evidence that recent changes in population-level incidence and survival of OPSCCs in the United States are caused by HPV infection. The overall rise in OPSCC incidence during 1984 to 2004 is largely explained by the increasing incidence of HPV-positive cancers, whereas incidence of HPV-negative cancers declined. Consequently, HPV prevalence in oropharyngeal tumors increased substantially from 16.3% during the 1980s to 72.7% during the 2000s. Significant improvements in OPSCC survival over time arise from the long-term survival advantage of HPV-positive patients.
Our study demonstrates how the knowledge and ability to classify phenotypically identical cancers as etiologically distinct can shed light on changes in population-level incidence and survival. The rapid changes in OPSCC morbidity and mortality during a relatively short period of 20 years perhaps arise from coincidental changes in smoking and sexual behaviors.
The declining incidence of HPV-negative OPSCCs parallels declines in smoking in the United States.23 In contrast, increasing incidence of HPV-positive OPSCCs perhaps arises from increased oral sex and oral HPV exposure over calendar time. Indeed, prevalence of genital herpes simplex virus 1 (HSV1), HSV2, and genital warts have increased among recent birth cohorts in the United States,24–26 accepted surrogates for oral sex, risky sexual behavior, and HPV exposure, respectively. The predominant rise in OPSCC incidence among the young is also consistent with changing HPV exposure among recent birth cohorts.10 However, the reasons for pronounced increases among men and whites remain unexplained.
Analogous increases in HPV-positive oropharyngeal tumors have been reported in Sweden.27 The unique aspects of our study are the use of multiple assays to determine tumor HPV status while accounting for degradation of DNA and RNA in specimens. By using the most sensitive assay for HPV detection in paraffin-embedded tissues (Inno-LiPA) and the most specific assay to determine HPV etiology in tumors (E6/E7 mRNA expression), we conclude that approximately 70% of OPSCCs are now caused by HPV infection.
Our data extend the previously reported survival benefit of HPV-positive OPSCCs 7–9,22 by showing that superior survival persists beyond 15 years after cancer diagnosis and that this survival benefit manifests at the population level. In addition, a novel observation of our study is the improving survival of HPV-positive patients with OPSCC in recent calendar periods. Our data support at least three sources for population-level increases in OPSCC survival during the late 1990s and the 2000s: first, the increasing proportion of HPV-positive cancers discussed herein; and second, increasing survival of HPV-positive patients with OPSCC potentially from reductions over time in tobacco exposure, an important predictor of survival.7 Indeed, median tobacco exposure among HPV-positive patients with OPSCC in U.S. cooperative group trials decreased from 29 pack-years during 1991 to 1997 to 14 pack-years during 2001 to 2005.7,28 Third, increasing survival of HPV-positive patients with OPSCC could be potentially from period effects, improved radiation delivery, and/or the introduction of concurrent chemoradiotherapy around 1999 to 2000.29 Indeed, we observed better survival among HPV-positive patients who were treated by radiation compared with those who were not, consistent with enhanced sensitivity of HPV-positive OPSCCs to chemoradiotherapy.22
The primary limitations of our study include the small size, nonrepresentativeness of the tested patients, and potential nongeneralizability of observations from Hawaii, Iowa, and LA to the US population. Nevertheless, because this study was performed within cancer registries, we were able to address nonrepresentativeness by reweighting our observed HPV prevalence to all patients with OPSCC within the three registries after accounting for differences in age, sex, race, and calendar years between the tested and untested patients. Notably, HPV prevalence in OPSCCs and incidence of HPV-positive OPSCCs increased across calendar periods, even after accounting for the nonrandom selection of patients, thus arguing against a major bias. The similarity in OPSCC incidence trends between Hawaii, Iowa, and LA and other SEER registries supports generalizability to the US population. In addition, we lacked detailed data on cancer therapy (including radiation method and dose or chemotherapy regimen), performance status, and smoking status of patients with OPSCC because these data are not collected by cancer registries.14 Nevertheless, our primary aims were to evaluate whether the previously reported survival benefit of HPV-positive patients with OPSCC manifests at the population level and to compare survival of HPV-positive and HPV-negative OPSCCs across calendar periods. Indeed, associations of specific therapies with survival of OPSCCs are best addressed in the context of clinical trials.8,9,22
The rapidly growing burden of HPV-positive OPSCCs in the United States has important public health and clinical implications. By 2020, the number of HPV-positive OPSCCs is expected to surpass the number of cervical cancers, the focus of prophylactic HPV vaccination. This focus is justified given the substantially higher burden of cervical precancers compared with invasive cervical cancers or OPSCCs in the United States. Indeed, invasive cervical cancer incidence rates would be substantially higher in the absence of screening. Nevertheless, the rising burden of HPV-positive OPSCCs argues for evaluation of the efficacy of vaccination to prevent oral HPV infections, particularly given the unavailability of screening for OPSCCs. The high efficacy of HPV vaccines in preventing extracervical infections among women (eg, vagina and vulva)30 and penile and anal infections among men31,32 implies that efficacy may be comparable against oral HPV infections. Assuming equivalent efficacy, reevaluation of the cost-effectiveness of male HPV vaccination may also be warranted, given the predominant burden of HPV-positive OPSCCs among men. Current cost-effectiveness analyses have underestimated the proportion of OPSCCs caused by HPV infection (approximately 31%) and have considered only the current burden of OPSCCs.33–36
HPV-positive OPSCCs will likely constitute a majority of all head and neck cancers in the United States in the next 20 years, highlighting the need for defined therapies for this patient population. Although these studies are underway, research is also needed to improve the historically poor survival of patients with HPV-negative OPSCC.
Supplementary Material
Footnotes
See accompanying editorial on page 4222; listen to the podcast by Dr Gillison at www.jco.org/podcast
Supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health; The James Comprehensive Cancer Center; The Ohio State University; and the Oral Cancer Foundation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Brenda Y. Hernandez, Merck (C); Maura L. Gillison, Merck (C), GlaxoSmithKline (C), Amgen (C), Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Brenda Y. Hernandez, Merck Research Funding: Brenda Y. Hernandez, Merck; Maura L. Gillison, Merck Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Anil K. Chaturvedi, Eric A. Engels, Nicolas Wentzensen, Maura L. Gillison
Financial support: Anil K. Chaturvedi, Maura L. Gillison
Provision of study materials or patients: Brenda Y. Hernandez, Marc T. Goodman, Maria Sibug-Saber, Wendy Cozen, Charles F. Lynch
Collection and assembly of data: Anil K. Chaturvedi, Brenda Y. Hernandez, Weihong Xiao, Esther Kim, Bo Jiang, Marc T. Goodman, Maria Sibug-Saber, Wendy Cozen, Lihua Liu, Charles F. Lynch, Richard C. Jordan, Sean Altekruse, Maura L. Gillison
Data analysis and interpretation: Anil K. Chaturvedi, Eric A. Engels, Ruth M. Pfeiffer, Weihong Xiao, William F. Anderson, Philip S. Rosenberg, Maura L. Gillison
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 2.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 3.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 4.Smith EM, Ritchie JM, Summersgill KF, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 11.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998-2003. Cancer. 2008;113:2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 12.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: Summary of a National Cancer Institute State of the Science Meeting, November 9-10, 2008, Washington, D.C. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 13.Goodman MT, Hernandez BY, Hewitt S, et al. Tissues from population-based cancer registries: A novel approach to increasing research potential. Hum Pathol. 2005;36:812–820. doi: 10.1016/j.humpath.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2009 Sub (1973-2007) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2007 Counties. http://seer.cancer.gov/seerstat/ [Google Scholar]
- 15.Koshiol J, Rottuno M, Gillison ML, et al. Assessment of human papillomavirus in lung tumor tissue. J Natl Cancer Inst. 2011;103:501–507. doi: 10.1093/jnci/djr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhry C, Sugar E, D'Souza G, et al. Two-week versus six-month sampling interval in a short-term natural history study of oral HPV infection in an HIV-positive cohort. PLoS One. 2010;5:e11918. doi: 10.1371/journal.pone.0011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- 18.Bray F, Møller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6:63–74. doi: 10.1038/nrc1781. [DOI] [PubMed] [Google Scholar]
- 19.Peto J, Decarli A, La Vecchia C, et al. The European mesothelioma epidemic. Br J Cancer. 1999;79:666–672. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson WF, Rosenberg PS, Menashe I, et al. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100:1804–1814. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Population Division, U.S. Census Bureau. Projections of the Population by Selected Age Groups and Sex for the United States: 2010 to 2050 (NP2008-T2) Release Date: August 14, 2008. http://www.census.gov/population/www/projections/2008projections.html.
- 22.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults: United States 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 24.Fleming DT, McQuillan GM, Johnson RE, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 25.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control (CDC) Condyloma acuminatum: United States, 1966-1981. MMWR Morb Mortal Wkly Rep. 1983;32:306–308. [PubMed] [Google Scholar]
- 27.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 28.Gillison ML, Zhang Q, Ang K, et al. Analysis of the effect of p16 and tobacco pack-years (p-y) on overall (OS) and progression-free survival (PFS) for patients with oropharynx cancer (OPC) in Radiation Therapy Oncology Group (RTOG) protocol 9003. J Clin Oncol. 2010;28(suppl):423s. abstr 5510. [Google Scholar]
- 29.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 30.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palefsky JM. Efficacy of the quadrivalent HPV vaccine to prevent anal intraepithelial neoplasia among young men who have sex with men. Proceedings of the 26th International Papillomavirus Conference; July 3-8, 2010; Montreal, Quebec, Canada. [Google Scholar]
- 33.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. doi: 10.1136/bmj.b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JJ. Focus on research: Weighing the benefits and costs of HPV vaccination of young men. N Engl J Med. 2011;364:393–395. doi: 10.1056/NEJMp1012246. [DOI] [PubMed] [Google Scholar]
- 36.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:500–507. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.