Abstract
Purpose
The survival of patients with non–small-cell lung cancer (NSCLC), even when resectable, remains poor. Several small studies suggest that occult metastases (OMs) in pleura, bone marrow (BM), or lymph nodes (LNs) are present in early-stage NSCLC and are associated with a poor outcome. We investigated the prevalence of OMs in resectable NSCLC and their relationship with survival.
Patients and Methods
Eligible patients had previously untreated, potentially resectable NSCLC. Saline lavage of the pleural space, performed before and after pulmonary resection, was examined cytologically. Rib BM and all histologically negative LNs (N0) were examined for OM, diagnosed by cytokeratin immunohistochemistry (IHC). Survival probabilities were estimated using the Kaplan-Meier method. The log-rank test and Cox proportional hazards regression model were used to compare survival of groups of patients. P < .05 was considered significant.
Results
From July 1999 to March 2004, 1,047 eligible patients (538 men and 509 women; median age, 67.2 years) were entered onto the study, of whom 50% had adenocarcinoma and 66% had stage I NSCLC. Pleural lavage was cytologically positive in only 29 patients. OMs were identified in 66 (8.0%) of 821 BM specimens and 130 (22.4%) of 580 LN specimens. In univariate and multivariable analyses OMs in LN but not BM were associated with significantly worse disease-free survival (hazard ratio [HR], 1.50; P = .031) and overall survival (HR, 1.58; P = .009).
Conclusion
In early-stage NSCLC, LN OMs detected by IHC identify patients with a worse prognosis. Future clinical trials should test the role of IHC in identifying patients for adjuvant therapy.
INTRODUCTION
Lung cancer remains the most frequent cause of cancer-related deaths worldwide, with an estimated survival of only 15% at 5 years.1 Even patients with stage I non–small-cell lung cancers (NSCLC) experience a 30% risk of recurrence after resection.2 However, outcomes have recently improved for specific groups of patients with NSCLC. Several randomized controlled trials now show that patients with lymph node (LN) metastases experience a survival benefit from the addition of chemotherapy after resection.3–5 Thus, it is increasingly important to identify patients with early-stage NSCLC who may benefit from combined-modality treatment.
Defining patients with early-stage cancer at increased risk for tumor progression is a major challenge. Efforts to detect the earliest dissemination of tumor before identification by routine pathologic or clinical methods, so-called occult metastases (OMs), led to studies in several cancers indicating that the detection of regional and/or hematogenous OMs can identify patients at increased risk for developing overt metastases.6–10 Variable terminology and definitions have been used to describe such small metastatic foci, including micrometastases, disseminated tumor cells, isolated tumor cells, circulating tumor cells, minimal residual disease, and OM. For this trial, we chose the term occult metastases (OMs) because it globally describes metastases that are not diagnosed by standard clinical and pathologic methods in all three compartments in the study.11
In NSCLC, several studies suggest that OMs identify patients who have a poor prognosis after resection. Sites of OM reported to have an adverse impact on survival in resectable NSCLC include the pleura (as diagnosed by lavage of the pleural space), the bone marrow (BM), and intrathoracic LNs.2,4,12–23 However, previous studies were small and retrospective, used varying methodologies to identify OM, and did not examine these three potential sites of OM simultaneously. We undertook this prospective multicenter clinical trial to determine the prevalence of OM in the pleural space, BM, and intrathoracic LNs in patients undergoing resection of NSCLC and to determine whether the presence of OM was associated with a worse survival.
PATIENTS AND METHODS
Eligibility Criteria
Eligible patients had known or suspected, previously untreated, resectable stage I to IIIb NSCLC. Patients were excluded if they had had other malignancies or an ipsilateral thoracotomy or thoracoscopy within the previous 5 years. Computed tomography of the chest and upper abdomen was required before registration to the study. Additional imaging studies were performed at the discretion of the treating physician but were not required. This trial was approved by the institutional review boards of all participating institutions, and all patients gave informed consent.
Study Interventions
At surgery, a tissue diagnosis of NSCLC was confirmed if this had not been done preoperatively. Patients went off study if they did not have NSCLC or had technically unresectable disease.
At thoracotomy or video-assisted thoracic surgery, a 3- to 4-cm long segment of rib was resected, placed into RPMI 1640 medium, and shipped at room temperature by overnight mail to the reference laboratory (University of Southern California [USC], Los Angeles, CA). After incision of the pleura, 250 mL of saline was instilled into the pleural cavity before manipulation of the lung, and the patient was gently rocked to ensure distribution throughout the pleural space. The lavage fluid was aspirated, and a 20-mL aliquot was added to each of two 50-mL tubes already containing 50% ethyl alcohol. A second lavage was performed after the pulmonary resection, and these specimens were shipped overnight to USC.
The primary tumor was removed via sublobar resection (wedge resection or segmentectomy), lobectomy, bilobectomy, or pneumonectomy, with or without bronchial sleeve resection and adjacent involved organs such as the chest wall or pericardium. A systematic LN sampling or dissection was required.24 LNs were labeled according to the Mountain-Dresler modification of the American Thoracic Society lymph node map.25 The primary tumor and the LNs were examined by the institutional pathologist using standard histologic techniques. Tumors were staged according to the sixth edition of the American Joint Commission on Cancer staging manual.26 All LNs (both N1 and N2) from patients with no evidence of LN metastases by hematoxylin and eosin staining (N0) were sent to USC and examined for OMs.
Postoperatively, all patients were observed until death or for 5 years. Patients were seen every 4 months during the first year, every 6 months during the second and third years, and annually thereafter. A history, physical examination, and chest x-ray were required at follow-up visits. Additional studies were performed at the discretion of the treating physician to evaluate signs and symptoms of recurrent disease. Because adjuvant therapy was not known to alter survival at the time this study was designed, we did not collect data on the use of adjuvant chemotherapy or radiotherapy.
Data Quality Control
Operative summaries and pathology reports for all patients entered onto the study were reviewed by the study chair (V.W.R.) to verify patient eligibility, tumor histology, the operation performed, the completeness of resection, and the tumor TNM stage.
Methods for Processing Pathology Specimens
Pleural lavage samples.
At USC, pleural lavage samples were processed according to standard cytologic techniques and were read by a single cytopathologist (S.E.M.).
BM specimen processing.
The BM was curetted into RPMI 1640, and cells were processed as previously described.17 Briefly, after density gradient separation, 1 million cells were centrifuged onto ProbeOn (Fisher Scientific, Hampton, NH) glass slides, air dried and acetone fixed, and stored at −20°C. Four slides (approximately 4 million mononuclear cells) from each specimen were assessed by immunohistochemistry (IHC) for OM. When less than 4 million mononuclear cells were recovered, all available material was assessed.
Cytokeratin IHC.
IHC was performed on BM and LN slides as previously described17,27 using anticytokeratin antibodies CAM 5.2 (Becton Dickinson, San Jose, CA) and AE-1 (Signet Laboratories, Dedham, MA). LN slides were deparaffinized and subjected to antigen retrieval.28 For both LN and BM slides, aviden-biotin IHC was performed, using as the chromogen Vector Red (Vector Laboratories, Burlingame, CA) for BM and AEC (Sigma-Aldrich, St Louis, MO) for LN. Positive controls for BM were cytospun breast cancer cell lines, and positive controls for LN were paraffin sections of NSCLC. In all cases, lymphoid cells served as internal controls.
Assessment of the BM and LN IHC.
All IHC-stained BM and LN slides were reviewed for the presence of cytokeratin-positive cells. All cells with the appropriate color (red for BM and red-brown for LN) were identified and assessed for morphologic characteristics of malignancy (size, nuclear pleomorphism, and increased nuclear-to-cytoplasmic ratio). All LN and BM slides in which any candidate cells were detected were rereviewed by a second pathologist; in addition, more than 10% of negative slides were randomly selected to be rereviewed by the second pathologist. All BM slides containing IHC-positive and/or suspicious cells were sent to the National Institutes of Health (NIH) for rereview by a cytopathologist. In instances in which the USC laboratory did not agree with the NIH assessment, an additional blinded review by a third pathologist was performed. Only samples in which two or more reviewers agreed that tumor cells were present were finally assessed as positive. The total number of tumor cells present was recorded for all positive samples. Concordance in interpretation was reached in all cases. Overall, the final interpretation agreed with the original interpretation from USC in 91% of samples; only 5% of samples showed major discordance (positive v negative) and all of these were resolved on further review.
For the LNs, more than 55% of slides underwent rereview. For slides in which there was not a consensus, the slides were rereviewed by both initial observers. In rare cases in which consensus was still not reached, a third pathologist served as the arbitrator. Only those slides in which two or more observers deemed OMs were present were assessed as positive. For all positive slides, the number of LNs containing OMs as well as OM number and location (capsular lymphatics, subcapsular sinus, medullary sinus, or parenchyma) were recorded and submitted to the American College of Surgeons Oncology Group Statistical Center. The pathologists at USC and at the NIH were blinded to all clinical information.
Statistical Considerations
The primary objective of the study was to evaluate the relationship between survival and markers of OM. The primary end point was overall survival, defined as the time period between patient registration and death.
Trial size computations are performed for a type I error probability of P = .05 (two-sided), an accrual rate of 300 patients per year for 4 years, and a follow-up interval of 5 years. The clinically consequential hazard ratio (HR; positive marrow death hazard rate over negative marrow death hazard rate) is considered to be in the range of 1.35.
For all patients, the estimated prevalence of an LN positive result by IHC is 20%. The estimated prevalence of a BM positive result is 30%. The estimated prevalence of a pleural fluid lavage positive result is expected to be between 20% and 30%. For a prevalence of 20% for OM with 900 patients enrolled, the study will have 90% power to detect an HR of 1.4.
Point estimates and 95% binomial CIs were calculated for each indicator of OM (LN, BM, and pre- and postpleural lavage). Cumulative survival probabilities were estimated using the Kaplan-Meier method, and a log-rank test was used to compare survival of groups of patients. Univariable and multivariable Cox proportional hazards regression models were used to compare groups and generate HRs and 95% CIs. Multivariable models were adjusted for age, sex, stage, size, and histology, as appropriate. In all cases, P < .05 was considered statistically significant.
RESULTS
Demographic Information
The study was activated on July 13, 1999, closed to accrual on March 14, 2004, and analyzed in June 2009 when all eligible patients could have been observed for at least 5 years. Of 1,310 patients registered, 263 were ultimately removed from the study for various reasons (eg, initially ineligible, final histology other than NSCLC), leaving 1,047 patients evaluable for study end points. A total of 114 patients were censored (lost to follow-up) before the final 5-year survival assessment (OM LN negative, n = 54; OM LN positive, n = 9). Table 1 and Appendix Table A1 (online only) show clinical and tumor characteristics. The median patient age was 67.2 years, and there were slightly more men (51.4%) than women. Lobectomy was the most common procedure (79.5%), and a complete resection (R0) was achieved in 95% of patients. The most common tumor histology was adenocarcinoma (50.0%), and the majority of patients (66%) had stage I tumors. All 29 tumors staged as IIIB were T4N0-2M0.
Table 1.
Patient Demographics and Surgical Procedure and Tumor Characteristics for Eligible Patients
| Demographic or Characteristic | No. of Patients (N = 1,047) | % |
|---|---|---|
| Age, years | ||
| Median | 67.2 | |
| Range | 33.6-89.5 | |
| Sex | ||
| Male | 538 | 51.4 |
| Female | 509 | 48.6 |
| Race | ||
| White | 959 | 91.6 |
| Hispanic/Latino | 11 | 1 |
| Black/African American | 60 | 5.7 |
| Asian | 14 | 1.3 |
| American Indian/Alaska Native | 1 | 0.1 |
| Other | 2 | 0.2 |
| Operation performed (pulmonary resection) | ||
| Exploration, no resection | 16 | 1.5 |
| Wedge resection | 24 | 2.3 |
| Segmentectomy | 79 | 7.5 |
| Lobectomy | 832 | 79.5 |
| Bilobectomy | 51 | 4.9 |
| Pneumonectomy | 71 | 6.8 |
| Additional components of resection | ||
| Chest wall resection ± reconstruction | 33 | 3.2 |
| Bronchial sleeve resection | 33 | 3.2 |
| Vascular sleeve resection/arterioplasty | 13 | 1.2 |
| Intrapericardial resection | 20 | 1.9 |
| Extent of resection | ||
| R0 | 996 | 95.1 |
| R1 | 31 | 3 |
| R2 | 20 | 1.9 |
| Tumor histology | ||
| Adenocarcinoma | 524 | 50 |
| Squamous cell carcinoma | 318 | 30.4 |
| Large cell | 54 | 5.2 |
| Adenosquamous | 14 | 1.3 |
| NSCLC, other subtype or otherwise unspecified | 137 | 13.1 |
| Pathologic stage | ||
| T stage | ||
| Missing* | 2 | 0.2 |
| T1 | 384 | 36.7 |
| T2 | 575 | 54.9 |
| T3 | 55 | 5.3 |
| T4 | 29 | 2.8 |
| TX | 2 | 0.2 |
| N stage | ||
| N0 | 739 | 70.6 |
| N1 | 171 | 16.3 |
| N2 | 129 | 12.3 |
| NX | 8 | 0.8 |
| Overall stage | ||
| IA | 324 | 30.9 |
| IB | 367 | 35.1 |
| IIA | 32 | 3.1 |
| IIB | 158 | 15.1 |
| IIIA | 137 | 13.1 |
| IIIB | 29 | 2.8 |
Abbreviation: NSCLC, non–small-cell lung cancer.
One was missing from the final operative and pathology reports, and one was missing as a result of lack of resection. Both patients had clinical T2 disease.
Prevalence of OM
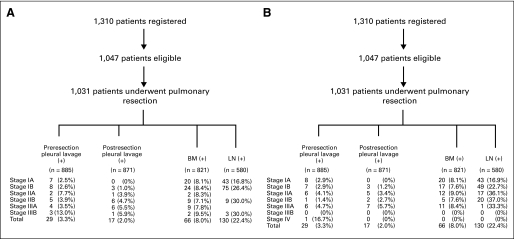
Representative images of IHC staining on BM and LN are shown in Figure 1. Figure 2 summarizes the results of pleural lavage cytology and of IHC staining on BM and LN in relationship to pathologic tumor stage based on the total number of technically adequate specimens in each category. With respect to BM specimens, low yields during the initial part of the study were related to participating surgeons submitting curettings of the rib and were corrected by subsequent submission of the entire rib segment for processing at USC. Only 29 patients had cytologically positive pleural lavage samples before resection, and only 17 patients had positive pleural lavage after resection, making it impossible to correlate this site of OM with survival. BM samples were positive for OMs in 66 (8.0%; 95% CI, 6.3% to 10.1%) of 821 patients with technically satisfactory specimens. LN OMs were found in 130 (22.4%; 95% CI, 19.1% to 26.0%) of 580 patients with histologic N0 disease.
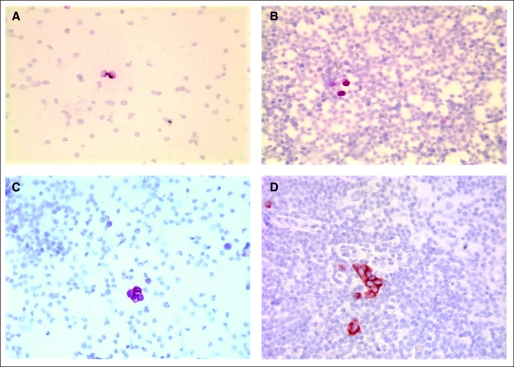
Fig 1.
Representative examples of cytokeratin immunohistochemistry in bone marrow (BM) and lymph nodes (LNs). (A) Negative BM with false-positive result. This is an example of two hematopoietic cells showing false-positive staining (red). The cells lack the size and nuclear characteristics of tumor cells. Here the nuclear membranes are smooth and the chromatin is finely granular and evenly distributed. Cells such as these were seen in virtually all samples, as well as in negative controls, and are considered nonspecific background. (B) BM with suspicious cells not defined as cancer. The cells in question (red) are more suggestive of tumor cells than the cells in panel A but lack definitive features of malignancy. The cells are slightly smaller than would be expected for tumor cells, and the nuclear-to-cytoplasmic ratio and nuclear features cannot be clearly seen. (C) BM with occult metastases (OMs). Note the four cells showing strong immunoreactivity (red staining). These cells are larger than the surrounding cells and have a scant amount of cytoplasm with large, irregular nuclei; they were confirmed as OMs by multiple independent reviews. (D) LN with OMs. Note the cluster of about nine cells and the two individual cells with strong cytokeratin immunoreactivity as evidenced by brown-red staining. The cells have the morphologic features of cancer cells, including large size compared with surrounding lymphoid cells, high nuclear-to-cytoplasmic ratio, dense irregular nuclei, and prominent nucleoli, and were confirmed as OM by multiple independent reviews.
Fig 2.
Schema summarizing the results of pleural fluid lavage cytology (before and after resection) and of immunostaining on bone marrow (BM) and lymph node (LN) samples in relationship to pathologic tumor stage according to the (A) sixth and (B) seventh editions of the American Joint Committee on Cancer lung cancer staging system.
The correlation of cytokeratin IHC staining results in BM and LN samples analyzed by pathologic tumor stage is shown in Appendix Table A2 (online only). Of 458 patients who had IHC performed on both BM and LN, only 10 patients (2.2%) had OM in both BM and LN. This number is too small to allow statistically valid comparison of this group to the patients who had OM in either site alone.
Survival Analysis
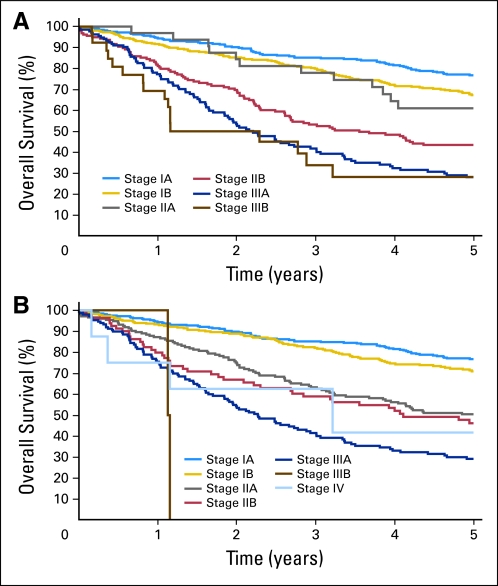
Currently, 663 patients (63.3%) remain alive, with a median follow-up time of 5 years (range, 0 to 5 years). Five-year overall survival by final pathologic stage (Appendix Fig A1, online only) ranged from 80% for stage IA tumors to 25% for stage III tumors.2
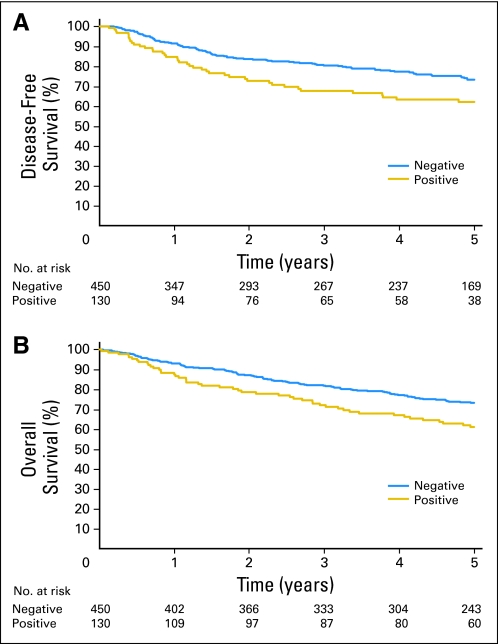
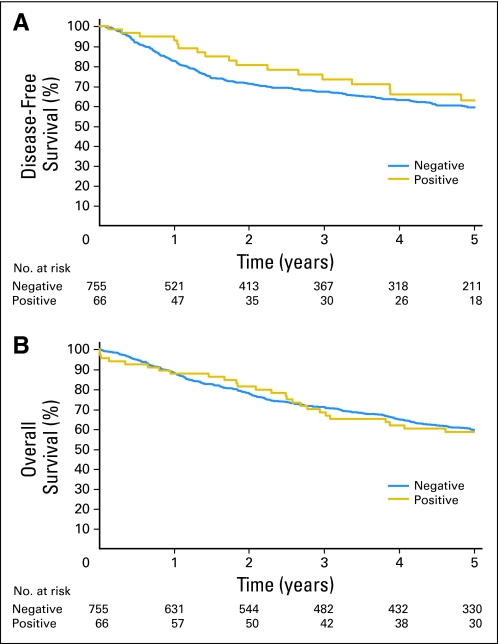
No significant difference in disease-free or overall survival was seen between patients who had OM in BM versus patients who did not (Appendix Fig A2, online only). However, a significant difference in both disease-free (HR, 1.63; 95% CI, 1.13 to 2.36; P = .009) and overall survival (HR, 1.59; 95% CI, 1.13 to 2.23; P = .007) was found in N0 patients who had OM in LN as opposed to patients who did not (Figs 3A and 3B). Neither the total number of OM-positive LNs (HR, 0.99; 95% CI, 0.98 to 1.0; P = .054) nor the location of IHC positivity within the nodes (HR, 1.03; 95% CI, 0.8 to 1.33; P = .825) seemed to be associated with a significantly worse overall survival. A statistically significant survival difference (Appendix Table A1) was observed when comparing stage IB patients with OM-positive LNs versus OM-negative LNs (HR, 1.82; 95% CI, 1.17 to 2.85; P = .01). No such difference was observed among patients with stage IA tumors. This is likely because the sample size of LN-positive tumors was almost twice as larger in the stage IB than the IA group (75 v 43 LN-positive tumors). It should be noted that this study was not designed or powered to detect a difference in these subgroups. By multivariable analysis (Appendix Table A3, online only), adjusted for patient age and sex and tumor histology, the presence of OM in LN had a significant adverse impact on disease-free survival (HR, 1.51; 95% CI, 1.04 to 2.19; P = .031) and overall survival (HR, 1.58; 95% CI, 1.13 to 2.22; P = .008).
Fig 3.
(A) Disease-free survival (P = .009) and (B) overall survival (P = .007) for patients with histologic N0 non–small-cell lung cancer who had occult metastases in lymph nodes by immunohistochemistry versus patients who did not.
This study was designed when the sixth edition of the American Joint Committee on Cancer lung cancer staging system was in effect. To determine whether our results were still valid within the current (seventh edition) staging system,29 all pathology reports were reviewed and tumors restaged accordingly (Fig 2B, Appendix Fig A1B, and Appendix Table A1). Exploratory analyses showed that the survival differences for OM-positive LN versus OM-negative LN tumors were still present (Table 2; Appendix Tables A4 and A5, online only).
Table 2.
Multivariate Analysis of Overall and Disease-Free Survival for Presence or Absence of OM in LN, Controlling for Patient Age, Sex, Tumor Stage,* and Histology
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| LN OM (negative v positive) | 1.58 | 1.13 to 2.22 | .008 |
| Age | 1.03 | 1.02 to 1.05 | < .001 |
| Sex | 0.66 | 0.48 to 0.91 | .013 |
| Stage | 1.48 | 1.29 to 1.70 | < .001 |
| Histology (squamous v adenocarcinoma v other) | .31 | ||
| Adenocarcinoma | 0.77 | 0.54 to 1.12 | |
| Other | 1.0 | 0.66 to 1.51 | |
| Disease-free survival | |||
| LN OM (negative v positive) | 1.51 | 1.04 to 2.19 | .031 |
| Age | 1.0 | 0.99 to 1.02 | .85 |
| Sex | 0.71 | 0.50 to 1.02 | .060 |
| Stage | 1.47 | 1.27 to 1.70 | < .001 |
| Histology (squamous v adenocarcinoma v other) | .86 | ||
| Adenocarcinoma | 0.91 | 0.60 to 1.37 | |
| Other | 1.01 | 0.63 to 1.62 |
Abbreviations: LN, lymph node; OM, occult metastases.
According to the sixth edition of the American Joint Committee on Cancer staging system.
DISCUSSION
Using techniques widely available to pathologists, this study shows that OMs in LN identify a group of patients with NSCLC at high risk for relapse and death after resection. Such patients may benefit from postoperative chemotherapy, which has been shown to improve the overall survival of patients with NSCLC with LN metastases.4,12
Cytologic detection of OM in pleural lavage has been investigated for more than 50 years with mixed results.13,14 A recent meta-analysis of 8,736 patients found that 511 patients (5.8%) had cytologically positive pleural lavage and that this independently predicted a poor survival. However, lavage technique was not standardized, and clinical follow-up in most institutions was less than 3 years.30 Our study allows a definitive estimate of the prevalence of positive pleural fluid cytology and shows that it is uncommon and thus cannot be correlated with survival.
OMs in BM are reported in several tumor types, especially early-stage breast cancer,6–8 where they occur in 15% to 60% of patients and are associated with a shorter time to recurrence. The impact on overall survival is not well established because previous studies are limited by small numbers of patients, short follow-up, and methodologic differences. Our results indicate that BM OMs in resectable NSCLC occur less frequently than previously reported, are unrelated to OMs in LN, and are not associated with a worse survival. Issues that may limit a general conclusion include the lower incidence of BM OMs in this study compared with others. Thus, it is possible that further work may show that BM OM is a predictor of lung cancer progression, as earlier studies (including those from our group) suggest.
LN OMs are reported to predict outcome in patients with breast, colon, prostate, and other cancers.8–10,27 Detection of OMs by IHC has been shown to be superior to rereview of multiple serial histologic sections.27 LN OMs examined by a variety of techniques have previously been reported in early-stage NSCLC15,16,19–23,31 at frequencies ranging from 4% to 70%, reflecting methodologic differences, case selection, and the fact that studies have been small and retrospective. We did not seek to repeat studies comparing IHC to serial hematoxylin and eosin–stained sections of LN. However, using rigorous pathologic criteria, the current study shows that LN OMs are frequently detected by IHC in tumors deemed N0 by standard histology and are associated with a significant decrease in survival, with the greatest impact in patients with stage IB disease, patients for whom it has been difficult to demonstrate the benefit of adjuvant chemotherapy using standard TNM staging.32,33
Consistent with our prior studies,17,27 we used the combination of the anticytokeratin antibodies AE-1 and CAM 5.2 because they recognize a spectrum of cytokeratins ubiquitously and highly expressed by virtually all tumor cells of simple epithelial origin, including NSCLC; facilitate detection of small numbers of tumor cells; and do not react with the normal constituents of BM or LN. Although reverse transcriptase polymerase chain reaction has been used to detect OM, this technique does not allow simultaneous morphologic evaluation of specimens and has been associated with false-positive results.34 A recent study showed that site-specific methylation of tumor-associated genes in LN identified patients with NSCLC at increased risk for progression.35 However, unlike IHC, these molecular assays are not yet available in routine clinical practice. Our results indicate that the IHC detection of OM in BM and LN is reproducible among different laboratories.
The three potential metastatic sites examined in this study differ widely from one another.36,37 The heterogeneity of individual tumor biology makes it unlikely that metastases would occur in one or more of these sites simultaneously, and our results confirm this. Although BM OMs were not associated with survival in this trial, recent improvements in techniques for OM detection38,39 warrant further work and could provide a better measure of the prognostic significance of BM OMs than was possible in the current study. The reasons why LN OMs, but not BM OMs, were associated with outcome in this study are unclear but may, in part, be a result of differences in incidence for these findings.
The association between LN OMs and survival has direct clinical implications. Our results clearly suggest that IHC should be routinely used to evaluate histologically negative LNs and to select patients with early-stage NSCLC for future trials of adjuvant chemotherapy.
Supplementary Material
Acknowledgment
We thank the patients and surgeons who participated in this trial; Drs Shan-Rong Shi, Janet Aleksanian, and Mohamed Alavi for expert review of the pathology slides; and Melody Owens for assistance in manuscript preparation.
Appendix
Z0040 participating surgeons and institutions (listed in order of patient accrual to study) were as follows: Landreneau, R.J., University of Pittsburgh, Pittsburgh, PA; Patterson, G.A., Washington University, Barnes Jewish Hospital, St Louis, MO; Inculet, R.I., London Health Sciences Center, London, Ontario, Canada; Jones, D.R., University of Virginia, Charlottesville, VA; Malthaner, R.A., London Health Sciences Center, London, Ontario, Canada; Rusch, V.W., Memorial Sloan-Kettering Cancer Center, New York, NY; Roberts, J.R., Vanderbilt University Medical Center, Nashville, TN; Goldberg, M., Fox Chase Cancer Center, Philadelphia, PA; Johnstone, D.W., Dartmouth Hitchcock Medical Center, Lebanon, NH; Nesbitt, J.C., Saint Thomas Hospital, Nashville, TN; Hazelrigg, J.R., Southern Illinois University School of Medicine, Carbondale, IL; Oaks, T.E., Wake Forest University, Baptist Medical Center, Greensboro, NC; Collins, M.P., Latter Day Saints, Hospital, Salt Lake City, UT; Daniel, T.M., University of Virginia, Charlottesville, VA; Meyers, B.F., Washington University, Barnes Jewish Hospital, St Louis, MO; Downey, R.J., Memorial Sloan-Kettering Cancer Center, New York, NY; Battafarano, R.J., University of Maryland, Greenebaum Cancer Center, Baltimore, MD; Keenan, R.J., Allegheny General Hospital, Pittsburgh, PA; Bains, M.S., Memorial Sloan-Kettering Cancer Center, New York, NY; Scott, W.J., Fox Chase Cancer Center Creighton University Medical Center, Omaha, NE; Cooper, J.D., Washington University, Barnes Jewish Hospital, St Louis, MO; Chmielewski, G.W., William Beaumont Hospital, Royal Oak, MI; Feins, R.H., University of Rochester Medical Center, Rochester, MN; Darling, G.E., University Health Network (UHN)-Toronto General Hospital, Toronto, Ontario, Canada; Kernstine, K.H., City of Hope, Los Angeles, CA; Waddell, T.K., Toronto General Hospital, Toronto, Ontario, Canada; Jablons, D.M., University of California San Francisco Medical Center, San Francisco, CA; Keshavjee, S., Toronto General Hospital, Toronto, Ontario, Canada; Weigel, T.L., University of Wisconsin, Madison, WS; Korst, R.J., Weill Medical College of Cornell University, New York, NY; Graeber, G.M., Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, WV; Johnston, M.R., UHN-Princess Margaret Hospital, Toronto, Ontario, Canada; Watson, T.J., University of Rochester Medical Center, Rochester, MN; Pierre, A.F., Toronto General Hospital, Toronto, Ontario, Canada; Ferguson, M.K., University of Chicago, Chicago, IL; Gasparri, M.G., Froedtert Hospital Medical College of Wisconsin, Milwaukee, WI; Reed, C.E., Medical College of South Carolina, Charleston, SC; Carp, N.Z., Lankenau Medical Research Center, Philadelphia, PA; Park, B.J., Memorial Sloan-Kettering Cancer Center, New York, NY; Ginsberg, R.J., Toronto General Hospital, Toronto, Ontario, Canada; Walker, G.T., Mobile Infirmary Medical Center, Mobile, AL; Shrager, J.B., Stanford University, Stanford, CA; Marshall, M.B., Georgetown University Hospital, Washington, DC; Davidson, B.S., Piedmount Hospital, Atlanta, GA; Bilfinger, T.V., State University of New York at Stony Brook, Stony Brook, NY; Rossi, N.P., University of Iowa Hospitals and Clinics, Iowa City, IA; Vallieres, E., University of Washington Medical Center, Seattle, WA; Handy, J.R., Providence Portland Medical Center, Portland, OR; Swisher, S.G., The University of Texas MD Anderson Cancer Center, Houston, TX; Roth, J.A., The University of Texas MD Anderson Cancer Center, Houston, TX; Roberts, P.F., University of California at Davis, Davis, CA; Strohl, J.A., Hurley Medical Center, McLaren Regional Medical Center, Flint, MI; Tsen, A.C., Providence Portland Medical Center, Portland, OR; Pechet, T.V., Thomas Jefferson University Hospital, Philadelphia, PA; Bold, R.J., University of California at Davis, Davis, CA; Rice, D.C., The University of Texas MD Anderson Cancer Center, Houston, TX; Keller, S.M., Beth Israel Medical Center, New York, NY; Putnam, J.B., Vanderbilt University Medical Center, Nashville, TN; Wood, D.E., University of Washington Medical Center, Seattle, WA; Howington, J.A., University of Cincinnati Medical Center, Cincinnati, OH; Block, M.I., Medical University of South Carolina, Charleston, SC; Levin, B.H., York Hospital, York, PA; Temes, G., Jewish Hospital, Cincinnati, OH; Douville, E.C., Providence Portland Medical Center, Portland, OR; Hinkamp, T.J., Alexian Brothers Medical Center, Hoffman Estates, IL; Ninan, M., Vanderbilt University Medical Center, Nashville, TN; Vaporciyan, A.A., The University of Texas MD Anderson Cancer Center, Houston, TX; Anderson, T.M., Roswell Park Memorial Hospital, Buffalo, NY; Cohen, R.G., Huntington Memorial Hospital, Pasadena, CA; Kaiser, L.R., University of Pennsylvania Cancer Center, Philadelphia, PA; Levine, E.A., Wake Forest University, Baptist Medical Center, Greensboro, NC; Reichert, R.A., Bayfront Outpatient Health Clinic, St Petersburg, FL; Kohman, L.J., State University of New York at Syracuse, Syracuse, NY; David, I.B., Holy Cross Hospital, Fort Lauderdale, FL; Maley, R.H., Allegany Cancer Center Network, Pittsburgh, PA; Shen, P., Wake Forest University, Baptist Medical Center, Greensboro, NC; Ott, G.Y., Providence Portland Medical Center, Portland, OR; Mitchell, J.D., Denver Veterans Administration Medical Center, Denver, CO; Dexter, E.U., State University of New York at Syracuse, Syracuse, NY; and Smythe, W.R., The University of Texas MD Anderson Cancer Center, Houston, TX.
Fig A1.
Overall survival for all eligible patients by pathologic stage, according to the (A) sixth and (B) seventh editions of the Joint Committee on Cancer lung cancer staging system.
Fig A2.
(A) Disease-free survival (P = .33) and (B) overall survival (P = .89) for patients who had occult metastases in bone marrow by immunohistochemistry versus patients who did not.
Table A1.
T Stage and Overall Stage for Eligible Patients According to the Seventh Edition of the American Joint Committee on Cancer Staging System
| Pathologic Stage | No. of Patients (N = 1,047) | % |
|---|---|---|
| T stage | ||
| Missing | 13 | 1.2 |
| T1* | 1 | 0.1 |
| T1a | 207 | 19.8 |
| T1b | 173 | 16.5 |
| T2a | 443 | 42.3 |
| T2b | 90 | 8.6 |
| T3 | 104 | 9.9 |
| T4 | 16 | 1.5 |
| Overall stage | ||
| Missing | 12 | 1.1 |
| IA | 324 | 30.9 |
| IB | 288 | 27.5 |
| IIA | 178 | 17.0 |
| IIB | 81 | 7.7 |
| IIIA | 153 | 14.6 |
| IIIB | 2 | 0.2 |
| IV | 9 | 0.9 |
Tumor measurement not stated on pathology reports; clinical T1, N0, and stage IA.
Table A2.
Correlation of Immunohistochemical Staining Results on BM and LN Samples, Analyzed by Pathologic Tumor Stage
| BM Status and Tumor Stage | LN Negative |
LN Positive |
Total |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. (N = 458) | % | |
| BM negative | ||||||
| Stage IA | 150 | 81.1 | 35 | 18.9 | 185 | 40.4 |
| Stage IB | 153 | 74.6 | 52 | 25.4 | 205 | 44.8 |
| Stage IIA | ||||||
| Stage IIB | 16 | 66.7 | 8 | 33.3 | 24 | 5.2 |
| Stage IIIA | ||||||
| Stage IIIB | 6 | 75 | 2 | 25 | 8 | 1.7 |
| BM positive | ||||||
| Stage IA | 12 | 85.7 | 2 | 14.3 | 14 | 3.1 |
| Stage IB | 13 | 65 | 7 | 35 | 20 | 4.4 |
| Stage IIA | ||||||
| Stage IIB | 1 | 50.0 | 1 | 50.0 | 2 | 0.4 |
| Stage IIIA | ||||||
| Stage IIIB | ||||||
| Total | 351 | 76.6 | 107 | 23.4 | 458 | |
Abbreviations: BM, bone marrow; LN, lymph node.
Table A3.
Comparison of Overall Survival by Tumor Stage (sixth edition of AJCC staging system) in Relationship to the Positive or Negative Status of OM in LN
| Stage/LN OM Status | No. | HR | 95% CI | 5-Year Overall Survival (%) | P* |
|---|---|---|---|---|---|
| Stage IA | |||||
| LN negative | 213 | 77.7 | |||
| LN positive | 43 | 1.03 | 0.50 to 2.10 | 78.2 | .94 |
| Stage IB | |||||
| LN negative | 209 | 0.83 | 0.41 to 1.69 | 73.7 | .61 |
| LN positive | 75 | 1.82 | 1.17 to 2.85 | 56.5 | .01† |
| Stage IIA | |||||
| LN negative | |||||
| LN positive | |||||
| Stage IIB | |||||
| LN negative | 21 | 42.9 | |||
| LN positive | 9 | 1.53 | 0.60 to 3.90 | 22.2 | .37 |
| Stage IIIA | |||||
| LN negative | |||||
| LN positive | |||||
| Stage IIIB | |||||
| LN negative | 7 | 42.9 (3 years) | |||
| LN positive | 3 | 50.0 | .38 |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; LN, lymph node; OM, occult metastases.
P value compares consecutive groups (ie, stage IA LN negative v stage IA LN positive; stage IA LN positive v stage IB LN negative, and so on).
Adjusted for age, sex, histology, and size (P < .001).
Table A4.
Comparison of Overall Survival by Tumor Stage (seventh edition of AJCC staging system) in Relationship to the Positive or Negative Status of OM in LN
| Stage/LN OM Status | No. | HR | 95% CI | 5-Year Overall Survival (%) | P* |
|---|---|---|---|---|---|
| Stage IA | |||||
| LN negative | 212 | 77.5 | |||
| LN positive | 43 | 1.02 | 0.50 to 2.09 | 78.2 | .95 |
| Stage IB | |||||
| LN negative | 167 | 0.98 | 0.47 to 2.04 | 77.2 | .96 |
| LN positive | 49 | 1.72 | 0.96 to 3.06 | 62.2 | .06† |
| Stage IIA | |||||
| LN negative | 30 | 0.85 | 0.39 to 1.86 | 59.9 | .69 |
| LN positive | 17 | 1.59 | 0.65 to 3.92 | 47.1 | .31 |
| Stage IIB | |||||
| LN negative | 34 | 0.98 | 0.44 to 2.18 | 45.1 | .96 |
| LN positive | 20 | 1.24 | 0.61 to 2.52 | 35.0 | .56 |
| Stage IIIA | |||||
| LN negative | 2 | 50.0 (at 2 years) | |||
| LN positive | 1 | ||||
| Stage IIIB | |||||
| LN negative | |||||
| LN positive | |||||
| Stage IV | |||||
| LN negative | 2 | 50.0 (at 3 years) | |||
| LN positive |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; LN, lymph node; OM, occult metastases.
P value compares consecutive groups (ie, stage IA LN negative v stage IA LN positive; stage IA LN positive v stage IB LN negative, and so on).
Adjusted for age, sex, histology, and size (P = .0077).
Table A5.
Multivariate Analysis of Overall and Disease-Free Survival for Presence or Absence of OM in LN, Controlling for Patient Age, Sex, Tumor Stage (seventh edition of AJCC staging system), and Histology
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| LN OM (negative v positive) | 1.47 | 1.05 to 2.08 | .027 |
| Age | 1.03 | 1.02 to 1.05 | < .001 |
| Sex | 0.67 | 0.48 to 0.93 | .017 |
| Stage | 1.49 | 1.29 to 1.71 | < .001 |
| Histology (squamous v adenocarcinoma v other) | .58 | ||
| Adenocarcinoma | 0.87 | 0.60 to 1.27 | |
| Other | 1.07 | 0.71 to 1.62 | |
| Disease-free survival | |||
| LN OM (negative v positive) | 1.43 | 0.99 to 2.08 | .057 |
| Age | 1.0 | 0.99 to 1.02 | .71 |
| Sex | 0.76 | 0.53 to 1.08 | .12 |
| Stage | 1.57 | 1.35 to 1.82 | < .001 |
| Histology (squamous v adenocarcinoma v other) | .92 | ||
| Adenocarcinoma | 1.04 | 0.69 to 1.59 | |
| Other | 1.11 | 0.66 to 1.77 |
Abbreviations: AJCC, American Joint Committee on Cancer; LN, lymph node; OM, occult metastases.
Footnotes
Listen to the podcast by Dr Kernstine at www.jco.org/podcasts
Supported in part by National Cancer Institute Grants No. U10 CA076001 and R01 CA84339 (R.J.C.) and University of California Tobacco-Related Disease Research Program Grant No. 8RT-0061 (R.J.C.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003901.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Valerie W. Rusch, Debra Hawes, Robbin G. Cohen, Richard J. Cote
Administrative support: Joe B. Putnam Jr
Collection and assembly of data: Valerie W. Rusch, Debra Hawes, Sue Ellen Martin, Rodney J. Landreneau, G. Alexander Patterson, Richard I. Inculet, David R. Jones, Richard A. Malthaner, Robbin G. Cohen, Joe B. Putnam Jr
Data analysis and interpretation: Valerie W. Rusch, Debra Hawes, Paul A. Decker, Andrea Abati, Karla Ballman, Joe B. Putnam Jr
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Decker RH, Wilson LD. Postoperative radiation therapy for non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2008;20:184–187. doi: 10.1053/j.semtcvs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Wakelee H, Chhatwani L. Adjuvant chemotherapy for resected non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2008;20:198–203. doi: 10.1053/j.semtcvs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Price KAR, Azzoli CG, Gaspar LE. Chemoradiation for unresectable stage III non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2008;20:204–209. doi: 10.1053/j.semtcvs.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Cote RJ, Rosen PP, Lesser ML, et al. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 7.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 8.Lugo TG, Braun S, Cote RJ, et al. Detection and measurement of occult disease for the prognosis of solid tumors. J Clin Oncol. 2003;21:2609–2615. doi: 10.1200/JCO.2003.01.153. [DOI] [PubMed] [Google Scholar]
- 9.Pagliarulo V, Hawes D, Brands FH, et al. Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J Clin Oncol. 2006;24:2735–2742. doi: 10.1200/JCO.2005.05.4767. [DOI] [PubMed] [Google Scholar]
- 10.Liefers GJ, Cleton-Jansen AM, van de Velde CJ, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:223–228. doi: 10.1056/NEJM199807233390403. [DOI] [PubMed] [Google Scholar]
- 11.Oxford, United Kingdom: Wiley-Blackwell; 2009. International Union Against Cancer: TNM Classification of Malignant Tumours (ed 7) [Google Scholar]
- 12.Harichand-Herdt S, Ramalingam SS. Targeted therapy for the treatment of non-small cell lung cancer: Focus on inhibition of epidermal growth factor receptor. Semin Thorac Cardiovasc Surg. 2008;20:217–223. doi: 10.1053/j.semtcvs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Spjut HJ, Hendrix VJ, Ramirez GA, et al. Carcinoma cells in pleural cavity washings. Cancer. 1958;11:1222–1225. doi: 10.1002/1097-0142(195811/12)11:6<1222::aid-cncr2820110618>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Kjellberg SI, Dresler CM, Goldberg M. Pleural cytologies in lung cancer without pleural effusions. Ann Thorac Surg. 1997;64:941–944. doi: 10.1016/s0003-4975(97)00817-5. [DOI] [PubMed] [Google Scholar]
- 15.Pantel K, Izbicki JR, Angstwurm M, et al. Immunocytological detection of bone marrow micrometastasis in operable non-small cell lung cancer. Cancer Res. 1993;53:1027–1031. [PubMed] [Google Scholar]
- 16.Passlick B, Izbicki JR, Kubuschok B, et al. Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small-cell lung cancer. J Clin Oncol. 1994;12:1827–1832. doi: 10.1200/JCO.1994.12.9.1827. [DOI] [PubMed] [Google Scholar]
- 17.Cote RJ, Beattie EJ, Chaiwun B, et al. Detection of occult bone marrow micrometastases in patients with operable lung carcinoma. Ann Surg. 1995;222:415–425. doi: 10.1097/00000658-199522240-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantel K, Izbicki J, Passlick B, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small cell lung cancer without overt metastases. Lancet. 1996;347:649–653. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z-L, Perez S, Holmes EC, et al. Frequency and distribution of occult micrometastases in lymph nodes of patients with non-small cell lung carcinoma. J Natl Cancer Inst. 1993;85:493–498. doi: 10.1093/jnci/85.6.493. [DOI] [PubMed] [Google Scholar]
- 20.Xi L, Coello MC, Litle VR, et al. A combination of molecular markers accurately detects lymph node metastasis in non-small cell lung cancer patients. Clin Cancer Res. 2006;12:2484–2491. doi: 10.1158/1078-0432.CCR-05-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda J, Inoue M, Okumura M, et al. Detection of occult tumor cells in lymph nodes from non-small cell lung cancer patients using reverse transcription-polymerase chain reaction for carcinoembryonic antigen mRNA with the evaluation of its sensitivity. Lung Cancer. 2006;52:235–240. doi: 10.1016/j.lungcan.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Saintigny P, Coulon S, Kambouchner M, et al. Real-time RT-PCR detection of CK19, CK7, and MUC1 mRNA for diagnosis of lymph node micrometastases in non small cell lung carcinoma. Int J Cancer. 2005;115:777–782. doi: 10.1002/ijc.20942. [DOI] [PubMed] [Google Scholar]
- 23.Vollmer RT, Herndon JE, II, D'Cunha J, et al. Immunohistochemical detection of occult lymph node metastases in non-small cell lung cancer: Anatomical pathology results from Cancer and Leukemia Group B trial 9761. Clin Cancer Res. 2003;9:5630–5635. [PubMed] [Google Scholar]
- 24.Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer. 2005;49:25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 26.American Joint Committee on Cancer. New York, NY: Springer-Verlag; 2002. AJCC Cancer Staging Manual (ed 6) [Google Scholar]
- 27.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer: International Breast Cancer Study Group. Lancet. 1999;354:896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 28.Shi S-R, Cote RJ, Chaiwun B, et al. Standardization of immunohistochemistry based on antigen retrieval technique for routine formalin-fixed tissue sections. Appl Immunohistochem. 1998;6:89–96. [Google Scholar]
- 29.American Joint Committee on Cancer. New York, NY: Springer; 2010. AJCC Cancer Staging Manual (ed 7) [Google Scholar]
- 30.Lim E, Clough R, Goldstraw P, et al. Impact of positive pleural lavage cytology on survival in patients having lung resection for non-small cell lung cancer: An international individual patient data meta-analysis. J Thorac Cardiovasc Surg. 2010;139:1441–1446. doi: 10.1016/j.jtcvs.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson AG, Graham ANJ, Pezzella F, et al. Does the use of immunohistochemistry to identify micrometastases provide useful information in the staging of node-negative non-small cell lung carcinomas? Lung Cancer. 1997;18:231–240. doi: 10.1016/s0169-5002(97)00065-2. [DOI] [PubMed] [Google Scholar]
- 32.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non–small-cell lung cancer: Updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strauss GM, Herndon JE, II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non–small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bostick PJ, Chatterjee S, Chi DD, et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–2640. doi: 10.1200/JCO.1998.16.8.2632. [DOI] [PubMed] [Google Scholar]
- 35.Brock MV, Hooker CM, Ota-Machida E, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358:1118–1128. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- 36.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 38.Zheng S, Lin H, Liu JQ, et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162:154–161. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 39.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.