Abstract
Although twin studies indicate clear genetic bases of autism spectrum disorder (ASD), the precise mechanisms through which genetic variations causally result in ASD are poorly understood. Individuals with 3 Mb and nested 1.5 Mb hemizygosity of the chromosome 22q11.2 represent genetically identifiable cases of ASD. However, because more than 30 genes are deleted even in the minimal deletion cases of 22q11.2 deficiency, the individual 22q11.2 gene(s) responsible for ASD remain elusive. Here, we examined the impact of constitutive heterozygosity of Tbx1, a 22q11.2 gene, on the behavioral phenotypes of ASD and characterized the regional and cellular expression of its mRNA and protein in mice. Congenic Tbx1 heterozygous (HT) mice were impaired in social interaction, ultrasonic vocalization, memory-based behavioral alternation, working memory and thigmotaxis, compared with wild-type (WT) mice. These phenotypes were not due to non-specific alterations in olfactory function, exploratory behavior, motor movement or anxiety-related behavior. Tbx1 mRNA and protein were ubiquitously expressed throughout the brains of C57BL/6J mice, but protein expression was enriched in regions that postnatally retain the capacity of neurogenesis, and in fact, postnatally proliferating cells expressed Tbx1. In postnatally derived hippocampal culture cells of C57BL/6J mice, Tbx1 levels were higher during proliferation than during differentiation, and expressed in neural progenitor cells, immature and matured neurons and glial cells. Taken together, our data suggest that Tbx1 is a gene responsible for the phenotypes of 22q11.2 hemizygosity-associated ASD possibly through its role in diverse cell types, including postnatally and prenatally generated neurons.
INTRODUCTION
Autism spectrum disorder (ASD) is behaviorally defined by a constellation of deficits in social interaction, language and communication and a range of behavior, interests and activities, and is comorbid with variable degrees of cognitive development. Owing to its early onset in childhood, it has a major negative impact on the development of children, but symptoms affect the lives of adolescents and adults as well. As the precise mechanisms underlying ASD remain poorly understood, mechanism-based therapies are not available.
Twin studies have amply implicated gene variations as the predominant causative factor (1,2), and there are genetically identifiable cases of ASD (3). Individuals with 22q11.2 hemizygosity exhibit social interaction deficits (4–9), delayed development of vocal volume, vocalization and language (10,11), repetitive behaviors (12) and impairments in cognition, including working memory (9,13–16). These deficits are symptomatic elements and comorbid traits of ASD, and in fact 14–50% of individuals with 22q11.2 hemizygosity tested for ASD have met diagnostic criteria (6,12,17–19), collectively totaling in 29% of 22q11.2 hemizygous cases in these studies. When screened for copy number variation (CNV) in individuals with ASD, 22q11.2 is one of many CNV sites (20–23).
A major obstacle in furthering our understanding of the genetic mechanisms of 22q11.2-associated ASD is that it is difficult to ascertain the precise manner by which individual genes cause ASD in humans. As hemizygosity of 22q11.2 minimally includes more than 30 genes (24), the impact of each of these genes on ASD cannot be isolated. Association of alleles of individual genes on the remaining copy of 22q11.2 with ASD determines how such alleles ‘modify’ phenotypes of 22q11.2 hemizygosity, but does not identify individual genes whose hemizygosity ‘causes’ phenotypes in humans.
We have focused on Tbx1, one of the 22q11.2 genes, for two reasons. First, we have previously identified a ∼200 kb 22q11.2 region, including Tbx1, whose gene dose alteration induces antipsychotic-responsive stereotyped behavior (25). Second, a rare case of Tbx1 mutation—not 22q11.2 hemizygosity—was associated with ASD in one family (26). TBX1 belongs to a phylogenetically conserved family of genes that share a common DNA-binding domain, the T-box. The human TBX1 protein and its mouse ortholog Tbx1 share a highly conserved amino acid sequence, and thus it is amenable to studies in the mouse.
In this study, we first evaluated the impact of constitutive Tbx1 heterozygosity on ASD-related behavioral phenotypes. Although any attempt to model ASD in animals is at best a proxy for the real events, a number of behavioral paradigms to model symptomatic elements have validity (27). Second, we identified the localization of Tbx1 protein in the mouse brain at the regional and cellular levels. Although Tbx1 mRNA steadily increases in the whole mouse brain sample during the postnatal period toward adulthood (26), the regional and cellular distributions of its protein product in the mouse brain are not known. Our data suggest that constitutive Tbx1 heterozygosity contributes to 22q11.2-associated ASD presumably through its expression in diverse brain regions, some of which include postnatally generated neurons.
RESULTS
Congenic Tbx1 heterozygous mice exhibit ASD-related behavioral phenotypes
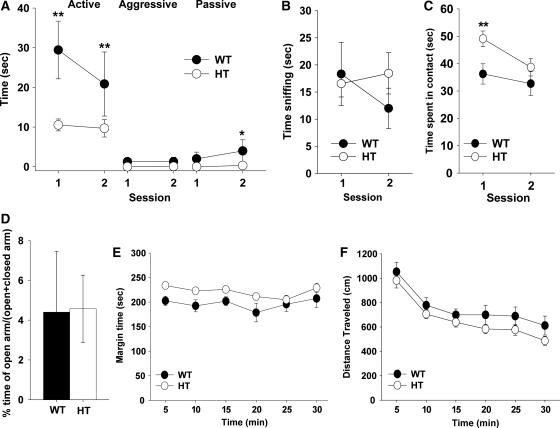
Congenic Tbx1 heterozygous (HT) mice exhibited lower levels of active and passive affiliative social interaction; aggressive behavior was rarely seen in our experimental setup (Fig. 1A). This phenotype was not due to a non-specific reduction in approach behaviors. HT and wild-type (WT) mice were indistinguishable in olfactory investigation of the non-mouse odor (i.e. peanut butter, Fig. 1B); HT mice initially exhibited higher levels of contact with a novel, non-mouse object (Fig. 1C), compared with WT mice. Social interaction includes an element of anxiety. HT and WT mice were indistinguishable in anxiety-related behaviors in the elevated plus maze (Fig. 1D), although HT mice exhibited a higher degree of thigmotaxis than did WT mice (Fig. 1E). The social interaction requires motor movement, but HT and WT mice were indistinguishable in motor activity in an open field (Fig. 1F).
Figure 1.
(A) Active affiliative, aggressive, and passive affiliative forms of social interaction. Time spent (mean ± SEM) in the three forms of social interaction in two 5 min sessions with an age-matched stimulus C57BL/6J mouse is shown. Although HT mice generally exhibited lower levels of social interaction than WT mice (genotype, F1,18 = 18.23, P = 0.0005), this effect depends on the form of social interaction and sessions (genotype × form, F2,36 = 6.67, P = 0.0034; genotype × form × session, F2,36 = 10.71, P = 0.0002). Asterisks indicate statistically significant differences between WT and HT mice at levels of 0.05 (*) and 0.01 (**), as determined by Newman–Keuls comparisons. WT, n = 7; HT, n = 13. (B) Olfactory investigation. Time spent (mean ± SEM) in sniffing a tube containing peanut butter is shown in two 5 min sessions. WT and HT mice were indistinguishable in sniffing (genotype, F1,13 = 0.24, P = 0.6313; session, F1,13= 0.59, P = 0.4575; genotype × session, F1,13 = 2.01, P = 0.1801). WT, n = 6; HT, n = 9. (C) Novel object contact. Time spent (mean ± SEM) in contacting a novel object is shown in two 5 min sessions. There was a statistically non-significant trend of higher levels of contact in HT mice than in WT mice at the first, but not second, session (genotype, F1,22 = 2.89, P = 0.103; session, F1,22= 13.79, P = 0.0012; genotype × session, F1,22 = 4.01, P = 0.0576). Post hoc comparisons confirmed this trend at levels of 0.01 (**), as determined by the Newman–Keuls test. WT, n = 9; HT, n = 15. (D) Anxiety-related behaviors in the elevated plus maze. The relative amounts (mean ± SEM) of time spent in the open arms versus both the open and closed arms of the elevated plus maze are shown. WT and HT mice were indistinguishable in the relative time spent in the open arms (t(19) = 0.052, P = 0.9587). A similar trend was seen in the frequency of visits to open arms (t(19) = 0.192, P = 0.8498; data not shown). WT, n = 8; HT, n = 13. (E and F) Motor activity in the inescapable open field. (E) Time (s, mean ± SEM) spent in the margin of the open field. Each tick on the x-axis represents a 5 min bin. HT mice spent more time in the margin area along the wall, compared with WT mice, whereas margin time fluctuated equally in WT and HT mice during the session (genotype, F1,27 = 6.33, P = 0.0181; session, F5,135= 2.69, P = 0.0238; genotype × time interval, F5,135 = 0.57, P = 0.7268). (F) Horizontal distance traveled (mean ± SEM) in the entire open field. WT and HT mice were indistinguishable and their spontaneous locomotor activity equally declined during a 30 min session (genotype, F1,27 = 2.08, P = 0.1604; session, F5,135 = 41.64, P < 0.0001; genotype × time interval, F5,135 = 0.303, P = 0.9107). WT, n = 10; HT, n = 19.
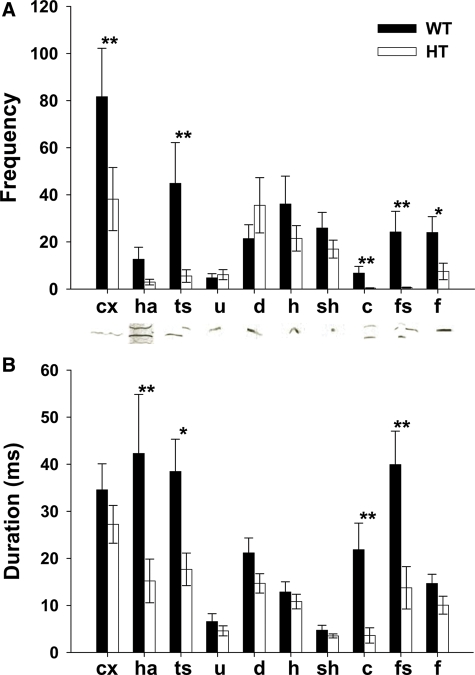
Compared with WT mice, HT mice exhibited vocalization less frequently in complex, two-syllable, composite, frequency steps and flat (Fig. 2A) and for shorter duration in harmonics, two-syllable, composite and frequency steps (Fig. 2B).
Figure 2.
Ultrasonic vocalization of pups during a 5 min separation from mothers at P7–8. (A) The frequency (mean ± SEM) and B) average duration (mean ± SEM) of each vocal call type are shown. Distinct categories of calls, as defined by Scattoni et al. (79), are indicated as: cx, complex; ha, harmonics; ts, two-syllable; u, upward; d, downward; h, hump (a.k.a., chevron); sh, shorts; c, composite; fs, frequency steps; f, flat. Typical sonograms are shown below each label. HT mice emitted ultrasonic vocalization in specific categories less frequently (genotype, F1,28 = 7.03, P = 0.013, category, F9,252 = 16.07, P < 0.0001; genotype × category, F1,252 = 3.52, P = 0.0004) and for shorter durations (genotype, F1,28 = 7.92, P = 0.0088, category, F9,252 = 10.66, P < 0.0001; genotype × category, F1,252 = 3.51, P = 0.0004), compared with WT mice. An asterisk indicates a statistically significant difference between WT and HT mice at levels of 0.05 (*) and 0.01 (**), as determined by Newman–Keuls comparisons. Because the homogeneity of variance was violated, data were transformed by square-root, and statistical analysis was applied. For clarity, the averages of raw data are shown. WT, n = 9; HT, n = 21.
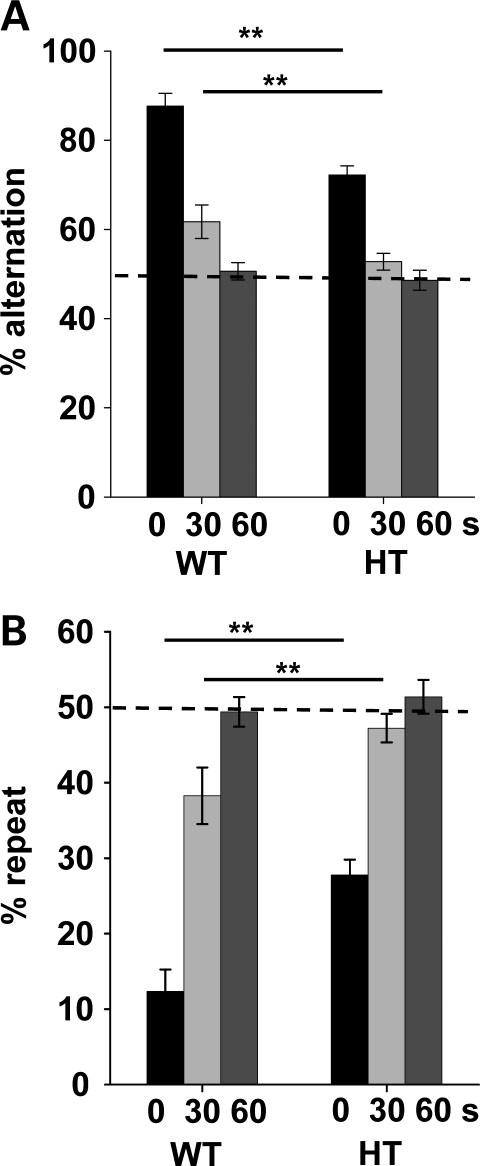
Compared with WT mice, congenic HT mice exhibited lower levels of spontaneous alternations at 0 and 30 s delays; both WT and HT mice reached a chance level at a 60 s delay (Fig. 3A). HT mice visited the same arms across trials more often than WT mice (Fig. 3B). Interestingly, this tendency was significantly different from WT mice when HT mice showed spontaneous alternation (e.g. 0 s delay) but not when they do not at 60 s delay (Fig. 3A and B).
Figure 3.
Spontaneous alternation in the T-maze. (A) The percentage of correctly alternated choices (mean ± SEM) is shown. (B) The percentage of repeated visits of the same arm (mean ± SEM) is shown. Mice were tested with 0, 30 and 60 s delays between trials. HT mice exhibited lower levels of spontaneous alternation at 0 and 30 s delay intervals; both genotypes exhibited a chance level choice at a 60 s delay interval (genotype, F1,23 = 12.51, P = 0.0018; delay, F2,46 = 105.89, P < 0.0001; genotype × delay, F2,46 = 4.79, P = 0.0128). Performance did not differ from 50% at 60 s delay (t(8) = 0.316, P = 0.7599) for WT mice and at 30 s (t(15) = 1.46, P = 0.1639) and 60 s delays (t(15) = 0.620, P = 0.5445) for HT mice, as determined by exploratory one-sample t-tests. Because repetitiveness percentages are reciprocals of the alternation percentages, the statistical values were identical. An asterisk indicates a statistically significant difference between WT and HT mice at 0.01 (**) at each delay (solid line), as determined by Newman–Keuls comparisons. The dotted horizontal line indicates 50%, i.e. chance. WT, n = 9; HT, n = 16.
Tbx1 is expressed throughout the mouse brain but is enriched in postnatally generated cells
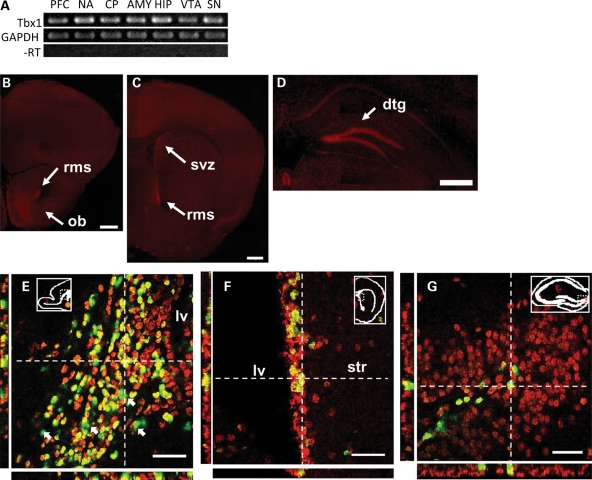
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) (Fig. 4A) showed that Tbx1 mRNA was expressed in all regions examined in C57BL/6J mice at 2 months of age.
Figure 4.
Regional and cellular distributions of Tbx1 in C57BL/6J mice. (A) RT-PCR analysis of Tbx1 mRNA in the prefrontal cortex (PFC), nucleus accumbens (NA), caudate-putamen (CP), amygdala (AMY), hippocampus (HIP), ventral tegmental area (VTA) and substantia nigra (SN) of 2-month-old C57BL/6J mice. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; -RT, omission of reverse transcriptase. (B–D) Immunofluorescent signals of Tbx1 protein at various anteroposterior levels in 2-month-old C57BL/6J mice. rms, rostral migratory stream; svz, subventricular zone; dtg, dentate gyrus. Scale bars, 500 μm. (E–G) Confocal images of Tbx1 expression (red) and BrdU (green) in the rostral migratory stream on a sagittal section (E), right subventricular zone (F) and the granule layer of the left dentate gyrus (G) of C57BL/6J mice. BrdU injections (50 mg/kg/day, intraperitoneal, 7 days) started at 5 weeks of age and mice were sacrificed 24 h after the last injection. BrdU-positive cells were colocalized with Tbx1-positive cells (yellowish green). Arrows in (E) indicate a few BrdU-positive, but Tbx1-negative cells. lv, lateral ventricle; str, striatum. Scale bars, 40 μm. Orthogonal projections of a point in space are shown as white, dotted crosshairs with the projected side views in the X–Z plane (below) and the Y–Z plane (left). The experiment was done in three mice, and data were consistent. The location of captured images is indicated by insets.
Although low levels of Tbx1 protein were ubiquitously seen in the whole brain of C57BL/6J mice, higher levels were present in the olfactory bulb (Fig. 4B), rostral migratory stream at the levels of olfactory bulb and nucleus accumbens (Fig. 4B and C), the subventricular zone (Fig. 4C) and the granular layer of the dentate gyrus (Fig. 4D).
Because these structures exhibit postnatal/adult neurogenesis (28), we next determined the localization of Tbx1 in postnatally proliferating cells. Almost all bromodeoxyuridine (BrdU)-positive cells (green) were colocalized (yellowish green) with Tbx1-positive cells (red) in the rostral migratory stream (Fig. 4E), the subventricular zone (Fig. 4F) and the subgranular zone of the dentate gyrus (Fig. 4G).
Because BrdU was given within a 7-day window and is expected to label a fraction of proliferating cells, there were BrdU-negative, Tbx1-positive cells within the regions known to exhibit postnatal neurogenesis (Fig. 4F and G). Tbx1 is also probably expressed in prenatally generated neurons, as Tbx1 was additionally seen in regions not known to exhibit postnatal/adult neurogenesis (Fig. 4B–D). On the other hand, there were a few BrdU-positive cells without detectable levels of Tbx1 staining in the rostral migratory stream (see arrows pointing at green cells in Fig. 4E), suggesting the possibility that Tbx1 expression might have declined after proliferation in these cells.
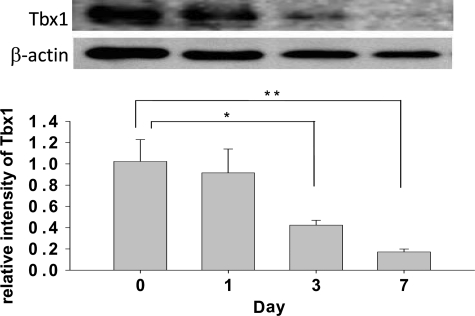
To confirm this possibility, we examined Tbx1 levels in proliferating and differentiated cells in hippocampal cell culture derived from the postnatal (P) day 0 hippocampus of C57BL/6J mice. Tbx1 levels were high during proliferation in the presence of epidermal growth factor (EGF), and declined as differentiation progressed following EGF withdrawal (Fig. 5).
Figure 5.
Tbx1 expression in postnatal hippocampal cell culture. Tbx1 protein during proliferation and differentiation of hippocampal cells. The relative intensity of Tbx1 signal (mean ± SEM) is plotted against days following EGF withdrawal (day, F3,12 = 6.81114, P = 0.0062). Asterisks indicate statistically significant differences from day 0, as determined by Newman–Keuls post hoc comparisons.
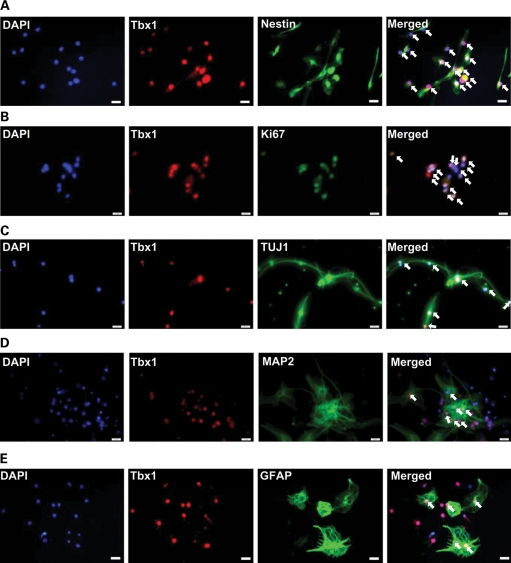
Although Tbx1 levels were very low during differentiation (see Fig. 5, bar graph, day 7), western blotting uses samples that include many cell types that may or may not express Tbx1, potentially underestimating the level of Tbx1. Thus, we histochemically examined Tbx1 in postnatally generated neurons during proliferation and differentiation. Tbx1 was expressed in neuronal progenitor cells (labeled by nestin) (Fig. 6A) during proliferation (labeled by Ki67) (Fig. 6B). Tbx1 was expressed in cells positive for neuron-specific class III β-tublin (Tuj1), an early neuronal marker (Fig. 6C) 7 days after EGF withdrawal. As Tuj1 is expressed in both immature and mature neurons, we also examined the colocalization of Tbx1 with microtubule-associated protein 2 (MAP2), a marker of mature neurons (29), at the time point when cells differentiate into mature neurons (i.e. 14 days after EGF withdrawal). Tbx1 was expressed in differentiated, mature neurons (Fig. 6D). Tbx1 was also expressed in glial fibrillary acidic protein (GFAP)-positive astroglia 7 days after EGF withdrawal (Fig. 6E). Tbx1 signals without MAP2 (Fig. 6D) or GFAP (Fig. 6E) are likely to represent glial cells and neurons, respectively. These data demonstrate that Tbx1 is present in proliferating cells, immature and mature neurons and glial cells of the postnatal origin.
Figure 6.
Tbx1 colocalization in postnatal hippocampal cell culture. (A and B) Tbx1 localization in postnatal progenitor cells of hippocampal cell culture during proliferation with EGF. The cell nucleus is visualized with the nuclear marker DAPI (blue). Tbx1 (red) is shown with the neural progenitor cell marker nestin (green) (A) or the cell proliferation marker Ki67 (green) (B). All Tbx1-positive cells are colocalized with nestin or Ki67, but the intensity of each color differed from cell to cell, thereby giving different tones of merged color. Tbx1 localization in postnatal progenitor cells of hippocampal cell culture during differentiation into neurons (C and D) and GFAP-positive glial cells (E) 7 (C and E) and 14 days (D) following EGF withdrawal. Tbx1 is shown with the neuronal markers TUJ1 and MAP2, the glial marker GFAP and the nucleus marker DAPI. The experiment was repeated two or three times. Scale bars, 20 μm.
DISCUSSION
Constitutive Tbx1 heterozygosity in mice genetically recapitulated constitutive human 22q11.2 hemizygosity at a single gene and isolated the impact of this 22q11.2 gene on behavior. Our analysis demonstrates that constitutive heterozygosity of Tbx1 results in ASD-related behavioral phenotypes, and that Tbx1 protein is ubiquitously expressed throughout the mouse brain, but is enriched in postnatally proliferating cells.
Defective social interaction, the core symptom of ASD, appears in childhood but persists into adolescence and adulthood (30). Mice begin to show signs of sexual maturation around 1 month of age (31), but many biological processes in the brain continue to change afterwards (32–36) until they reach mature adulthood at 3 months of age (37). Congenic HT mice were impaired in affiliative social interaction at 2 months of age without confounding alterations in aggression, olfactory investigation or motor behavior. This behavioral phenotype in social interaction is not due to a non-specific reduction in exploratory behavior, as HT mice showed elevated levels of interaction with a non-mouse object. Interestingly, this phenotype in interaction with an object is consistent with the fact that children with ASD often prefer to interact with non-human objects.
HT mice were not impaired in the elevated plus maze, but exhibited higher levels of thigmotaxis in the inescapable open field. This apparent discrepancy might be due to a floor effect. Because WT and HT mice exhibited robust anxiety-related behaviors in our elevated plus maze, as shown by low levels (∼4%) of time spent in the open arms of the elevated plus maze, it might have been difficult to detect a further reduction in time spent in the open arms. However, much lower levels of time in the open arms have been demonstrated in various mouse strains (38). Alternatively, although these two tasks reflect the level of anxiety, stress levels are much lower when mice have a choice to escape from an open arm to a closed arm in the elevated plus maze than when they do not in the inescapable open field (39,40). Although anxiety is not the symptomatic element of ASD in itself, it is highly prevalent and exacerbated by stress in children, adolescents and adults with 22q11.2 deletion (5,16,41–43). Tbx1 deficiency might additionally increase the vulnerability to stress-related anxiety in 22q11.2 deletion cases.
When mouse pups are separated from mothers, they emit ultrasonic vocalization, which elicits their retrieval by mothers. This behavior is regarded as a form of social communication in rodents (44). Compared with WT pups, HT pups were impaired in complex patterns of vocalization (e.g. complex, harmonics, two-syllable, composite and frequency steps); WT and HT were indistinguishable in simple patterns (e.g. upward, downward, hump and short). This deficit pattern in vocalization might signify an autism-related phenotype in pups. One caution is that, like 22q11.2-deletion patients (45,46), constitutive Tbx1-deficient mice show hearing impairments (47) and velopharyngeal dysfunction (47–50). It could be that Tbx1 HT mice emit less vocal calls partly due to these physical abnormalities. However, HT mice selectively showed lower levels of vocal sounds in complex vocal patterns only, and were indistinguishable from WT mice in simple vocal patterns; HT mice emitted even more frequently—although statistically non-significantly—upward and downward vocal sounds. These considerations notwithstanding, this is not an issue of whether this mouse faithfully recapitulates the ASD-related phenotypes—it does—but what processes underlie defective vocal sounds in both 22q11.2-deletion patients and Tbx1 HT mice.
Children and adolescents with 22q11.2 hemizygosity—like idiopathic autism—are weak in working memory (9,13–16). Tbx1 HT mice showed lower levels of spontaneous alternation in the T-maze. Given the lack of anxiety-related phenotypes in a similar choice paradigm (i.e. elevated plus maze), the deficit in spontaneous alternation is unlikely to be a result of altered anxiety levels. Although spontaneous alternation requires working memory to recall an arm previously visited (51), performance could also be negatively impacted by a repetitive behavioral tendency. Interestingly, when HT mice showed a sign of memory at 0 s delay, they had a higher degree of repetitive choices than WT mice; at a 60 s delay, HT and WT mice were indistinguishable in repetitiveness and HT mice did not increase the degree of repetitiveness beyond 50% (Fig. 3B). These data suggest that the repetitive behavioral tendency manifests itself in the form of memory-guided behavior, but not as a simple motor repetitiveness, in HT mice. Interestingly, individuals with idiopathic ASD have difficulty in inhibiting context-inappropriate behavior based on working memory, which is thought to underlie actions and verbalizations that are inappropriate in timing or to the circumstances; they are not impaired in simple response inhibition that are not dependent on memory (52,53).
A gene dose alteration of a ∼200 kb 22q11.2 region, including Tbx1, induces antipsychotic-responsive repetitive motor behavior (25), and 22q11.2-associated ASD includes motor stereotypy (12). We did not find motor stereotypy in HT mice. As deficiency in Sept5, another gene in the 200 kb region, impairs social interaction but does not cause motor stereotypy either (54), Gnb1l and Gp1bβ, the remaining genes encoded in the segment, might contribute to motor stereotypy. A corollary of the existing data so far (25,54,55) is that some but not all 22q11.2 genes contribute to behavioral phenotypes and each of these genes affects its own set of behaviors, which could partially overlap or be totally distinct in their phenotypic targets.
Tbx1 protein was present in regions not known to have postnatal/adult neurogenesis (e.g. prefrontal cortex and amygdala) as well as in postnatally proliferating cells in the rostral migratory stream, subventricular zone and hippocampus. Tbx1 deficiency could cause signaling alterations in prenatally or postnatally generated mature neurons in the mouse brain. Alternatively or additionally, Tbx1 deficiency might alter prenatal/postnatal/adult neurogenesis. However, not all of the behavioral phenotypes of congenic Tbx1 HT mice are likely to occur through low levels of Tbx1 in postnatally generated neurons or alterations in postnatal/adult neurogenesis. First, our data show that Tbx1 could additionally be expressed in cells that differentiate into glial cells. As various environmental stimuli (e.g. enriched environment, physical activity and electroconvulsive seizures) induce reactive glial proliferation in the prefrontal cortex and amygdala of adult rats and mice (56–60), Tbx1 might be additionally involved in reactive glial proliferation in areas with no known postnatal/adult neurogenesis. Second, the phenotype in ultrasonic vocalization was detected at P7–8, a time point too early for postnatally generated neurons to mature and function. Third, spontaneous alternation in the T- or Y-maze is not affected by inhibition of adult neurogenesis (61,62) (but see 63). Fourth, inter-gender interaction (but not intra-gender affiliative social interaction) in female mice is ‘increased’ (not decreased) by inhibition of adult neurogenesis in the subventricular zone (64). However, as inhibition of adult neurogenesis was targeted at 2 months of age or older in these studies, the role of earlier, postnatal neurogenesis in these ASD-related behaviors remains unexplored. A future challenge is to selectively target Tbx1 deletion in prenatally or postnatally generated neurons and glial cells, and determine the precise role this transcription factor plays in ASD-related behavioral phenotypes and postnatal/adult neurogenesis.
Individuals with 22q11.2 hemizygosity are also diagnosed—at elevated rates compared with the general population—with schizophrenia (41,65–70). A recent estimate indicates that one-fourth of individuals with 22q11.2 deletion are diagnosed with schizophrenia (71). Defective social cognition, poverty of speech and working memory deficits are prodromal symptoms and symptomatic elements of schizophrenia (72,73). When individuals with 22q11.2 hemizygosity reach adulthood, defective working memory is worse in those with schizophrenia than in those without it (15). Higher and increasing levels of anxiety are a risk factor for later developing schizophrenia in 22q11.2 cases (74). Our data suggest a tantalizing hypothesis that Tbx1 deficiency underlies specific prodromal and symptomatic elements of schizophrenia, as well as ASD. Such an action of a gene is consistent with the suggestion that the way genes influence neuropsychiatric disorders does not necessarily follow the DSM-based diagnostic boundary (55,75,76).
MATERIALS AND METHODS
Mouse
We generated congenic Tbx1 HT and WT mice by backcrossing non-congenic Tbx1 HT mouse (77) to C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) for 10 generations. Congenic, male Tbx1 WT and HT mice were behaviorally tested at 2 months of age. Because ultrasonic vocalization is detectable at P7–8 but is rare and difficult to detect before and after this time window (44), this behavior was recorded at P7–8 in congenic, female Tbx1 WT and HT mice. For anatomical analyses, male C57BL/6J mice were used at 5 weeks or 2 months of age. For cell culture, C57BL/6J mice were sacrificed at P0. Animal handling and use followed a protocol approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine, in accordance with NIH guidelines.
Behavioral analysis
Social interaction
Our experimental protocol (54,55) was designed to optimally elicit affiliative social interaction and minimize aggressive behaviors. Age-matched, male C57BL/6J inbred mice (Jackson Laboratory) were paired with either WT or HT mice.
Olfactory investigation
A plastic home cage (28.5 cm long × 18 cm wide and 13 cm high) was divided into two compartments (19.5 cm long and 9 cm long) with a partition wall. A 1.5 ml Eppendorf tube containing peanut butter (50% in water) was attached on the wall of the smaller compartment 2.5 cm above the floor. The lid of the tube had seven holes to release the odor. Two 3 min sessions were given with a 30 min intersession interval. All activity was recorded with a digital camera. An observer blinded to genotype and experimental groups scored the amount of time each mouse spent sniffing the odorant-containing tube.
Novel object contact
An individual mouse was placed in a new home cage for 30 min. The mouse was then moved into another home cage that contained a modified falcon tube (3 cm diameter × 8.5 cm length) during two 5 min sessions with a 30 min intersession interval. The amount of time each mouse spent contacting the novel object, in the forms of sniffing and exploratory investigation, was analyzed. Otherwise, the procedure was identical to that of olfactory investigation.
Elevated plus maze
The apparatus and procedure for this anxiety-related behavior were identical to those described in our previous studies (54,55,78).
Motor activity
Motor activity was recorded for 30 min in an inescapable open field (Truscan, Coulbourn Instruments, Allentwon, PA, USA) (54,55,78).
Ultrasonic vocalization
Each pup was separated from its mother and placed onto a plastic tray with cage bedding at P7–8. The tray was placed in a styrofoam box with the ultrasonic microphone (Avisoft UltraSoundGate condenser microphone capsule CM16, Avisoft Bioacoustics, Berlin, Germany) on the lid 12.5 cm above the tray. This microphone is sensitive to frequencies between 10 and 200 kHz. Immediately after each pup was placed onto the tray, the vocalization sound was recorded and analyzed for 5 min at 300 kHz sampling rate (format 16 bit) using AVISOFT SASLABPRO (version 5.1.0). For acoustical analysis, a fast Fourier transformation (FFT) was conducted. Spectrograms were generated with an FFT length of 256 points and a time window overlap of 50% (100% frame, flat-top window). The spectrogram was produced at a frequency resolution of 488 Hz and a time resolution of 1 ms. A lower cut-off frequency was set at 20 kHz. The frequency and average duration of 10 distinct types of calls (79) were analyzed.
Spontaneous alternation
A T-maze was used to evaluate spontaneous alternation, as described in our previous publications (54,55).
Anatomical Analysis
Semiquantitative RT-PCR
Total RNA was extracted from the prefrontal cortex, nucleus accumbens, caudate-putamen, hippocampus, amygdala, ventral tegmental area and substantia nigra of 2-month-old C57BL/6J mice using RNeasy Mini Kit (QIAGEN, Valencia, CA, USA). DNA was eliminated by on-column treatment with RNase-Free DNase I (QIAGEN). A total of 250 μg RNA was used for cDNA synthesis by SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Primers used were: Tbx1, forward, ATGATCTCCGCCGTGTCTAG, reverse, CGTGGGGAACATTCGTCTGCCTG, band size, 420 bp; Gapdh, forward, ACCACCATGGAGAAGGC, reverse, GGCATGGACTGTGGTCATGA, band size, 234 bp. RT-PCR was run for 35 cycles.
Immunofluorescence
C57BL/6J mice were sacrificed at 2 months of age and their brains were coronally or sagittally sectioned at 10 μm using a cryostat and placed onto silane glass slides. Sections were fixed for 5 min with 4% paraformaldehyde in phosphate-buffered saline (PBS) with Tween 20 (0.2%), washed in PBS three times, treated with 0.1% Triton X in PBS for 15 min, washed with PBS three times, blocked for 1 h with 10% fetal bovine serum in PBS and incubated at 4°C overnight with a polyclonal rabbit anti-Tbx1 antibody (1:50 or 1:100, Abcam, see Supplementary Material, Figure S1, for the selectivity of the antibody). Sections were then incubated for 90 min with donkey anti-rabbit IgG conjugated with Texas red (1:200, Jackson ImmunoResearch, West Grove, PA, USA) and cover-slipped.
BrdU labeling
C57BL/6J mice were given the thymidine analog BrdU (50 mg/kg/day, intraperitoneal, 10 mg/ml) for 7 days starting at the age of 5 weeks, and were killed 24 h after the last injection. Following the basic procedure (see Immunofluorescence), sections were incubated for 48 h at 4°C with a cocktail of rabbit anti-Tbx1 antibody (1:100, Abcam) and a mouse anti-BrdU (1:12, Roche, Indianapolis, IN, USA). The sections were incubated with a cocktail of goat anti-rabbit IgG conjugated with Alexa Fluor-594 (red, 1:200, Invitrogen) for Tbx1, and goat anti-mouse IgG conjugated with Alexa Fluor-488 (green, 1:200, Invitrogen) for BrdU in PBS for 90 min at room temperature. Fluorescent signals were detected using confocal microscopy (Nikon, Melville, NY, USA).
Cell culture preparation
We followed our standard procedure with modifications (80). Briefly, hippocampal tissues were dissected out from the brains of C57BL/6J mice at P0. Cells were maintained in a culture medium containing EGF; cell differentiation was initiated by removing EGF from the culture. Cells were culled and fixed for western blotting and immunofluorescent staining, respectively, while they were maintained in an EGF-containing medium or 1, 2, 7 or 14 days after the removal of EGF from the culture. We used our routine western blotting protocol (39) to quantitatively evaluate Tbx1 expression in postnatally derived and cultured hippocampal cells. We used a rabbit polyclonal antibody to Tbx1 (1:500, Abcam, Cambridge, MA, USA) and goat anti-rabbit horseradish peroxidase-conjugated IgG secondary antibody (1:5000, Pierce, Rockford, IL, USA). Additionally, immunofluorescent staining was used to evaluate Tbx1 expression in various cell types, according to our routine procedure (80). Briefly, we used rabbit anti-Tbx1 antibody (1:100, Abcam), mouse anti-nestin antibody (1:50, BD Pharmingen, Franklin Lakes, NJ, USA), mouse anti-Ki67 antibody (1:100, Abcam), mouse anti-Tuj1 antibody (1:100, Sigma), mouse anti-MAP2 antibody (1:100, Thermo Scientific) and mouse anti-GFAP antibody (1:200, Sigma). These markers were then labeled with goat anti-rabbit IgG conjugated with Alexa Fluor-594 (1:200, Invitrogen) for Tbx1, and goat anti-mouse IgG conjugated with Alexa Fluor-488 (1:200, Invitrogen) for nestin, Ki67, Tuj1 MAP2, and GFAP.
Statistical analysis
Group means were compared using analysis of variance, followed by Newman–Keuls post hoc comparisons. When only two groups were compared, we used the t-test. A probability of 0.05 or less was considered to be significant.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
This work was supported by the National Institute of Health (HD05311), NARSAD Independent Investigator Award and the Maltz Foundation to N.H. and funds from the Smoking Research Foundation grant to Y.W.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Bernice Morrow for providing us with the original line of Tbx1 HT breeders and Mr D. Scott and Ms K.M. Harper for their valuable comments on an early draft of this article.
REFERENCES
- 1.Steffenburg S., Gillberg C., Hellgren L., Andersson L., Gillberg I.C., Jakobsson G., Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J. Child Psychol. Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey A., Le C.A., Gottesman I., Bolton P., Simonoff E., Yuzda E., Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol. Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 3.Schaaf C.P., Zoghbi H.Y. Solving the autism puzzle a few pieces at a time. Neuron. 2011;70:806–808. doi: 10.1016/j.neuron.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Golding-Kushner K.J., Weller G., Shprintzen R.J. Velo-cardio-facial syndrome: language and psychological profiles. J. Craniofac. Genet. Dev. Biol. 1985;5:259–266. [PubMed] [Google Scholar]
- 5.Baker K.D., Skuse D.H. Adolescents and young adults with 22q11 deletion syndrome: psychopathology in an at-risk group. Br. J. Psychiatry. 2005;186:115–120. doi: 10.1192/bjp.186.2.115. [DOI] [PubMed] [Google Scholar]
- 6.Niklasson L., Rasmussen P., Oskarsdottir S., Gillberg C. Chromosome 22q11 deletion syndrome (CATCH 22): neuropsychiatric and neuropsychological aspects. Dev. Med. Child Neurol. 2002;44:44–50. doi: 10.1017/s0012162201001645. [DOI] [PubMed] [Google Scholar]
- 7.Swillen A., Devriendt K., Legius E., Eyskens B., Dumoulin M., Gewillig M., Fryns J.P. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J. Med. Genet. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiley-Brabeck K., Sobin C. Social skills and executive function deficits in children with the 22q11 deletion syndrome. Appl. Neuropsychol. 2006;13:258–268. doi: 10.1207/s15324826an1304_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodin M., Wang P.P., Aleman D., Donald-McGinn D., Zackai E., Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet. Med. 2001;3:34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Solot C.B., Knightly C., Handler S.D., Gerdes M., Donald-McGinn D.M., Moss E., Wang P., Cohen M., Randall P., LaRossa D., Driscoll D.A. Communication disorders in the 22Q11.2 microdeletion syndrome. J. Commun. Disord. 2000;33:187–203. doi: 10.1016/s0021-9924(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 11.Solot C.B., Gerdes M., Kirschner R.E., Donald-McGinn D.M., Moss E., Woodin M., Aleman D., Zackai E.H., Wang P.P. Communication issues in 22q11.2 deletion syndrome: children at risk. Genet. Med. 2001;3:67–71. doi: 10.1097/00125817-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Kates W.R., Antshel K.M., Fremont W.P., Shprintzen R.J., Strunge L.A., Burnette C.P., Higgins A.M. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am. J. Med. Genet. A. 2007;143A:2642–2650. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- 13.Campbell L.E., Azuma R., Ambery F., Stevens A., Smith A., Morris R.G., Murphy D.G., Murphy K.C. Executive functions and memory abilities in children with 22q11.2 deletion syndrome. Aust. N. Z. J. Psychiatry. 2010;44:364–371. doi: 10.3109/00048670903489882. [DOI] [PubMed] [Google Scholar]
- 14.Sobin C., Kiley-Brabeck K., Daniels S., Khuri J., Taylor L., Blundell M., Anyane-Yeboa K., Karayiorgou M. Neuropsychological characteristics of children with the 22q11 deletion syndrome: a descriptive analysis. Child Neuropsychol. 2005;11:39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Amelsvoort T., Henry J., Morris R., Owen M., Linszen D., Murphy K., Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophr. Res. 2004;70:223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Lewandowski K.E., Shashi V., Berry P.M., Kwapil T.R. Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:27–36. doi: 10.1002/ajmg.b.30379. [DOI] [PubMed] [Google Scholar]
- 17.Fine S.E., Weissman A., Gerdes M., Pinto-Martin J., Zackai E.H., Donald-McGinn D.M., Emanuel B.S. Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. J. Autism Dev. Disord. 2005;35:461–470. doi: 10.1007/s10803-005-5036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vorstman J.A., Morcus M.E., Duijff S.N., Klaassen P.W., Heineman-de Boer J.A., Beemer F.A., Swaab H., Kahn R.S., van Engeland E.H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 19.Antshel K.M., Aneja A., Strunge L., Peebles J., Fremont W.P., Stallone K., AbdulSabur N., Higgins A.M., Shprintzen R.J., Kates W.R. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion) J. Autism Dev. Disord. 2007;37:1776–1786. doi: 10.1007/s10803-006-0308-6. [DOI] [PubMed] [Google Scholar]
- 20.Szatmari P., Paterson A.D., Zwaigenbaum L., Roberts W., Brian J., Liu X.Q., Vincent J.B., Skaug J.L., Thompson A.P., Senman L., et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall C.R., Noor A., Vincent J.B., Lionel A.C., Feuk L., Skaug J., Shago M., Moessner R., Pinto D., Ren Y., et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christian S.L., Brune C.W., Sudi J., Kumar R.A., Liu S., Karamohamed S., Badner J.A., Matsui S., Conroy J., McQuaid D., et al. Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biol. Psychiatry. 2008;63:1111–1117. doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai G., Edelmann L., Goldsmith J.E., Cohen N., Nakamine A., Reichert J.G., Hoffman E.J., Zurawiecki D.M., Silverman J.M., Hollander E., et al. Multiplex ligation-dependent probe amplification for genetic screening in autism spectrum disorders: efficient identification of known microduplications and identification of a novel microduplication in ASMT. BMC Med. Genomics. 2008;1:50. doi: 10.1186/1755-8794-1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaikh T.H., Kurahashi H., Saitta S.C., O'Hare A.M., Hu P., Roe B.A., Driscoll D.A., Donald-McGinn D.M., Zackai E.H., Budarf M.L., Emanuel B.S. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum. Mol. Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- 25.Hiroi N., Zhu H., Lee M., Funke B., Arai M., Itokawa M., Kucherlapati R., Morrow B., Sawamura T., Agatsuma S. A 200-kb region of human chromosome 22q11.2 confers antipsychotic-responsive behavioral abnormalities in mice. Proc. Natl Acad. Sci. USA. 2005;102:19132–19137. doi: 10.1073/pnas.0509635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paylor R., Glaser B., Mupo A., Ataliotis P., Spencer C., Sobotka A., Sparks C., Choi C.H., Oghalai J., Curran S., et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc. Natl Acad. Sci. USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawley J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Scheffler B., Walton N.M., Lin D.D., Goetz A.K., Enikolopov G., Roper S.N., Steindler D.A. Phenotypic and functional characterization of adult brain neuropoiesis. Proc. Natl Acad. Sci. USA. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigman M. The Emanuel Miller Memorial Lecture 1997. Change and continuity in the development of children with autism. J. Child Psychol. Psychiatry. 1998;39:817–827. [PubMed] [Google Scholar]
- 31.Bronson F.H., Dagg C.P., Snell G.D. Reproduction. In: Green E.L, editor. Biology of the Laboratory Mouse. New York: Dover Publications, Inc.; 2007. online publication. [Google Scholar]
- 32.Counotte D.S., Li K.W., Wortel J., Gouwenberg Y., Van Der Schors R.C., Smit A.B., Spijker S. Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. Eur. J. Neurosci. 2010;32:1452–1460. doi: 10.1111/j.1460-9568.2010.07404.x. [DOI] [PubMed] [Google Scholar]
- 33.Cressman V.L., Balaban J., Steinfeld S., Shemyakin A., Graham P., Parisot N., Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J. Comp. Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham M.G., Bhattacharyya S., Benes F.M. Increasing interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb. Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- 35.He J., Crews F.T. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol. Biochem. Behav. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Markham J.A., Morris J.R., Juraska J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Flurkey K., Currrer J.M., Harrison D.E. The mouse in aging research. In: Fox J.G., editor. The Mouse in Biomedical Research. Vol. 3. Burlington, MA: Elsevier; 2007. pp. 637–672. [Google Scholar]
- 38.Moy S.S., Nadler J.J., Young N.B., Perez A., Holloway L.P., Barbaro R.P., Barbaro J.R., Wilson L.M., Threadgill D.W., Lauder J.M., et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H., Lee M., Agatsuma S., Hiroi N. Pleiotropic impact of constitutive fosB inactivation on nicotine-induced behavioral alterations and stress-related traits in mice. Hum. Mol. Genet. 2007;16:820–836. doi: 10.1093/hmg/ddm027. [DOI] [PubMed] [Google Scholar]
- 40.Misslin R., Herzog F., Koch B., Ropartz P. Effects of isolation, handling and novelty on the pituitary–adrenal response in the mouse. Psychoneuroendocrinology. 1982;7:217–221. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 41.Bassett A.S., Hodgkinson K., Chow E.W., Correia S., Scutt L.E., Weksberg R. 22q11 deletion syndrome in adults with schizophrenia. Am. J. Med. Genet. 1998;81:328–337. [PMC free article] [PubMed] [Google Scholar]
- 42.Swillen A., Vogels A., Devriendt K., Fryns J.P. Chromosome 22q11 deletion syndrome: update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am. J. Med. Genet. 2000;97:128–135. doi: 10.1002/1096-8628(200022)97:2<128::aid-ajmg4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Beaton E.A., Simon T.J. How might stress contribute to increased risk for schizophrenia in children with chromosome 22q11.2 deletion syndrome? J. Neurodev. Disord. 2011;3:68–75. doi: 10.1007/s11689-010-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scattoni M.L., Crawley J., Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scambler P.J. The 22q11 deletion syndromes. Hum. Mol. Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- 46.Piran S., Bassett A.S., Grewal J., Swaby J.A., Morel C., Oechslin E.N., Redington A.N., Liu P.P., Silversides C.K. Patterns of cardiac and extracardiac anomalies in adults with tetralogy of Fallot. Am. Heart J. 2011;161:131–137. doi: 10.1016/j.ahj.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao J., Kochilas L., Nowotschin S., Arnold J.S., Aggarwal V.S., Epstein J.A., Brown M.C., Adams J., Morrow B.E. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- 48.Jerome L.A., Papaioannou V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 49.Vitelli F., Morishima M., Taddei I., Lindsay E.A., Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum. Mol. Genet. 2002;11:915–922. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- 50.Kelly R.G., Jerome-Majewska L.A., Papaioannou V.E. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum. Mol. Genet. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- 51.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 52.O'Hearn K., Asato M., Ordaz S., Luna B. Neurodevelopment and executive function in autism. Dev. Psychopathol. 2008;20:1103–1132. doi: 10.1017/S0954579408000527. [DOI] [PubMed] [Google Scholar]
- 53.Kana R.K., Keller T.A., Minshew N.J., Just M.A. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol. Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki G., Harper K.M., Hiramoto T., Sawamura T., Lee M., Kang G., Tanigaki K., Buell M., Geyer M.A., Trimble W.S., et al. Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum. Mol. Genet. 2009;18:1652–1660. doi: 10.1093/hmg/ddp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki G., Harper K.M., Hiramoto T., Funke B., Lee M., Kang G., Buell M., Geyer M.A., Kucherlapati R., Morrow B., et al. Over-expression of a human chromosome 22q11.2 segment including TXNRD2, COMT, and ARVCF developmentally affects incentive learning and working memory in mice. Hum. Mol. Genet. 2009;18:3914–3925. doi: 10.1093/hmg/ddp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehninger D., Wang L.P., Klempin F., Romer B., Kettenmann H., Kempermann G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okuda H., Tatsumi K., Makinodan M., Yamauchi T., Kishimoto T., Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- 58.Ongur D., Pohlman J., Dow A.L., Eisch A.J., Edwin F., Heckers S., Cohen B.M., Patel T.B., Carlezon W.A. Electroconvulsive seizures stimulate glial proliferation and reduce expression of Sprouty2 within the prefrontal cortex of rats. Biol. Psychiatry. 2007;62:505–512. doi: 10.1016/j.biopsych.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Wennstrom M., Hellsten J., Tingstrom A. Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat amygdala. Biol. Psychiatry. 2004;55:464–471. doi: 10.1016/j.biopsych.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Madsen T.M., Yeh D.D., Valentine G.W., Duman R.S. Electroconvulsive seizure treatment increases cell proliferation in rat frontal cortex. Neuropsychopharmacology. 2005;30:27–34. doi: 10.1038/sj.npp.1300565. [DOI] [PubMed] [Google Scholar]
- 61.Hernandez-Rabaza V., Llorens-Martin M., Velazquez-Sanchez C., Ferragud A., Arcusa A., Gumus H.G., Gomez-Pinedo U., Perez-Villalba A., Rosello J., Trejo J.L., et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 62.Saxe M.D., Battaglia F., Wang J.W., Malleret G., David D.J., Monckton J.E., Garcia A.D., Sofroniew M.V., Kandel E.R., Santarelli L., et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl Acad. Sci. USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madsen T.M., Kristjansen P.E., Bolwig T.G., Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 64.Feierstein C.E., Lazarini F., Wagner S., Gabellec M.M., de Chaumont F., Olivo-Marin J.C., Boussin F.D., Lledo P.M., Gheusi G. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front. Behav. Neurosci. 2010;4:176. doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shprintzen R.J., Goldberg R., Golding-Kushner K.J., Marion R.W. Late-onset psychosis in the velo-cardio-facial syndrome. Am. J. Med. Genet. 1992;42:141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- 66.Pulver A.E., Nestadt G., Goldberg R., Shprintzen R.J., Lamacz M., Wolyniec P.S., Morrow B., Karayiorgou M., Antonarakis S.E., Housman D. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J. Nerv. Ment. Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Murphy K.C., Jones L.A., Owen M.J. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch. Gen. Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 68.Gothelf D., Presburger G., Zohar A.H., Burg M., Nahmani A., Frydman M., Shohat M., Inbar D., Aviram-Goldring A., Yeshaya J., et al. Obsessive-compulsive disorder in patients with velocardiofacial (22q11 deletion) syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;126:99–105. doi: 10.1002/ajmg.b.20124. [DOI] [PubMed] [Google Scholar]
- 69.Karayiorgou M., Morris M.A., Morrow B., Shprintzen R.J., Goldberg R., Borrow J., Gos A., Nestadt G., Wolyniec P.S., Lasseter V.K. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc. Natl Acad. Sci. USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green T., Gothelf D., Glaser B., Debbane M., Frisch A., Kotler M., Weizman A., Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 71.Bassett A.S., Marshall C.R., Lionel A.C., Chow E.W., Scherer S.W. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum. Mol. Genet. 2008;17:4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chow E.W., Watson M., Young D.A., Bassett A.S. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr. Res. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Debbane M., Glaser B., David M.K., Feinstein C., Eliez S. Psychotic symptoms in children and adolescents with 22q11.2 deletion syndrome: neuropsychological and behavioral implications. Schizophr. Res. 2006;84:187–193. doi: 10.1016/j.schres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 74.Gothelf D., Feinstein C., Thompson T., Gu E., Penniman L., Van Stone E., Kwon H., Eliez S., Reiss A.L. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am. J. Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- 75.Hyman S.E. Can neuroscience be integrated into the DSM-V? Nat. Rev. Neurosci. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- 76.Hiroi N., Scott D. Constitutional mechanisms of vulnerability and resilience to nicotine dependence. Mol. Psychiatry. 2009;14:653–667. doi: 10.1038/mp.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long J.M., Laporte P., Merscher S., Funke B., Saint-Jore B., Puech A., Kucherlapati R., Morrow B.E., Skoultchi A.I., Wynshaw-Boris A. Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics. 2006;7:247–257. doi: 10.1007/s10048-006-0054-0. [DOI] [PubMed] [Google Scholar]
- 78.Agatsuma S., Lee M., Zhu H., Chen K., Shih J.C., Seif I., Hiroi N. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum. Mol. Genet. 2006;15:2721–2731. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- 79.Scattoni M.L., Gandhy S.U., Ricceri L., Crawley J.N. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiramoto T., Kanda Y., Satoh Y., Takishima K., Watanabe Y. Dopamine D2 receptor stimulation promotes the proliferation of neural progenitor cells in adult mouse hippocampus. Neuroreport. 2007;18:659–664. doi: 10.1097/WNR.0b013e3280bef9d3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.