Abstract
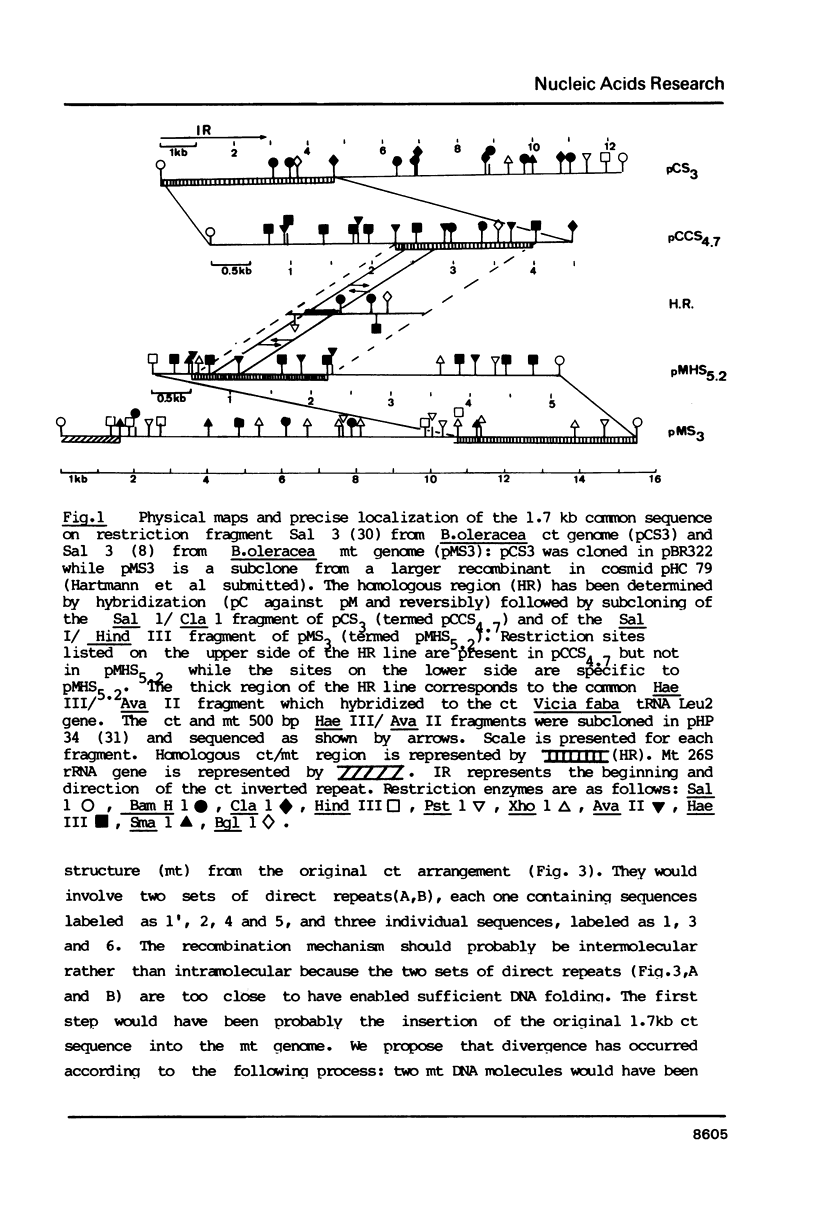
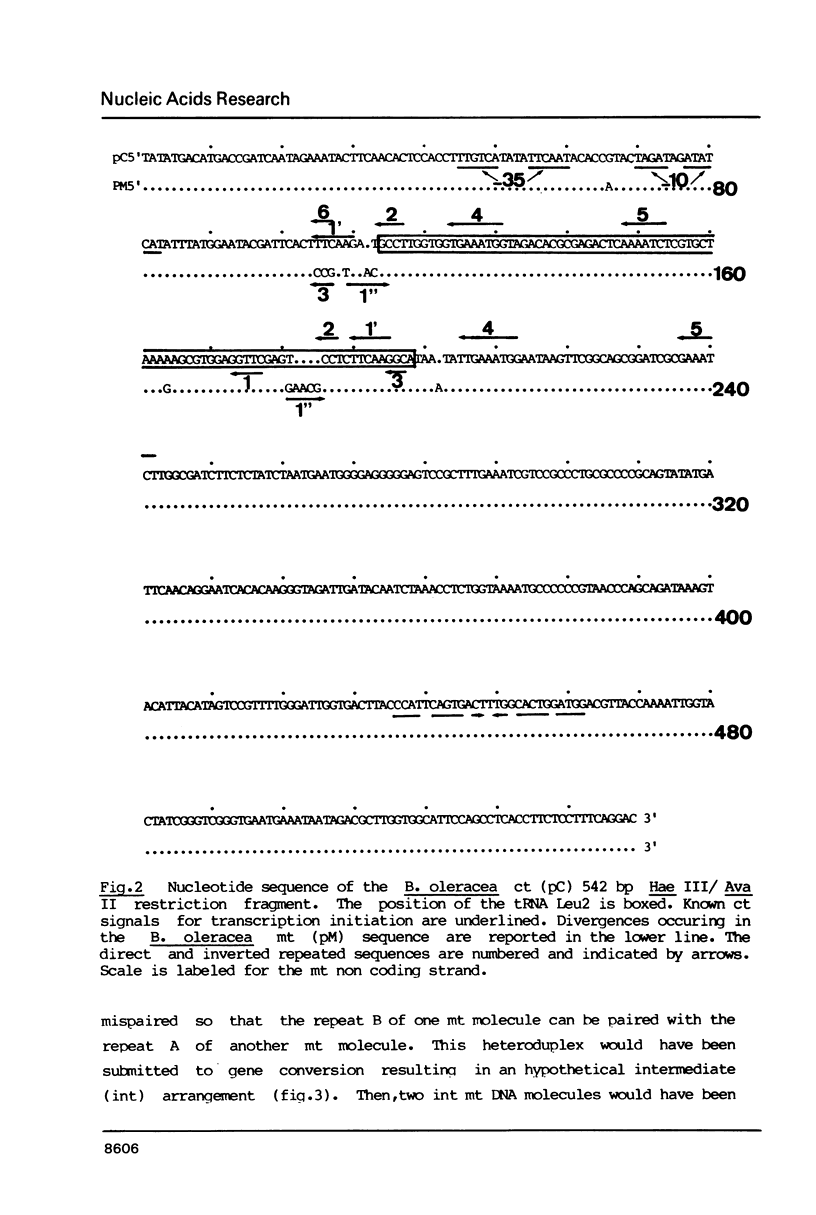
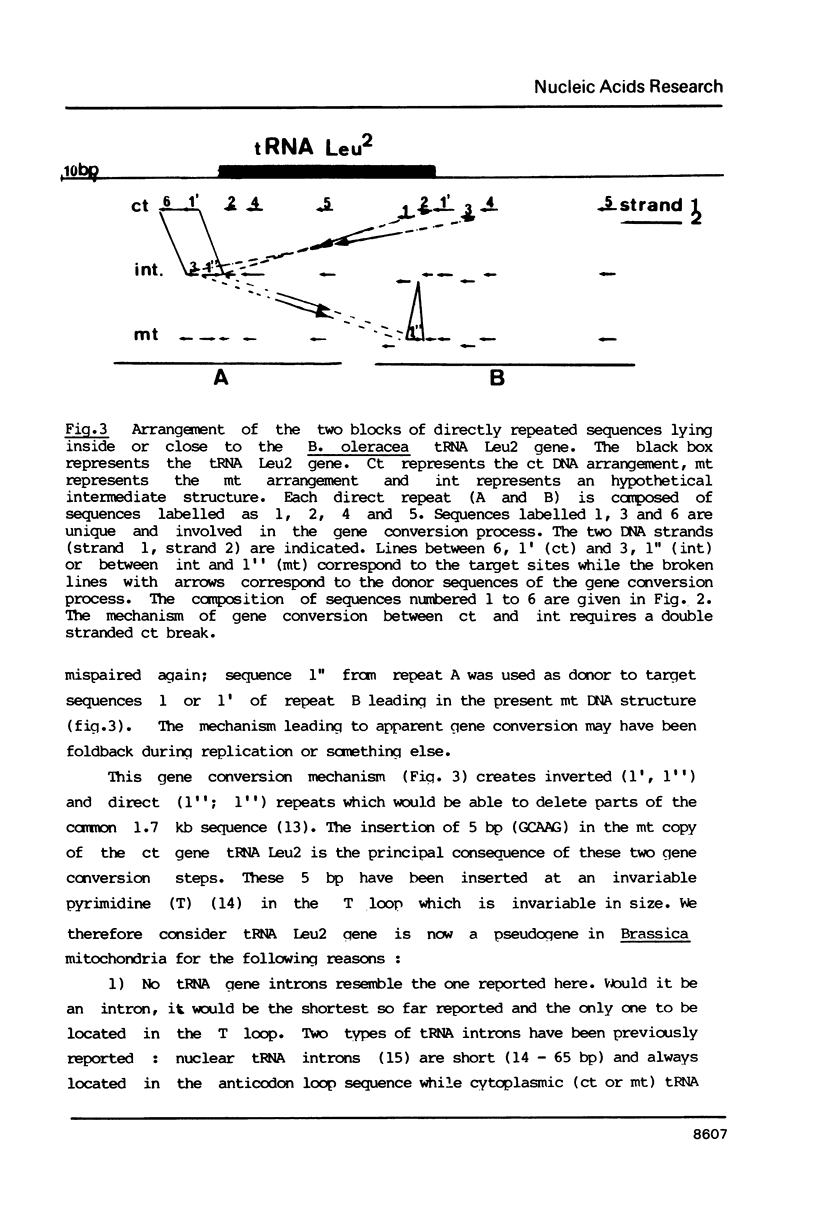
We have characterized a 1.7 kb sequence, containing a tRNA Leu2 gene shared by the ct and mt genomes of Brassica oleracea. The two sequences are completely homologous except in two short regions where two distinct gene conversion events have occurred between two sets of direct repeats leading to the insertion of 5 bp in the T loop of the mt copy of the ct gene. This is the first evidence that gene conversion represents the initial evolutionary step in inactivation of transferred ct genes in the mt genome. We also indicate that organelle DNA transfer by organelle fusion is an ongoing process which could be useful in genetic engineering.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bonnard G., Weil J. H., Steinmetz A. The intergenic region between the Vicia faba chloroplast tRNA(CAALeu) and tRNA(UAALeu) genes contains a partial copy of the split tRNA(UAALeu) gene. Curr Genet. 1985;9(5):417–422. doi: 10.1007/BF00421614. [DOI] [PubMed] [Google Scholar]
- Deno H., Kato A., Shinozaki K., Sugiura M. Nucleotide sequences of tobacco chloroplast genes for elongator tRNAMet and tRNAVal (UAC): the tRNAVal (UAC) gene contains a long intron. Nucleic Acids Res. 1982 Dec 11;10(23):7511–7520. doi: 10.1093/nar/10.23.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Promiscuous DNA--chloroplast genes inside plant mitochondria. Nature. 1982 Oct 21;299(5885):678–679. doi: 10.1038/299678a0. [DOI] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., West S. C., Stasiak A. Role of RecA protein spiral filaments in genetic recombination. Nature. 1984 May 17;309(5965):215–219. doi: 10.1038/309215a0. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Posakony J. W., Grula J. W., Roberts J. W., Xin J. H., Britten R. J., Davidson E. H. Mitochondrial DNA sequences in the nuclear genome of Strongylocentrotus purpuratus. J Mol Biol. 1983 Apr 25;165(4):609–632. doi: 10.1016/s0022-2836(83)80270-8. [DOI] [PubMed] [Google Scholar]
- Kourilsky P. Genetic exchanges between partially homologous nucleotide sequences: possible implications for multigene families. Biochimie. 1983 Feb;65(2):85–93. doi: 10.1016/s0300-9084(83)80178-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nomiyama H., Fukuda M., Wakasugi S., Tsuzuki T., Shimada K. Molecular structures of mitochondrial-DNA-like sequences in human nuclear DNA. Nucleic Acids Res. 1985 Mar 11;13(5):1649–1658. doi: 10.1093/nar/13.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Almeida M. L., Guillemaut P., Keith G., Canaday J., Weil J. H. Primary structure of three leucine transfer RNAs from bean chloroplast. Biochem Biophys Res Commun. 1980 Jan 15;92(1):102–108. doi: 10.1016/0006-291x(80)91525-9. [DOI] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G., Levings C. S., Timothy D. H. Identification of two methionine transfer RNA genes in the maize mitochondrial genome. Plant Physiol. 1984 Dec;76(4):1079–1082. doi: 10.1104/pp.76.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Gegenheimer P., Abelson J. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell. 1983 Feb;32(2):525–536. doi: 10.1016/0092-8674(83)90472-5. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. A modified pBR322 vector with improved properties for the cloning, recovery, and sequencing of blunt-ended DNA fragments. Gene. 1982 Feb;17(2):189–196. doi: 10.1016/0378-1119(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. F., Schnare M. N., Gray M. W. Pronounced structural similarities between the small subunit ribosomal RNA genes of wheat mitochondria and Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):493–497. doi: 10.1073/pnas.81.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Palmer J. D. Extensive and widespread homologies between mitochondrial DNA and chloroplast DNA in plants. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1946–1950. doi: 10.1073/pnas.81.7.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]



