Abstract
Leptin signaling in the hypothalamus is obligatory for normal food intake and body weight homeostasis. It is now well established that besides the signal transducer and activator of transcription-3 (STAT3) pathway, several non-STAT3 pathways mediate leptin signaling in the hypothalamus. We have previously demonstrated that leptin stimulates phosphodiesterase-3B (PDE3B) activity in the hypothalamus, and PDE3 inhibitor cilostamide reverses anorectic and bodyweight reducing effects of leptin. Recently, we have demonstrated that cilostamide reversed the leptin-induced increase in proopiomelanocortin (POMC) gene expression in the hypothalamus. Because POMC and neuropeptide Y (NPY) neurons are thought to be the major targets of leptin signaling in the hypothalamus, to establish the physiological role of the PDE3B pathway it is important to demonstrate if PDE3B is expressed in these neurons. To this end we examined co-localization of PDE3B with POMC and NPY neurons using immunocytochemistry in POMC-GFP and NPY-GFP mice, respectively. Results showed that PDE3B was highly localized throughout the various hypothalamic sites including the arcuate nucleus (ARC), ventromedial nucleus, dorsomedial nucleus, ventral premammillary nucleus, paraventricular nucleus, and lateral hypothalamus. Importantly, almost all NPY (91.7%) and POMC (97.7%) neurons co-expressed PDE3B. These results suggest a direct role of the PDE3B pathway in mediating leptin signaling in the POMC and NPY neurons –a potential mechanism of leptin signaling in the hypothalamus.
Keywords: phosphodiesterase-3B, POMC, NPY, hypothalamus, leptin
Introduction
Leptin, the product of the obese gene [39], is secreted by the adipocytes and signals nutritional status to key regulatory centers in the hypothalamus and it has emerged as an important signal regulating energy homeostasis [13–15,33]. Central or peripheral leptin administration decreases food intake and body weight in a variety of animals [13, 34]. The deletion of leptin receptor (LEPR) in neurons leads to an obese phenotype [8], and transgenic supplementation of the LEPR in neurons of Leprdb/db mice results in an amelioration of the obese phenotype [20]. Besides its role in energy homeostasis, leptin also plays important role in reproduction, bone growth and immuno functions [37]. Importantly, most, if not all, of these functions of leptin are mediated at the level of the hypothalamus. Thus, understanding the mechanism of leptin signaling is very important. Several lines of evidence suggest that besides the classical Janus-kinase 2 (JAK2)-signal transducer and activator of transcription-3 (STAT3) pathway [4,14,15,35,36], leptin signaling in the hypothalamus is mediated through various non-STAT3 pathways including including AMP-activated protein kinase (AMPK) [22], mammalian target of rapamycin (mTOR) [10], forkhead protein (FOXO1) [5,17], phosphatidylinositol 3-kinase (PI3K) [23,40], and SHP2-GRB2-Ras-Raf-MAPK (mitogen-activated protein kinase) [2,6,7,38]. We have demonstrated that leptin action is also mediated through an insulin-like signaling pathway involving stimulation of PI3K and phosphodiesterase 3B (PDE3B) activities and reduction in cAMP levels in the hypothalamus [40]. Furthermore, cilostamide, a selective PDE3 inhibitor, reverses the anorectic and body weight reducing effect of leptin [40]. While these results suggest a potential role of the PDE3B pathway in mediating leptin action in the hypothalamus, the physiological role of this pathway of leptin signaling in energy homeostasis remains relatively unknown.
Towards establishing the physiological role of the PDE3B pathway, we have recently demonstrated that PDE3B inhibitor reversed the leptin-induced increase in proopiomelanocortin (POMC) and neurotensin gene expression in the hypothalamus [30]. However, it is still unknown whether leptin directly activates PDE3B in these and other leptin target neurons, a possibility that could be demonstrated if PDE3B is expressed in these neurons. Besides POMC neurons, neuropeptide Y (NPY) neurons in the hypothalamus play a significant role in energy homeostasis, and both POMC and NPY neurons are the major targets of leptin action in the hypothalamus [1,9,12,26,28,33]. Specifically, leptin inhibits NPY gene expression and induces POMC gene expression in the hypothalamus. Thus, to further establish the physiological role of the PDE3B pathway of leptin signaling, we tested the hypothesis that PDE3B is expressed in the POMC and NPY neurons. To this end, we performed dual-label immunocytochemistry (ICC) with a specific PDE3B antibody and GFP (green fluorescence protein) antibody to examine PDE3B co-localization in hypothalamic POMC and NPY neurons in POMC-GFP and NPY–GFP mice, respectively.
Materials and methods
Transgenic mouse lines expressing GFP in either NPY (NPY-GFP) or POMC (POMC-GFP) neurons were kindly provided by Dr Jeffrey Friedman (The Rockefeller University, New York, NY), and were maintained in our animal facility in a light (lights on 0500 h to 1900 h) and temperature (22 °C)-controlled room with food (Pelleted Purina rodent chow) and water available ad libitum. The procedures used herein were according to an approved Institutional Animal Care and Use Committee protocol.
Adult male mice (NPY-GFP, n = 4; POMC-GFP, n = 3) were injected stereotaxically with colchicine (1 μl, 20 μg/μl) into the lateral cerebroventricle under pentobarbital anesthesia using an ultra micro-II injection pump (Precision Instruments). Forty-eight hours later, mice were deeply anesthetized with pentobarbital, and perfused transcardially with 0.9% saline (RT) followed by ice-cold 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4. The brains were quickly removed from each perfused animal, post-fixed in the same fixative overnight at 4 °C, and cryoprotected in 25% sucrose solution until they sank. The brains were then frozen on dry ice and kept at −80 °C until sectioning. Five series of coronal 25 μm free-floating sections were cut through the mediobasal hypothalamus on a freezing microtome (Leica Sliding Microtome), and stored in cryoprotectant at −20 °C until use.
To demonstrate PDE3B localization in various hypothalamic nuclei, adult male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, Maine) were injected with recombinant murine leptin (5 mg/kg body weight, i.p; A.F. Parlow, NHPP, Torrance, CA) followed 60 minutes later by transcardial perfusion with saline and 4% paraformaldehyde. The brain was processed for sectioning as described above. Free-floating sections were pretreated with 1.8% H2O2 in 50 mM KPBS (potassium phosphate buffered saline) for 20 min followed by several washes and incubation in 0.05% glycine for 10 min at RT. Sections were then blocked with 5% normal rabbit serum +1% BSA + 0.4% Triton X-100 and incubated with goat anti-PDE3B (1:500, FabGennix Inc., Frisco, TX) at 4 °C for 48 hr. After washing, sections were incubated with biotinylated rabbit anti-goat secondary antibody (1:1200, 90 min at RT, Vector Laboratories), then washed again and incubated in avidin-biotin complex (Vectastatin, ABC Elite kit, Vector Laboratories) for 90 minutes at RT. Immunoreactive PDE3B was visualized with diaminobenzidine hydrochloride (DAB, Sigma) reaction. Finally, sections were mounted on superfrost slide (Fisher Scientific, Pittsburgh), dried overnight, rehydrated and then dehydrated with increasing concentrations of ethanol, washed in xylene and mounted with DPX. Visualization of immunostaining detected with DAB was performed using a Leica DMRBE microscope (Brodersen Instruments, Co, Valencia, PA), MicroPublisher 5.0 RTV QImaging color digital camera and Bioquant NOVA PRIME Program (BIOQUANT Image Analysis Corporation, Nashville, TN).
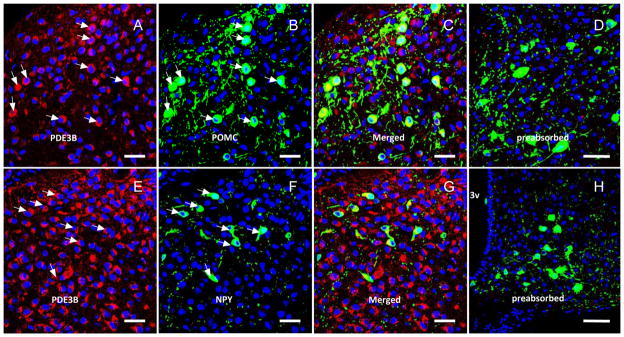
For dual-label ICC for PDE3B and GFP (for NPY or POMC), ICC for PDE3B was performed first followed by GFP staining. Free floating tissue sections were pretreated with 1% NaOH and 1% H2O2 in H2O for 20 min. Sections were then blocked for 1 h and incubated with goat anti-PDE3B (1:500, FabGennix Inc.) at 4 °C for 48 h, followed by washing and incubation with Cy3-conjugated donkey anti-goat secondary antibody (1:800, 1 hour RT). Sections were washed and then incubated with Alexa 488-conjugated rabbit anti-GFP (1:1500, Invitrogen). Finally, sections were stained with DRAQ5 (fluorescence DNA dye, 1:2000), mounted on Superfrost slides (Fisher Scientific, Pittsburgh, PA) using Fluoromount-G (Southern Biotech), and visualized with an Olympus FluoView Confocal Microscope for green GFP (POMC or NPY), red PDE3B expressing neurons and blue nuclear stain. The specificity of the PDE3B antibody was validated in the following manner. First, pre-absorption of primary antibody with the PDE3B peptide used as immunogen blocked all staining in both single- and dual-label ICC procedures (Fig. 2, preadsorbed). Second, substitution of isotypic serum for primary antibody eliminated all staining (Fig. 1).
Fig. 2.
Double ICC for PDE3B (red) and GFP-POMC (upper panels, A–C) and GFP-NPY (lower panels, F–G) in the ARC of POMC-GFP or NPY-GFP mice. Blue = nuclear stain. Arrows indicate co-localization. 3v = third ventricle. Note that preabsorption of PDE3B antibody with PDE3B peptide used as immunogen blocked all PDE3B staining as shown in the right panels (D, H). Scale bar = 50 μm
Fig. 1.
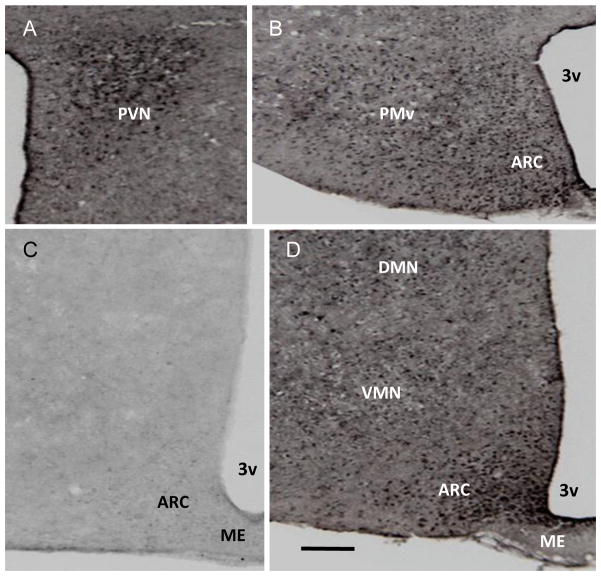
Bright-field photograph showing phosphodiesterase 3B (PDE3B) immunoreactive cells in mouse hypothalamus. (A) PDE3B-ir positive cells in the paraventricular nucleus (PVN). (B) PDE3B-ir positive cells in the ventral premammiliary nucleus (PMv) and arcuate nucleus (ARC) at bregma −2.46 mm. (C) Immunocytochemical reaction with substitution of isotypic serum for PDE3B primary antibody in a section through the median eminence (ME)-ARC area at bregma −1.46 mm. (D) PDE3B-ir positive cells in the ARC, ventromedial nucleus (VMN) and dorsomedial nucleus (DMN) at bregma −1.94 mm. 3v = third ventricle. Scale bar = 100 μm
Sections were scanned at 1024 × 1024 pixels, 40× objective 0.2micron pixel size, using two or three color image collection (488 nm laser, 543 nm, 633 nm) together with appropriate dichroics and barrier filters. Image planes throughout the depth of the specimen were collected and the neurons expressing both GFP and PDE3B (yellow color in merged images) were counted on at least ten different sections through the entire rostro-caudal extent of the ARC of each brain using the MetaMorph software (Molecular Devices, Sunny Vale, CA). Co-expression values were calculated as percentages of the total number of POMC- or NPY-positive cells expressing PDE3B. All values were expressed as means ± standard error (SE).
Results
As reported previously in rat [28], PDE3B+ve cells were localized in various nuclei in the mouse hypothalamus including the arcuate nucleus (ARC), ventromedial nucleus (VMN), dorsomedial nucleus (DMN), paraventricular nucleus (PVN), ventral premammillary nucleus (PMv) and lateral hypothalamic areas (LH) (Fig. 1 and data not shown). Among these nuclei, PDE3B was expressed highly in the ARC followed by PVN and PMv. GFP+ve cells identifying the POMC and NPY neurons in POMC-GFP and NPY-GFP mice, respectively, were distributed throughout the ARC as described previously [11,25]. Systematic examination of sections through the rostro-caudal axis of the ARC showed expression of PDE3B (red) in POMC and NPY neurons (green) as shown by the development of yellow color in the merged figures (Fig. 2). Analysis of various sections throughout the ARC in four NPY-GFP and three POMC- GFP mice showed that PDE3B was expressed in almost all POMC and NPY neurons (Fig 3).
Fig. 3.
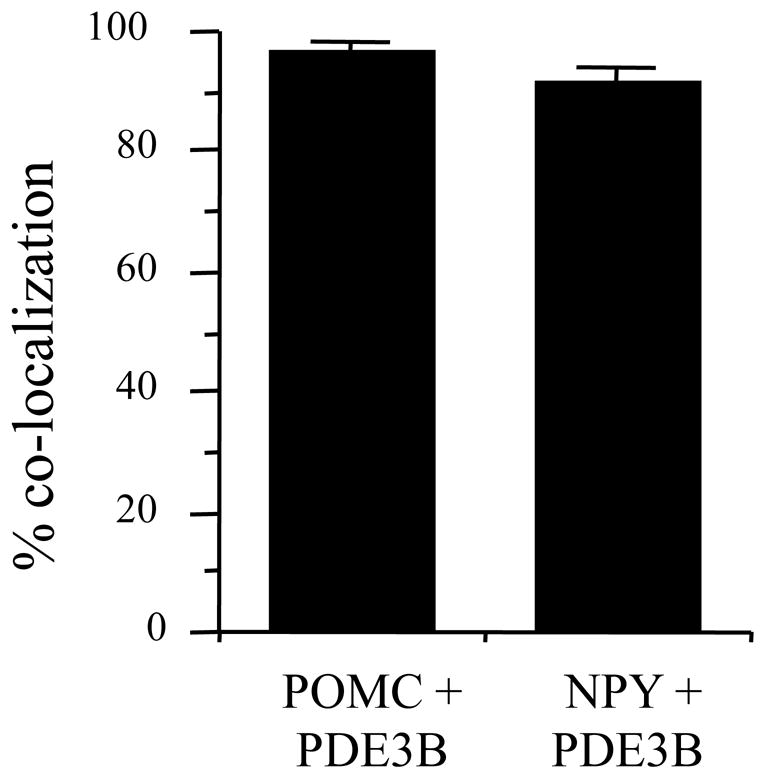
Percent of POMC and NPY neurons co-localized with PDE3B in the hypothalamus of POMC-GFP and NPY-GFP mice. Values represent the mean ± SEM for 3 and 4 animals in POMC-GFP and NPY-GFP groups, respectively.
Discussion
The present study shows that PDE3B is expressed in POMC and NPY neurons and in those hypothalamic nuclei that have been implicated in energy homeostasis. These results further support a role of PDE3B in energy homeostasis particularly in leptin signaling in the hypothalamus.
Leptin signaling in hypothalamic neurons, particularly in POMC and NPY neurons, is critical for normal energy homeostasis [1,13,28,33]. Understanding the mechanism of leptin signaling in these neurons is important toward identifying any defect in a particular signaling pathway during the development of diet-induced obesity, which could be targeted for therapeutic approaches. Cumulative evidence suggests that besides the STAT3 pathway, several non-STAT3 pathways are integral part of the leptin-signaling network in the hypothalamus that regulates energy homeostasis [28,31]. Our pharmacological studies have identified PDE3B pathway as one of the non-STAT3 pathways of leptin signaling in the hypothalamus in that PDE3 inhibition reverses the anorectic and body weight reducing effects of leptin [40]. In addition, reversal of the leptin-induced STAT3 activation in the hypothalamus by PDE3 inhibition demonstrates a cross talk between the PDE3B and STAT3 pathways of leptin signaling [40]. We have also reported that the PI3K-PDE3B-cAMP pathway but not the STAT3 pathway of leptin signaling in the hypothalamus was impaired during the development of leptin resistance in POMC and NPY neurons following chronic central leptin infusion [27,29,32]. However, the physiological role of hypothalamic PDE3B signaling in energy homeostasis is not clearly understood.
Thus to begin to examine the physiological role of PDE3B signaling in the hypothalamus, in the present study we sought to examine if PDE3B was localized in POMC- and NPY-expressing neurons, two important neuronal subtypes that have been established to play significant role not only in leptin signaling but also in overall energy homeostasis [33]. The finding of PDE3B expression in almost all POMC- and NPY-expressing neurons in the arcuate nucleus together with previous report of leptin receptor expression in these neurons [3] are in favor of a direct role of PDE3B pathway in transducing leptin action in these neurons. Our recent demonstration that PDE3 inhibition by cilostamide reverses the leptin-induced stimulation of hypothalamic POMC and neurotensin (NT) gene expression [30], and the finding that leptin suppresses ghrelin-induced NPY neurons by activation of the PI3K-PDE3B pathway [18] are in line with this possibility.
In addition to PDE3B co-localization in POMC and NPY neurons, we also documented PDE3B expression in those hypothalamic nuclei including the ARC, VMN, DMN, PMv, LH and PVN that are known to express leptin receptor and have been implicated in food intake and body weight regulation. This finding suggests the possibility of PDE3B playing a role in leptin signaling in these nuclei. Whereas the specific role of the PDE3B pathway in mediating leptin signaling in various hypothalamic nuclei is not clearly understood, the role of this pathway in mediating leptin signaling in POMC, NPY and NT neuronal activities is becoming apparent [30]. Also, the demonstration that leptin blocks glucocorticoid-induced endocannabinoid biosynthesis and suppression of excitation in the PVN via a PDE3B-mediated reduction in cAMP levels [21] suggests a role of PVN PDE3B in leptin signaling and energy homeostasis. It is noteworthy that insulin signaling in hypothalamic neurons including that in POMC and NPY neurons plays a critical role in energy homeostasis [16,19,24]. Thus, PDE3B expression in POMC and NPY neurons and in various hypothalamic nuclei along with our preliminary finding that insulin increases PDE3B activity in the mouse hypothalamus [A. Sahu and M. Sahu, unpublished] may suggest a potential role of PDE3B in mediating insulin action in the hypothalamus.
In summary, we have demonstrated expression of PDE3B in POMC and NPY neurons and in various hypothalamic nuclei that have been implicated in energy homeostasis. This study along with reversal of leptin-induced POMC and NT gene expression by PDE3 inhibition suggest an important role of the PDE3B pathway in mediating action of leptin and other metabolic signals including insulin in these and other hypothalamic neurons.
Highlights.
PDE3B pathway plays an important role in mediating hypothalamic action of leptin.
This study shows expression of PDE3B in POMC and NPY neurons in the hypothalamus.
PDE3B is also expressed in the hypothalamic nuclei implicated in energy homeostasis.
Direct PDE3B signaling appears to mediate leptin action in POMC and NPY neurons.
Acknowledgments
This work was supported by NIH RO1 Grants DK61499 and DK78068. Thanks are due to Dr. Jeffrey M. Friedman (Rockefeller University, New York) for providing POMC-GFP and NPY-GFP mice, and to Dr. A. F. Parlow and the NIDDK National Hormone & Pituitary Program, Torrance, CA, for supplying the recombinant murine leptin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 3.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 4.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 10.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 11.DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 12.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 14.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 15.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 18.Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251–2263. doi: 10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- 19.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 21.Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 23.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 24.Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-Mediated Inhibition of Proopiomelanocortin Neurons in the Arcuate Nucleus Shows Enhanced Desensitization in ob/ob Mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 26.Sahu A. Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology. 1998;139:795–798. doi: 10.1210/endo.139.2.5909. [DOI] [PubMed] [Google Scholar]
- 27.Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 28.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Sahu A. Effects of chronic central leptin infusion on proopiomelanocortin and neurotensin gene expression in the rat hypothalamus. Neurosci Lett. 2008;440:125–129. doi: 10.1016/j.neulet.2008.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu A. A role of phosphodiesterase-3B pathway in mediating leptin action on proopiomelanocortin and neurotensin neurons in the hypothalamus. Neurosci Lett. 2010;479:18–21. doi: 10.1016/j.neulet.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–210. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol. 2005;17:720–726. doi: 10.1111/j.1365-2826.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 34.Tang-Christensen M, Havel PJ, Jacobs RR, Larsen PJ, Cameron JL. Central administration of leptin inhibits food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab. 1999;84:711–717. doi: 10.1210/jcem.84.2.5458. [DOI] [PubMed] [Google Scholar]
- 35.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 36.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 37.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]