Abstract
The mobility of elements within plants contributes to a plant species' tolerance of nutrient deficiencies in the soil. The genetic manipulation of within-plant nutrient movement may therefore provide a means to enhance plant growth under conditions of variable soil nutrient availability. In these experiments tobacco (Nicotiana tabacum) was engineered to synthesize sorbitol, and the resultant effect on phloem mobility of boron (B) was determined. In contrast to wild-type tobacco, transgenic tobacco plants containing sorbitol exhibit a marked increase in within-plant B mobility and a resultant increase in plant growth and yield when grown with limited or interrupted soil B supply. Growth of transgenic tobacco could be maintained by reutilization of B present in mature tissues or from B supplied as a foliar application to mature leaves. In contrast, B present in mature leaves of control tobacco lines could not be used to provide the B requirements for new plant growth. 10B-labeling experiments verified that B is phloem mobile in transgenic tobacco but is immobile in control lines. These results demonstrate that the transgenic enhancement of within-plant nutrient mobility is a viable approach to improve plant tolerance of nutrient stress.
The inorganic nutrients required for plant growth can be provided to growing tissues either in the xylem, driven by transpirational water flow, or in the phloem, associated with the sink-driven movement of organic solutes from source to sink tissues. The rate of supply of inorganic nutrients to growing tissues is a primary determinant of plant growth, and inadequate nutrient supply results in growth impairment and the development of deficiency symptoms. The relative contribution of phloem and xylem transport to the supply of nutrient elements varies from species to species and differs for each element. N, P, K, S, and Mg are readily transported in either the xylem or the phloem (phloem-mobile nutrients), whereas Ca, and, in most species, B have limited mobility and can only be supplied to growing tissues in the xylem (phloem-immobile nutrients).
The relative mobility of an element within a plant has important physiological and agricultural implications. Immobile elements present in mature tissues cannot be retranslocated to supply the needs of developing tissues and must therefore be available in the soil at all times. For elements with limited within-plant mobility, the absence of soil nutrient supply results in rapid inhibition of meristematic growth (particularly reproductive growth). This is especially critical for elements required in high amounts in growing tissues, of which B and Ca are the most relevant examples. The ability of a plant species to survive or to yield optimally during a period of nutrient stress is therefore a consequence of both its ability to obtain nutrients from the soil under limiting conditions and the extent to which the nutrients can be supplied through redistribution from other plant tissues.
B plays an important role in the formation of plant cell walls, and, as a consequence, it is critical for plant growth (Matoh, 1997). Historically, B has been considered to have only limited phloem mobility (Oertli and Richardson, 1970) and the removal of B from the growth medium frequently results in rapid inhibition of plant growth (Loomis and Durst, 1992). B deficiency is a widespread agricultural problem that results in yield and quality loss in many crop species worldwide (Shorrocks, 1997). Perhaps the most important manifestation of B deficiency is the reduction in seed set and fruit yields that have been observed in diverse agricultural regions (Dell and Huang, 1997). Because B cannot readily be redistributed within the plant in most species, even a brief disruption of soil nutrient supply results in growth depression and yield loss, the extent of which is dependent upon the duration of the deficiency and the stage of plant growth at which it occurs (Dell and Huang, 1997).
Recently, it has been demonstrated that the mobility of B varies greatly among species (Brown and Hu, 1996). The biochemical basis of these species differences and the resulting physiological effects and agronomic consequences are now well described (Brown and Shelp, 1997). Evidence suggests that the principal factor that confers phloem B mobility to a plant species is the synthesis of sugar alcohols and the subsequent transport of the B-sugar alcohol complex in the phloem to sink tissues (Hu et al., 1997). The capacity to use B present in mature tissues is now known to enhance species tolerance to transient B deficiency in the growth medium (Brown and Hu, 1996). To further demonstrate the critical role of sugar alcohols in B transport and to determine if the capacity for within-plant B mobility could be transferred to species in which B is normally immobile, we engineered tobacco (Nicotiana tabacum) with the gene for sorbitol production, S6PDH, and determined the resultant effect on sorbitol production, B mobility, and tolerance of tobacco to B stress.
MATERIALS AND METHODS
Plant Growth and Treatment
Three tobacco (Nicotiana tabacum) lines were used: SR1, wild-type tobacco; A4, tobacco transformed with the antisense gene construct for S6PDH; and S11, the tobacco line transformed with the sorbitol-synthesizing sense construct (Tao et al., 1995). Lines A4 and SR1 served as controls; lines A4 and S11 are identical in all regards except the orientation of the S6PDH coding region with respect to the cauliflower mosaic virus 35S promoter.
Homozygous seed of each tobacco line were germinated and then grown in vermiculite for 4 weeks with adequate supply of all nutrients, including 0.05 μg mL−1 B. At 4 weeks, plants were transferred to hydroponic solution (one-half-strength Hoagland solution [Hoagland and Arnon, 1950], minus B) and the following treatments were imposed: (a) continual supply of 0.05 μg mL−1 B in the rooting medium; (b) 0 μg mL−1 B, received no B in the rooting medium; and (c) “foliar”-treated plants, received biweekly foliar applications of B to three mature leaves (described below) with no B supplied in the root nutrient medium.
At the time of foliar B application, the three mature leaves were immersed for 10 s in 100 μg mL−1 B solution as 10B-enriched boric acid (99.43% 10B:0.57% 11B) with 0.05% (v/v) L-77 as surfactant. Care was taken so that contamination of B to the stem/petiole or drip of the B solution was avoided. The foliar B application was made three times. B analysis was performed by inductively coupled plasma MS (Elan 5000, Perkin-Elmer SCIEX, Norwalk, CT), as previously described (Brown and Hu, 1996). Plant appearance was closely monitored; 8 weeks after transfer to hydroponic solutions, plants were harvested, and growth, reproductive performance, and tissue analysis for various parameters was performed.
There were six replicate plants in each treatment group. Sorbitol production was determined by GC-MS (Greve and Labavitch, 1993) in mature leaf discs of all lines, significant sorbitol concentrations were detected in line S11 (800 ± 100 nmol g−1 fresh weight) but no detectable sorbitol could be found in either control (SR1) or antisense lines (A4).
RESULTS AND DISCUSSION
Tobacco lines that received continuous adequate B in the rooting medium (0.05 μg mL−1B) showed no sign of B deficiency at any time during the experiment; no differences in final dry weight, flower abortion, or seed yield were evident at harvest among any of the tobacco lines (Table I; Fig. 1).
Table I.
Flower abortion and plant dry weight at harvest
| Treatment | Line | Total Dry Wt | Flower Abortion |
|---|---|---|---|
| g | % | ||
| 0 μg mL−1 B | S11 | 22.8 ± 4.3 | 44 ± 15 |
| SR1 | 18.7 ± 2.8 | 52 ± 5 | |
| A4 | 18.5 ± 2.3 | 54 ± 8 | |
| Foliar | S11 | 26.3 ± 7.2 | 22 ± 9 |
| SR1 | 16.4 ± 3.3 | 57 ± 12 | |
| A4 | 17.3 ± 3.9 | 43 ± 5 | |
| 0.05 μg mL−1 B | S11 | 32.2 ± 4.3 | 24 ± 5 |
| in solution | SR1 | 35.5 ± 6.0 | 19 ± 3 |
| A4 | 37.5 ± 6.9 | 17 ± 3 |
Three tobacco lines, S11 (sorbitol producing) and SR1 and A4 (control lines producing no sorbitol), were grown for 28 d with complete nutrient solution, including adequate B (0.0 μg mL−1), and then transferred to nutrient solutions supplied with 0 μg mL−1 B, no B added to the medium; foliar B, 100 μg mL−1 solutions of 10B (as boric acid with 99.43% 10B:0.57% 11B atomic composition) applied to three mature leaves at 2-week intervals; or 0.05 μg mL−1 B supplied to the rooting medium. Percentage of abortion was determined as the number of aborted flowers/total number of initiated flowers × 100. Percentage of abortion and plant dry weight were determined 8 weeks after transfer to hydroponic solution.
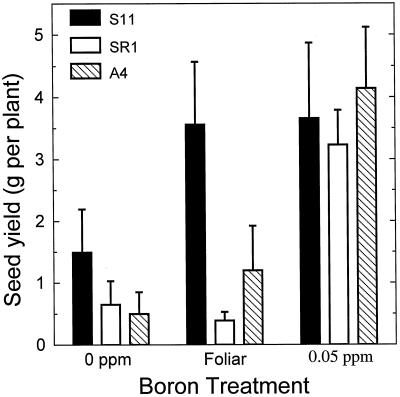
Figure 1.
Seed yield of tobacco lines (S11, SR1, and A4) grown for 28 d with adequate B and then transferred to 0 μg mL−1 B, 0.05 μg mL−1 B supplied to the roots, or 100 μg mL−1 B supplied to three mature leaves. Seed yield was determined 56 d after transfer to treatment solutions. Values represent means ± se of six replicates.
In tobacco plants deprived of all B in the medium, the control lines SR1 and A4 rapidly developed chlorosis, demonstrated enhanced flower abortion, and, at harvest, exhibited significantly decreased growth and seed yield compared with plants grown with adequate B (Table I; Figs. 1 and 2). In comparison with plants continually supplied with B in the rooting medium, seed yield in lines A4 and SR1 were reduced by 90% and 80%, respectively, whereas plant growth was reduced by 50% in both tobacco lines. Visual symptoms of B deficiency were first apparent 2 weeks after treatment imposition in SR1 and A4 lines, but were not evident in the S11 line until 5 weeks after transfer to treatment solutions. The delayed appearance of B deficiency symptoms in the S11 line was also reflected in the 20% greater plant weight, 10% less flower abortion, and a greater than 100% increase in seed yield over either SR1 or A4 lines grown under the same conditions (Table I; Fig. 1). The first symptom of B deficiency in tobacco was the development of mild chlorosis in young leaves, and this was followed by flower abortion and incomplete expansion of young leaves (Fig. 2).
Figure 2.
B deficiency symptoms in the control tobacco line SR1 (left) and transgenic tobacco (S11), supplied with B as a foliar application to three mature leaves. Symptoms exhibited in SR1 include flower bud abortion, deformation, reduced elongation, and chlorosis of young leaves. The control tobacco line A4 exhibited symptoms similar to those observed in SR1 (not shown). No symptoms of B deficiency were observed in growing tissues of transgenic tobacco line S11.
When B was supplied solely as foliar applications to three mature leaves, a marked difference in symptom expression, growth, and yield among the tobacco lines was observed (Table I). Foliar-fed S11 showed no signs of chlorosis in young, growing tissues, and did not exhibit enhanced flower abortion at any time during the experiment, whereas control (SR1 and A4) lines developed marked B deficiency symptoms within 2 weeks of treatment imposition (Fig. 2). Growth and final seed yield of foliar-fed A4 and SR1 lines did not differ significantly from plants receiving 0 μg mL−1 B treatment, illustrating that foliar B supply was ineffective at supplying B for plant growth in these lines. Growth and seed yield of the foliar-fed line S11 line, however, was equal to the growth of plants that received a continuous adequate supply of B, suggesting that foliar B was effective at supplying the B requirements of this line (Table I; Fig. 1).
Flower abortion was the most pronounced symptom of B deficiency and was observed in all tobacco lines receiving 0 μg mL−1 B (although it was delayed in S11). Flower abortion was also prevalent in tobacco lines that do not produce sorbitol (SR1 and A4) when foliar B was the sole B source provided. Flower abortion occurred at all stages of flower and pod development, and there was no clear sensitivity to any particular stage of development (Fig. 2). A close correlation between the percentages of flower abortion and final seed yield suggests that flower abortion was the primary determinant of yield. No difference in the total number of flowers initiated (aborted sites plus flowers and plus pods) among any of the lines or treatments was observed, suggesting that sufficient localized B supplies existed to support flower initiation, but not necessarily continued flower retention (results not shown). Pollen viability was tested in all lines and treatments. All plants had similar and adequate levels of pollen germination (>85%, results not shown).
The delayed appearance of B deficiency in the sorbitol-producing tobacco line S11, grown without root B supply, and the capacity for the S11 tobacco line to grow optimally when supplied only with foliar B suggests that B present in mature S11 leaves is phloem mobile and that this mobility confers tolerance to B deprivation. To verify that B was indeed mobile in S11 but not in the SR1 or A4 lines, movement of foliar-applied 10B was assessed using isotopically enriched foliar B application. If foliar-applied 10B is mobilized from leaves receiving foliar 10B and transported to supply the B needs of growing tissues, then the relative abundance of 10B in these tissues should increase above the natural abundance. In control tobacco (A4 and SR1) lines the application of foliar 10B did not alter the abundance of 10B in young tissues, flowers, or seeds in comparison with untreated plants. In contrast, foliar 10B application to the S11 line resulted in a marked increase in 10B abundance in both young leaves and seeds (Table II).
Table II.
Within-plant mobility of foliar-applied 10B
| Line | 10B Excess in Top Leaf | 10B Excess in Seed |
|---|---|---|
| S11 | 56.4 ± 9.4 | 156 ± 25 |
| SR1 | 1.4 ± 1.1 | 0.3 ± 1.1 |
| A4 | 3.5 ± 1.2 | −1.2 ± 1.5 |
Isotopically enriched boric acid (99.43% 10B:0.57% 11B) was applied to three mature leaves of each tobacco line. B mobility was determined as a percentage of 10B atom excess (ratio of 10B/11B in treatment minus ratio of 10B/11B in control × 100). Experimental details are as described in Table I.
The greater 10B atom percentage enrichment in the seeds of line S11 compared with the young leaves of S11 demonstrates that the seeds received a greater proportion of their B requirement from the foliar treatment than did the leaves. This is probably a consequence of the later development of the seeds, which did not occur until much of the B originally present in the plant had been depleted. As the level of retranslocatable B declines in tobacco tissues, the dependence on foliar 10B supply increases, resulting in proportionally greater 10B enrichment in the seeds.
These results clearly demonstrate that the introduction of the gene for sorbitol production confers B mobility to tobacco. These results, in combination with previous evidence of B mobility in sugar-alcohol-producing species and the isolation of the B-sorbitol complexes from the phloem sap of peach, conclusively prove that sorbitol facilitates B mobility in higher plants (Brown and Hu, 1996). The genetically enhanced B mobility in transgenic tobacco demonstrated here clearly facilitated the maintenance of plant growth in the absence of B in the rooting medium. Apparently, the metabolic disruption imposed by the production of sorbitol in transgenic tobacco was insufficient to negatively affect plant performance. This is in contrast to recent reports suggesting that higher levels of mannitol production in tobacco can result in significant negative effects on plant growth and development (Sheveleva et al., 1997). The level of sorbitol produced in these current experiments is significantly lower than those reported elsewhere, suggesting that maintaining a low level of gene expression is critically important to ensure optimal B mobility while avoiding disruptive changes to cellular metabolism.
This research has practical relevance to production agriculture and to understanding the role of B in plant growth and reproduction. Here we have demonstrated the critical importance of B for reproduction in plants (Dell and Huang, 1997) and illustrated how a temporary reduction in soil B availability can have profound effects on yield, particularly when the deficiency occurs during flowering. This is in agreement with previous research in many agricultural regions of temperate and subtropical Asia, where reproductive B deficiency is recognized as a primary cause of seed set failure in wheat, rice, and canola (Dell and Huang, 1997; Rerkasem and Jamjod, 1997).
This reproductive failure is most prevalent during times of drought, cool temperatures, and high humidity that often occur at the time of flowering, and is probably the result of interrupted B supply from the soil to the developing reproductive tissues. Because B is immobile in these species, B present in mature leaves is not available to support reproductive growth, and soil fertilizer applications are ineffective. In these species the introduction of the gene for sorbitol production would provide the ability to use the B present in mature tissues and may be sufficient to overcome short-term B deficiencies and prevent yield loss. Fertilization strategies would also be markedly simplified, because B fertilization early in plant development could provide the B requirements at flowering. The results presented here for tobacco clearly suggest that this is feasible.
It is provocative that the introduction of the single gene for sorbitol production is sufficient to facilitate not only the production of sorbitol but also its phloem loading, transport, and the utilization of the transported B by reproductive tissues. The mechanism by which the B-sorbitol complex is transported is unknown. These results imply, however, that tobacco has retained an evolutionary capacity to transport and metabolize sorbitol, that transmembrane B-sorbitol transport occurs by purely passive mechanisms, or that there is a symplastic pathway from mature leaf cells to the phloem that allows for the movement of the B-sorbitol complex without the requirement for transmembrane passage. Each of these suggestions has intriguing physiological and evolutionary implications.
CONCLUSION
Plant tolerance of nutrient deficiencies is an active area of research that has largely focused on identifying the molecular mechanisms involved in obtaining nutrients from nutrient-poor soils. Although identifying the mechanisms of nutrient uptake is clearly essential to our understanding of plant physiology, the role of within-plant nutrient mobility in plant tolerance to nutrient deficiencies has received scant attention. The results presented here address this subject and represent a novel approach to enhancing plant tolerance to B deficiency. This approach may be applicable to a wide range of elements with limited within-plant mobility.
ACKNOWLEDGMENTS
We thank Meiru Wu, Agnes Nyomora, and Sandy Uratsu for their assistance.
Abbreviation:
- S6PDH
sorbitol-6-phosphate dehydrogenase
Footnotes
This work was supported by the U.S. Department of Agriculture (grant no. CSRS 9801010).
LITERATURE CITED
- Brown PH, Hu H. Phloem mobility of boron is species dependent: evidence for phloem mobility in sorbitol rich species. Ann Bot. 1996;77:497–505. [Google Scholar]
- Brown PH, Shelp BJ. Boron mobility in plants. Plant Soil. 1997;193:85–101. [Google Scholar]
- Dell B, Huang L. Physiological response of plants to low boron. Plant Soil. 1997;193:103–120. [Google Scholar]
- Greve C, Labavitch JM. Cell wall metabolism in ripening fruit. V. Analysis of cell wall synthesis in ripening tomato pericarp tissue using a d-[U-13C] glucose tracer and gas chromatography-mass spectrometry. Plant Physiol. 1993;97:1456–1461. doi: 10.1104/pp.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Experiment Station Circular 347. The College of Agriculture, Berkeley, CA: University of California; 1950. [Google Scholar]
- Hu H, Penn SG, Lebrilla CB, Brown PH. Isolation and characterization of soluble boron complexes in higher plants. The mechanism of phloem mobility of boron. Plant Physiol. 1997;113:649–655. doi: 10.1104/pp.113.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WD, Durst RW. Chemistry and biology of boron. Biofactors. 1992;3:229–239. [PubMed] [Google Scholar]
- Matoh T. Boron in plant cell walls. Plant Soil. 1997;193:59–70. [Google Scholar]
- Oertli JJ, Richardson WF. The mechanism of boron immobility in plants. Physiol Plant. 1970;23:108–116. [Google Scholar]
- Rerkasem B, Jamjod S. Genotypic variation in plant response to low boron and implications for plant breeding. Plant Soil. 1997;193:169–180. [Google Scholar]
- Sheveleva EV, Bohnert HJ, Richard JG. Metabolic engineering of polyol production in Nicotiana tabacum (abstract no. 1559) Plant Physiol. 1997;114:S-298. [Google Scholar]
- Shorrocks VM. The occurrence and correction of boron deficiency. Plant Soil. 1997;193:121–148. [Google Scholar]
- Tao R, Uratsu SL, Dandekar AM. Sorbitol synthesis in transgenic tobacco with apple cDNA encoding NADP-dependent sorbitol-6-phosphate dehydrogenase. Plant Cell Physiol. 1995;36:525–532. doi: 10.1093/oxfordjournals.pcp.a078789. [DOI] [PubMed] [Google Scholar]