Abstract
Arrestins were identified as mediators of G protein-coupled receptor (GPCR) desensitization and endocytosis. However, it is now clear that they scaffold many intracellular signaling networks to modulate the strength and duration of signaling by diverse types of receptors—including those relevant to the Hedgehog, Wnt, Notch, and TGFβ pathways—and downstream kinases such as the MAPK and Akt/PI3K cascades. The involvement of arrestins in many discrete developmental signaling events suggests an indispensable role for these multifaceted molecular scaffolds.
Introduction
Across development and phylogeny, a relatively small number of core signaling pathways are consistently reutilized for the patterning, growth, differentiation, and homeostasis of embryonic and adult tissues. In recent years, multifunctional adaptor proteins known as arrestins have come to be appreciated as important mediators of many of these pathways, including the Hedgehog (Hh), Wingless, Notch, and TGFβ pathways. The arrestins constitute a small family of gene products that were originally discovered as molecules that desensitize, or turn off, classical G protein-coupled receptor (GPCR) signaling. Upon ligand binding, GPCRs undergo conformational changes that allow them to be recognized by the family of GPCR kinases (GRKs) that phosphorylate the receptors on their intracellular loops and C-terminal tails (Premont and Gainetdinov, 2007). Arrestin binding to the receptors is generally enhanced by GRK-mediated phosphorylation of multiple sites on the inner surface of the receptors. This modification leads to β-arrestin recruitment, which sterically interdicts further signaling to G proteins, thus leading to the classical phenomenon of receptor desensitization. Arrestins can also mediate endocytosis of receptors, leading to numerous physiological outcomes including receptor degradation, receptor recycling, and the generation of “signalosomes” where arrestins scaffold various proteins to potentiate distinct downstream signaling events.
There are four members of the arrestin family. Visual arrestin (arrestin 1) is localized to retinal rods and cones, whereas X-arrestin (arrestin 4) is found exclusively in retinal cones. β-arrestins 1 and 2 (arrestins 2 and 3, respectively) are ubiquitously distributed. All of the proteins are closely related (70% sequence identity) and the sequence similarity between the β-arrestins is highly conserved across species (Gurevich and Gurevich, 2006). Arrestins contain N- and C-terminal domains consisting almost entirely of antiparallel beta sheets. The two domains, moreover, share a very similar fold despite the absence of any sequence similarity. This domain is known as an “arrestin fold.” It should also be noted that despite a lack of extensive conservation in primary amino acid sequence, Vps26 (which is part of a retromer subcomplex involved in recognition of cargo at the endosome and retrograde transport to the Golgi complex; Collins et al., 2008) and mammalian arrestins appear to have similar structures, and share not only the aforementioned arrestin fold, but a polar core as well (Shi et al., 2006). It is tempting to speculate that the arrestin fold is one of the structural signatures for the regulation of membrane trafficking in the cell.
Over the past decade, evidence has emerged that the β-arrestins play much wider roles in biology than previously imagined. They serve as multifunctional adaptors and scaffolds that mediate the clathrin-dependent endocytosis of diverse 7 transmembrane-spanning receptors (7TMRs, which include classical GPCRs), subserve a growing list of signaling functions, act as adaptors for various E3 ubiquitin ligases, and have many other roles (Table 1). Thus, in addition to their originally discovered role as desensitizers of G protein-mediated signaling, the β-arrestins serve as signal transducers in their own right. As shown in Table 1, the range of 7TMR-coupled signaling systems that are engaged through the β-arrestins has grown rapidly, as has the list of cellular processes that are regulated by these β-arrestin-mediated pathways. Several recent reviews have covered this very exciting, dynamic, and rapidly expanding field of research (Defea, 2008; DeWire et al., 2007; Ma and Pei, 2007).
Table 1.
β-arrestin-Dependent Signaling Pathways Engaged by GPCRs
AT1AR, angiotensine type 1A receptor; α2aAR, α2a-adrenergic receptor; β1(2)AR, β1(2)-adrenergic receptor; AIP4, atrophin-interacting protein 4; DGK, diacylglycerol kinase; D1(2)R, D1(2) dopamine receptor; eNOS, endothelial nitric oxide synthase; FLNA, filamin A; FSH-R, follicle-stimulating hormone receptor; GHS-R1a, ghrelin receptor growth hormone secretagogue receptor type 1a; GLP-1R, glucagon-like peptide-1 receptor; 5-HT2AR, serotonin 2A receptor; 5-HT 2C receptor, serotoinin 2C receptor; JNK3, c-Jun N-terminal kinase 3; LIMK, LIM domain kinase; LPAR, lysophosphatidic acid receptor; mGluR III, group III metabotropic glutamate receptor; M1R, M1 muscarinic acetylcholine receptor; MT1R, MT1 melatonin receptor; Mnk1, MAP kinase-interacting kinase 1; Nedd4, neural precursor cell expressed, developmentally downregulated 4; OR, opioid receptor; OX1R, orexin 1 receptor; P2YR, purinergic receptor; PAFR, platelet activating factor receptor; PAR-1, protease activated receptor-1; PDE, phosphodiesterase; PIP5K, phosphatidylinositol 4-phosphate 5-kinase; PGE2R, prostaglandin E2 receptor; PTH1R, type I parathyroid hormone receptor; PP2A, protein phosphatase 2A; USP33, ubiquitin specific peptidase 33; V2R, arginine vasopressin receptor 2; XGnRHR, Xenopus gonadotropin-releasing hormone receptor.
Recently, an even more surprising development has been the growing list of publications that document roles for the β-arrest-ins in signaling and/or endocytosis of noncanoncical families of cellular receptors and transporters (Table 2). These include non-receptor and receptor tyrosine kinases (RTKs), nonclassical 7TMRs like Smoothened (Smo) and Frizzled (Fz), ion channel receptors, and cytokine receptors. As with the GPCRs, many of these molecules interact with the β-arrestins in a ligand- or stimulus-dependent fashion. Moreover, many of these newly discovered interactions are pertinent to developmental biology, regulating cellular proliferation, differentiation, and apoptosis. This rapidly growing area has received much less attention and serves as the focus of this review.
Table 2.
Emerging β-arrestin-Coupled Receptors and Signaling Events
| Receptor | β-arrestin-Dependent Signaling Activity | Reference |
|---|---|---|
| Frizzled | recruitment of Dsh, endocytosis, activation of β-catenin, convergent extension movement | Bryja et al., 2007; Bryja et al., 2008; Chen et al., 2001; Chen et al., 2003b; Kim and Han, 2007; Kim et al., 2008a |
| IGF1R | endocytosis, ERK activation, Mdm2-mediated receptor ubiquitination, desensitization | Dalle et al., 2002; Dalle et al., 2001; Girnita et al., 2005; Girnita et al., 2007; Hupfeld et al., 2005; Lin et al., 1998 |
| Insulin receptor | interaction with Akt and Src | Luan et al., 2009 |
| Notch | ubiquitination of Notch | Mukherjee et al., 2005 |
| Smoothened | internalization, translocation to primary cilia, transcriptional activation | Chen et al., 2004; Kovacs et al., 2008; Meloni et al., 2006; Wilbanks et al., 2004 |
| TβRIII | internalization/endocytosis, downregulation of Smad and p38 signaling, Cdc42 activation | Chen et al., 2003a; Finger et al., 2008; Mythreye and Blobe, 2009 |
| Endoglin | ERK activation | Lee and Blobe, 2007 |
β-arrestin Gene Knockouts
Considering the numerous ways in which β-arrestins are involved in the regulation and control of important receptors and signaling molecules, it would seem likely that genetic ablation of arrestins would have significant developmental consequences. The Drosophila melanogaster genome encodes a single arrestin gene, kurtz (krz), which is essential for development and survival (Roman et al., 2000). Krz is ubiquitously expressed during early embryogenesis and is later concentrated in the central nervous system. Krz is also highly expressed in fat bodies, and krz mutants form melanotic tumors due to the disaggregation of fat bodies (Roman et al., 2000). The expression of Krz in the neuronal tissue of fly larvae is essential for viability (Roman et al., 2000). Continued research in Drosophila demonstrating that Krz is required for receptor desensitization and olfactory sensitivity suggests that many of the classical functions of arrestins are conserved between multiple species (Ge et al., 2006; Johnson et al., 2008).
β-arrestin 1 knockout mice develop normally, but β-arrestin 1 is required for normal adrenergic response (Conner et al., 1997). β-arrestin 2 knockout mice develop normally as well, but display increased analgesia in response to morphine (Bohn et al., 1999) due to misregulated internalization and desensitization of the μ-opiod receptor (Bohn et al., 2000). Over the past decade, numerous examples of supersensitive or diminished 7TMR signaling have been demonstrated in β-arrestin 1 or 2 knockout mice (DeWire et al., 2007; Kohout and Lefkowitz, 2003). Even with impaired signaling in numerous pathways, mice with either β-arrestin 1 or β-arrestin 2 ablated appear healthy and function normally unless challenged. However, unpublished studies indicate that simultaneous ablation of both β-arrestins in mice leads to multiple developmental abnormalities incompatible with postnatal survival (C.L.D., J.J.K., J. Klingensmith, and R.J.L, unpublished data). These phenotypes suggest that each β-arrestin can functionally compensate for the other in the developing mouse embryo. Indeed, in several receptor systems, β-arrestins can perform interchangeable functions in signaling pathways (DeWire et al., 2007).
Emerging β-arrestin-Coupled Receptors
Smoothened
Smo is a member of the 7TMR family that controls activation of the Hh signaling pathway. Modulation of Smo and its downstream signaling cascades may undergo species-specific regulation (Huangfu and Anderson, 2006). Notably, the C-terminal cytoplasmic tail of Smo differs significantly between species, which may provide clues to differing mechanisms of regulation and signal transduction (Varjosalo et al., 2006). In general, Smo activity is repressed by the 12 membrane-spanning receptor Patched (Ptc). Ptc acts as a receptor for the Hh family of ligands, which are morphogens that regulate everything from Drosophila larval segmentation to mammalian organogenesis and stem cell maintenance (Ingham and McMahon, 2001; Jiang and Hui, 2008). Binding of Hh ligands to Ptc relieves its repression of Smo, which allows activation of downstream signaling cascades (Murone et al., 1999). Typical 7TMRs are known to associate and signal through heterotrimeric G proteins, earning the name GPCRs. After activation, these receptors interact with β-arrestins in a ligand-dependent and usually phosphorylation-dependent manner (Kohout and Lefkowitz, 2003; Zhang et al., 1997). In the case of Smo, G protein coupling has been examined for more than a decade, after an initial study implicating G protein activity in zebrafish Hh signaling (Hammerschmidt and McMahon, 1998). However, the involvement of G protein-mediated signaling activity downstream of Smo has remained controversial. Recently, a study has shown the necessity of Gαi for proper Hh signaling in Drosophila (Ogden et al., 2008). Additionally, other GPCR-associated signaling proteins, the β-arrestins and GRK-2, have been identified as important modulators of Hh signaling, lending insight into the mechanisms of Smo regulation (Figure 1).
Figure 1. β-arrestins Mediate Vertebrate Hedgehog Signaling.
(1) Upon Hh binding, Ptc-mediated repression of Smo is released, resulting in (2) phosphorylation of Smo by GRK-2 and formation of a complex between Smo, β-arrestins, and the molecular motor Kif3A. (3) The Smo-β-arrestin-Kif3A complex translocates Smo to the primary cilium where (4) Smo cleaves Gli into its active form. (5) Active Gli then translocates down the primary cilium and (6) into the nucleus where it activates transcription of downstream targets.
In 2004, two studies revealed the importance of β-arrestin 2 and GRK-2 in the Hh pathway (Chen et al., 2004; Wilbanks et al., 2004). One study showed that knocking down β-arrestin 2 protein levels in zebrafish embryos phenocopied Smo mutants: ventrally curved bodies, underdeveloped heads, partial cyclopia, and loss of some midline tissues (resulting in characteristic U-shaped somites) all indicate Hh pathway defects (Wilbanks et al., 2004). Subsequent studies of zebrafish GRK-2 knockdown obtained analogous results (Philipp et al., 2008). In mammalian cells, Smo activation recruits β-arrestin 2 to the cell membrane where it promotes endocytosis of Smo via clathrin-coated pits through a GRK-2-dependent mechanism (Chen et al., 2004). In fact, GRK-2 phosphorylates Smo, potentiating its interaction with β-arrestin 2, and thus enhancing its signaling activity (Meloni et al., 2006). Thus, in both zebrafish embryos and in mammalian cell culture, β-arrestin 2 and GRK-2, key regulators of GPCRs, also serve as key regulators of a nonclassical 7TMR, Smo.
However, β-arrestins are not only important mediators of 7TMR internalization and subcellular localization (Lefkowitz and Whalen, 2004; Shenoy and Lefkowitz, 2003); they also serve as scaffolding proteins that mediate distinct signaling and subcellular trafficking events (Lefkowitz et al., 2006). Vertebrate Smo translocates to primary cilia during pathway activation (Corbit et al., 2005; Huangfu and Anderson, 2005), and this translocation event is mediated by members of the intraflagellar transport (IFT) complex (May et al., 2005), which are in turn required for proper regulation of Hh signaling. The kinesin-2 motor complex, an anterograde molecular motor that transports protein complexes to and within cilia and flagella (Hirokawa, 2000), is involved in Hh signaling (Huangfu et al., 2003). Specifically, one kinesin-2 subunit, Kif3A, has been shown to be essential for Hh signaling in mammalian systems (Huangfu and Anderson, 2006). Interestingly, β-arrestin was recently found to bind Kif3A (Xiao et al., 2007) and localize to primary cilia (Kovacs et al., 2008; Molla-Herman et al., 2008). In fact, both β-arrestin 1 and 2 are necessary for the formation of a “translocation complex” between Smo and Kif3A that promotes activity-dependent localization of Smo to primary cilia and activation of downstream transcriptional targets in mammalian cells (Kovacs et al., 2008), suggesting an intricate scaffolding mechanism that regulates Hh signaling.
The association between β-arrestin and GRK-2 function in Hh signaling and ciliary localization of Smo may provide important insight into evolutionary differences between vertebrate and invertebrate Hh signaling. While β-arrestins, GRK-2, and primary cilia are important for Hh signal transduction in vertebrates, these functions are not conserved across metazoans. Primary cilia are rare in Drosophila, and the Drosophila arrestin, Krz, has not been implicated in Hh signaling. The C-terminal region of Smo that is phosphorylated by GRK-2 for β-arrestin recruitment and signaling is divergent between vertebrates and invertebrates. In fact, the residues phosphorylated by GRK-2 are absent from Drosophila Smo. A recent study has revealed that Drosophila Smo is unable to translocate to primary cilia and activate mammalian Hh pathway signaling in cultured Smo null cells (Chen et al., 2009b). On the other hand, the same study demonstrated that other portions of the Hh signaling pathway are evolutionarily conserved. Consequently, it appears that the roles of the β-arrestins are divergent between species, and may only be necessary for Smo function in vertebrate systems. Supporting this idea, a study of zebrafish has shown that loss of IFT ablates primary cilia and dampens Hh signaling in the developing animal, though downstream transcriptional activities do not appear to require primary cilia absolutely (Huang and Schier, 2009). The fact that β-arrestins are required for localization of Smo to primary cilia, combined with the data from these studies, suggest that β-arrestins may function as upstream regulators of the Hh pathway specifically in vertebrates.
It was recently shown that Smo regulates a noncanonical Src-dependent pathway responsible for the mediation of axon guidance (Yam et al., 2009). Perhaps β-arrestins serve as a link between Smo and c-Src, as both have been identified as β-arrestin interacting proteins (Luttrell et al., 1999). It will be interesting to determine whether β-arrestins may regulate yet to be identified noncanonical signaling events downstream of Smo.
Frizzled and Wingless
Wingless is the founding member of the Wnt family of secreted glycoproteins that signal through a distinct family of 7TMRs termed Fz receptors (Logan and Nusse, 2004). The Wnts regulate cell proliferation, differentiation, and polarity at various stages of development (Moon et al., 2004). In the absence of a Wnt ligand, the Axin scaffold allows glycogen synthase kinase 3β (GSK3β) to phosphorylate cytoplasmic β-catenin, inducing its degradation. However, during so-called “canonical” Wnt signaling, Wnts bind to Fz, Disheveled (Dsh) proteins are activated, and Axin/GSK3β action on β-catenin is prevented. β-catenin, thus stabilized, accumulates in the cytoplasm, and can translocate into the nucleus, where it binds to TCF/LEF-transcription factors and promotes transcriptional activation (Figure 2).
Figure 2. β-arrestins Are Involved in Both Canonical and Noncanonical Wnt Signaling.
(1) In canonical Wnt signaling, in the absence of receptor stimulation, the Axin-APC complex degrades β-catenin and strongly represses transcriptional activity. (2) Upon Wnt binding to Fz, β-arrestins bind to the receptor through Dsh, thus sequestering Axin and GSK3 away from β-catenin, (3) leading to its stabilization. (4) β-arrestins also act to internalize Fzs into endosomes while (5) β-catenin is free to translocate into the nucleus and initiate transcription. (6) During noncanonical Wnt signaling, β-arrestin complexes with Dsh and AP-2 after Wnt binding to Fz. (7) Subsequently, β-arrestin activates the GTPases RhoA and Rac1 leading to (8) ROCK activation, (9) actin reorganization and convergent extension, or (10) JNK activation.
In noncanonical Wnt signaling, Fz activates two prominent β-catenin-independent pathways. In the Wnt/Planar Cell Polarity (PCP) pathway, Wnts signal through Fz to the small GTPases, Rho and Rac, to promote changes in the actin cytoskeleton (Schlessinger et al., 2009) (Figure 2). In a second noncanonical Wnt pathway, Wnts promote the release of intracellular calcium and negatively regulate the canonical Wnt/β-catenin pathway (Kohn and Moon, 2005).
β-arrestins have been implicated as important mediators of both canonical and noncanonical Wnt signaling, but in different capacities. It has been reported that heterotrimeric G proteins couple to Fzs in both the canonical and noncanonical pathways (Katanaev et al., 2005; Wang et al., 2006a), and, consequently, it is not surprising that β-arrestins act downstream of these receptors. β-arrestin 1 was initially found to interact with phosphorylated Dsh1 and Dsh2 (Chen et al., 2001), and in turn was shown to synergistically enhance LEF-mediated transcriptional activity when coexpressed ectopically with either Dsh1 or Dsh2. β-arrestin 2 interacts with both Axin and Dsh after Wnt3A stimulation of mouse embryo fibroblasts (MEFs) (Bryja et al., 2007), thus suggesting that it has an essential role in scaffolding this critical interaction, resulting in inactivation of GSK3β and subsequent stabilization of β-catenin (Figure 2). On the other hand, during Wnt5A-stimulated noncanonical signaling, phosphorylated active Dsh2 binds β-arrestin 2 and recruits it to the cell membrane (Chen et al., 2003b), where it regulates the endocytosis of Fz4. β-arrestin 2 requires Dsh to interact with Fz for internalization, unlike endocytosis of Smo, in which β-arrestin 2 binds to Smo directly. Interestingly, this is the only receptor reported to recruit β-arrestin that requires an intermediary binding partner. There is also evidence in other cellular contexts that Wnt ligands can stimulate PI3K-mediated activation of Akt (Kawasaki et al., 2007; Kim and Han, 2007) and/or ERK signaling cascades (Kim et al., 2004; Yun et al., 2005). These signaling molecules are well known to be regulated by β-arrestins (DeWire et al., 2007) and may further support the notion that β-arrestins play important roles in the activation of canonical and noncanonical Wnt signaling.
The importance of β-arrestins for canonical and noncanonical Wnt signaling in vivo has been demonstrated by a series of studies in Xenopus. Morpholino-mediated β-arrestin 2 knockdown in Xenopus embryos reduces β-catenin activation and blocks Wnt8- or Dsh-induced axis duplication (Bryja et al., 2007), indicating inhibition of the canonical Wnt signaling pathway. β-arrestin 2 does not affect β-catenin-dependent secondary axis differentiation, suggesting a requirement for β-arrestin 2 in canonical Wnt signaling downstream of casein kinase (CK) and Dsh, but upstream of β-catenin. β-arrestin 2 is also required for convergent extension (CE) during Xenopus axis elongation, a classical model of noncanonical Wnt/PCP signaling. β-arrestin-2-deficient mesoderm fails to polarize and intercalate with wild-type mesoderm at the midline (Kim et al., 2008a). β-arrestin may collaborate with the PCP pathway to activate RhoA and regulate convergent extension movement (Figure 2), supporting previous findings that β-arrestins are required for RhoA activation in cell culture (Barnes et al., 2005). In Xenopus CE, β-arrestin 2 works cooperatively with the RTK Ryk to mediate the endocytosis of Fz7 and Dsh after Wnt11 stimulation (Kim et al., 2008a). Subsequent studies have indicated that this effect is mediated through activation of Rac1 (Bryja et al., 2008). These data indicate that β-arrestins play crucial roles in both canonical and noncanonical Wnt signaling, yet their involvement is most likely tightly regulated within specific arms of these pathways.
An intriguing link between the Wnt pathways and the β-arrestins may be found at primary cilia, where both canonical and non-canonical Wnt pathway members are enriched (Eggenschwiler and Anderson, 2007). Indeed, β-arrestins are concentrated in the primary cilia (Kovacs et al., 2008) and at the centrosome or basal bodies of the cilia (Molla-Herman et al., 2008) as are other Wnt signaling molecules such as Dsh (Park et al., 2008), APC, β-catenin (Corbit et al., 2008), GSK3β (Thoma et al., 2007), and Inversin (Watanabe et al., 2003). Primary cilia are required in vivo for noncanonical (specifically PCP) Wnt signaling (Ross et al., 2005). Conversely, the disassembly of primary cilia results in increased levels of β-catenin activation and signaling (Cano et al., 2004; Corbit et al., 2008; Lin et al., 2003). However, it should be noted that recent studies suggest that primary cilia are not required for canonical Wnt signaling in developing mice or zebrafish (Huang and Schier, 2009; Ocbina et al., 2009). Perhaps primary cilia or basal bodies act as microdomains (Christensen et al., 2008) that facilitate regulation of specific signaling intermediates, but that are not required for all aspects and variations of a given signaling pathway. If so, it seems possible that β-arrestins in or near primary cilia and the centrosomes would play a role in this unique microdomain-dependent regulation of these Wnt subpathways.
The TGFβ Superfamily and Cytokine Signaling
The transforming growth factor β (TGFβ) superfamily contains over 30 secreted proteins that include the TGFβs, bone morphogenic proteins (BMPs), growth and differentiation factors (GDFs), activins, and several other members important for development and homeostasis (Feng and Derynck, 2005; Gordon and Blobe, 2008). They are involved in numerous developmental processes including gastrulation and germ-layer specification, axis formation, left-right asymmetry, patterning, and various stages of organogenesis (Wu and Hill, 2009). The TGFβ ligands signal through type I and type II TGFβ family serine/threonine kinase receptors. The numerous permutations of type I receptor/type II receptor/ligand combinations possible within the TGFβ superfamily of ligands and receptors creates a remarkable diversity of signaling outcomes (Feng and Derynck, 2005). Activated type I receptors phosphorylate Smad transcriptional mediators, promoting their accumulation in the nucleus where they bind DNA and regulate gene transcription (Massague and Wotton, 2000). Smad-dependent mechanisms are the most widely studied downstream mediators of TGFβ signaling; however, recent studies have also delineated several Smad-independent signaling pathways downstream of TGFβ superfamily receptors (Zhang, 2009) involving a number of well characterized signaling cascades including various mitogen activated protein kineases (MAPKs), PI3K/Akt, and Rho-like GTPases. As β-arrestins have been implicated in the regulation of all of these pathways (DeWire et al., 2007), it may not be surprising that β-arrestin functions extend beyond the 7TMR systems discussed above to cytokine receptors, including those involved in TGFβ signaling.
The type III TGFβ receptor (TβRIII or betaglycan) is a coreceptor that contributes to TGFβ signaling through mechanisms that have yet to be fully understood. β-arrestin 2 binds TβRIII (Chen et al., 2003a) and mediates its clathrin-independent/lipid raft pathway-dependent internalization (Finger et al., 2008). This β-arrestin-2-mediated internalization event downregulates TGFβ signaling, including both Smad phosphorylation and Smad-independent p38 phosphorylation (Finger et al., 2008) (Figure 3). Recent studies have shown that β-arrestin 2 also mediates TβRIII-induced activation of Cdc42 (Mythreye and Blobe, 2009) and inhibition of NF-κB-dependent cell migration (You et al., 2009). The β-arrestin-2-dependent activation of Cdc42 is reminiscent of β-arrestin-1-mediated activation of Rho after stimulation of the Angiotensin Type 1A Receptor (AT1AR) (Barnes et al., 2005) and requirements for β-arrestin 2 and p38 for pseudopodia extension and chemotaxis (Ge et al., 2003; Hunton et al., 2005). In this context, it is interesting to note the diversity of mechanisms by which TβRIII/β-arrestin 2 complexes influence TGFβ superfamily signals. Whereas β-arrestin-induced internalization attenuates TGFβ signaling, it potentiates signaling by the type I BMP receptor, ALK6. However, TβRIII acts in a β-arrestin-independent manner to retain the closely related type I BMP receptor, ALK3, on the cell surface (Lee et al., 2009).
Figure 3. β-arrestins Regulate Multiple Aspects of TGFβ Superfamily Signaling.
(1) Upon ligand binding to the TβRI, -RII, and -RIII type receptors, (2) β-arrestin binds TβRIII (3) while TbRII phophorylates TβRI, which in turn activates R-Smads. (4) Activated R-Smads complex with co-Smads and (5) translocate into the nucleus where they initiate transcription. (6) Meanwhile, β-arrestin internalizes TβRIII, (7) thus attenuating TGFβ-mediated activation of R-Smads. (8) Additionally, TβRIII is able to activate p38 in a Smad-independent manner after TGFβ binding and (9) β-arrestin-mediated internalization of TβRIII attenuates this signaling as well. (10) Downstream of TGFβ-binding, β-arrestin is essential for the activation of Cdc42, which is responsible for (11) actin reorganization leading to (12) chemotaxis and filipodial extension.
The endothelial-specific TGFβ receptor family member endoglin also interacts with β-arrestin 2, leading to its internalization (Lee and Blobe, 2007). The ability of endoglin to antagonize TGFβ-dependent ERK activation is β-arrestin 2 dependent (Lee and Blobe, 2007). In fact, the β-arrestin 2/endoglin interaction is responsible for sequestering ERK in the cytoplasm and subsequently preventing cell migration without affecting Smad-dependent signaling. Again, it appears that β-arrestins may be involved in Smad-dependent and -independent signaling mechanisms downstream of TGFβ receptors. While retention of ERK in the cytoplasm by β-arrestins may attenuate certain ERK signaling cascades in the nucleus, it has previously been shown that cytosolic ERK has distinct substrates and functions from nuclear ERK (Ahn et al., 2004; Tohgo et al., 2002). Consequently, specific β-arrestin-mediated subcellular localization of ERK downstream of TGFβ receptors, and other β-arrestin-linked receptors, may prove to be significant in multiple developmental pathways.
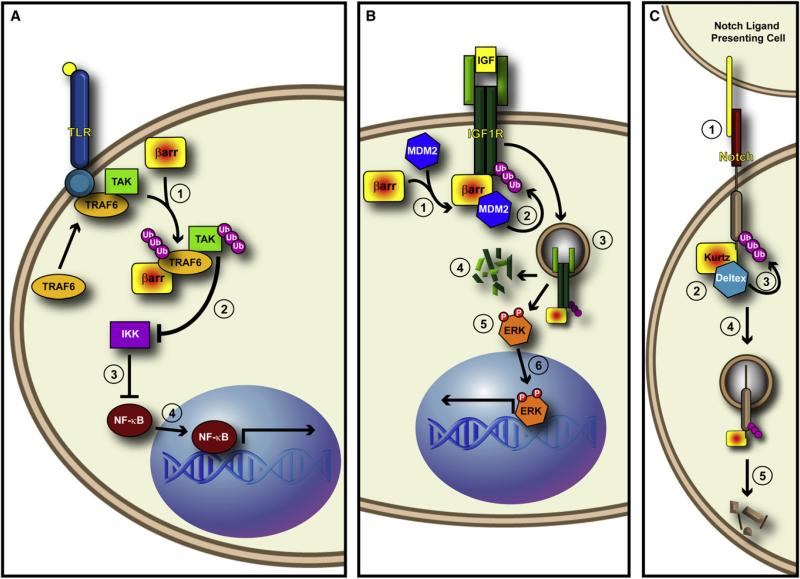
Further evidence for the involvement of β-arrestins in cytokine signaling comes from a series of studies involving the activation of TGFβ-activated kinase 1 (TAK1) by the E3 ligase tumor necrosis factor receptor-associated factor 6 (TRAF6). TAK1 is one of the activating MAP kinase kinase kinases that were originally found to be activated by TGFβ signaling (Yamaguchi et al., 1995). Later it was shown to be involved in BMP-dependent mesoderm induction in Xenopus (Shibuya et al., 1998) and proper formation of vasculature in developing mouse embryos (Jadrich et al., 2006). TGFβ treatment induces TRAF6 to polyubiquitinate itself and TAK1, resulting in p38/JNK pathway activation and apoptosis (Sorrentino et al., 2008; Yamashita et al., 2008). Indeed, direct binding of TRAF6 to TGFβ receptors is required for apoptosis in murine hepatocytes and epithelial to mesenchymal transition (EMT) in mouse mammary epithelial cells (Yamashita et al., 2008). That said, the TRAF6/TAK1 complex mediates activation of JNK and NF-κB not only by TGFβ (Shim et al., 2005), but also by the Toll-like receptor-inter-leukin 1 receptor (TLR-IL-1R) (Wang et al., 2006c). β-arrestin 2 is necessary for the auto K63-linked polyubiquitination of TRAF6 in response to TLR-IL-1R stimulation (Wang et al., 2006c) (Figure 4A). These studies raise the possibility that β-arrestin 2 may serve as an E3 ligase adaptor for TRAF6 toward TAK1, and may serve to regulate diverse cytokine receptor pathways, including Smad-independent TGFβ signaling, ultimately controlling ERK, JNK, p38 and TAK1.
Figure 4. β-arrestins Are Multifunctional Signaling Adaptors.
(A) β-arrestin promotes the ubiquitination of TRAF6. (1) Upon binding of ligand to the Toll-like Receptor, β-arrestin binds to and promotes the autoubiquitination of TRAF6. This autoubiquitination event is required for downstream activation of TAK and subsequent activation of NF-κB signaling. (2) It may be that β-arrestin-mediated ubiquitination of TRAF6 leads to activation of TAK, which inhibits IKK, (3) thus freeing NF-κB from IKK-mediated inhibition. (4) This would then allow NF-kB translocation into the nucleus and activation of transcription.
(B) β-arrestin acts as an E3 ligase adaptor in response to IGF stimulation. (1) After IGF binds to the tetrameric IGF1R, β-arrestin recruits Mdm2 to the receptor. (2) Mdm2 ubiquitinates IGF1R, thus (3) leading to its internalization. (4) Once internalized, IGF1R is degraded by the protoesome, and (5) β-arrestin mediates the activation of ERK from internalized “signalosomes.” (6) ERK then translocates to the nucleus and activates transcription.
(C) Krz mediates the ubiquitination and degradation of Drosophila Notch. (1) A Notch ligand on the Notch-ligand presenting cell binds Notch. (2) This event triggers the formation of a complex between Krz and the E3 ligase Dx. (3) Krz brings Dx to Notch and promotes Notch ubiquitination, (4) which leads to the Krz-dependent internalization of Notch, and (5) its subsequent degradation.
The IGF-1 Receptor
The role of β-arrestins also extends to RTKs. The Insulin-like growth factors (IGF-1 and IGF-2) are peptides that bind two cell surface receptors (IGF1R and IGF2R) and signal through a number of accessory proteins (Laviola et al., 2007). The IGF-1 receptor is a heterotetrameric protein with two transmembrane domains whose cytoplasmic substituents act as tyrosine kinases. IGF binding initiates signaling cascades that include the PI3K/Akt and Ras/Raf/MEK/ERK pathways, which regulate cell growth, survival, and differentiation (Siddle et al., 2001). IGF signaling has been implicated in fetal growth and the growth and maintenance of various organs, including but not limited to the central nervous system, bone, skeletal muscle, and the pancreas (Dupont and Holzenberger, 2003). Furthermore, aberrant IGF1R signaling has been shown to play important roles in tumorigenesis (Chitnis et al., 2008).
β-arrestins were found to regulate the endocytosis and mitogenic signaling of IGF1R more than a decade ago (Lin et al., 1998). Much like a typical 7TMR, it was shown that β-arrestin 1 and β-arrestin 2 associated with IGF1R after ligand stimulation and positively mediate ERK phosphorylation (Figure 4B). IGF1R was later discovered to utilize both G proteins and β-arrestins to mediate signaling in response to IGF-1 stimulation, even though it is not a 7TMR (Dalle et al., 2001). IGF1R signaling is dependent upon β-arrestin 1, whereas that by the closely related insulin receptor (IR) is not. Intriguingly, it was recently found that stimulation with insulin nonetheless initiates a signaling complex between β-arrestin 2, Akt, and Src that plays a role in insulin sensitivity (Luan et al., 2009).
Though the mechanisms underlying the β-arrestin 2 interaction with IR signaling have yet to be elucidated, the consequences of formation of the IGF1R-β-arrestin 1 complex have been more thoroughly examined. When β-arrestin 1 binds the IGF1R, it recruits the E3 ubiquitin ligase Mdm2 to the receptor, leading to its proteosomal degradation (Girnita et al., 2005). Mdm2 also ubiquitinates β-arrestin 1 in a reversible fashion (Girnita et al., 2007); a modification that has been shown to regulate subcellular trafficking of several receptors and signalosomes (Shenoy and Lefkowitz, 2003). IGF1-induced Mdm2-meditated ubiquitination of β-arrestin 1 promotes the intracellular trafficking of β-arrestin 1 to signalosomes that mediate ERK signaling by the IGF1R (Figure 4B), and, in turn, control cell cycle progression even in the absence of RTK signaling (Girnita et al., 2007). Interestingly, IGF stimulation enhances the dephosphorylation of β-arrestin 1 (Hupfeld et al., 2005), which results in an active form of β-arrestin 1 at the cell membrane. This active β-arrestin 1 plays a direct role in desensitizing IGF1R G protein-coupled signaling (Dalle et al., 2002). This suggests that β-arrestin 1 modulates two separate signaling arms from the IGF1R, similar to its regulation of many 7TMRs, RTKs and ion channels (DeWire et al., 2007). The significant involvement of the β-arrestins in IGF and Insulin-mediated signaling pathways suggests that β-arrestins may be involved in many other hormone-related developmental and homeostatic signaling pathways.
Notch
Notch, a single transmembrane spanning receptor, was first identified in flies and worms as a mediator of lateral inhibition, controlling the allocation of cell fate and the balance between cell proliferation and differentiation. These receptors are characterized by EGF-like repeats in the extracellular domain. Misregulation of the four mammalian Notch receptors has been implicated in numerous diseases and developmental abnormalities (Harper et al., 2003). Canonical Notch ligands are transmembrane proteins that bind Notch receptors and initiate juxtacrine signaling via a complicated sequence of proteolytic cleavage events culminating in g-secretase-dependent production of a free Notch intracellular domain (NICD), which translocates into the nucleus to serve as a transcriptional coregulator (Kopan and Ilagan, 2009).
Interestingly, while there are four mammalian Notch receptors, there is only one unique Notch in Drosophila melanogaster. That singular Notch is regulated by the unique nonvisual arrestin homolog Krz. In D. melanogaster one effect of ligand stimulation is to recruit the E3 ligase Deltex (Dx) to associate with Notch (Takeyama et al., 2003) and influence signaling (Matsuno et al., 1995). However, the specific mechanism by which Dx mediated Notch signaling remained unknown until it was shown that Notch, Dx, and Krz form a complex, and that Krz promotes Dx E3 ligase activity toward Notch, leading to its ubiquitination and degradation (Mukherjee et al., 2005) (Figure 4C), reminiscent of β-arrestin-mediated ubiquitination of other receptors. However, this work begs the question of how Krz and Dx promote Notch signaling, and whether this interaction may both positively and negatively regulate Notch signaling. Furthermore, it will be inter esting to investigate whether β-arrestins are also involved in vertebrate Notch signaling.
β-arrestin-Dependent Pathways Converge on Common Cytoplasmic Effectors
In recent years it has become evident that β-arrestins serve as molecular scaffolds for a myriad of signaling proteins (DeWire et al., 2007). It should come as no surprise that many of these signaling molecules lie downstream of receptors such as Smo, the Frzs, the TβRs, and many others. The proper functioning and tight regulation of these cascades has been shown to be essential for development of numerous organisms.
β-arrestins and Ubiquitination
More than just a regulatory mechanism for protein degradation, the ubiquitin system controls complex cellular events such as cell division, protein trafficking, and signal transduction, by additions of specific linkages of ubiquitin moieties to signaling proteins (Mukhopadhyay and Riezman, 2007). Numerous receptors and proteins important for proper development, including β-catenin, Notch, and NF-κB, are subject to regulation by ubiquitination. The TGFβ and Hh pathways, as well as cell cycle progression and apoptosis, are known to be tightly regulated by ubiquitination. A functional role of β-arrestins in ubiquitination was initially identified for the β2-adrenergic receptor (β2AR). The β2AR is a prototypical GPCR that mediates multiple physiological outcomes such as increased heart rate, vasodilation, and pupillary dilation, among others. Stimulation of the β2AR leads to Gs-dependent activation of adenylyl cyclase, an enzyme that forms the second messenger cAMP. Desensitization of β2ARs is conferred by GRK-dependent phosphorylation, followed by the recruitment of β-arrestin. The RING ubiquitin ligase Mdm2 catalyzes transient receptor-stimulated ubiquitination of β-arrestin 2, which is required for clathrin-mediated β2AR internalization (Shenoy et al., 2001). Ubiquitinated β-arrestin recruits certain endocytic components, such as clathrin and AP-2, leading to rapid receptor internalization; however, the mechanisms by which ubiquitination of β-arrestin enhances β-arrestin/clathrin-dependent endocytosis remain to be elucidated (Shenoy, 2007).
Interestingly, ubiquitinated β-arrestin has a higher affinity than nonubiquitinated β-arrestin for many receptors and downstream signaling intermediates, such as ERK (Shenoy et al., 2007). Subsequent deubiquitination of β-arrestin 2 by the deubiquitinase USP33 dissociates the receptor-β-arrestin complex (Shenoy et al., 2009). The internalized 7TMR is either degraded in the lysosomal compartment or resensitized by dephosphorylation and recycled to the plasma membrane. These trafficking processes are orchestrated by β-arrestins, which can recruit either the HECT ubiquitin ligase neural precursor cell expressed, developmentally downregulated 4 (Nedd4) for receptor degradation (Shenoy et al., 2008), or the deubiquitinases USP33 and USP20 for β-arrestin deubiquitination and receptor recycling (Berthouze et al., 2009). In other contexts, β-arrestin 2 appears to recruit unknown ubiquitin ligases to the Gs-coupled Vasopressin V2 Receptor (V2R), facilitating its ubiquitination and degradation (Martin et al., 2003). β-arrestin 1 associates with the HECT ubiquitin ligase atrophin-interacting protein 4 (AIP4) to downregulate the Gi/o-coupled chemokine receptor CXCR4 (Bhandari et al., 2007).
In the last several years, β-arrestins have emerged as general adaptors for diverse groups of E3 ubiquitin ligases (Table 3) to facilitate ubiquitination of their substrates (Shenoy, 2007). We have already mentioned this idea in the context of Mdm2 regulation of the IGF1R (Girnita et al., 2005) and of Notch regulation by Dx and Krz (Mukherjee et al., 2005). Cytosolic molecules are also targeted for ubiquitination in a β-arrestin-dependent manner (Li et al., 2009; Salcedo et al., 2006). Furthermore, recent studies in yeast have shown that arrestin-related trafficking adaptors (ARTs) regulate turnover of plasma membrane proteins, such as the arginine transporter Can1 and the lysine transporter Lyp1 (Lin et al., 2008). Despite lacking sequence homology with β-arrestins, ARTs contain a short amino acid motif, the “arrestin motif,” within the predicted arrestin fold that is conserved in the mammalian arrestin family and interacts with the HECT E3 ligase Rsp5 (a homolog of Nedd4 family proteins). Through this interaction, ARTs lead to internalization and degradation of amino acid transporters in an ubiquitination-dependent manner (Lin et al., 2008). These findings suggest that arrestins are bona fide ubiquitin ligase adaptors, and that this characteristic of arrestins is remarkably conserved even among distantly related proteins (i.e., ARTs) in species as divergent as yeast and mammals.
Table 3.
E3 Ubiquitin Ligases Scaffolded by β-arrestins
| E3 ubiquitin ligase | Type | Targets | Reference |
|---|---|---|---|
| AIP4 | HECT | CXCR4 | Bhandari et al., 2007 |
| Deltex | RING | Notch | Mukherjee et al., 2005 |
| Mdm2 | RING | IGF1R, Androgen Receptor, PDE4D5, GRK-2, β2AR | Girnita et al., 2005; Lakshmikanthan et al., 2009; Salcedo et al., 2006; Shenoy et al., 2001 |
| NEDD4 | HECT | β2AR | Shenoy et al., 2008 |
The MAP Kinases
Many extracellular growth factors and stresses converge on the MAPKs to control cellular proliferation, survival, and differentiation. It is acutely important for the strength and kinetics of these signals that they be tightly regulated. While classical G protein-dependent ERK activation is rapid and transient, recent studies demonstrate that β-arrestins produce a slower, but more persistent response, perhaps allowing intricate regulation.
ERK
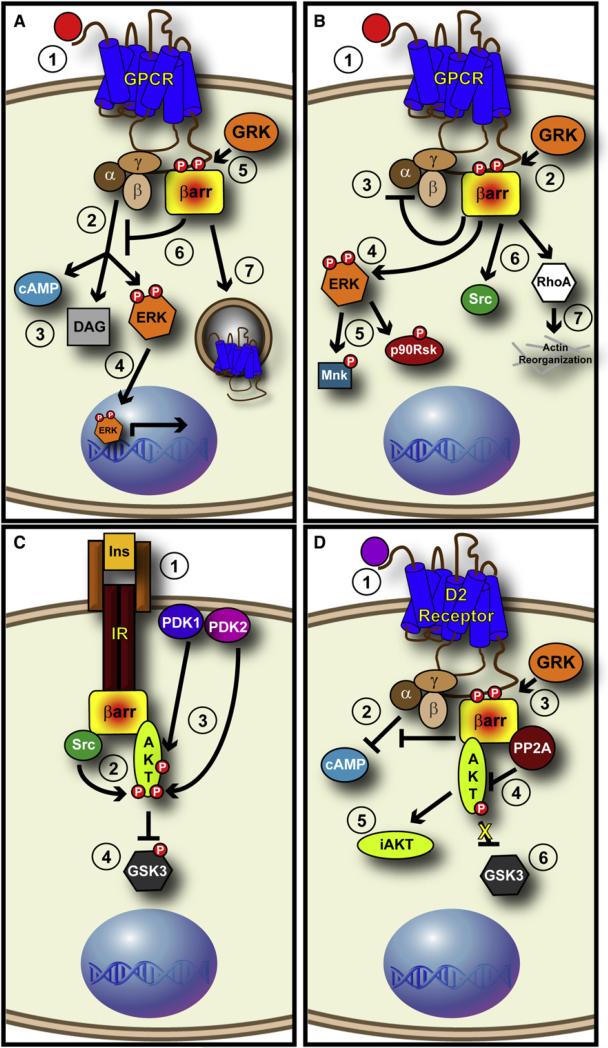
The ERK MAPK is the archetype for β-arrestin-mediated signaling. Following agonist stimulation, GPCRs activate MAPK through both Gα and Gβγ subunit-dependent mechanisms (Luttrell and Luttrell, 2003). This activated MAPK signal is rapid and transient because it is quickly quenched by β-arrestin-mediated desensitization of the receptor (Figure 5A). In a discrete yet related process, β-arrestins scaffold the MAP kinase signaling molecules, MAP kinase kinase kinase (Raf-1), MAP kinase kinase (MEK1), and MAP kinase (ERK), leading to phosphorylation and activation of ERK 1/2 (DeWire et al., 2007) (Figure 5B). G protein-mediated ERK activation is generally rapid but transient, leading primarily to nuclear signaling via activation of transcription factors. In contrast, β-arrestin-mediated ERK responses are slower and more persistent, generally retaining the activated kinases in the cytosol. This not only prevents ERK from phosphorylating nuclear substrates, but results in increased phosphorylation of cytosolic substrates, prolonging signaling events (Figure 5B). This intricate balance between G protein-dependent and β-arrestin-dependent ERK activation regulates the strength, kinetics, and localization of the ERK/MAPK signaling cascade.
Figure 5. β-arrestins Act as Signaling Scaffolds.
(A) GPCRs generate rapid and transient ERK, which is attenuated by β-arrestins. (1) As ligand (red circle) binds to the extracellular domain of a GPCR, G proteins are activated (2) and generate second messengers such as cAMP and DAG, while (3) also stimulating the rapid and transient phosphorylation of ERK. (4) This “G protein-stimulated” ERK translocates into the nucleus and modulates transcription. Meanwhile, (5) GRKs phosphorylate the C-terminal tail of receptors, thus (6) recruiting β-arrestins that sterically inhibit G protein binding, thus attenuating generation of second messengers. (7) β-arrestins also assist in the internalization of receptors, thus leading to their downregulation.
(B) β-arrestins act as signaling adaptors and activate cytosolic ERK in a persistent fashion. (1) Upon ligand binding, (2) β-arrestins are recruited to the receptor and (3) block G protein-mediated signaling. (4) β-arrestins are able to stimulate the phosphorylation of ERK, which leads to a slowly developing, persistent pool of “β-arrestin-stimulated” ERK that resides exclusively in the cytosol and phosphorylates cytoplasmic substrates such as (5) Mnk and p90Rsk. (6) β-arrestins are also able to stimulate proteins such as c-Src and (7) RhoA, leading to a wide range of responses.
(C) β-arrestin positively regulates Akt in response to Insulin Receptor Stimulation. (1) Bindng of Insulin (Ins) to the Insulin Receptor (IR) triggers the binding of β-arrestin 2 to the receptor. (2) β-arrestin scaffolds c-Src to Akt, causing the phosphorylation of Akt by c-Src. (3) This phosphorylation allows PDK1 and PDK2 phosphorylation events that (4) inhibit GSK3β.
(D) β-arrestins negatively regulate Akt in response to Dopamine Receptor Stimulation. (1) Upon binding of Dopamine (purple circle) to the D2 Dopamine Receptor, (2) G proteins inhibit cAMP generation and (3) β-arrestin is recruited to the receptor to relieve this repression. (4) Not only does β-arrestin block further G protein-mediated signaling, it also scaffolds PP2A to Akt, (5) thus leading to the dephosphorylation of Akt, and the generation of an inactive Akt (iAkt). (6) This leads to the activation of GSK3β.
An association with β-arrestin-dependent ERK activation has been described for many 7TMRs including the angiotensin II type 1A receptor (AT1AR) and β-adrenergic receptors (Table 1), among others (DeWire et al., 2007). The physiological implications of β-arrestin-dependent ERK activation have recently begun to come into focus. For example, in primary vascular smooth muscle cells the AT1AR-β-arrestin-ERK signaling complex uses distinct effectors to block apoptosis via BAD phosphorylation (Ahn et al., 2009), stimulate translation through Mnk1 phosphorylation of the translation initiation factor eIF4E (DeWire et al., 2008), and trigger S-phase entry and cell proliferation with EGFR (Kim et al., 2009). These latter mitogenic responses are very common among receptors that activate ERK during β-arrestin-mediated endocytosis (Lin et al., 1998). Thus, β-arrestin-dependent ERK activation regulates several events that are important for the intricate regulation of developmental processes.
JNK Family
JNK3 is another of the many MAPKs activated by β-arrestin signaling pathways. The JNK family of protein kinases (stress-activated protein kinases) phosphorylate Jun-family components of the AP-1 transcription factor complex, thus increasing AP-1 transcriptional activity (Davis, 2000). β-arrestin 2 acts as a scaffold for JNK3 and its upstream kinases ASK1 and MKK4 (McDonald et al., 2000). Stimulation of the AT1AR leads to the formation of this β-arrestin 2-scaffold complex on endosomal structures containing AT1AR, resulting in phosphorylated, active JNK3 accumulation in the cytosol (McDonald et al., 2000), much like the ERK cascade discussed above. However, it is still unknown which substrates are targeted by β-arrestin 2-activated JNK3 in the cytosol. This question is of considerable interest, as JNK3 is strongly implicated in AP-1-dependent neurotoxicity. Mice lacking JNK3 exhibit reduction in both seizure activity and hippocampal neuron apoptosis in the kainate-induced stroke model (Yang et al., 1997). Since β-arrestin 2 is also highly expressed in the hippocampus, it seems likely that this activity is β-arrestin 2-dependent.
p38
β-arrestin-mediated signaling also leads to p38 phosphorylation and activation (Bruchas et al., 2006; Gong et al., 2008; Miller et al., 2003; Sun et al., 2002), though it has not directly been shown that β-arrestins scaffold either p38 or its upstream kinases. Substrates of p38 include transcription factors, such as p53 or activating transcription factor 2 (ATF2), and protein kinases, including MAPK-activated kinase 2 (MK2) and Mnk1. Activation of p38α is normally associated with inhibition of cell cycle progression both at the G1/S and G2/M transitions, leading to antiproliferative effects (Wagner and Nebreda, 2009). So far, the principal contexts in which β-arrestin seems to regulate p38 activation are after receptor stimulation. For example, stimulation of β2AR causes phosphorylation and activation of p38, which is attenuated by β-arrestin1 RNAi (Gong et al., 2008), and the β-arrestin-dependent chemotactic response to stimulation of the chemokine receptor CXCR4 is prevented by pharmacological p38 inhibitors (Sun et al., 2002).
Differential Regulation of Akt by β-arrestins
Depending on the type of stimuli and the relevant receptors, β-arrestins can scaffold different sets of molecules that determine distinct, even opposite effects on the same signaling cascade. One such example is Akt signaling, which is either activated or suppressed by β-arrestin-recruited c-Src or protein phosphatase 2A (PP2A), respectively (Figures 5C and 5D).
A physiological role for β-arrestin-mediated c-Src recruitment and its effects on Akt signaling was recently identified (Luan et al., 2009). In canonical insulin-mediated Akt activation, stimulation of the insulin receptor, an RTK, ultimately leads to the generation of phosphatidylinositol (3,4,5) trisphosphate (PIP3). PIP3 binds to the pleckstrin homology (PH) domain of Akt and phosphoinositide-dependent kinases (PDKs), which then phosphorylate and activate proximal Akt on the membrane. Recently, it was revealed that β-arrestin 2 regulates insulin action by scaffolding a complex-containing insulin receptor, c-Src, and Akt (Luan et al., 2009). This complex allows c-Src to phosphorylate Akt on Tyr 315 and Tyr 326, which are required for the subsequent phosphorylation of Akt at Thr 308 and Ser 473 by PDK1 and PDK2, respectively (Figure 5C). Accordingly, β-arrestin 2 knockout mice are insulin resistant, and overexpression of β-arrestin 2 in mice causes increased insulin sensitivity (Luan et al., 2009).
On the other hand, β-arrestin 2 can deactivate Akt by the formation of a β-arrestin 2/PP2A/Akt complex upon stimulation of the D2-class dopamine receptors (Beaulieu et al., 2005). PP2A dephosphorylates Akt, blocking Akt function (Figure 5D). Akt inactivation releases GSK3β from repressive phosphorylation by Akt, resulting in GSK3β activation. Lithium is a GSK3β inhibitor (Klein and Melton, 1996) commonly used for the clinical management of psychiatric diseases such as schizophrenia and bipolar disorder; however, lithium may exert its therapeutic actions via diverse mechanisms (Beaulieu et al., 2004). Interestingly, lithium was recently shown to disrupt the signaling complex of Akt, β-arrestin 2, and PP2A, promoting Akt activity and greater inhibition of GSK3β (Beaulieu et al., 2008). Accordingly lithium action on behavior is abrogated in β-arrestin 2 knockout mice where GSK3β is not inhibited (Beaulieu et al., 2008).
These studies provide examples of how β-arrestins can affect two different outcomes in the same signaling cascade (e.g., Akt), dependent on the type of ligand and its relevant receptor. These differing combinations of ligand-receptor interactions determine which β-arrestins are recruited and which protein complexes are scaffolded by β-arrestins. Moreover, such differential regulation by β-arrestins is implicated in human disorders such as type II diabetes and psychiatric disorders (Beaulieu et al., 2005; Luan et al., 2009). Pharmacological manipulation of selective β-arrestin-dependent complexes (e.g., by lithium) may provide novel therapeutic benefits in such disorders (Beaulieu et al., 2008).
Concluding Remarks
Recent studies have prompted a rapidly growing appreciation of the roles of β-arrestins as both mediators of desensitization and 7TMR signaling. In addition, it has become evident that receptors from outside the 7TMR family, such as receptor serine/threonine kinases, tyrosine kinases, and cytokine receptors, also exploit β-arrestin-mediated scaffolding to regulate a downstream signaling response. As β-arrestins continue to emerge as broadly functioning adaptors for signaling molecules such as protein kinases, ubiquitin ligases, and other signal transducers, the roles that these versatile molecules play during development will continue to expand and be defined. As reviewed above, β-arrestins play critical roles in the precise regulation of many signaling pathways, such as responses to Hh, Wnts, and TGFβs. Future analyses of the diverse pheno-types seen in Drosophila krz mutants, and in mouse tissues lacking both β-arrestin 1 and 2, therefore represent a promising approach to the discovery of new functions for nonvisual arrest-ins in signaling and development.
ACKNOWLEDGMENTS
R.J.L. is an investigator with the Howard Hughes Medical Institute (HHMI). J.J.K., M.R.H., and J.K. are supported by the NIH. We acknowledge grant support from the National Institutes of Health (HL16037 and HL70631). C.L.D. is supported by an NSF Graduate Research Fellowship. We thank Donna Addison and Elizabeth Hall for excellent administrative assistance. We also thank Gerald Blobe, Sudha Shenoy, Melanie Philipp, Seungkirl Ahn, Wei Chen, Arun Shukla, Jinpeng Sun, and Minyong Chen for critical reading of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J. Biol. Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J. Biol. Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bondi CD, McKeon RM, Bennett JM, Ignatius PF, Brydon L, Jockers R, Melan MA, Witt-Enderby PA. MT1 melatonin receptor internalization underlies melatonin-induced morphologic changes in Chinese hamster ovary cells and these processes are dependent on Gi proteins, MEK 1/2 and microtubule modulation. J. Pineal Res. 2008;44:288–298. doi: 10.1111/j.1600-079X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J. Biol. Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:6690–6695. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Schambony A, Cajanek L, Dominguez I, Arenas E, Schulte G. Beta-arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep. 2008;9:1244–1250. doi: 10.1038/embor.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc. Natl. Acad. Sci. USA. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camina JP, Lodeiro M, Ischenko O, Martini AC, Casanueva FF. Stimulation by ghrelin of p42/p44 mitogen-activated protein kinase through the GHS-R1a receptor: role of G-proteins and beta-arrestins. J. Cell. Physiol. 2007;213:187–200. doi: 10.1002/jcp.21109. [DOI] [PubMed] [Google Scholar]
- Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- Caunt CJ, Finch AR, Sedgley KR, Oakley L, Luttrell LM, McArdle CA. Arrestin-mediated ERK activation by gonadotropin-releasing hormone receptors: receptor-specific activation mechanisms and compartmentalization. J. Biol. Chem. 2006;281:2701–2710. doi: 10.1074/jbc.M507242200. [DOI] [PubMed] [Google Scholar]
- Charest PG, Oligny-Longpre G, Bonin H, Azzi M, Bouvier M. The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell. Signal. 2007;19:32–41. doi: 10.1016/j.cellsig.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc. Natl. Acad. Sci. USA. 2001;98:14889–14894. doi: 10.1073/pnas.211572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003a;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003b;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- Chen J, Rusnak M, Lombroso PJ, Sidhu A. Dopamine promotes striatal neuronal apoptotic death via ERK signaling cascades. Eur. J. Neurosci. 2009a;29:287–306. doi: 10.1111/j.1460-9568.2008.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009b;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr. Top. Dev. Biol. 2008;85:261–301. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- Collins BM, Norwood SJ, Kerr MC, Mahony D, Seaman MN, Teasdale RD, Owen DJ. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic. 2008;9:366–379. doi: 10.1111/j.1600-0854.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ. Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J. Biol. Chem. 2001;276:15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- Dalle S, Imamura T, Rose DW, Worrall DS, Ugi S, Hupfeld CJ, Olefsky JM. Insulin induces heterologous desensitization of G-protein-coupled receptor and insulin-like growth factor I signaling by down-regulating beta-arrestin-1. Mol. Cell. Biol. 2002;22:6272–6285. doi: 10.1128/MCB.22.17.6272-6285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Defea K. Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br. J. Pharmacol. 2008;153(Suppl 1):S298–S309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Kim J, Whalen EJ, Ahn S, Chen M, Lefkowitz RJ. Beta-arrestin-mediated signaling regulates protein synthesis. J. Biol. Chem. 2008;283:10611–10620. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J, Holzenberger M. IGF type 1 receptor: a cell cycle progression factor that regulates aging. Cell Cycle. 2003;2:270–272. [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Finger EC, Lee NY, You HJ, Blobe GC. Endocytosis of the type III transforming growth factor-beta (TGF-beta) receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor down-regulation. J. Biol. Chem. 2008;283:34808–34818. doi: 10.1074/jbc.M804741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J. Biol. Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- Ge H, Krishnan P, Liu L, Krishnan B, Davis RL, Hardin PE, Roman G. A Drosophila nonvisual arrestin is required for the maintenance of olfactory sensitivity. Chem. Senses. 2006;31:49–62. doi: 10.1093/chemse/bjj005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ, Larsson O. {beta}-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J. Biol. Chem. 2005;280:24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Vasilcanu D, Girnita A, Lefkowitz RJ, Larsson O. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J. Biol. Chem. 2007;282:11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- Goel R, Phillips-Mason PJ, Raben DM, Baldassare JJ. alpha-Thrombin induces rapid and sustained Akt phosphorylation by beta-arrestin1-dependent and -independent mechanisms, and only the sustained Akt phosphorylation is essential for G1 phase progression. J. Biol. Chem. 2002;277:18640–18648. doi: 10.1074/jbc.M108995200. [DOI] [PubMed] [Google Scholar]
- Gong K, Li Z, Xu M, Du J, Lv Z, Zhang Y. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J. Biol. Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, McMahon AP. The effect of pertussis toxin on zebrafish development: a possible role for inhibitory G-proteins in hedgehog signaling. Dev. Biol. 1998;194:166–171. doi: 10.1006/dbio.1997.8796. [DOI] [PubMed] [Google Scholar]
- Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ. Notch signaling in development and disease. Clin. Genet. 2003;64:461–472. doi: 10.1046/j.1399-0004.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Stirring up development with the heterotrimeric kinesin KIF3. Traffic. 2000;1:29–34. doi: 10.1034/j.1600-0854.2000.010105.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, receptor-specific interaction of human P2Y receptors with beta-arrestin-1 and 2. J. Biol. Chem. 2008;283:30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, Milligan G, Patel DD, Lefkowitz RJ. Beta-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol. Pharmacol. 2005;67:1229–1236. doi: 10.1124/mol.104.006270. [DOI] [PubMed] [Google Scholar]
- Hupfeld CJ, Resnik JL, Ugi S, Olefsky JM. Insulin-induced beta-arrestin1 Ser-412 phosphorylation is a mechanism for desensitization of ERK activation by Galphai-coupled receptors. J. Biol. Chem. 2005;280:1016–1023. doi: 10.1074/jbc.M403674200. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Capobianco L, D'Ancona GM, Picascia A, De Blasi A. Regulation of lysophosphatidic acid receptor-stimulated response by G-protein-coupled receptor kinase-2 and beta-arrestin1 in FRTL-5 rat thyroid cells. J. Endocrinol. 2002;174:103–110. doi: 10.1677/joe.0.1740103. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jadrich JL, O'Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Yan Z, Feng J. Activation of group III metabotropic glutamate receptors attenuates rotenone toxicity on dopaminergic neurons through a microtubule-dependent mechanism. J. Neurosci. 2006;26:4318–4328. doi: 10.1523/JNEUROSCI.0118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev. Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Tift FW, McCauley A, Liu L, Roman G. Functional characterization of kurtz, a Drosophila non-visual arrestin, reveals conservation of GPCR desensitization mechanisms. Insect Biochem. Mol. Biol. 2008;38:1016–1022. doi: 10.1016/j.ibmb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, et al. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol. Endocrinol. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Torii K, Yamashita Y, Nishizawa K, Kanekura K, Katada M, Ito M, Nishimoto I, Terashita K, Aiso S, Matsuoka M. Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell. Signal. 2007;19:2498–2506. doi: 10.1016/j.cellsig.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Cho JY, Kim KS, Lee SJ, Lee KH, Choi KY. Drosophila PI3 kinase and Akt involved in insulin-stimulated proliferation and ERK pathway activation in Schneider cells. Cell. Signal. 2004;16:1309–1317. doi: 10.1016/j.cellsig.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J. Cell Biol. 2008a;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. USA. 2008b;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J. Biol. Chem. 2009;284:11953–11962. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol. Biol. Cell. 2008;19:4640–4650. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanthan V, Zou L, Kim JI, Michal A, Nie Z, Messias NC, Benovic JL, Daaka Y. Identification of betaArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc. Natl. Acad. Sci. USA. 2009;106:9379–9384. doi: 10.1073/pnas.0900258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr. Pharm. Des. 2007;13:663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- Lee NY, Blobe GC. The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J. Biol. Chem. 2007;282:21507–21517. doi: 10.1074/jbc.M700176200. [DOI] [PubMed] [Google Scholar]
- Lee NY, Kirkbride KC, Sheu RD, Blobe GC. The TGF-{beta} Type III Receptor Mediates Distinct Subcellular Trafficking and Downstream Signaling of ALK3 and ALK6 Receptors. Mol. Biol. Cell. 2009 doi: 10.1091/mbc.E09-07-0539. in press. 10.1091/mbc.E1009-1007-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ. beta-arrestins: traffic cops of cell signaling. Curr. Opin. Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Li X, Baillie GS, Houslay MD. Mdm2 Directs the Ubiquitination of {beta}-Arrestin-sequestered cAMP Phosphodiesterase-4D5. J. Biol. Chem. 2009;284:16170–16182. doi: 10.1074/jbc.M109.008078. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lin FT, Daaka Y, Lefkowitz RJ. beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J. Biol. Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc. Natl. Acad. Sci. USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- Luo J, Busillo JM, Benovic JL. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol. Pharmacol. 2008;74:338–347. doi: 10.1124/mol.107.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell DK, Luttrell LM. Signaling in time and space: G protein-coupled receptors and mitogen-activated protein kinases. Assay Drug Dev. Technol. 2003;1:327–338. doi: 10.1089/15406580360545143. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl. Acad. Sci. USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J. Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J. Biol. Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J. Biol. Chem. 2003;278:45954–45959. doi: 10.1074/jbc.M308285200. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995;121:2633–2644. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J. Immunol. 2006;176:7039–7050. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J. Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler-Reya RJ, Lefkowitz RJ, Caron MG. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol. Cell. Biol. 2006;26:7550–7560. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem. J. 2005;387:573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J. Biol. Chem. 2003;278:21663–21671. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, Boularan C, Ghossoub R, Scott MG, Burtey A, Zarka M, Saunier S, Concordet JP, Marullo S, Benmerah A. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS ONE. 2008;3:e3728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual beta-arrestin. Nat. Cell Biol. 2005;7:1191–1201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, de Sauvage FJ. Hedgehog signal transduction: from flies to vertebrates. Exp. Cell Res. 1999;253:25–33. doi: 10.1006/excr.1999.4676. [DOI] [PubMed] [Google Scholar]
- Mythreye K, Blobe GC. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc. Natl. Acad. Sci. USA. 2009;106:8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- Nelson CD, Kovacs JJ, Nobles KN, Whalen EJ, Lefkowitz RJ. Beta-arrestin scaffolding of phosphatidylinositol 4-phosphate 5-kinase Ialpha promotes agonist-stimulated sequestration of the beta2-adrenergic receptor. J. Biol. Chem. 2008;238:21093–21101. doi: 10.1074/jbc.M800431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature. 2008;456:967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol. Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Philipp M, Fralish GB, Meloni AR, Chen W, MacInnes AW, Barak LS, Caron MG. Smoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinase. Mol. Biol. Cell. 2008;19:5478–5489. doi: 10.1091/mbc.E08-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Maudsley S, Daaka Y, Luttrell LM, Lefkowitz RJ. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, He J, Davis RL. kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics. 2000;155:1281–1295. doi: 10.1093/genetics/155.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Salcedo A, Mayor F, Jr., Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006;25:4752–4762. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, Pierotti V, Storez H, Lindberg E, Thuret A, Muntaner O, Labbe-Jullie C, Pitcher JA, Marullo S. Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and beta-arrestins. Mol. Cell. Biol. 2006;26:3432–3445. doi: 10.1128/MCB.26.9.3432-3445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circ. Res. 2007;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J. Biol. Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J. Biol. Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J. Biol. Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J. Biol. Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc. Natl. Acad. Sci. USA. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Rojas R, Bonifacino JS, Hurley JH. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat. Struct. Mol. Biol. 2006;13:540–548. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors. Biochem. Soc. Trans. 2001;29:513–525. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. USA. 2008;105:6614–6619. doi: 10.1073/pnas.0710402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J. Biol. Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Aguiar RC, Gu L, He C, Freeman GJ, Kutok JL, Aster JC, Shipp MA. The BAL-binding protein BBAP and related Deltex family members exhibit ubiquitin-protein isopeptide ligase activity. J. Biol. Chem. 2003;278:21930–21937. doi: 10.1074/jbc.M301157200. [DOI] [PubMed] [Google Scholar]
- Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat. Cell Biol. 2007;9:588–595. doi: 10.1038/ncb1579. [DOI] [PubMed] [Google Scholar]
- Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev. Cell. 2006;10:177–186. doi: 10.1016/j.devcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry. 2006;45:9374–9385. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell. Signal. 2006a;18:934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J. Biol. Chem. 2006b;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2006c;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, 3rd, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol. Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- You HJ, How T, Blobe GC. The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis. 2009;30:1281–1287. doi: 10.1093/carcin/bgp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J. Cell Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels. 1997;5:193–199. [PubMed] [Google Scholar]
- Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]