Abstract
Background and Purpose
The aging brain demonstrates frequent MRI and pathological evidence of cerebral microbleeds, which are often associated with cerebral amyloid angiopathy. In order to develop new therapeutic strategies for this disorder, we studied cerebral microhemorrhage in a well-characterized mouse model of cerebral amyloid angiopathy.
Methods
Tg 2576 mice were studied at ages ranging from 2 to 21 months. Spontaneous and induced microscopic bleeding was analyzed with and without passive anti-amyloid immunization regimen and dietary supplementation of ischemic stroke prevention medication dipyridamole.
Results
Areas of microhemorrhage were easily demonstrated, and were significantly more prominent in oldest mice and in animals treated with anti-amyloid immunotherapy. Dipyridamole supplementation in diet generated plasma levels greater than 790ng/ml, within the range seen clinically. Dipyridamole treatment did not worsen frequency and size of cerebral microscopic bleeding.
Conclusions
The Tg2576 mouse is a useful model to study progression and modification of spontaneous and immunotherapy-induced cerebral microhemorrhage. Absence of microhemorrhage worsening with dipyridamole treatment suggests a potential therapeutic role of this agent when ischemic and microhemorrhagic lesions coexist.
Keywords: Alzheimer’s, Amyloid angiopathy, Animal models, Antiplatelet drugs, Basic science, Experimental, Intracerebral hemorrhage, Microcirculation
INTRODUCTION
There is increasing recognition of the high prevalence of cerebral microbleeds in the aging population. Over the age of 60 years, MRI evidence of cerebral microbleeds is present in approximately 20% of the population; this number rises to nearly 40% for individuals over the age of 80 (1). Moreover, post-mortem pathology studies demonstrate microscopic brain hemorrhages occurring at the capillary level in the vast majority of individuals over the age of 70 (2,3).
There are at least two key issues that emanate from these microbleed observations. First, there is the necessity for establishing animal models of this disorder that will enable investigators to delineate pathophysiological mechanisms of microbleed formation. Second, new treatments and treatment modifications are needed to address the microbleeds themselves.
The issue of microbleeds treatment is of necessity complex. Microbleeds are not only common, but they do not occur in a neurovascular vacuum. Microbleeds may coexist with other forms of cerebrovascular disease. Cerebral white matter disease, which has at least a partial microvascular origin (4), has a strong association with cerebral microbleeds in the presence of cerebral amyloid angiopathy (5). Moreover, ischemic stroke may coexist with cerebral microbleeds, leading to a profound challenge in stroke neurology: How to protect the patient against ischemic stroke while not worsening the brain hemorrhage potential of cerebral microbleeds? The concept of “mixed cerebrovascular disease” has thus been established in order to encompass the wide spectrum of ischemic and hemorrhagic cerbrovascular disease, both clinical and subclinical (6).
It is against this background that we initiated the current study in which we analyzed microhemorrhage in a well-characterized mouse model of Alzheimer’s disease and cerebral amyloid angiopathy. The Tg2576 transgenic mouse has been extensively studied in the course of investigations of Alzheimer’s disease-like amyloid deposition (7). In the current study, we have used this mouse model to investigate the development of brain microhemorrhage, as well as initiate new therapeutic strategies directed at the microhemorrhages themselves. We thus utilized the Tg2576 mouse model, alone and modified by passive immunization (8,9), with and without the ischemic stroke prevention medication dipyridamole (10). The establishment of reliable animal models of microhemorrhage and related therapeutic interventions should have translational value in the clinical efforts directed against mixed cerebrovascular disease.
METHODS
All animal studies were performed in accordance with IACUC-approved protocols at UC Irvine.
Animals and Treatment Protocols
Experiment 1
In order to determine achievable plasma levels with dipyridamole supplementation, 2-month-old female Tg2576 mice (n=6) were treated with dipyridamole-supplemented high fat diet (HFD, 40% fat, 40% carbohydrate and 20% protein, with dipyridamole 5g/kg chow) for 2 weeks. Age-matched female wild-type (WT) littermates were used as controls. HFD was used due to poor oral intake by mice when bitter tasting dipyridamole was incorporated into a standard diet.
Experiment 2
12-month-old female Tg2576 mice were divided into 2 groups: 1) Tg2576-Control (Control) mice (n=9) fed HFD; 2) Tg2576- + dipyridamole (DIP) mice (n=11) fed HFD supplemented with dipyridamole (5 g/kg of chow). Animals were treated for 3 months.
Experiment 3
21-month-old Tg2576 were divided into 4 groups: 1) Tg2576-Control mice (n=9) fed HFD; 2) Tg2576 +DIP mice (n=11) fed HFD supplemented with dipyridamole (5 g/kg of chow); 3) Tg2576- mice (n=9) on HFD were treated with anti-Aβ40 antibodies clone 1313 (10mg/kg, i.p., weekly) (Abody); 4) Tg2576 +DIP mice (n=9) treated with anti-Aβ40 antibodies clone 1313 (10mg/kg, i.p., weekly) (DIP+Abody). There were two males with seven or nine female mice in each of the four groups. Mice were pretreated with the diets for 2 weeks before the antibody injections, which continued for 9 weeks together with the diet supplement.
Behavioral Test and Blood Pressure Monitoring
Cognition was assessed at end of treatment using the Novel Object Recognition task as described (11). Blood pressure was assessed noninvasively in conscious restrained mice by the tail cuff technique using the CODA System (Kent Scientific) for 3 days at the end of the experiment.
Immunohistochemistry
PBS perfused 4% paraformaldehyde fixed brain sections were immunostained for Aβ using 6E10 and 4G8 mouse monoclonal antibodies (Signet Laboratories, Dedham, MA) at 1:1,000 dilution, as described (11, 12).
Hemorrhage Detection
Determination of hemorrhages was performed using Prussian blue staining of ferric acid, as previously described (12). Coronal sections of experimental and control mouse brains were collected from 3 different planes of the brain (about 2.5 mm apart). The sections were mounted on Superfrost Plus microscopic slides (Fisher Scientific, Pittsburg, PA). Staining was performed using Prussian blue solution, ie, freshly made 5% potassium hexacyanoferrate trihydrate and 5% hydrochloric acid (Sigma, MO). 30 minutes later, sections were rinsed in water, and counterstained with Nuclear Fast Red (Sigma, MO), dehydrated and covered using DPX (BDH Laboratory Supplies, England). Hemorrhage profiles were counted by three independent observers using Olympus BX40 microscope at 20x original magnification. For each brain section, the average number and size of Prussian blue-positive deposits was calculated. We used a scale of 1 to 4 to determine the size of microhemorrhage, where 1 was 1–5 grains of iron or small microvessel involvement; 2 was multiple grains of iron and microvessel involvement; 3 was several positive microvessels in one area; and 4 was large blood vessel involvement. Results were averaged between investigators and presented as a mean±SEM.
Beta Amyloid (Aβ) ELISA
ELISA of detergent soluble and 70% formic acid soluble Aβ brain fractions was performed using our established assay, as previously described (11, 12)
Statistical Analysis
All data are expressed as mean ± SEM. The statistical evaluation was carried out using Student's t-test or analysis of variance (ANOVA). Following significant ANOVAs, multiple post hoc comparisons were performed using Bonferroni’s test.
RESULTS
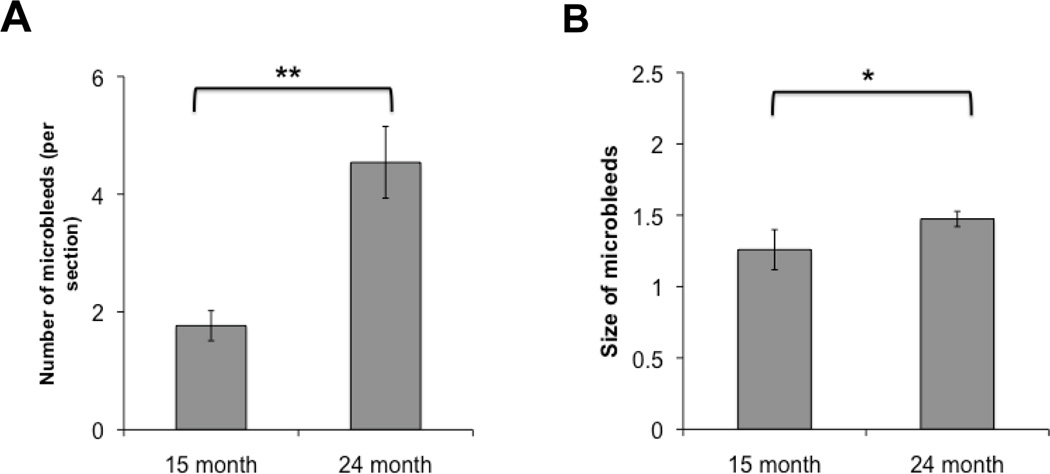
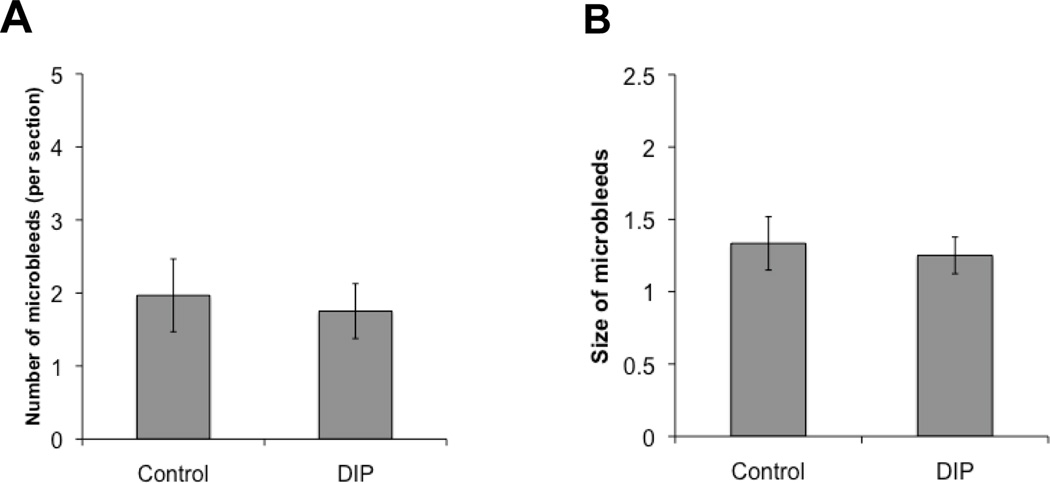
Tg 2576 mice showed progressive accumulation of microhemorrhages with aging, which ranged from 1.76±0.25 microhemorrhages per brain section in 15-month old mice to 4.54±0.6 microhemorrhages in 24-month old animals (Fig. 1A). The average size of spontaneous microhemorrhage also significantly increased with age (Fig 1B). Dipyridamole supplementation in high fat diet was well tolerated by mice. After 2 weeks, plasma levels of dipyridamole reached 794±246ng/ml and 793±97ng/ml in WT and Tg2576 mice, respectively. We then studied the effects of three months dipyridamole-supplemented diet on blood pressure, object recognition, and Aβ40 and Aβ42 in 15 month old mice. In these studies, there were no effects of dipyridamole on novel object recognition, blood pressure, and Aβ40 and Aβ42 levels (data not shown). The latter were measured in both detergent-soluble and detergent-insoluble brain fractions. In addition, there was no significant difference in spontaneous microhemorrhage size or number observed in mice receiving dipyridamole-supplemented diet (Figure 2). There was also no significant difference in microhemorrhage size and number between animals fed high fat diet and animals (N=9) fed standard (10% fat, 70% carbohydrate, and 20% protein) diet for three months (data not shown).
FIGURE 1.
Tg2576 mice progressively accumulate microhemorrhages with aging. Number (A) and size (B) of microhemorrhages assessed in 15- and 24-month old Tg2576 mice fed regular mouse chow. (**- P<0.01; * - P< 0.05)
FIGURE 2.
Dipyridamole diet (5 g/kg of chow) does not affect the number (A) nor the size (B) of spontaneous microhemorrhages in 15-month old Tg2576 mice receiving 3 months dipyridamole (DIP).
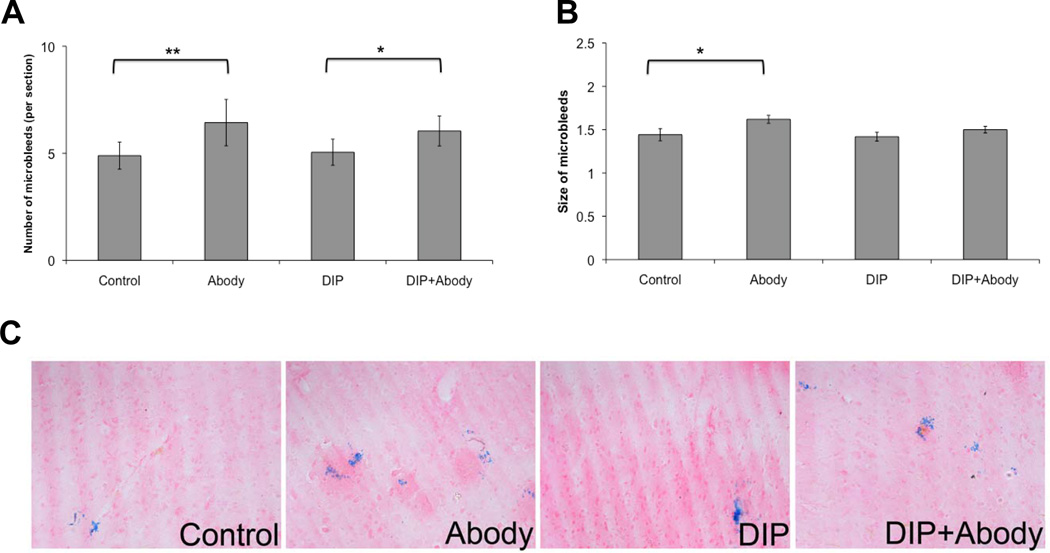
Finally, we studied the effects of eleven weeks dipyridamole-supplemented diet on aged (21 months old) mice, with and without passive immunization to Aβ40. The latter was achieved by nine weekly injections beginning two weeks after the start of the experiments. There were no significant effects of dipyridamole on novel object recognition and biochemical levels of Aβ40 and Aβ42, and there were no significant male-female differences in dipyridamole effects (data not shown). There was a significant increase in the number and size of microhemorrhages in antibody-treated mice on control diet (Figure 3). Antibody-treated mice fed dipyridamole-supplemented diet demonstrated significant increase in number, but not size, of microhemorrhages (Figure 3).
FIGURE 3.
Immunotherapy effects on number (A) and size (B) of microhemorrhages. Twenty-one-month-old mice were treated for 11 weeks with control (high fat) diet, or with diet supplemented with dipyridamole (DIP; 5 g/kg chow). Two weeks after initiation of the diet, mice were given anti-Aβ40 antibody (Abody, 10mg/kg, i.p., weekly for 9 weeks). Significantly increased number of microhemorrhages after immunotherapy was seen with both diets, and significantly increased microhemorrhage size with control diet only. Representative photomicrographs were taken from cortex (C). Magnification: ×100. (**- P<0.01; * - P< 0.05).
DISCUSSION
Cerebral microscopic bleeding was easily demonstrable in this transgenic mouse model of cerebral amyloid angiopathy, with microhemorrhages more than twice as frequent in elderly compared to younger adult mice. These microhemorrhages tended to be exacerbated by our passive immunotherapy regimen. Treatment with dipyridamole did not worsen microhemorrhages, and the significantly increased microhemorrhage size of immunized mice was not seen after dipyridamole treatment. Plasma dipyridamole levels achievable were within the range encountered clinically, ie, 0.5–2.0ug/ml (13).
The Tg2576 mouse expresses mutant amyloid precursor protein (APP) from which pathogenic beta-amyloid fragments 1–40 and 1–42 are easily generated (7). Tg2576 is characterized by predictable progression of band-like vascular amyloid deposits in leptomeningeal and cortical arteries and arterioles, notable by age 12 months (14,15). Immunotherapy directed at beta-amyloid fragments tends to lessen parenchymal beta-amyloid but may worsen vascular amyloid deposition and exacerbate microhemorrhages, perhaps by direct antibody targeting of vascular wall (9,16).
The relative ease of demonstrating cerebral microhemorrhages makes the Tg2576 mouse an attractive model to study progression and treatment of these lesions. The latter issue is of particular importance where there is coexistence of ischemic and microhemorrhagic lesions, the typical picture of mixed cerebrovascular disease (6). Use of platelet agents, particularly aspirin, has been associated with higher prevalence of microbleeds on MRI (17) and increased risk of intracerebral hemorrhage in patients with cerebral amyloid angiopathy (18). Dipyridamole, shown to be effective as monotherapy for prevention of ischemic stroke (10), has therapeutic actions largely directed at the vessel wall, rather than platelets alone (19); these vessel wall effects include vasodilation via cGMP potentiation, anti-oxidant and anti-inflammatory actions, and enhancement of endothelial nitric oxide (19). Moreover, acute treatment with dipyridamole at the time of reperfusion tends to reduce hemorrhagic conversion of ischemic stroke in a rodent model of experimental stroke (20). Findings of our study are limited by lack of aspirin treatment arm. Nevertheless, absence of microhemorrhage worsening with dipyridamole treatment in this model suggests a potential role for this agent in patients with coexisting ischemic and microhemorrhagic disease.
Acknowledgements
We thank Dr. Wolfgang Eisert for assistance.
Sources of Funding
NIH NS20989 (MF), NIH AG020241 (DHC), NIH AG00538 (DHC), NIH NS050895 (DHC); Alzheimer’s Association grant IIRG 91822 (DHC); NIH summer student fellowship GM-69337 (CV); and a research grant from Boehringer-Ingelheim (MF & DHC). Aβ peptides and anti-Aβ monoclonal antibodies were provided by the UCI Alzheimer’s Disease Research Center funded by NIH P50AG16573.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Fisher has received support from Boehringer-Ingelheim (research grants, speakers’ bureau, honoraria) and Otsuka Pharmaceutical Company (research grant and honoraria). Dr. Cribbs has received support from Boehringer-Ingelheim (research grant).
References
- 1.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 2.Cullen KM, Kocsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M, French S, Ji P, Kim RC. Cerebral microbleeds in the elderly: a pathological analysis. Stroke. 2010;41:2782–2785. doi: 10.1161/STROKEAHA.110.593657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 5.Chen YW, Gurol ME, Rosand J, Vismanathan A, Rakich SM, Groover TR, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher M. The challenge of mixed cerebrovascular disease. Ann N Y Acad Sci. 2010;1207:18–22. doi: 10.1111/j.1749-6632.2010.05758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depeotes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasilevko V, Passos GF, Quiring D, Head E, Kim RC, Fisher M, et al. Aging and cerebrovascular dysfunction: contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Ann N Y Acad Sci. 2010;1207:58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 11.Parachikova A, Vasilevko V, Cribbs DH, LaFerla F, Green K. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restores cognition but not significantly affect tau hyperphosphorylation. J Alzheimers Dis. 2010;21:527–542. doi: 10.3233/JAD-2010-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitazawa M, Vasilevko V, Cribbs DH, LaFerla FM. Immunization with amyloid-beta attenuates inclusion body myositis-like myopathology and motor impairment in a transgenic mouse model. J Neuroscience. 2009;29:6132–6141. doi: 10.1523/JNEUROSCI.1150-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derendorf H, VanderMaelen CP, Brickl R-S, MacGregor TR, Eisert W. Dipyridamole bioavailability in subjects with reduced gastric acidity. J Clin Pharmacol. 2005;45:845–850. doi: 10.1177/0091270005276738. [DOI] [PubMed] [Google Scholar]
- 14.Robbins EM, Betensky RA, Domnitz SB, Purcell SM, Garcia-Alloza M, Greenberg SM, et al. Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer Disease. J Neuroscience. 2006;26:365–371. doi: 10.1523/JNEUROSCI.3854-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han BH, Zhou M, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, et al. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: Contribution of soluble and insoluble amyloid-β peptide, partial restoration via γ-secretase inhibition. J Neuroscience. 2008;28:13542–13550. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrushina I, Ghochikyan A, Mkrtichyan M, Mamikonyan G, Movsesyan N, Ajdari R, et al. Mannan-Abeta28 conjugate prevents Abeta-plaque deposition, but increases microhemorrhages in the brains of vaccinated Tg2576 (APPsw) mice. J Neuroinflammation. 2008;5:42. doi: 10.1186/1742-2094-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: The Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. doi: 10.1001/archneurol.2009.42. [DOI] [PubMed] [Google Scholar]
- 18.Biffi A, Halpin A, Towfighi A, Gilson A, Busi K, Rost N, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HH, Liao JK. Translational therapeutics of dipyridamole. Arterioscler Thromb Vasc Biol. 2008;28:s39–s42. doi: 10.1161/ATVBAHA.107.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Bonilla L, Sosti V, Campos M, Penalba A, Boada C, Sumalla M, et al. Effects of acute post-treatment with dipyridamole in a rat model of focal cerebral ischemia. Brain Research. 2011;1373:211–220. doi: 10.1016/j.brainres.2010.12.005. [DOI] [PubMed] [Google Scholar]