Abstract
Increased expression of the astrocytic intermediate filament protein glial fibrillary acidic protein (GFAP) is a characteristic of astrogliosis. This process occurs in the brain during aging and neurodegeneration and coincides with impairment of the ubiquitin proteasome system. Inhibition of the proteasome impairs protein degradation; therefore, we hypothesized that the increase in GFAP may be the result of impaired proteasomal activity in astrocytes. We investigated the effect of proteasome inhibitors on GFAP expression and other intermediate filament proteins in human astrocytoma cells and in a rat brain model for astrogliosis. Extensive quantitative RT-PCR, immunocytochemistry, and Western blot analysis resulted unexpectedly in a strong decrease of GFAP mRNA to <4% of control levels [Control (DMSO) 100±19.2%; proteasome inhibitor (epoxomicin) 3.5±1.3%, n=8; P≤0.001] and a loss of GFAP protein in astrocytes in vitro. We show that the proteasome alters GFAP promoter activity, possibly mediated by transcription factors as demonstrated by a GFAP promoter-luciferase assay and RT2 Profiler PCR array for human transcription factors. Most important, we demonstrate that proteasome inhibitors also reduce GFAP and vimentin expression in a rat model for induced astrogliosis in vivo. Therefore, proteasome inhibitors could serve as a potential therapy to modulate astrogliosis associated with CNS injuries and disease.—Middeldorp, J., Kamphuis, W., Sluijs, J. A., Achoui, D., Leenaars, C. H. C., Feenstra, M. G. P., van Tijn, P., Fischer, D. F., Berkers, C., Ovaa, H., Quinlan, R. A., Hol, E. M. Intermediate filament transcription in astrocytes is repressed by proteasome inhibition.
Keywords: glial fibrillary acidic protein, astrogliosis, protein degradation, neurodegenerative diseases
Increased expression of the intermediate filaments (IFs) glial fibrillary acidic protein (GFAP) and vimentin and the reexpression of nestin are the hallmarks of astrogliosis. This process occurs in the aging brain and is also induced by neurodegenerative processes present in diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD) (1), and Alexander’s disease (AxD) (2, 3). GFAP is the major IF protein in mature astrocytes and forms an important part of the IF cytoskeleton of the astrocyte. Multiple isoforms of GFAP are expressed in the human brain (4,5,6,7), of which the canonical form is GFAPα. Both protein and mRNA levels of GFAP have been shown to increase with age (8, 9) and neurodegeneration (10, 11).
Next to an increase in GFAP levels, it has also been shown that the ubiquitin proteasome system (UPS) is impaired in the aged brain and in AD (12, 13), PD (14), and AxD (15). The UPS is the principal cellular protein degradation system that tags and targets short-lived proteins, for instance, transcription factors, as well as aberrant proteins, such as incomplete, missense, and misfolded proteins, for destruction (16). The UPS modulates many regulatory proteins that control cellular processes such as the inflammatory response, the cell cycle, cell growth, signal transduction, and cellular differentiation (17, 18). The UPS is present in all living cells in both the cytoplasm and the nucleus and has been identified in almost every species from archaebacteria to human. The proteasome is a multicatalytic protease that comprises a 20S core and a 19S lid. The ubiquitinated target protein is unfolded and fed into the core of the proteasome, where the protein is degraded by the three different enzymatic activities: trypsin-like, chymotrypsin-like, and peptidyl-glutamyl peptide hydrolase-like (19).
It is generally presumed that the accumulation of nondegraded proteins resulting from inhibition of the proteasome will affect cellular homeostasis. However, proteasomal inhibition may also affect cellular processes via mechanisms other than protein accumulation. It has been suggested that there is a direct link between proteasome inhibition and protein synthesis (20, 21), resulting in either induced or impaired protein synthesis. Indeed, aging (22) and some diseases, e.g., AD (23), are associated with decreased proteasome activity and decreased ribosome function and protein synthesis. The proteasome has been implicated in controlling expression at the level of transcription (24,25,26). Regulation of gene transcription is a complex interplay between transcriptional regulators, general transcription factors, and chromatin structures, which can all be influenced by the UPS, more specifically through modulation of DNA binding and through the proteolysis of transcription factors (27, 28). Furthermore, recent studies have suggested that the UPS links histone modifications to transcriptional activation (29, 30). So far, most evidence has been obtained in yeast, and the relevance of the UPS in the regulation of transcription in mammalian cells is inferred mainly from these studies.
In many pathological situations, proteasome activity is impaired. Because astrogliosis and proteasome inhibition often occur mutually in disease conditions, we hypothesized that impaired proteasomal activity in astrocytes contributes to the increase in IFs such as GFAP. The first indication came from work on AxD, which is caused by missense mutations in GFAP (31). It has been suggested that proteasome inhibition can be both a possible cause as well as a consequence of the mutant GFAP accumulation (15) in this disease. In the current study we investigated the effect of proteasome inhibition on GFAP expression in human astrocytoma cells and in a rat brain model for astrogliosis. In contrast to our expectation, the data showed that inhibition of the proteasome results in a decrease of GFAP protein levels in astrocytes in vitro and in the rat brain in vivo. We provide evidence that inhibition of proteasome activity directly reduces GFAP transcription, thus leading to a rapid decline in GFAP gene expression. Our studies also show that astrogliosis as a result of invasive brain damage can be prevented by treatment with proteasome inhibitors.
MATERIALS AND METHODS
Cell lines and inhibitors
Human glioblastoma cell line U343 was a gift from Dr. James T. Rutka (University of Toronto, Toronto, ON, Canada) and the U251 and SNB19 glioblastomas were a gift from Dr. B. de Leeuw (Erasmus Medical Center, Rotterdam, The Netherlands). These cell lines were cultured in high-glucose DMEM with Ham’s F10 (1:1), supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin (all Invitrogen-Life Technologies, Carlsbad, CA, USA) at 37°C with 5% CO2. The human cervical epithelial carcinoma cell line HeLa and the neuroblastoma cell line SH-SY5Y were cultured in high-glucose DMEM, supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin, at 37°C with 5% CO2. HeLa and SH-SY5Y cells stably expressing the proteasome reporter construct UbG76V-GFP (32, 33) were cultured similarly. These green fluorescent protein (GFP) reporter cell lines were selected in the presence of geneticin (0.3 mg/ml for HeLa and 0.2 mg/ml for SH-SY5Y cells; Sigma-Aldrich Corp., St. Louis, MO, USA) and screened for GFP fluorescence on administration of proteasome inhibitors. Cells were treated with four different proteasome inhibitors: two irreversible inhibitors, epoxomicin (Biomol, Plymouth Meeting, PA, USA) and lactacystin (Sigma-Aldrich Corp.), and two reversible inhibitors, MG132 (Affinity Research, Exeter, UK) and MG262 (Boston Biochem, Cambridge, MA, USA). To verify proteasome specificity, inhibitors of other proteolytic activities were used: chloroquine (Sigma-Aldrich Corp.) to inhibit lysosomal degradation, bacitracin (Sigma-Aldrich Corp.) to interfere with the insulin-degrading enzyme, and Ala-Ala-Phe-chloromethylketone (AAF-cmk, Bachem, Bubendorf, Switzerland) to inhibit tripeptidyl peptidase II (TPPII). All chemical inhibitors were dissolved in DMSO, except for chloroquine, which was dissolved in water.
Flow cytometry
GFP fluorescence was determined by flow cytometric analysis. Three days before the analysis, SH-SY5Y cells and HeLa cells, both stably expressing the UbG76V-GFP reporter, were cultured in 6-well plates. The cells were treated with proteasome inhibitor 1 d before flow cytometric analysis was performed. Cell suspensions were fixed in 4% formalin in PBS (137 mM NaCl, 2.7 mM KCl, 1.8 mM KH2PO4, and 4 mM Na2HPO4, pH 7.4) and resuspended in PBS-0.5% BSA (Roche Diagnostics, Mannheim, Germany). GFP could be directly visualized. Analysis was performed on at least 20,000 cells/sample with a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA), and data were analyzed using CellQuest Pro 5.2 software (BD Biosciences).
Profiling proteasome activity with fluorescent probe
To confirm proteasome inhibition in U343 cells after treatment with various inhibitors and at different concentrations, we used a close analog of the bodipyFL fluorescent proteasome activity probe (34) that has identical spectral characteristics. To assess the proteasome activity, cells cultured on coverslips were washed in DMEM-Ham’s F10 medium (1:1, without serum and antibiotics) before the cells were incubated with the fluorescent proteasome probe (500 nM) in the medium for 3 h at 37°C. After that, the cells were rinsed in medium and incubated in the same medium for another hour at 37°C. Finally, the cells were washed with PBS, fixed in 4% paraformaldehyde (PFA), and washed again with PBS. The coverslips were embedded with Vectashield with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA, USA) and inspected by fluorescence microscopy (Leica DMRE; Leica Microsystems, Wetzlar, Germany). Probe fluorescence was visualized with FITC filter settings. Images were recorded by a digital camera using fixed exposure settings. Quantification was performed using the ImageJ (NIH, Bethesda, MD, USA) program by outlining the cellular circumference and obtaining the average density value.
Plasmids and transfections
To demonstrate that the proteasome in U343 cells was inhibited after overnight treatment with 100 nM epoxomicin, cells were transfected with UbG76V-GFP, a reporter for proteasome activity, which was developed by Dantuma et al.(32) and has been shown to be stabilized on proteasome inhibition (33, 35). The pGfa2-luciferase reporter gene construct was kindly provided by Michael Brenner (University of Alabama at Birmingham, Birmingham, AL, USA), and was made by cloning the gfa2 promoter from pGfa2-CAT (formerly called pGfaCAT-2; ref. 36) in the BglII-HindII sites of luciferase reporter vector pGL3 (Promega Corp., Madison, WI, USA). This construct encompasses the human GFAP promoter fused to a luciferase reporter gene, which enables the measurement of GFAP promoter activity. A Renilla luciferase control reporter vector (Promega Corp.) was cotransfected as an internal control. Cell culture medium was refreshed 2 h before lipofectamine (Invitrogen) transfection, according to the manufacturer’s protocol. For the GFAP promoter activity measurements, cells were cotransfected with pGfa2-luciferase (0.8 μg/well) and pRL-TK (Renilla) (1 ng/well) in a 24-well plate. Culture medium was refreshed 4 h after transfection, and 1 d later, epoxomicin (final concentration 100 nM) or DMSO (final concentration 0.01%) was added to the culture medium for ∼16 h.
Cells transfected with UbG76V-GFP were viewed with a fluorescence microscope (Zeiss Axiovert 10; Carl Zeiss, Oberkochen, Germany), and images were made with a digital camera (Canon Powershot G6; Canon, Inc., Tokyo, Japan) to show accumulation of GFP after proteasome inhibition. For luciferase measurements, the cells were rinsed with PBS and lysed in 100 μl of reporter lysis buffer (Promega Corp.) on a rocking table for 15 min at room temperature (RT). The cell lysate was transferred to a tube and centrifuged for 30 s at 20,000 g. The supernatant was collected in a new tube and stored at −80°C. Per sample, 5 μl was measured for luciferase activity using the luciferase reporter assay system (Promega Corp.) and a luminometer (Berthold Technologies, Bad Wildbad, Germany).
RNA isolation, reverse transcription, and real-time quantitative PCR (Q-PCR)
For RNA isolation the cells were rinsed in PBS, scraped from the well plate in PBS, and centrifuged for 5 min at 1000 g. Supernatant was removed, and the pellet was stored at −20°C until RNA was isolated. Then 1 ml of TRIzol reagent (Invitrogen) was added to the cell pellet. Total RNA was isolated according to the manufacturer’s protocol. The resulting RNA pellet was dissolved in distilled water and stored at −80°C. The RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Subsequently, 2 μg of RNA was treated with DNase I (Invitrogen) and reverse-transcribed with SuperScript II RT (Invitrogen), according to the manufacturer’s protocol, using random hexanucleotides (Roche Diagnostics). The cDNA was stored at −20°C for later use in the Q-PCR reaction. Q-PCR was performed in 96-well plates, with a final volume of 20 μl/well using the SYBR Green PCR kit (Applied Biosystems, Inc., Foster City, CA, USA). Each reaction volume contained 10 μl of SYBR Green mix (2× concentrated), 6 μl of H2O, 1 μl of cDNA sample, and 3 μl of primer mix (sense and antisense primers, each 2 pmol/μl) (see Supplemental Table 1 for primer sequences). The plate was sealed and spun for 1 min at 200 g before the Q-PCR program was started with the following cycling conditions: 2 min at 50°C; 10 min at 95°C; 15 s at 95°C, and 1 min at 60°C for 40 cycles. After the amplification protocol a dissociation curve was constructed by ramping the temperature from 60 to 90°C. The resulting Ct values were converted to absolute amounts of cDNA present in the sample (E-Ct) (37). To correct for differences in cDNA amounts between samples, we normalized the target PCR to the geomean values of PCRs on a set of reference genes. Transcript levels of E2 ubiquitin ligase (E2Ubi), RNA polymerase II (RNA pol II), and 18S ribosomal RNA (18S) were most stable and were used for normalization.
3-(4,5-Dimethylthizol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cell viability assay
Cells were seeded onto 24-well plates and were treated with proteasome inhibitors or DMSO for the indicated time periods. Subsequently, culture medium was replaced by serum-free medium without phenol, and 50 μl of MTT solution (5 mg/ml in PBS) was added to each well and incubated at 37° for 4 h. After that, medium was removed, and 500 μl of DMSO was added to each well and incubated for 5–10 min to dissolve the formazan crystals. Two times 50 μl was transferred to a 96-well plate, and absorbance was measured at a wavelength of 570 nm on a microplate reader (Molecular Devices Corp, Sunnyvale, CA, USA).
Immunocytochemistry
U343 cells, cultured on gelatin-coated coverslips, were rinsed with PBS and fixed in 4% PFA in PBS. After fixation, the cells were rinsed again with PBS and incubated with Supermix (0.05 M Tris, 0.9% NaCl, 0.25% gelatin, and 0.5% Triton X-100, pH 7.4) for 10 min at RT. The cells were then incubated with anti-GFAP, anti-vimentin, anti-nestin, or anti-heat shock protein 70 (Hsp70) antibodies (see Table 1 for details) in Supermix overnight at 4°C. Subsequently, the cells were rinsed with Tris-buffered saline (TBS) (100 mM Tris-HCl, pH 7.4, and 150 mM NaCl) after incubation with Cy3- or Cy2-labeled secondary antibodies (1:400; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) in Supermix for 1 h at RT. Finally, the cells were rinsed 3 times with TBS and once with 0.05 M Tris and were coverslipped in Vectashield with DAPI (Vector Laboratories Inc.).
TABLE 1.
Detailed information on primary antibodies used in this study
| Primary antibody | Manufacturer | Catalog no. | Concentration |
|---|---|---|---|
| GFAP, rabbit polyclonal | DakoCytomation, Glostrup, Denmark | Z0334 | 1:2000 (ICC) |
| 1:50,000 (WB) | |||
| Vimentin, chicken polyclonal | Chemicon International Inc., Temecula, CA, USA | AB5733 | 1:2500 |
| Nestin, mouse monoclonal | Chemicon International Inc. | MAB5326 | 1:1500 |
| Hsp70, mouse monoclonal | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | sc-24 | 1:400 |
| NeuN, mouse monoclonal | Chemicon International Inc. | MAB377 | 1:250 |
| S100β, rabbit polyclonal | DakoCytomation | Z0311 | 1:6000 |
ICC, immunocytochemistry; WB, Western blot.
For immunostaining of the rat brain sections, blocking was performed by preincubation with 10% normal serum and 0.4% Triton X-100 in 0.05 M phosphate buffer (0.05 M Na2HPO4 and 0.05 M NaH2PO4·H2O, pH 7.4) for 1 h. Subsequently, the sections were incubated with the primary antibody diluted in 0.05 M phosphate buffer containing 3% normal serum and 0.4% Triton X-100. Incubations with the primary antibodies against GFAP, vimentin, S100β, neuronal nuclear protein (NeuN), and Hsp70 (see Table 1 for details) were carried out overnight at RT. Slides were rinsed and incubated with F(ab′)2-Cy3 secondary antibodies for 1 h (1 μg/ml; Jackson ImmunoResearch Laboratories). Sections were washed and coverslipped in Vectashield with DAPI (Vector Laboratories Inc.).
Both the U343 cells and rat brain sections were visualized on a Leica DMRE fluorescence microscope. Digital images were collected of the area around the implanted probes with identical settings for the areas around the perfused probe (experimental − control) and for the area around the contralateral nonperfused probe.
Western blot
Protein was isolated from U343 cells treated with epoxomicin or DMSO by homogenization with lysis buffer (0.1 M NaCl, 0.01 M Tris-HCl, pH 7.6, and 1 mM EDTA, pH 8.0) supplemented with a protease inhibitor cocktail (Roche Diagnostics). Cell lysates were sonicated twice for 10 s and centrifuged for 3 min at 20,000 g. Supernatant and pellet fraction were collected and dissolved in 2× or 1× loading buffer (2×: 100 mM Tris, 4% SDS, 20% glycerol, 200 mM DTT, and 0.006% bromphenol blue), respectively, and boiled for 5 min. Subsequently, they were run on a 7.5% SDS-PAGE gel and blotted semidry on nitrocellulose. Blots were probed overnight with polyclonal anti-GFAP (Table 1) in Supermix. The next day, the blots were washed with TBS-T (TBS with 0.2% Tween 20) and incubated with secondary antibody anti-rabbit IRDye800 in Supermix (1:5000; Rockland Immunochemicals for Research, Gilbertsville, PA, USA) for 1 h at RT. After 3 washes in TBS, bands were visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Quantification of GFAP protein levels was performed using Odyssey software (version 2.1) by measuring the integrated intensity with subtracted background of GFAP protein bands. These integrated intensities were divided by the total amount of protein in the samples, which was measured either with a Bradford assay (soluble protein) or by Coomassie gel quantification (insoluble protein). Protein percentages were calculated for epoxomicin-treated cells compared with the DMSO control.
Statistical analysis
To demonstrate whether statistically significant differences existed among different groups, the nonparametric Kruskal-Wallis test was used. For all target genes in the Q-PCR studies, the statistical analysis was performed on the normalized values.
RT array
Two plates of the RT2 Profiler PCR array for human transcription factors (PAHS-075 A; SuperArray Bioscience Corporation, Frederick, MD, USA) were used to compare Q-PCR validated cDNA samples of an overnight 100 nM treatment with epoxomicin and a vehicle control. cDNA equivalent to 1 μg of total RNA was used for each plate. The cDNA was mixed with the RT2 SYBR Green/ROX Q-PCR Master Mix, and 25 μl was added to each of the wells containing different primers. The plate was run under the same conditions as described above. The outcome was normalized against the set of reference genes used for the Q-PCR. Analysis using the references genes present on the array yielded a comparable outcome.
In silico analyses
We used Genomatix software (Genomatix Software GmbH, Munich, Germany; www.genomatix.de). The RefSeqs of GFAP, vimentin, and nestin (human) were used as input for the MatInspector, Gene2Promoter, and FrameWorker tools searching for promoter regions in individual transcripts and for promoter regions common to the three genes (38). The target sequence of GFAP was adjusted to 2163 bp upstream and 47 bp downstream of transcriptional start sites reflecting the promoter sequence of pGfa2-luc.
Animals and surgery
Experimentally naive male Wistar rats (Harlan/CPB-WU, body weight of 250–350 g at time of the experiment) were kept in a temperature- and humidity-controlled room. Animals were housed in Macrolon cages with sawdust bedding and had ad libitum access to food and water. Microdialysis probes were placed bilaterally in the prefrontal cortex (PFC) at an angle of 12°, as described in detail previously (39), or in the striatum at an angle of 0° (coordinates in millimeters from bregma: anterior +1.0, lateral +2.5, and ventral −6.0). The exposed membrane length of the Hospal AN69 dialysis membrane (outer diameter 0.32 mm in watery medium) was 4 mm for the PFC and 3 mm for the striatum. After surgery, a subcutaneous injection of 0.075 mg/kg buprenorphine (Temgesic; Schering-Plough, Reckitt Benckiser, UK) was given, and rats were housed separately.
Microdialysis
Microdialysis was started ≤7 d (n=10) or >7 d (n=4) after probe implantation. Only one of the two implanted probes was connected to a Tsumura (TCS 2-23) quartz-lined dual channel swivel (Pronexus, Skärholmen, Sweden) and to a perfusion pump using flexible PEEK (Upchurch Scientific, Oak Harbor, WA, USA), and all connections were made using small pieces of PVC tubing (inner diameter 0.38 mm; Watson-Marlow Inc., Wilmington, MA, USA). Before the experimental perfusions, the probes were perfused for 2 d with Ringer’s solution (145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 1 mM MgCl2). For the experimental procedure, the perfused Ringer’s solution contained either 0.025% DMSO or 250 nM epoxomicin-0.025% DMSO. Solutions were perfused at a rate of 2 μl/min, and when the flow of the first probe was found to be obstructed in attempting to start the microdialysis, the second probe was used. Animals were perfused continuously for 72 h. The flow from the outlet was collected to monitor whether the flow was uninterrupted. Animals did not show changes in their overall behavior during perfusion.
Tissue processing
After 72 h of perfusion, the animals were disconnected from the microdialysis system. Animals were anesthetized by an intraperitoneal injection of 1.0 ml of Nembutal and transcardially perfused with 150 ml of PBS, followed by 500 ml of freshly prepared 4% PFA in PBS at pH 7.2. After perfusion, the dental acrylic head mount including the microdialysis probes was pulled away from the skull. The brain was isolated, and a 5-mm-thick coronal slice was cut around the area with the probe position. The slice was cryoprotected overnight in PBS containing 15% sucrose, frozen on powdered dry ice, and stored at −80°C until sectioning. Serial 10-μm cryosections were cut and thaw-mounted on SuperFrost Plus slides. Sections were dried for 1 h and stored at −20°C. Immunocytochemistry was performed as described above.
RESULTS
IFs GFAP, vimentin, and nestin are expressed in U343 cells
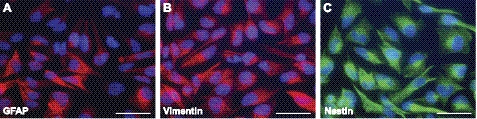
To investigate whether the U343 astrocytoma cell line is useful to study the effect of proteasome inhibition on GFAP and other IF proteins, the expression of IF proteins involved in astrogliosis was analyzed in these cells. Fluorescent staining clearly showed GFAP, vimentin, and nestin immunoreactivity (IR) in all U343 cells (Fig. 1). Although it was reported previously that U343 cells do not express nestin (40), nestin-positive cytoskeletal filaments were evident in this study, similar to GFAP- and vimentin-positive filaments. Moreover, Q-PCR showed the presence of nestin transcripts (Fig. 4D).
Figure 1.
Intermediate filament expression in U343 astrocytoma cells. The cytoskeletal distribution of GFAP (A), vimentin (B), and nestin (C) IR was clearly present in U343 cells. Scale bars = 50 μm.
Figure 2.
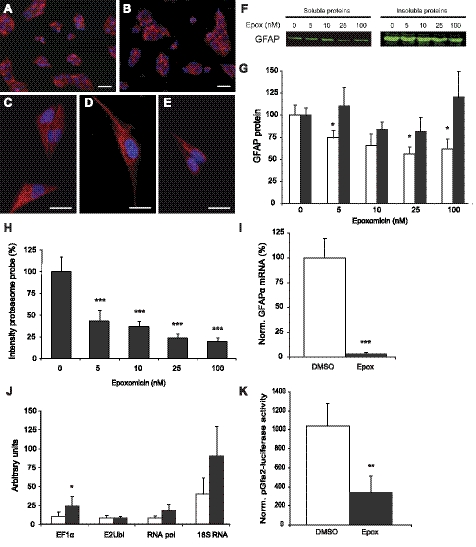
Epoxomicin in a concentration of 100 nM is sufficient to inhibit proteasome activity. A) Flow cytometric analysis of HeLa and SH-SY5Y cell lines stably expressing UbG76V-GFP, a proteasome reporter construct, showed that at a concentration of 100 nM epoxomicin, 95% of all cells accumulated the reporter, indicating a strong inhibition of proteasome activity (n=3 independent experiments). Data are presented as means ± se. B, C) U343 cells transfected with a proteasome reporter construct UbG76V-GFP. GFP was degraded without proteasome inhibition (B). After overnight treatment with 100 nM epoxomicin (epox), GFP accumulated in the cells (C). D, E) Fluorescent proteasome activity probe showed fluorescence in all U343 cells treated with DMSO (D) in contrast to U343 cells treated with 100 nM epoxomicin, which hardly showed fluorescence (E). This means that proteasome activity is strongly decreased after treatment with 100 nM epoxomicin. Scale bars = 100 μm (B, C); 50 μm (D, E).
Figure 3.
Proteasome inhibition showed no clear effect on protein levels but strongly decreased GFAP mRNA expression. A–E) GFAP staining in U343 cells. No clear difference was found in GFAP IR between U343 cells treated with DMSO (A) or 100 nM epoxomicin (B). Most cells showed a GFAP cytoskeleton throughout the cytoplasm (C, D); however, in some 100 nM epoxomicin-treated cells, the GFAP cytoskeleton was less pronounced (E). Scale bars = 50 μm (A, B); 25 μm (C–E). F) Example of a Western blot analysis of lysates from U343 cells treated with a range of epoxomicin (epox) concentrations. Soluble and insoluble fractions were blotted separately and probed for GFAP. G) Western blot quantification showed a decrease in GFAP protein levels in soluble protein fraction (□) when treated with various concentrations of epoxomicin; 5, 25, and 100 nM resulted in a significant decrease (P≤0.05) (n=4 for 25 nM; n=5 for all other concentrations). No significant changes in GFAP protein levels were found in insoluble protein fractions (▪) (n=5 for all concentrations). H) Increased epoxomicin concentration resulted in a dose-dependent decrease in proteasome activity when quantified with a fluorescent proteasome activity probe. Activity was decreased to 20% of control values with 100 nM epoxomicin (n=4). I) Normalized GFAP mRNA levels measured with quantitative RT-PCR. Overnight treatment with 100 nM epoxomicin resulted in a clear and significant decrease of GFAP mRNA levels (P≤0.001) to ∼3.5% of control values (n=8). J) Arbitrary units representing mRNA levels of four reference genes showed no significant decrease after overnight proteasome inhibition with 100 nM epoxomicin (n=14). EF1α mRNA levels were significantly increased. E2Ubi, RNA pol II, and 18S mRNA levels were used to normalize mRNA levels of other genes in other experiments. K) Luciferase activity measurement from U343 cells transfected with a construct of luciferase coupled to the human GFAP promoter (pGfa2-luc). Proteasome inhibition with 100 nM epoxomicin for 16 h significantly decreased luciferase activity (P≤0.009, n=8). □, DMSO-treated U343 cells; ▪, U343 cells treated with 100 nM epoxomicin for 16 h (I–K). Data are presented as means ± se between individual experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Norm., normalized.
Figure 4.
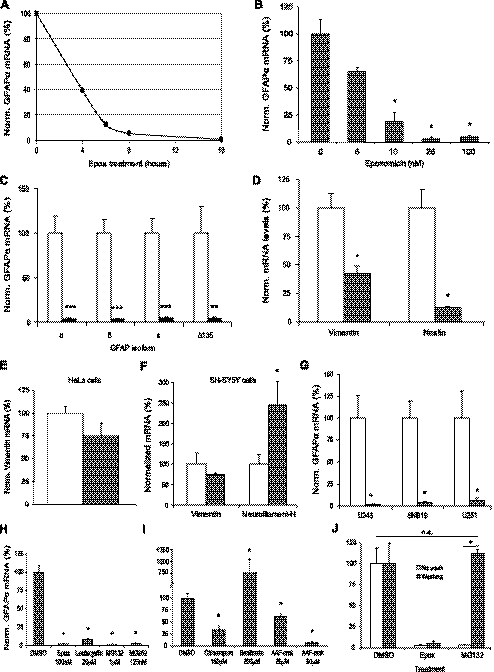
Specificity of GFAP mRNA down-regulation. A) Decrease in GFAP mRNA is time-dependent with respect to epoxomicin (epox) treatment. After 4 h of epoxomicin treatment, GFAP mRNA levels have already decreased to ∼40% compared with those in untreated U343 cells (n=1). B) Decrease in GFAP mRNA is dependent on concentration of epoxomicin used. GFAP mRNA is already significantly decreased when 10 nM epoxomicin is used, and GFAP mRNA levels decrease further to <5% of untreated cells with epoxomicin concentrations of 25 nM or higher (n=4). C) Transcript levels of all GFAP isoforms expressed in U343 cells were significantly down-regulated after proteasome inhibition (n=8 for GFAP-α, -δ, and -κ; n=5 for GFAPΔ135). D) Vimentin and nestin mRNA levels were significantly down-regulated by proteasome inhibition with 100 nM epoxomicin in U343 cells to 43 and 13% of control values, respectively (n=5). E) In HeLa cells, vimentin mRNA levels were not significantly decreased by proteasome inhibition with 100 nM epoxomicin (n=3). F) In SH-SY5Y neuroblastoma cells, vimentin mRNA levels were not significantly decreased by proteasome inhibition with 100 nM epoxomicin. NFH mRNA levels in this cell line were significantly up-regulated to ∼250% when treated with 100 nM epoxomicin, compared with untreated cells (n=3). G) GFAP mRNA levels significantly decrease after proteasome inhibition with 100 nM epoxomicin in three different astrocytoma cell lines (n=4). C–G) □, DMSO-treated U343 cells; ▪, U343 cells treated with 100 nM epoxomicin for 16 h. H) Four different proteasome inhibitors, both irreversible (epoxomicin and lactacystin) and reversible (MG132 and MG262) significantly decreased GFAP mRNA levels in U343 cells after treatment overnight (n=3). I) Inhibitors of other proteolytic activities present in U343 cells significantly up-regulated (bacitracin) or down-regulated (chloroquine, AAF-cmk) GFAP mRNA levels (n=3). J) Down-regulation of GFAP mRNA levels after proteasome inhibition was reversible. GFAP mRNA levels significantly increased to ∼100% when medium containing a reversible proteasome inhibitor (MG132) was replaced for medium without inhibitor (n=3). With irreversible inhibitor, epoxomicin GFAP mRNA levels remained low. □, unwashed cells; ▪, washed-out cells. Data presented means ± se between individual experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Norm., normalized.
Inhibition of the proteasome by epoxomicin
The optimal concentration of the proteasome inhibitor epoxomicin needed to inhibit the activity of the proteasome was determined in HeLa and SH-SY5Y cell lines stably transfected with UbG76V-GFP, a reporter for proteasomal activity. UbG76V-GFP is normally targeted to the proteasome for degradation, but when the proteasome is inhibited, the fusion protein is no longer degraded, and the GFP accumulates. Flow cytometric analysis of these cell lines treated for 16 h with various concentrations of epoxomicin showed that in both cell lines 100 nM was sufficient to almost completely inhibit the proteasome activity (Fig. 2A). In addition, this concentration was shown to sufficiently inhibit the proteasome in U343 astrocytoma cells, because all U343 cells transfected with the UbG76V-GFP proteasome reporter construct (−50% transfection efficiency) accumulated GFP when treated with 100 nM epoxomicin (Fig. 2B, C). In addition, we used a fluorescent probe (34) that specifically binds to the active proteolytic sites of the proteasome. The labeling observed after overnight DMSO treatment (Fig. 2D) was almost completely absent after overnight treatment with 100 nM epoxomicin (Fig. 2E), indicating that the core proteasome proteolytic activity became inactive because of epoxomicin treatment. The absence of probe labeling was not the result of cell death, as indicated with an MTT assay (Fig. 5C).
Figure 5.
No cell death, but increased heat shock protein expression after proteasome inhibition overnight. A, B) DAPI staining did not show any increase in nuclear condensation after proteasome inhibition with 100 nM epoxomicin (epox) for 16 h (B) compared with DMSO-treated U343 cells (A). C) An MTT assay revealed no significant changes in cell viability after treatment with proteasome inhibitors epoxomicin (n=4) and lactacystin (n=3) for 16 h, compared with DMSO-treated cells. D) Hsp70 mRNA levels were significantly increased after overnight treatment with proteasome inhibitors epoxomicin (100 nM), lactacystin (20μM), MG132 (1 μM), and MG262 (125 nM) (n=3). E, F) Hsp70 IR was clearly increased after 100 nM epoxomicin treatment (F) compared with DMSO treatment (E). G) Hsp70 levels increased significantly when lactacystin concentrations exceeded 20 μM. Because GFAP transcript levels were already maximally reduced at 20 μM, the induced stress response does not seem to influence the effect on GFAP mRNA (n=1). Scale bars = 50 μm. Data represent means ± se between individual experiments. *P ≤ 0.05. Norm., normalized.
Proteasome inhibition in U343 cells resulted in a strong reduction of GFAP mRNA
Next we determined the effect of proteasome inhibition on GFAP protein levels. The U343 cells were treated overnight with 100 nM epoxomicin or 0.01% DMSO as a control and were immunostained for GFAP. No clear change in GFAP IR was observed between DMSO- and epoxomicin-treated cells (Fig. 3A, B). At greater magnification, a clear filamentous network was visible throughout the cytoplasm in the DMSO-treated cells (Fig. 3C). In the 100 nM epoxomicin-treated cells this was also seen in many cells (Fig. 3D), although the GFAP network often appeared less pronounced (Fig. 3E). We quantified the changes in GFAP protein levels using Western blot. It is known that the turnover of filament proteins such as GFAP shows a biphasic decay, including a fast-decaying pool with a half-life of 12–18 h and a slow-decaying pool exhibiting a half-life of ∼8 d (41). Most likely, this fast-decaying pool contains the soluble forms of these proteins, whereas the slow-decaying pool consists of the filamentous, insoluble forms. To distinguish between these two assembly states, a soluble supernatant fraction and an insoluble pellet fraction were loaded onto the gel. Quantification of these Western blots, an example of which is presented in Fig. 3F, showed a slight decrease in relative GFAP protein levels in the soluble fraction (Fig. 3G, □), which was significant for 5 nM (75%, P≤0.047), 25 nM (56%, P≤0.037), and 100 nM epoxomicin (63%, P≤0.028). No significant changes were found in the insoluble fraction after 16 h (Fig. 3G, ▪), which was in line with our expectations, considering the long half-life of insoluble filamentous IF proteins. The relative proteasome activity in U343 cells treated with the concentrations of epoxomicin used in the Western blot experiment was quantified using the fluorescent proteasome activity probe (Fig. 3H), which confirmed that proteasome activity gradually decreased with increasing concentrations.
Because proteasomal activity is involved in the regulation of gene expression (24,25,26), we studied the effect of epoxomicin on GFAP mRNA levels. Relative amounts of GFAP mRNA were measured with quantitative RT-PCR after overnight treatment with 100 nM epoxomicin. Remarkably, GFAP transcript levels were profoundly decreased after proteasome inhibition down to less than 5% of the levels in the control condition (Fig. 3I; P≤0.001). For comparison, transcript levels of several reference genes that are not related to IFs were measured in the same samples (Fig. 3J). Epoxomicin treatment had no effect on the expression level of E2Ubi, RNA pol II, and 18S, although elongation factor 1α (EF1α) was significantly up-regulated (P≤0.02). These results indicate that either GFAP mRNA stability or GFAP gene expression is strongly regulated by proteasome activity.
To investigate whether the effect of proteasome inhibition on the GFAP mRNA levels is caused by a direct regulation of the GFAP promoter, U343 cells were transfected with a construct driving the luciferase gene under the human GFAP promoter (pGfa2-luc). Renilla luciferase was used as an internal control, because proteasome inhibition is known to decrease the activity of luciferase in general (42). The luciferase activity measured from pGfa2-luc after normalization with the Renilla luciferase was significantly decreased by proteasome inhibition (Fig. 3K) (P≤0.008). This result showed that the GFAP promoter after proteasome inhibition exhibits less than one third of its activity compared with the control condition. All together, these data strongly indicate that proteasome inhibition results in a rapid decrease in GFAP transcription, as a result of a less active promoter.
Specificity of GFAP mRNA down-regulation
The strong reduction of the GFAP transcript levels was studied in more detail. GFAP mRNA was measured at different time points after epoxomicin treatment, and the effect of different concentrations of epoxomicin was studied. The decrease in GFAP mRNA was dependent on the duration of treatment (Fig. 4A). After 4 h of 100 nM epoxomicin treatment, the GFAP mRNA levels were decreased to 40%, whereas an overnight (16 h) treatment resulted in a nearly complete down-regulation of GFAP mRNA. In addition, the GFAP mRNA down-regulation was also shown to be epoxomicin concentration-dependent (Fig. 4B). A concentration of 5 nM epoxomicin already showed a drop in GFAP mRNA, and a significant decrease in GFAP mRNA levels was reached when concentrations of 10 nM epoxomicin (P≤0.021) or higher were applied for 16 h. GFAP mRNA levels were decreased down to ∼5% of control levels with 25 nM (P≤0.019) and 100 nM (P≤0.021) epoxomicin.
Multiple splice variants are derived from the GFAP gene (43), i.e., GFAPα, GFAPΔ135, GFAPΔ164, GFAPΔexon6 (5), GFAPδ (6, 7), and GFAPκ (4). We investigated whether proteasome inhibition altered the GFAP splicing pattern in U343 cells. GFAPδ mRNA and protein were shown previously to be clearly expressed in U343 IF networks (44); the GFAPΔ164/GFAPΔexon6 proteins (recognized by a GFAP+1 antibody) (5), however, are not expressed (data not shown). The mRNA expression of the GFAP isoforms was investigated after proteasome inhibition. The GFAPΔ164 and GFAPΔexon6 mRNA could not be detected in U343 cells (not shown), which is in accordance with their absence at the protein level. GFAPα, GFAPδ, GFAPκ, and GFAPΔ135 mRNA levels were decreased significantly (Fig. 4C), and the ratio between the different isoforms remained constant (not shown), indicating that overnight epoxomicin treatment did not change GFAP splicing.
Because GFAP is not the only IF protein present in U343 cells, we tested whether other IF transcripts involved in gliosis were also decreased upon proteasome inhibition. Q-PCR revealed a significant decrease of both vimentin to ∼43% of the level found in controls and nestin mRNA levels to ∼13% after overnight epoxomicin treatment (Fig. 4D) (P≤0.017). This result demonstrates that the proteasomal activity regulates the mRNA levels of all IFs involved in gliosis in U343 cells with the down-regulation of GFAP to levels less than 5% being most pronounced. Vimentin is an IF protein that is present in many cell lines. We tested whether vimentin down-regulation on proteasome inhibition was specific for astrocytoma cells or present in other cell lines as well. A Q-PCR for vimentin was performed on HeLa cells (Fig. 4E) and SH-SY5Y neuroblastoma cells (Fig. 4F), in which the neuron-specific IF protein neurofilament-H (NFH) was also tested. These experiments showed no significant down-regulation of mRNA expression of any of the genes tested, although a small decrease in vimentin mRNA was detected in both cell lines and a significant up-regulation of NFH to 244% was measured in the neuroblastoma cells (P≤0.05). To exclude the possibility that the effect of proteasome inhibition on IF transcription was present solely in U343 astrocytoma cells, a Q-PCR was performed on astrocytoma cell lines U251 and SNB19 (Fig. 4G). Although the overall GFAP expression level was lower in these cells compared with that in U343 cells (data not shown), a significant down-regulation was found after proteasome inhibition in both astrocytoma cell lines (P≤0.021). This result indicates that the regulation of IF transcription through proteasome inhibition is astrocyte-specific.
Next we addressed specificity aspects of the observed effect of epoxomicin on GFAP mRNA levels. U343 cells were treated with several other proteasome activity inhibitors (Fig. 4H) and inhibitors of other proteolytic enzymes (Fig. 4I). Overnight treatments with lactacystin, MG132, and the boronate analog of MG132, MG262, all resulted in a clear and significant reduction of GFAP mRNA comparable to the effects observed with epoxomicin. U343 cells were treated with either chloroquine, an inhibitor of lysosomal degradation, bacitracin, an inhibitor of insulin-degrading enzyme, or AAF-cmk, an inhibitor of TPPII (Fig. 4I). Chloroquine and AAF-cmk treatment both showed a significant down-regulation of GFAP mRNA to ∼33% of control levels (P≤0.05), but the reduction was not as profound as that of the proteasome inhibitors (P≤0.05). In contrast, treatment with bacitracin resulted in a significant increase of GFAP mRNA to ∼760% (P<0.05). In conclusion, any effect on GFAP mRNA levels found with other proteases is much smaller or opposite from the effect of the proteasome inhibitors.
Epoxomicin is known to be an irreversible inhibitor of the proteasome, whereas the inhibitory effect of MG132 is reversible. Cells were treated overnight with either epoxomicin or MG132, after which the culture medium was refreshed with medium without inhibitors. Cells were harvested 24 h later, and RNA was isolated (Fig. 4J). The epoxomicin-treated cells showed a significant and irreversible decrease in GFAP mRNA expression, whereas washout of the reversible inhibitor MG132 caused the significantly decreased levels of GFAP mRNA (P≤0.046) to recover to levels not different from control levels. This result is in agreement with the pharmacological profile of the two inhibitors (45, 46). Taken together, these results clearly show that proteasome inhibition is involved in the decreased GFAP mRNA expression.
Induced cell death and stress response are not responsible for the decrease in IF mRNA expression
Proteasome inhibition is known to cause an up-regulation of heat shock proteins, including Hsp70 (47, 48), and in the long term to induce cell death (49). We studied whether these effects of proteasome inhibition in U343 astrocytoma cells could be responsible for the effect on the GFAP mRNA expression. Cell death after proteasome inhibition can be detected by studying nuclear condensation, a classic feature of apoptosis. In U343 cells treated with 100 nM epoxomicin overnight, no evident condensed nuclei were observed with DAPI staining (Fig. 5A, B). To quantify the viability of the cells, an MTT assay was performed after treatment with the proteasome inhibitors epoxomicin and lactacystin. No significant reduction of cell viability was observed after an overnight treatment with either proteasome inhibitor (Fig. 5C).
To investigate whether heat shock proteins were induced, Q-PCR with Hsp70-specific primers was performed on cDNA samples from previous experiments. As expected, a highly significant up-regulation of Hsp70 mRNA levels was found after proteasome inhibition with several proteasome inhibitors (Fig. 5D) (P≤0.05). We could confirm this effect at the protein level; no Hsp70 IR could be detected in DMSO-treated cells in contrast to epoxomicin-treated cells (Fig. 5E, F). Proteasome inhibition with lactacystin resulted in a remarkably lower up-regulation of Hsp70 mRNA than that with other proteasome inhibitors (Fig. 5D), whereas lactacystin was as efficient in reducing the GFAP mRNA expression as epoxomicin (Fig. 4H). In addition, a concentration range of lactacystin showed that at a concentration of 10 μM GFAP mRNA levels were substantially decreased, and Hsp70 mRNA levels were not increased yet (Fig. 5G), showing that an increase in Hsp70 transcript levels or a more general heat shock response is not likely to be the cause for the decrease in GFAP transcript levels. Moreover, we measured the expression of BiP, an important component of the endoplasmic reticulum stress response. Although BiP mRNA levels were significantly increased by tunicamycin treatment, no change was found after epoxomicin treatment (data not shown), which indicates that endoplasmic reticulum stress was not induced.
Various transcription factors are regulated by the proteasome in U343 cells
The cell culture studies have provided extensive evidence for the specificity of proteasome inhibition on GFAP transcription in U343 astrocytoma cells. Because it has become more evident that the proteasome has a fundamental role in regulating transcription, partly by regulating transcription factor half-life (24, 50), we investigated whether proteasome inhibition regulates (astrocytoma) specific transcription factors that could alter IF transcription in these cells.
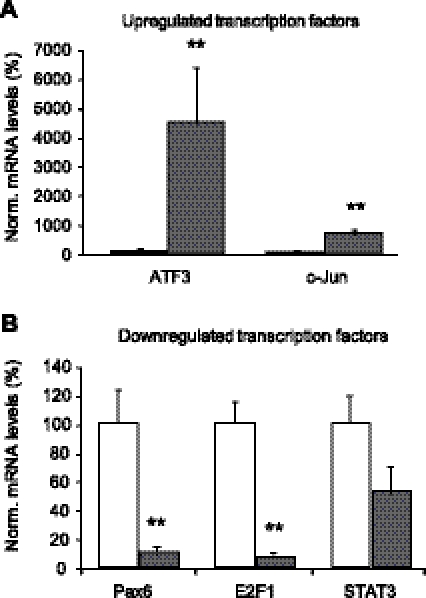
To study whether proteasome inhibition has a general effect on the levels of transcription factors we used an RT-PCR array to assess the 100 nM epoxomicin-induced changes in transcript levels of 84 transcription factors implicated in various regulatory pathways. A total of 76 transcription factors could be detected in U343 cells; 20 showed an up-regulation of >1.5-fold and 20 showed a down-regulation of >1.5-fold (see Supplemental Table 2 for RT-PCR array results). We validated the changes in expression for several transcription factors for which a relation with the proteasome (51,52,53) or involvement in GFAP expression or astrocyte biology was known (54,55,56,57). The changes for up-regulated ATF3 and c-Jun transcript levels and down-regulated PAX6, E2F1, and STAT3 transcript levels were confirmed by Q-PCR on a set of five DMSO-treated and five epoxomicin-treated samples (Fig. 6A, B). STAT3 mRNA, which showed a decrease of 1.7-fold in the PCR array, was down-regulated in the epoxomicin-treated samples; however, this result was not significant. These results suggest that many transcription factors may be involved in the alteration of GFAP promoter activity.
Figure 6.
Proteasome inhibition regulates transcription factors that influence GFAP transcript levels via its promoter. A) Q-PCR results validate significant up-regulation of transcription factors ATF3 and c-Jun (P≤0.009, n=5), which was shown in RT array (Supplemental Table 2). B) Q-PCR results validate significant down-regulation of transcription factors PAX6 and E2F1 (P≤0.009, n=5). STAT3 was also down-regulated; however, this result was not statistically significant. Data represent means ± se between individual experiments. **P≤0.01. Norm., normalized.
To more specifically identify which transcription factors can bind the GFAP promoter as well as the vimentin and nestin promoter, we performed an in silico analysis of the promoter regions of GFAP, vimentin, and nestin to unravel a common transcription binding profile for these three genes (Genomatix Software GmbH). Using the GFAP promoter sequence as target, MatInspector revealed 456 binding sites for transcription factors. Restricting the target input to the promoter region present in the pGfa2-luc revealed 106 potential transcription factors from 58 different transcription factor families. Because proteasome inhibition also reduced the transcript levels of vimentin and nestin, a functional assessment of binding sites was carried out by the Gene2Promoter and FrameWorker tools to find common elements. Gene2Promoter identified 20 transcription factor binding sites that are present in GFAP, vimentin, and nestin. Applying the “tissue filter” nervous system reduced this number to binding sites for six transcription factor families: V$CREB (n=14 different transcription factors), V$EGRF (n=7), V$ETSF (n=16), V$NR2F (n=12), V$PARF (n=5), and V$PAX6 (n=5). Altogether there were 59 different transcription factors, so even under stringent settings the number of potential candidates remains high and does not allow drawing of conclusions as to which transcription factors play a key regulatory role.
Astrogliosis is decreased after proteasome inhibition in vivo
Because filamentous GFAP and other IF proteins have a half-life of ∼8 d, only longer periods of reduced mRNA levels will have substantial effects on the existing IF network. Treatment of U343 cells with high concentrations of proteasome inhibitors for >3 d is toxic to the cells, whereas low concentrations did not lead to impaired proteasome activity (data not shown). Therefore, we studied the effects of chronic proteasome inhibition on GFAP protein levels in a rat model for induced astrogliosis. It is known that in rats the implantation of a microdialysis probe in the hippocampus causes buildup of gliosis in an area around the probe after 24–48 h, reducing the drainage. This layer of GFAP IR and astrocytic hypertrophy is most prominent after 72 h but does not progress after that (58, 59). We made use of this model of astrogliosis by perfusing the microdialysis probe, implanted in either the PFC or striatum, with epoxomicin. This approach enables the delivery of the proteasome inhibitor precisely at the core of the astrogliosis. Five days after implantation, GFAP-positive reactive astrocytes were observed around the control probe that was perfused with 0.025% DMSO in Ringer’s solution for 72 h (result not shown), comparable to the contralateral nonperfused probe (Fig. 7A, B). In contrast, around the probe perfused with 250 nM epoxomicin (in DMSO-Ringer’s solution) for 72 h, a clear reduction in GFAP IR was observed (Fig. 7C, D). Only a few filaments and “squiggles” (60) were labeled by the GFAP antibody (Fig. 7C, arrowheads). The reduction in GFAP IR was most notably in a zone up to 300 μm distant from the probe. Beyond this zone, GFAP-positive astrocytes appeared normal, indicating the extent of the epoxomicin diffusion in these conditions. These results show that inhibition of the proteasome can prevent the increase in GFAP levels associated with astrogliosis. To substantiate this result, we stained adjacent sections for vimentin, also known to be up-regulated in astrogliosis. Vimentin IR clearly showed a rim of reactive astrocytes around the DMSO and nonperfused probe position (Fig. 7E) but was virtually absent when the probe was perfused with epoxomicin (Fig. 7F). In a number of experiments, the start of the probe perfusion with epoxomicin was delayed to >7 d after probe implantation. In these brains, no clear effect on the expression of GFAP or vimentin was observed between the nonperfused and epoxomicin-perfused probe site with either GFAP or vimentin staining (results not shown), suggesting that at this point gliosis had already progressed to a stage at which IF gene expression was not susceptible to epoxomicin anymore. To prove that the reduction in GFAP and vimentin IR is not due to astrocyte cell death, the sections were stained for S100β, another marker for mature astrocytes specific for a family of calcium-binding proteins (Fig. 7G, H). No evident differences were found between the nonperfused and epoxomicin-perfused probe site. S100β IR was clearly visible in astrocytes around the epoxomicin-perfused probe site, similar to the control site. To show that neurons in the area around the probe were not affected by epoxomicin, NeuN staining was performed. A similar number of neurons could be detected around both the nonperfused and the epoxomicin-perfused probe (Fig. 7I, J). Immediately surrounding the probe site, neurons were completely absent in both the epoxomicin-perfused and nonperfused conditions (Fig. 7I, J, arrowheads).
Figure 7.
Proteasome inhibition decreased astrogliosis in rat brain. Rat brains were perfused with a microdialysis probe into PFC or striatum. Brains were isolated after 72 h of perfusion starting ≤7 d after probe implantation. Probes were either left nonperfused or were perfused with 250 nM epoxomicin (n=7). Images are from a rat that was perfused for 72 h with epoxomicin, starting 4 d after probe implantation into PFC. Dotted lines indicate area where probe was located. Images are rotated for presentation purposes, placing the midline below the probe. Results were comparable among all rats studied. A) GFAP IR showed astrogliosis around area where nonperfused microdialysis probe was located. Arrows indicate area of astrogliosis around probe site. B) At higher magnification, reactive GFAP-positive astrocytic processes are clearly visible. C) No astrogliosis was found around epoxomicin-perfused probe and only a little GFAP IR is seen in several filaments and “squiggles” (arrowheads). Cell layers further away from probe looked normal. D) At higher magnification, no clear GFAP-positive astrocytic processes are visible around epoxomicin-perfused probe site. Only a punctate staining pattern is present, indicating loss of GFAP cytoskeletal fibers. E) Vimentin IR also showed astrogliosis around nonperfused probe site, similar to GFAP. Asterisk marks area that looks normal, without astrogliosis. F) Vimentin IR was clearly decreased after epoxomicin perfusion around probe site, as with GFAP. G, H) S100β clearly stained astrocytes around nonperfused (E) and epoxomicin-perfused (F) probe sites. I, J) NeuN staining showed normal-looking neurons located around both nonperfused (I) and epoxomicin-perfused (J) probe site. Immediately adjacent to probe site, NeuN-negative band was present in both conditions, as indicated by dashed line. K) Hsp70 IR was not detected around nonperfused probe site. L) Hsp70 IR was clearly up-regulated around epoxomicin-perfused site, indicating area of brain that was penetrated by epoxomicin. Scale bars = 100 μm (A, C, E–L); 50 μm (B, D).
Hsp70 IR, which was highly up-regulated after epoxomicin treatment in vitro, was also greatly enhanced in the epoxomicin-perfused rat brain in all cell types present in that region (Fig. 7L). Hardly any Hsp70 was detected around the nonperfused probe site (Fig. 7K), whereas a clear zone of Hsp70 IR cells was visible around the epoxomicin-perfused probe site overlapping with the reduced GFAP staining. This ring may mark the area of epoxomicin diffusion from the probe into the rat brain. Although heat shock proteins are known to be up-regulated at sites of inflammation, no signs of increased inflammation were detected with a CD11b antibody around the epoxomicin-perfused probe site compared with the control site (not shown). These results suggest that proteasome inhibitors in vivo can locally prevent GFAP and vimentin protein accumulation without obvious damage to the surrounding tissue.
DISCUSSION
Astrogliosis and impaired proteasome activity both occur in the aging brain and in neurodegenerative diseases, such as AD and PD. This occurrence has led us to speculate that the UPS might contribute to the regulation of astrogliosis, for instance, in the regulation of IF components such as GFAP. In this study we show that in contrast to our expectations, proteasome inhibition does not induce an accumulation of GFAP protein but rather induces a sharp decrease in the transcript levels of all expressed GFAP isoforms and other cytoplasmic IF protein transcripts in human astrocytoma cells in vitro. In line with these findings, we observed that proteasome inhibition prevents the accumulation of GFAP and vimentin in reactive astrocytes in the rat brain in vivo, thereby suppressing reactive gliosis.
The reduction of GFAP gene expression as a result of proteasome inhibition is highly significant and specific for astrocytes. Biochemically distinct proteasome inhibitors decreased GFAP mRNA levels to less than 5% of the control levels. Several inhibitors of other proteolytic enzymes also affected GFAP transcript levels (Fig. 4H, I), but the effect was far less pronounced than that with epoxomicin and other proteasome inhibitors. The small but significant down-regulation by chloroquine, an inhibitor of lysosomal degradation, could be explained by recent findings from Sprangers et al.(61), who showed in eukaryotic cell extracts and in preparations of purified 20S archaeal proteasomes that chloroquine can inhibit proteasome function as well. Another explanation could be that lysosomes also regulate factors important for GFAP transcription but to a lesser extent. The significant effect by the TPPII inhibitor AAF-cmk was less unexpected, because it is known that TPPII may substitute for some proteasome functions (62).
Previous studies in aging rodent and human brains have pointed out that increased GFAP protein could result from either transcriptional or post-transcriptional regulation (8, 63,64,65). The results of the pGfa2-luc experiment (Fig. 3K) and the RT-PCR array (Supplemental Table 2) show that the proteasome alters the transcription of GFAP and other IF proteins, most likely by affecting the levels of specific transcription factors that act on the promoter. Our study is one of a few that link proteasome activity with transcription in mammalian cells, and we suggest that this is achieved by regulation of transcription factor levels (50, 66,67,68,69). By using the Genomatix Web tool, potential transcription factors were revealed that could act on the promoters of different IF genes at the same time, which might occur during astrogliosis when GFAP, vimentin, and nestin are up-regulated. This analysis revealed 6 potential transcription factor families that cover in total 59 different transcription factors. Four of these transcription factors (CREB, c-Jun, EGR-1, and PAX6) were indeed found to be regulated by the proteasome, as was shown by the RT-PCR array (Supplemental Table 2). The most interesting transcription factor is EGR-1, which was strongly decreased on proteasome inhibition (17-fold). This regulation by the proteasome corresponds to previous studies, which showed that EGR-1 can regulate the proteasome and vice versa(70,71,72). Furthermore, various studies suggest a relation between increased EGR-1 expression and increased GFAP expression (73, 74) and a role in astrogliosis (75). However, it needs to be emphasized that the promoter sequences contain many putative binding sites for a large number of transcription factors. Clearly, further investigation is needed to unravel which transcription factors are involved in IF transcription in astrogliosis.
The significant down-regulation of GFAP expression after proteasome inhibition was not in line with our expectations. In diseases in which impaired proteasome activity is confirmed, GFAP protein levels are generally up-regulated in reactive astrocytes (15). The decrease in proteasome activity in AD described by others, however, was measured in whole brain homogenates (12, 76, 77), without discriminating between the different cell types present. Moreover, ubiquitin immunoreactivity was found mostly in inclusion bodies within neurons (78,79,80) but not in astrocytes. Therefore, it could be argued that in these diseases the proteasome is not inhibited in astrocytes but might rather be activated. Tentatively, a more active proteasome in astrocytes may indicate that in AD these cells are trying to degrade Aβ, the main constituent of amyloid plaques in the brains of patients with AD (81,82,83). Evidence for this line of reasoning has to come from animal models with astrocyte-specific proteasome activity impairment. Moreover, our results put studies using systemic administration of proteasome inhibitors in a new perspective. For example, it has been reported that systemic administration of epoxomicin in rats can induce a model of PD (84). Although attempts to replicate this model have not always been successful, no attention has gone to astrocytes, which are likely to also play a role in the disease process. In our opinion, it would be important to investigate neuron-specific proteasome inhibition in animal models to study the role of the UPS in neurodegenerative diseases.
The profound down-regulation of the transcript levels of GFAP, vimentin, and nestin by proteasome inhibitors offers a new approach to impede astrogliosis, which may be beneficial for certain disease situations. Studies using GFAP−/−Vim−/−-knockout mice (85), a model for reduced astrogliosis, show that reactive astrocytes are a main component in brain injury, stroke, neurodegeneration, and the physiological aging of the brain. There is ongoing debate whether reactive gliosis is beneficial or detrimental to the surrounding neurons (86, 87). As it is increasingly being recognized that astrocytes not only provide a general support to neurons but are also essential for synaptic transmission and synchronizing neuronal signals, it may be that the transition from quiescent astrocytes to reactive cells may affect their normal functional role and hence be damaging to neurons (88,89,90). In agreement with such a detrimental role, the hypertrophy of astrocytes was shown to coincide with cognitive decline, implying that reactive gliosis negatively influences cognition (11, 91). GFAP−/−Vim−/− mice and cultured astrocytes from these mice have shown that astrocytes without IF proteins show improved axonal sprouting and functional recovery after spinal cord injury (92) and improved synaptic regeneration in mice conducted with entorhinal cortex lesions (93). Furthermore, the retinal environment in GFAP−/−Vim−/−-knockout mice was more permissive for retinal transplants compared with that in wild-type mice (94), and in old GFAP−/−Vim−/−-knockout mice, cell proliferation and neurogenesis in the hippocampal dentate gyrus were increased (95). The beneficial effects of reactive gliosis appear to be limited to the acute phase of brain injury (96, 97).
Taken together, these findings indicate that inhibition of proteasomal activity may be a method to reduce astrogliosis and could serve as a potential therapy to treat CNS injuries in the future. Our research proposes that novel CNS-penetrant proteasome inhibitors could be considered as a treatment for astrogliosis, particularly in conditions in which gliosis prevents recovery of neuronal function. Notably, the proteasome inhibitor bortezomib has been registered for treatment of multiple myeloma (98) as a first-in-class drug and is currently also being investigated in clinical trials as a potential candidate in the treatment of gliomas (99). Because proteasome inhibitors could also cause negative effects in other cell types surrounding reactive astrocytes, it would be beneficial to study the mechanism behind IF protein regulation in greater detail to find more specific targets for the development of therapeutic agents.
Supplementary Material
Acknowledgments
This research was supported by the Hersenstichting Nederland (13F05.08 to E.M.H.) and the Internationale Stichting Alzheimer Onderzoek (04511 to E.M.H.). We thank J. Rutka (University of Toronto, Toronto, ON, Canada) for the U343 glioblastoma cells; B. de Leeuw (Erasmus University Medical Center, Rotterdam, The Netherlands) for kindly providing us with U251 and SNB19 glioblastoma cells; O. Meijer (Leiden University Medical Center, Leiden, The Netherlands) for the Renilla construct; and M. Brenner (University of Alabama at Birmingham, Birmingham, AL, USA) for the pGfa2-luciferase construct. We thank Lourens Nonkes, Laura Koster, and Rick Wassing for their technical assistance during cell culture, flow cytometric analysis, and animal surgery, respectively.
References
- Cotrina M. L., Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- Borrett D., Becker L. E. Alexander’s disease A disease of astrocytes. Brain. 1985;108(Pt. 2):367–385. doi: 10.1093/brain/108.2.367. [DOI] [PubMed] [Google Scholar]
- Duckett S., Schwartzman R. J., Osterholm J., Rorke L. B., Friedman D., McLellan T. L. Biopsy diagnosis of familial Alexander’s disease. Pediatr Neurosurg. 1992;18:134–138. doi: 10.1159/000120652. [DOI] [PubMed] [Google Scholar]
- Blechingberg J., Holm I. E., Nielsen K. B., Jensen T. H., Jorgensen A. L., Nielsen A. L. Identification and characterization of GFAPκ, a novel glial fibrillary acidic protein isoform. Glia. 2007;55:497–507. doi: 10.1002/glia.20475. [DOI] [PubMed] [Google Scholar]
- Hol E. M., Roelofs R. F., Moraal E., Sonnemans M. A., Sluijs J. A., Proper E. A., de Graan P. N., Fischer D. F., van Leeuwen F. W. Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol Psychiatry. 2003;8:786–796. doi: 10.1038/sj.mp.4001379. [DOI] [PubMed] [Google Scholar]
- Roelofs R. F., Fischer D. F., Houtman S. H., Sluijs J. A., Van Haren W., van Leeuwen F. W., Hol E. M. Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia. 2005;52:289–300. doi: 10.1002/glia.20243. [DOI] [PubMed] [Google Scholar]
- Nielsen A. L., Holm I. E., Johansen M., Bonven B., Jorgensen P., Jorgensen A. L. A new splice variant of glial fibrillary acidic protein, GFAPε, interacts with the presenilin proteins. J Biol Chem. 2002;277:29983–29991. doi: 10.1074/jbc.M112121200. [DOI] [PubMed] [Google Scholar]
- Nichols N. R., Day J. R., Laping N. J., Johnson S. A., Finch C. E. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- Goss J. R., Finch C. E., Morgan D. G. Age-related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging. 1991;12:165–170. doi: 10.1016/0197-4580(91)90056-p. [DOI] [PubMed] [Google Scholar]
- Porchet R., Probst A., Bouras C., Draberova E., Draber P., Riederer B. M. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics. 2003;3:1476–1485. doi: 10.1002/pmic.200300456. [DOI] [PubMed] [Google Scholar]
- Ingelsson M., Fukumoto H., Newell K. L., Growdon J. H., Hedley-Whyte E. T., Frosch M. P., Albert M. S., Hyman B. T., Irizarry M. C. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- Keller J. N., Hanni K. B., Markesbery W. R. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- De Vrij F. M., Fischer D. F., van Leeuwen F. W., Hol E. M. Protein quality control in Alzheimer’s disease by the ubiquitin proteasome system. Prog Neurobiol. 2004;74:249–270. doi: 10.1016/j.pneurobio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Olanow C. W., McNaught K. S. Ubiquitin-proteasome system and Parkinson’s disease. Mov Disord. 2006;21:1806–1823. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- Tang G., Xu Z., Goldman J. E. Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem. 2006;281:38634–38643. doi: 10.1074/jbc.M604942200. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Pickart C. M., Cohen R. E. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- Ding Q., Dimayuga E., Markesbery W. R., Keller J. N. Proteasome inhibition induces reversible impairments in protein synthesis. FASEB J. 2006;20:1055–1063. doi: 10.1096/fj.05-5495com. [DOI] [PubMed] [Google Scholar]
- Jiang H. Y., Wek R. C. Phosphorylation of the α-subunit of the eukaryotic initiation factor-2 (eIF2α) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- Rattan S. I. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol. 1996;31:33–47. doi: 10.1016/0531-5565(95)02022-5. [DOI] [PubMed] [Google Scholar]
- Ding Q., Markesbery W. R., Chen Q., Li F., Keller J. N. Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld K. L., Brown C. R., Casolari J. M., Komili S., Silver P. A. Genomic association of the proteasome demonstrates overlapping gene regulatory activity with transcription factor substrates. Mol Cell. 2006;21:861–871. doi: 10.1016/j.molcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Dembla-Rajpal N., Seipelt R., Wang Q., Rymond B. C. Proteasome inhibition alters the transcription of multiple yeast genes. Biochim Biophys Acta. 2004;1680:34–45. doi: 10.1016/j.bbaexp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Fleming J. A., Lightcap E. S., Sadis S., Thoroddsen V., Bulawa C. E., Blackman R. K. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc Natl Acad Sci U S A. 2002;99:1461–1466. doi: 10.1073/pnas.032516399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford J. R., Deshaies R. J. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat Cell Biol. 2003;5:845–850. doi: 10.1038/ncb1003-845. [DOI] [PubMed] [Google Scholar]
- Muratani M., Tansey W. P. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- Ezhkova E., Tansey W. P. Proteasomal ATPases link ubiquitylation of histone H2B to methylation of histone H3. Mol Cell. 2004;13:435–442. doi: 10.1016/s1097-2765(04)00026-7. [DOI] [PubMed] [Google Scholar]
- Lee D., Ezhkova E., Li B., Pattenden S. G., Tansey W. P., Workman J. L. The proteasome regulatory particle alters the SAGA coactivator to enhance its interactions with transcriptional activators. Cell. 2005;123:423–436. doi: 10.1016/j.cell.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Li R., Johnson A. B., Salomons G., Goldman J. E., Naidu S., Quinlan R., Cree B., Ruyle S. Z., Banwell B., D'Hooghe M., Siebert J. R., Rolf C. M., Cox H., Reddy A., Gutierrez-Solana L. G., Collins A., Weller R. O., Messing A., van der Knaap M. S., Brenner M. Glial fibrillary acidic protein mutations in infantile, juvenile, and adult forms of Alexander disease. Ann Neurol. 2005;57:310–326. doi: 10.1002/ana.20406. [DOI] [PubMed] [Google Scholar]
- Dantuma N. P., Lindsten K., Glas R., Jellne M., Masucci M. G. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat Biotechnol. 2000;18:538–543. doi: 10.1038/75406. [DOI] [PubMed] [Google Scholar]
- Lindsten K., de Vrij F. M., Verhoef L. G., Fischer D. F., van Leeuwen F. W., Hol E. M., Masucci M. G., Dantuma N. P. Mutant ubiquitin found in neurodegenerative disorders is a ubiquitin fusion degradation substrate that blocks proteasomal degradation. J Cell Biol. 2002;157:417–427. doi: 10.1083/jcb.200111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkers C. R., van Leeuwen F. W., Groothuis T. A., Peperzak V., van Tilburg E. W., Borst J., Neefjes J. J., Ovaa H. Profiling proteasome activity in tissue with fluorescent probes. Mol Pharm. 2007;4:739–748. doi: 10.1021/mp0700256. [DOI] [PubMed] [Google Scholar]
- Van Tijn P., de Vrij F. M., Schuurman K. G., Dantuma N. P., Fischer D. F., van Leeuwen F. W., Hol E. M. Dose-dependent inhibition of proteasome activity by a mutant ubiquitin associated with neurodegenerative disease. J Cell Sci. 2007;120:1615–1623. doi: 10.1242/jcs.03438. [DOI] [PubMed] [Google Scholar]
- Besnard F., Brenner M., Nakatani Y., Chao R., Purohit H. J., Freese E. Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein. J Biol Chem. 1991;266:18877–18883. [PubMed] [Google Scholar]
- Dijk F., Kraal-Muller E., Kamphuis W. Ischemia-induced changes of AMPA-type glutamate receptor subunit expression pattern in the rat retina: a real-time quantitative PCR study. Invest Ophthalmol Vis Sci. 2004;45:330–341. doi: 10.1167/iovs.03-0285. [DOI] [PubMed] [Google Scholar]
- Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Van der Meulen J. A., Joosten R. N., de Bruin J. P., Feenstra M. G. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cereb Cortex. 2007;17:1444–1453. doi: 10.1093/cercor/bhl057. [DOI] [PubMed] [Google Scholar]
- Rutka J. T., Ivanchuk S., Mondal S., Taylor M., Sakai K., Dirks P., Jun P., Jung S., Becker L. E., Ackerley C. Co-expression of nestin and vimentin intermediate filaments in invasive human astrocytoma cells. Int J Dev Neurosci. 1999;17:503–515. doi: 10.1016/s0736-5748(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Goldman J. E. Synthesis and turnover of cytoskeletal proteins in cultured astrocytes. J Neurochem. 1984;42:166–174. doi: 10.1111/j.1471-4159.1984.tb09713.x. [DOI] [PubMed] [Google Scholar]
- Deroo B. J., Archer T. K. Proteasome inhibitors reduce luciferase and β-galactosidase activity in tissue culture cells. J Biol Chem. 2002;277:20120–20123. doi: 10.1074/jbc.C200173200. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Brenner M., Goldman J. E., Messing A. GFAP and its role in Alexander disease. Exp Cell Res. 2007;313:2077–2087. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng M. D., Wen S. F., Gibbon T., Middeldorp J., Sluijs J. A., Hol E. M., Quinlan R. A. GFAP filaments can tolerate the incorporation of assembly-compromised GFAP-δ, but with consequences for filament organization and αB-crystallin association. Mol Biol Cell. 2008;19:4521–4533. doi: 10.1091/mbc.E08-03-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsberg L. B., Galiani D., Dantes A., Amsterdam A., Dekel N. The proteasome is involved in the first metaphase-to-anaphase transition of meiosis in rat oocytes. Biol Reprod. 2000;62:1270–1277. doi: 10.1095/biolreprod62.5.1270. [DOI] [PubMed] [Google Scholar]
- Meng L., Mohan R., Kwok B. H., Elofsson M., Sin N., Crews C. M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. T., Goldberg A. L., Nigam S. K. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- Kawazoe Y., Nakai A., Tanabe M., Nagata K. Proteasome inhibition leads to the activation of all members of the heat-shock-factor family. Eur J Biochem. 1998;255:356–362. doi: 10.1046/j.1432-1327.1998.2550356.x. [DOI] [PubMed] [Google Scholar]
- Ding Q., Keller J. N. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001;77:1010–1017. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- Lassot I., Latreille D., Rousset E., Sourisseau M., Linares L. K., Chable-Bessia C., Coux O., Benkirane M., Kiernan R. E. The proteasome regulates HIV-1 transcription by both proteolytic and nonproteolytic mechanisms. Mol Cell. 2007;25:369–383. doi: 10.1016/j.molcel.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Choi K., Lee J., Choi C. Divergent effect of proteasome inhibition on interleukin-1β and tumor necrosis factor α signaling in human astroglial cells. FEBS Lett. 2007;581:4691–4696. doi: 10.1016/j.febslet.2007.08.065. [DOI] [PubMed] [Google Scholar]
- Lim J. H., Chang Y. C., Park Y. B., Park J. W., Kwon T. K. Transcriptional repression of E2F gene by proteasome inhibitors in human osteosarcoma cells. Biochem Biophys Res Commun. 2004;318:868–872. doi: 10.1016/j.bbrc.2004.04.103. [DOI] [PubMed] [Google Scholar]
- Zimmermann J., Erdmann D., Lalande I., Grossenbacher R., Noorani M., Furst P. Proteasome inhibitor induced gene expression profiles reveal overexpression of transcriptional regulators ATF3, GADD153, and MAD1. Oncogene. 2000;19:2913–2920. doi: 10.1038/sj.onc.1203606. [DOI] [PubMed] [Google Scholar]
- Gadea A., Schinelli S., Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Imura T., Song B., Qi J., Ao Y., Nguyen T. K., Korsak R. A., Takeda K., Akira S., Sofroniew M. V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima M., Nornes H. O., Sato K., Neuman T. Overexpression of E2F1 in astrocytes leads to neoplastic transformation and changes in expression of retinoblastoma family members. J Neurosci Res. 1996;46:108–113. doi: 10.1002/(SICI)1097-4547(19961001)46:1<108::AID-JNR13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sakurai K., Osumi N. The neurogenesis-controlling factor, Pax6, inhibits proliferation and promotes maturation in murine astrocytes. J Neurosci. 2008;28:4604–4612. doi: 10.1523/JNEUROSCI.5074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H., Diemer N. H. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol. 1987;74:234–238. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- Shuaib A., Xu K., Crain B., Siren A. L., Feuerstein G., Hallenbeck J., Davis J. N. Assessment of damage from implantation of microdialysis probes in the rat hippocampus with silver degeneration staining. Neurosci Lett. 1990;112:149–154. doi: 10.1016/0304-3940(90)90194-e. [DOI] [PubMed] [Google Scholar]
- Prahlad V., Yoon M., Moir R. D., Vale R. D., Goldman R. D. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers R., Li X., Mao X., Rubinstein J. L., Schimmer A. D., Kay L. E. TROSY-based NMR evidence for a novel class of 20S proteasome inhibitors. Biochemistry. 2008;47:6727–6734. doi: 10.1021/bi8005913. [DOI] [PubMed] [Google Scholar]
- Geier E., Pfeifer G., Wilm M., Lucchiari-Hartz M., Baumeister W., Eichmann K., Niedermann G. A giant protease with potential to substitute for some functions of the proteasome. Science. 1999;283:978–981. doi: 10.1126/science.283.5404.978. [DOI] [PubMed] [Google Scholar]
- Riol H., Tardy M., Rolland B., Levesque G., Murthy M. R. Detection of the peripheral nervous system (PNS)-type glial fibrillary acidic protein (GFAP) and its mRNA in human lymphocytes. J Neurosci Res. 1997;48:53–62. [PubMed] [Google Scholar]
- Sabbatini M., Barili P., Bronzetti E., Zaccheo D., Amenta F. Age-related changes of glial fibrillary acidic protein immunoreactive astrocytes in the rat cerebellar cortex. Mech Ageing Dev. 1999;108:165–172. doi: 10.1016/s0047-6374(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Goldsmith S. K., Morgan T. E., Stone D. J., Finch C. E. Transcription supports age-related increases of GFAP gene expression in the male rat brain. Neurosci Lett. 1996;215:107–110. [PubMed] [Google Scholar]
- Bhat K. P., Turner J. D., Myers S. E., Cape A. D., Ting J. P., Greer S. F. The 19S proteasome ATPase Sug1 plays a critical role in regulating MHC class II transcription. Mol Immunol. 2008;45:2214–2224. doi: 10.1016/j.molimm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hattori T., Ohoka N., Inoue Y., Hayashi H., Onozaki K. C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene. 2003;22:1273–1280. doi: 10.1038/sj.onc.1206204. [DOI] [PubMed] [Google Scholar]
- Koues O. I., Dudley R. K., Truax A. D., Gerhardt D., Bhat K. P., McNeal S., Greer S. F. Regulation of acetylation at the MHC-II proximal promoter by the 19S proteasomal ATPase Sug1. Mol Cell Biol. 2008;28:5837–5850. doi: 10.1128/MCB.00535-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Wani G., Yao J., Patnaik S., Wang Q. E., El-Mahdy M. A., Praetorius-Ibba M., Wani A. A. The ubiquitin-proteasome system regulates p53-mediated transcription at p21waf1 promoter. Oncogene. 2007;26:4199–4208. doi: 10.1038/sj.onc.1210191. [DOI] [PubMed] [Google Scholar]
- Bae M. H., Jeong C. H., Kim S. H., Bae M. K., Jeong J. W., Ahn M. Y., Bae S. K., Kim N. D., Kim C. W., Kim K. R., Kim K. W. Regulation of Egr-1 by association with the proteasome component C8. Biochim Biophys Acta. 2002;1592:163–167. doi: 10.1016/s0167-4889(02)00310-5. [DOI] [PubMed] [Google Scholar]
- Fernandez S., Garcia-Garcia M., Torres-Aleman I. Modulation by insulin-like growth factor I of the phosphatase PTEN in astrocytes. Biochim Biophys Acta. 2008;1783:803–812. doi: 10.1016/j.bbamcr.2007.10.020. [DOI] [PubMed] [Google Scholar]
- James A. B., Conway A. M., Morris B. J. Regulation of the neuronal proteasome by Zif268 (Egr1) J Neurosci. 2006;26:1624–1634. doi: 10.1523/JNEUROSCI.4199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins S., Mayo W., Vallee M., Hancock D., Le M. M., Simon H., Abrous D. N. Effect of aging on the basal expression of c-Fos, c-Jun, and Egr-1 proteins in the hippocampus. Neurobiol Aging. 1997;18:37–44. doi: 10.1016/s0197-4580(96)00206-0. [DOI] [PubMed] [Google Scholar]
- Mack K. J., Kriegler S., Chang S., Chiu S. Y. Transcription factor expression is induced by axonal stimulation and glutamate in the glia of the developing optic nerve. Brain Res Mol Brain Res. 1994;23:73–80. doi: 10.1016/0169-328x(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Beck H., Semisch M., Culmsee C., Plesnila N., Hatzopoulos A. K. Egr-1 regulates expression of the glial scar component phosphacan in astrocytes after experimental stroke. Am J Pathol. 2008;173:77–92. doi: 10.2353/ajpath.2008.070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn R. J., Pemberton A. J., Royle H. J., Spackman R. W., Smith E., Jennifer R. A., Steverding D. Trypanocidal effect of α′,β′-epoxyketones indicates that trypanosomes are particularly sensitive to inhibitors of proteasome trypsin-like activity. Int J Antimicrob Agents. 2004;24:286–289. doi: 10.1016/j.ijantimicag.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Lopez S. M., Morelli L., Castano E. M., Soto E. F., Pasquini J. M. Defective ubiquitination of cerebral proteins in Alzheimer’s disease. J Neurosci Res. 2000;62:302–310. doi: 10.1002/1097-4547(20001015)62:2<302::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Layfield R., Lowe J., Bedford L. The ubiquitin-proteasome system and neurodegenerative disorders. Essays Biochem. 2005;41:157–171. doi: 10.1042/EB0410157. [DOI] [PubMed] [Google Scholar]
- Lowe J., Blanchard A., Morrell K., Lennox G., Reynolds L., Billett M., Landon M., Mayer R. J. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson’s disease, Pick’s disease, and Alzheimer’s disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and Mallory bodies in alcoholic liver disease. J Pathol. 1988;155:9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Mayer R. J., Tipler C., Arnold J., Laszlo L., Al-Khedhairy A., Lowe J., Landon M. Endosome-lysosomes, ubiquitin and neurodegeneration. Adv Exp Med Biol. 1996;389:261–269. doi: 10.1007/978-1-4613-0335-0_33. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T., Loike J. D., Brionne T. C., Lu E., Anankov R., Yan F., Silverstein S. C., Husemann J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Pihlaja R., Koistinaho J., Malm T., Sikkila H., Vainio S., Koistinaho M. Transplanted astrocytes internalize deposited β-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56:154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- Thal D. R., Schultz C., Dehghani F., Yamaguchi H., Braak H., Braak E. Amyloid β-protein (Aβ)-containing astrocytes are located preferentially near N-terminal-truncated Aβ deposits in the human entorhinal cortex. Acta Neuropathol. 2000;100:608–617. doi: 10.1007/s004010000242. [DOI] [PubMed] [Google Scholar]
- McNaught K. S., Perl D. P., Brownell A. L., Olanow C. W. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol. 2004;56:149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- Pekny M., Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Myer D. J., Gurkoff G. G., Lee S. M., Hovda D. A., Sofroniew M. V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Laird M. D., Vender J. R., Dhandapani K. M. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Kang J., Jiang L., Goldman S. A., Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Vesce S., Rossi D., Brambilla L., Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- Volterra A., Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Kashon M. L., Ross G. W., O'Callaghan J. P., Miller D. B., Petrovitch H., Burchfiel C. M., Sharp D. S., Markesbery W. R., Davis D. G., Hardman J., Nelson J., White L. R. Associations of cortical astrogliosis with cognitive performance and dementia status. J Alzheimers Dis. 2004;6:595–604. doi: 10.3233/jad-2004-6604. [DOI] [PubMed] [Google Scholar]
- Menet V., Prieto M., Privat A., Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci U S A. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U., Li L., Pekna M., Berthold C. H., Blom S., Eliasson C., Renner O., Bushong E., Ellisman M., Morgan T. E., Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouchi R., Takeda M., Yang L., Wilhelmsson U., Lundkvist A., Pekny M., Chen D. F. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6:863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- Larsson A., Wilhelmsson U., Pekna M., Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP−/−)Vim−/− mice. Neurochem Res. 2004;29:2069–2073. doi: 10.1007/s11064-004-6880-2. [DOI] [PubMed] [Google Scholar]
- Li L., Lundkvist A., Andersson D., Wilhelmsson U., Nagai N., Pardo A. C., Nodin C., Ståhlberg A., Aprico K., Larsson K., Yabe T., Moons L., Fotheringham A., Davies I., Carmeliet P., Schwartz J. P., Pekna M., Kubista M., Blomstrand F., Maragakis N., Nilsson M., Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- San Miguel J. F., Schlag R., Khuageva N. K., Dimopoulos M. A., Shpilberg O., Kropff M., Spicka I., Petrucci M. T., Palumbo A., Samoilova O. S., Dmoszynska A., Abdulkadyrov K. M., Schots R., Jiang B., Mateos M. V., Anderson K. C., Esseltine D. L., Liu K., Cakana A., van de Velde H., Richardson P. G., VISTA Trial Investigators Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Kubicek G. J., Werner-Wasik M., Machtay M., Mallon G., Myers T., Ramirez M., Andrews D., Curran W. J., Jr, Dicker A. P. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies [E-pub ahead of print] Int J Radiat Oncol Biol Phys. 2008 doi: 10.1016/j.ijrobp.2008.08.050. 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.