Abstract
Arginase1 and nitric oxide synthase2 (NOS2) utilize L-arginine as a substrate, with both enzymes expressed at high levels in the asthmatic lung. Inhibition of arginase in ovalbumin-exposed C57BL/6 mice with the transition state inhibitor Nω-hydroxy-nor-L-arginine (nor-NOHA) significantly increased total L-arginine content in the airway compartment. We hypothesized that such an increase in L-arginine content would increase the amount of nitric oxide (NO) being produced in the airways and thereby decrease airway hyper-reactivity and eosinophilic influx. We further hypothesized that despite arginase inhibition, NOS2 knockout (NOS2−/−) mice would be unable to up-regulate NO production in response to allergen exposure and would demonstrate higher amounts of airway hyper-reactivity and eosinophilia under conditions of arginase inhibition than C57BL/6 animals. We found that administration of nor-NOHA significantly decreased airway hyper-reactivity and eosinophilic airway inflammation in ovalbumin-exposed C57BL/6 mice, but these parameters were unchanged in ovalbumin-exposed NOS2−/− mice.
Arginase1 protein content was increased in mice exposed to ovalbumin, an effect that was reversed upon nor-NOHA treatment in C57BL/6 mice. Arginase1 protein content in the airway compartment directly correlated with the degree of airway hyper-reactivity in all treatment groups. NOS2−/− mice had a significantly greater arginase1 and arginase2 concentrations compared to their respective C57BL/6 groups, indicating that inhibition of arginase may be dependent upon NOS2 expression. Arginase1 and 2 content were not affected by nor-NOHA administration in the NOS2−/− mice.
We conclude that L-arginine metabolism plays an important role in the development of airway hyper-reactivity and eosinophilic airway inflammation. Inhibition of arginase early in the allergic inflammatory response decreases the severity of the chronic inflammatory phenotype. These effects appear to be attributable to NOS2, which is a major source of NO production in the inflamed airway, although arginase inhibition may also be affecting the turnover of arginine by the other NOS isoforms, NOS1 and NOS3. The increased L-arginine content in the airway compartment of mice treated with nor-NOHA may directly or indirectly, through NOS2, control arginase expression both in response to OVA exposure and at a basal level.
Keywords: Asthma, L-arginine, Nitric Oxide, Eosinophil, Inflammation, nor-NOHA, airway hyper-reactivity
Introduction
There are three isoforms of nitric oxide synthase (NOS) present in the lung, NOS1, NOS2 and NOS3. These enzymes convert L-arginine into L-citrulline and nitric oxide (NO). Two isoforms of arginase in the lung, arginase1 and arginase2, convert L-arginine into L-ornithine and urea, and are proposed to be in competition with the NOS enzymes for the substrate, L-arginine., Three of these enzymes, arginase1, arginase2, and NOS2, are up-regulated in the mouse model of allergic airway inflammation [Takemoto et al., 2007; Zimmermann et al., 2003] and the arginase enzymes have been implicated in the regulation of NO production, utilizing L-arginine via their alternative metabolic pathway [King et al., 2004; Ricciardolo, 2003]. Increased production of NO by the inflamed lung is thought to act as an important protective mechanism that helps limit lung injury (Ckless et al., 2007).
Despite the higher affinity of NOS enzymes for L-arginine compared to the arginases (NOS Km approximately 2–20 μM vs. arginase Km approximately 1–5 mM [Wu and Morris, 1998]), arginase appears to be capable of regulating NO production by causing regional substrate depletion. One mechanism by which arginase might modulate the production of NO is by enzyme localization, creating a dependency on substrate diffusion and substrate compartmentalization [Shen et al., 2005] that may result in non-freely exchanging L-arginine pools in the cell [Closs et al., 2000; Topal et al., 2006]. Heightened arginase activity also creates fluctuations in L-arginine concentration affecting circulating plasma L-arginine concentration [Morris et al., 2004]. Low concentrations of plasma L-arginine may have a significant effect on the NOS2-catalyzed production of NO. In macrophages, the NOS2 isoform has been shown to be dependent upon the extracellular L-arginine pool when intracellular L-arginine depletion occurs [Iniesta et al., 2001; Escobales et al., 2000; Martin et al., 2005; Nicholson et al., 2001]. The effects of arginase activity on substrate limitation may also be isoform specific as arginase1, which is strongly up-regulated in allergic inflammation in mice, regulates NOS2 activity [El-Gayar et al., 2003]. Thus, there is a great deal of interest in the potential use of arginase inhibitors as a mechanism to increase nitric oxide (NO) production during inflammation. Arginase inhibitors have been used successfully in rodent models of hepatic ischemia-reperfusion injury [Jeyabalan et al., 2008; Reid et al., 2007], endothelial dysfunction [Bagnost et al., 2008; Berkowitz et al., 2003], Leishmania infection [Iniesta et al., 2001; 2002], and in a guinea pig model of airway hyper-reactivity [Maarsingh et al., 2008]. Preliminary studies in C57BL/6 mice indicated a reduction of airway inflammation after administration of the arginase inhibitor Nω-hydroxy-nor-L-arginine (nor-NOHA) [Bratt et al., 2009].
Recent studies from our laboratory have shown that NOS2−/− mice are highly susceptible to airway inflammation in response to allergen exposure [Bratt et al., 2009], with significantly increased inflammatory cells present in the bronchoalveolar lavage as compared to C57BL/6 mice. We have also reported [Bratt et al., 2009] that arginase1 is expressed at very low (or undetectable) levels in isolated airways obtained from NOS2−/− or C57BL/6 mice exposed to filtered air, and that the arginase1 isoform is expressed at relatively high levels in isolated airways from mice of both strains (but especially the NOS2−/− animals) exposed to ovalbumin aerosol. Arginase2, on the other hand, is expressed in airways of unexposed mice of both strains and its expression is not increased in the airways of C57BL/6 mice exposed to ovalbumin. Expression of arginase2 in the airways of NOS2−/− mice exposed to ovalbumin is significantly increased relative to values in NOS2−/− mice exposed only to filtered air, but the extent of this increase (3-fold) is dwarfed by the relative increase in arginase1 (42-fold) in airways of mice exposed to ovalbumin.
In the present study, to further examine the role of arginase in the development of allergen-induced airway disease, we tested the hypothesis that arginase inhibition would increase L-arginine content in the conducting airways of the lung, resulting in reduced airway hyper-reactivity and airway obstruction by the influx of inflammatory cells into the airway, two characteristic symptoms of asthma. We examined the effects of arginase inhibition using the specific arginase inhibitor nor-NOHA on L-arginine concentration in the airway compartment of C57BL/6 mice exposed to ovalbumin (OVA). We next examined how altering L-arginine concentration by arginase inhibition affected the development of airway hyper-reactivity and inflammatory cell influx and the arginase content in the airways, and whether correlations existed between arginase1 content and either inflammatory cell influx or altered lung function. Finally, we examined the effect of arginase activity in the absence of NOS2 on these parameters and the relationship between NOS2 activity and arginase1 content by performing parallel studies using a NOS2 knockout (NOS2−/−) strain of mice derived from the C57BL/6 strain.
Materials and Methods
Mice
All procedures with mice were performed in accordance with an IACUC approved protocol. Specific pathogen-free C57BL/6 male mice, aged 6–8 weeks, were purchased from Charles River Laboratory (Wilmington, MA). NOS2−/− mice, on a C57BL/6 background designated C57BL/6 Ai-[KO]NOS2 N5 [Kenyon et al., 2002], were purchased from Taconic Laboratories, Germantown, N.Y., and breeding colonies were established from the initial mice using an embryo transfer method. All mice were maintained in a HEPA-filtered laminar flow cage rack with a 12-hour light/dark cycle and allowed free access to food (Purina Rodent Chow) and water. Mice were housed and cared for by the veterinary staff of Animal Resource Services at UC Davis in AALAC-accredited facilities and were routinely screened for health status by serology and histology by the veterinary animal resources facility. Mice were euthanized at the end of an experiment with an intraperitioneal (IP) overdose of pentobarbital.
Ovalbumin aerosol exposures
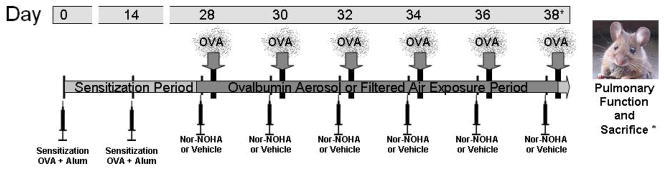
The procedures used have been described in detail previously [Bratt et al., 2009]. Briefly, mice (ovalbumin aerosol-exposed and control) were sensitized by IP injections of 10 μg/0.1 ml of ovalbumin (OVA, 98% pure, endotoxin-free, Sigma, St. Louis, MO) in PBS, pH 7.4, with alum adjuvant [Temelkovski et al., 1998] on days 1 and 15 of the experiment. Starting on day 29 and continuing every other day for a period of two weeks, animals received either an IP injection of 0.1 ml PBS or 100 μg/0.1 ml of Nω-hydroxy-nor-L-arginine (~4 mg/Kg, nor-NOHA, Bachem Bioscience, Inc., King of Prussia, PA) in PBS thirty minutes prior to exposure to OVA aerosol (10 mL X 1% OVA in PBS). Control animals were allowed to breathe filtered air while their matched experimental animals were exposed to OVA aerosol for 30 minutes (Exposure protocol: Figure 1). Aerosol was delivered with a side-stream nebulizer (Invacare, Elyria, OH) and air compressor (Invacare, Sanford, FL). Mass concentration and aerodynamic size distributions for this methodology were determined with a Mercer-type cascade impactor [Kenyon et al., 2003].
Figure 1.
Sensitization and exposure schedule. Day of procedure listed above with injections administered 30 minutes prior to ovalbumin aerosol (OVA) exposure. Pulmonary function testing and sacrifice occurred on day 38, 1–3 hours after exposure.
Lung compliance and resistance measurements
Dynamic compliance and resistance of the respiratory system were measured using a plethysmograph for restrained mice. (Buxco Inc., Troy, NY). Mice were deeply anesthetized and sedated with medetomidine, 0.75 mg/kg (Domitor, Orion Pharma, Finland) and tiletamine/zolpidem, 37.5 mg/kg (Telazol, Fort Dodge Laboratories, Fort Dodge, IA), and ventilated at 7–8 cc/kg with a mouse ventilator (MiniVent, Harvard Apparatus, Cambridge, MA) for the duration of the procedure. Compliance and resistance measurements were made at baseline, using 3-minute average values, and immediately after a series of 3-minute exposures to nebulized aerosols of saline or methacholine (0, 0.5, 1.0 and 2.0 mg/ml), as described in detail by Kenyon et al. [2003].
Measurement of exhaled NO
A 5-minute sample of exhaled gases was collected from the cannulated mice through the ventilator exhalation port immediately after insertion of the mouse into the plethysmograph. Samples were collected in a specially constructed mylar bag and exhaled NO was measured by chemiluminescent assay using a Sievers Nitric Oxide analyzer (Sievers Inst., Boulder, CO).
Airway inflammatory cell populations
Mice were euthanized with an IP injection of phenobarbital and dilantin. The lungs were lavaged using two 1-ml aliquots of PBS, pH 7.4, with each aliquot passaged twice. Cells were pelleted at 2,500 rpm for 10 minutes, and the supernantant was removed and stored at −80°C for subsequent analysis. Red blood cells were lysed using ACK lysis buffer (0.15 M NH4Cl, 1mM KHCO3, 0.1mM EDTA, pH 7.3) and centrifuged at 2,500 rpm for an additional 10 minutes; the subsequent pellet was suspended in 0.5 ml PBS.
Total live cell number in the lung lavage fluid was determined using a hemocytometer and trypan blue exclusion; 100 μl of the remaining cell suspension was processed onto slides using a cytocentrifuge at 1,650 rpm for 15 minutes for determination of the cell differential present. Slides were air dried, stained with a Hema3 stain set (eosin Y and methylene blue/azure A) as described in the manufacturer’s instructions (Fisher Scientific, Kalamazoo, MI), and sealed using Cytoseal 60 (Richard-Allen Scientific, Kalamazoo, MI). Cell percent differentials were calculated by counting 10 fields at 400X under a 40X objective (10X eyepiece) and classifying cell types as alveolar macrophage, neutrophil, eosinophil, lymphocyte, or “other” based upon standard morphological characteristics and staining profile. Totals of each cell type per ml of bronchoalveolar lavage fluid were calculated using the differential percentages.
Western blot analysis of tissue
Isolated airway preparations were obtained by dissecting the lobar bronchi and daughter airways from the left lung under a dissecting microscope to give a preparation of the larger airways distal to the carina consisting of the bronchus through the third generation bronchi separated from adhering parenchyma. The dissected airways were sonicated in a homogenization buffer containing 0.1% SDS, protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 574 uM phenylmethanesulfonyl fluoride (PMSF) in PBS. Homogenates were centrifuged at 10,000 rpm for 20 minutes and the resulting supernatant was stored at −80° C until further use.
The western blot procedure used is described in Kenyon et al. [2008]. Antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) unless otherwise stated. In brief, total protein concentration of homogenate samples was determined using the Micro BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Samples containing 20 μg of protein were incubated at 65°C for 15 min, electroporated under reducing conditions, and transferred to a polyvinyldene difluoride (PVDF) membrane. Membranes were blocked in 5% dry milk in PBS for 1 hr at 25°C, then incubated in 0.4 μg/ml of goat, anti-mouse Arg1, 0.4 μg/ml of goat, anti-mouse Arg2, or 0.4 μg/ml of rabbit anti-human α-actinin overnight at 4°C, then incubated for 1 hour at 25°C in 40 ng/ml of horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG (Pierce Biotechnology, Rockford, IL) or 40 ng/ml of horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (Pierce Biotechnology, Rockford, IL). Bands were visualized using the Immobilon western chemiluminescent HRP substrate kit (Millipore, Billerica, MA) and images captured using Image Reader LAS-3000 version 2.1 (FUJIFILM, Cypress, CA) and analyzed using Image J 1.38x software (NIH, USA). Band intensities were normalized to actinin values for the same sample.
Measurement of L-arginine in the conducting airways
Methods are described in detail in Kenyon et al. [2008]. In brief, airway homogenate aliquots containing 50 μg of total protein were deproteinized using acetone (4:1 [vol/vol] acetone: tissue) and evaporated to dryness. The samples were then analyzed for basic amino acid content by reverse-phase HPLC after derivatization with phenylisothiocyanate (PITC) [Sarwar and Botting, 1990].
Statistical analyses
Data are presented as mean values ± SEM. Data were analyzed using unpaired values compared by two-tailed Student’s t-test or one-way ANOVA with Tukey’s post test where appropriate using the Prism 4.0 or 5.0 software package (Graphpad, Inc., San Diego, CA), with statistical significance determined as a P value of 0.05 or less.
Results
Effect of arginase inhibition on the concentration of L-arginine in isolated airways from C57BL/6 mice
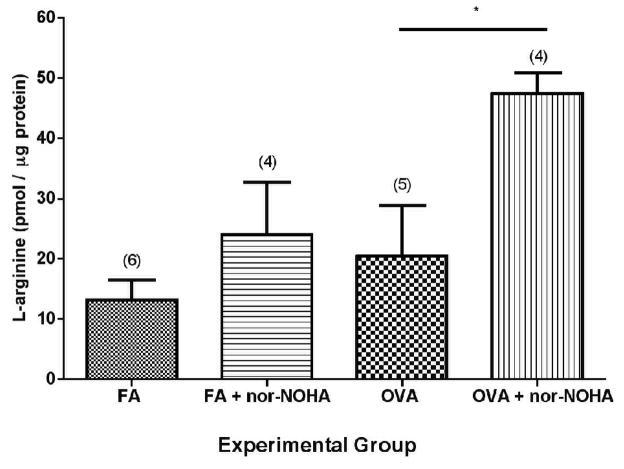
We first asked whether the dose of nor-NOHA administered was effective in vivo. To answer this question, we asked whether administration of nor-NOHA using the protocol described increased the concentration of L-arginine in isolated airway homogenates from C57BL/6 mice (Figure 2). In the mice administered OVA + nor-NOHA, the concentration of L-arginine was increased significantly compared to mice receiving OVA alone (p=0.029). Thus, we could conclude that the dose and pharmacokinetics of nor-NOHA was effective in raising the concentration of L-arginine in the lungs of mice administered this arginase inhibitor.
Figure 2.
Effect of arginase inhibition (nor-NOHA) on L-arginine concentration in isolated airways from C57BL/6 mice. Administration of nor-NOHA significantly increased the cellular L-arginine content of the airway compartment of OVA-exposed mice. L-Arginine concentration was calculated as pmol per μg total protein with data represented as mean ± SEM and the number of animals analyzed per group (n) indicated above the columns in parentheses. * p=0.029.
Effect of arginase inhibition on airway inflammation
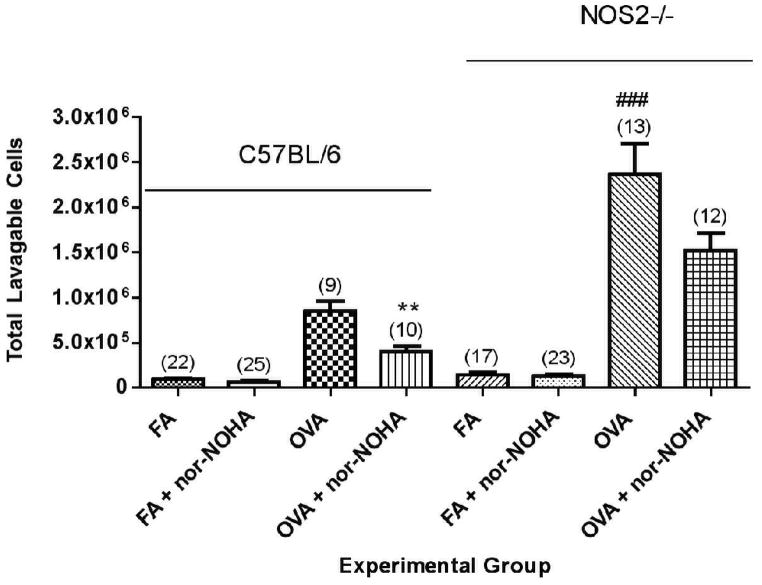
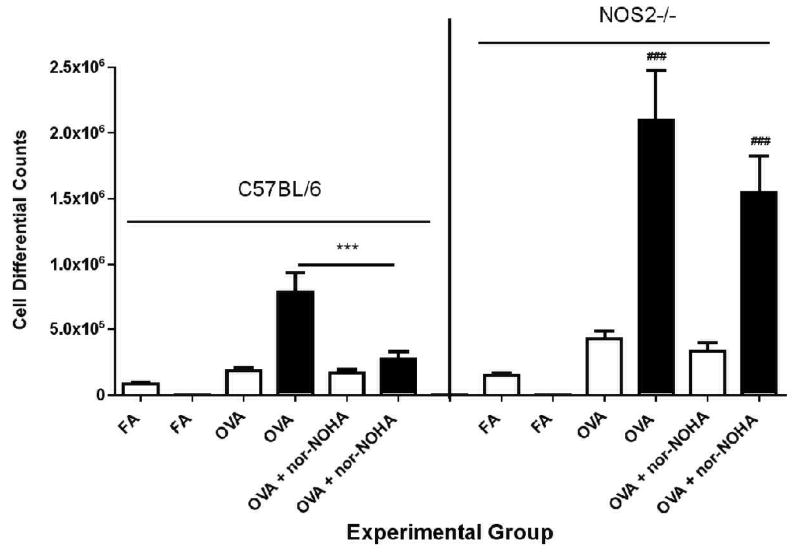
OVA exposure significantly increased the number of total cells in the lavage fluid of C57BL/6 mice (Figure 3). We observed significantly lower numbers of inflammatory cells present in the lavage fluid of OVA + nor-NOHA animals compared to OVA-exposed animals administered PBS alone. Cell differential analysis revealed a significant (P<0.01) reduction in the total number of eosinophils (black bars) in the lavage fluid cell population (Figure 4), with the total eosinophil fraction of the inflammatory cell population reduced from 76.6% (OVA + PBS) to 54.8% (OVA + nor-NOHA).
Figure 3.
Effect of arginase inhibition on total inflammatory cell number present in lavage fluid in C57BL/6 or NOS2−/− mice exposed to either filtered air (FA) or OVA aerosol. Significant increases in lavage fluid total cell counts were observed in all groups exposed to OVA compared to their corresponding treatment-matched filtered air controls. Administration of nor-NOHA significantly reduced the total airway inflammatory cell burden in OVA-exposed mice compared to vehicle-administered C57BL/6 mice. NOS2−/− mice exposed to OVA had a significantly greater inflammatory cell burden in comparison to the corresponding C57BL/6 mouse groups. Data are presented as mean ± SEM with N, the number of mice in each group, indicated above the column in parentheses. **p<0.01 compared to OVA; ###p<0.01 compared to the corresponding C57BL/6 treatment groups.
Figure 4.
Effect of arginase inhibition on lavage fluid cell differentials in C57BL/6 or NOS2−/− mice exposed to filtered air or to OVA aerosol. There were no significant differences between the OVA FA or the NOS2−/− FA groups, which are represented graphically as “FA Combined” in the figure. Eosinophils were not detectable in FA-exposed mice of either strain (as shown in the figure). In the OVA-exposed groups, macrophages (empty bar) and eosinophils (black bar) were the predominant cell types present in the lavage fluid . Arginase inhibition significantly reduced the number of eosinophils in OVA-exposed C57BL/6 mice, but not in NOS2−/− animals. NOS2−/− mice had significantly more total eosinophils after exposure to OVA than the corresponding C57BL/6 groups. Data are represented as mean ± SEM. ***p=<0.001 compared to OVA alone. ###p<0.01 compared to the corresponding C57BL/6 treatment groups.
NOS2−/− mice had consistently greater numbers of inflammatory cells in their lavage fluid compared to treatment-matched C57BL/6 groups (Figure 3). However, in contrast to the C57BL/ 6 mice, the NOS2−/−mice administered OVA + nor-NOHA did not have a significant difference in lavage fluid total cell counts (Figure 3) or the percent of eosinophils (Figure 4) compared to NOS2−/− mice administered OVA alone (p=0.0947).
Effect of arginase inhibition on arginase expression in isolated airways
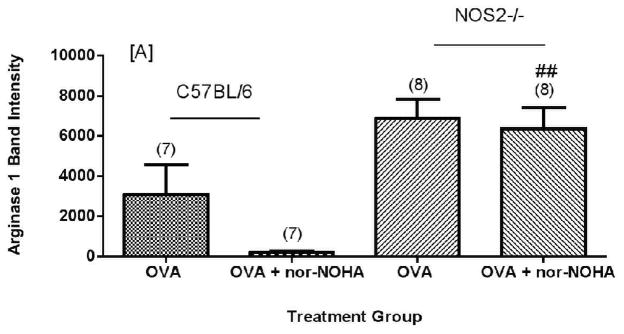
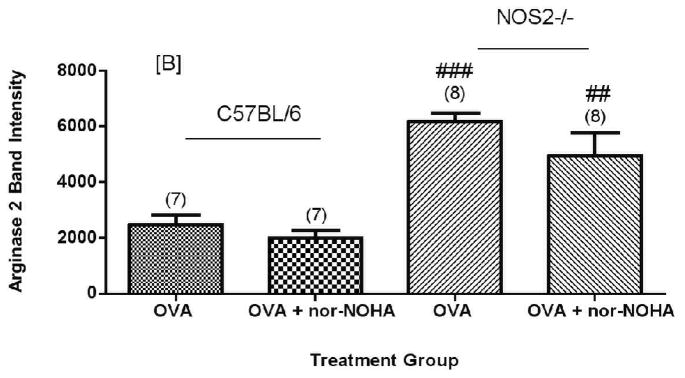
None of the FA control animals expressed arginase1 protein at significant levels as compared to mice exposed to OVA; for most of these control animals arginase1 was not detectable by Western blots (data not shown). Exposure to OVA increased (Figure 5A) arginase1 expression in both C57BL/6 and NOS2−/− mice. C57BL/6 mice administered OVA + nor-NOHA had an apparently lower (P = 0.07, two tailed test) arginase1 protein content compared to animals given OVA alone (Figure 5A). There was variability in C57BL/6 arginase1 content within the OVA alone group despite the fact that the matched mice received the same treatment and exposure schedule. Repeated independent experiments verified this variability in the OVA-treated mice (Figure 6). Arginase1 protein expression in the isolated airways was less variable in the OVA + nor-NOHA treated group as measured by relative intensity units (normalized to actinin). In contrast to arginase1, arginase2 content was consistent in all of the C57BL/6 mice (Figures 5B and 6B). Arginase2 content increased by about 3-fold in the NOS2−/− mice exposed to OVA, but the arginase2 content was not affected by nor-NOHA administration in the OVA-exposed mice (Figures 5B and 6B).
Figure 5.
Effect of arginase inhibition on Arginase1 [A] or Arginase2 [B] content in the airways of C57BL/6 or NOS2−/− mice exposed to OVA aerosol. NOS2−/− animals show significantly greater Arg1 and Arg2 expression in all treatment groups compared to their corresponding C57BL/6 treatment group (p<0.01) except Arg1 OVA. Administration of nor-NOHA apparently decreased Arg1 content in the airways (p=0.0795; two-tailed test). ##p<0.01, ###p<0.001 compared to treatment-matched C57BL/6 mice.
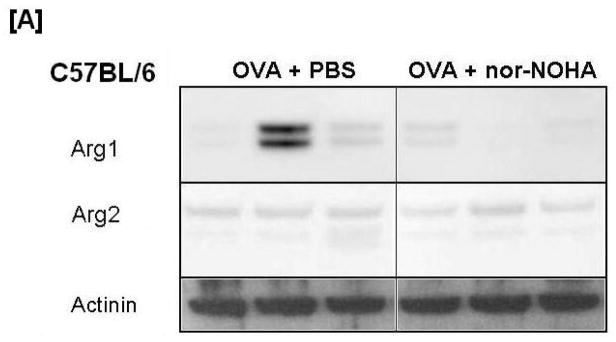
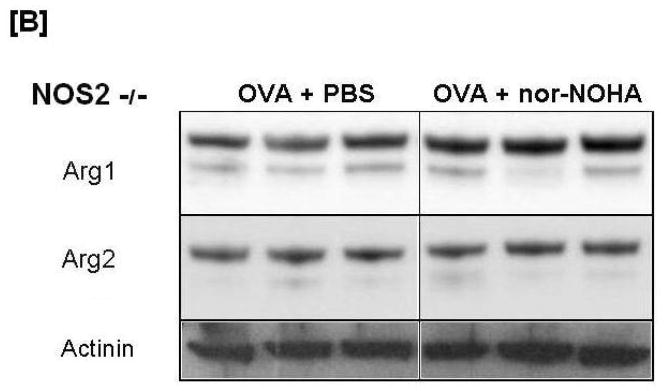
Figure 6.
Effect of arginase inhibition on Arginase1 [A] and Arginase2 [B] content in Western blot images from C57BL/6 and NOS2−/− mice. Representative images of each treatment group are displayed with actinin content displayed below as a loading control. Variability of Arginase1 protein content in the C57BL/6 OVA group was observed in all 8 animals examined. This variability was not observed in any other treatment group.
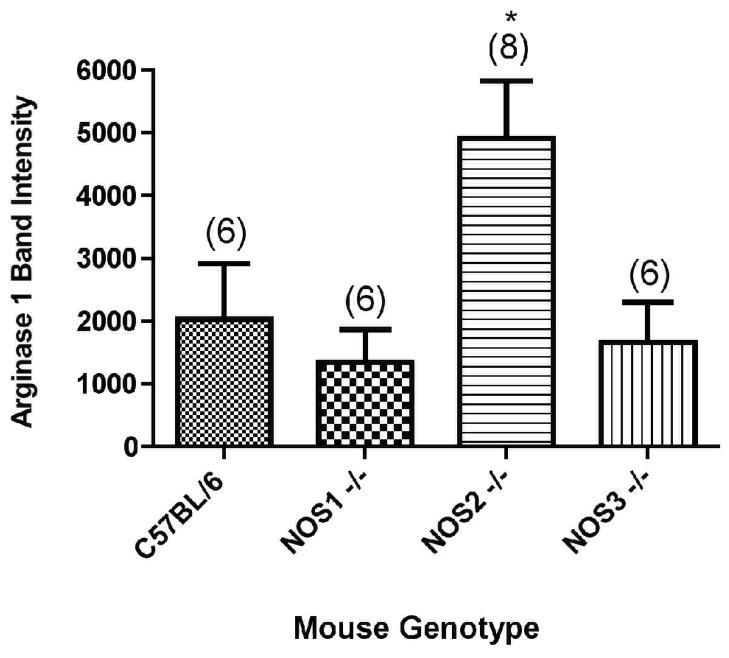
Nor-NOHA administration (OVA + nor-NOHA) did not affect arginase1 protein content in NOS2−/− animals exposed to OVA (Figures 5A and 6A). When the arginase1 and arginase2 protein content were compared in NOS2−/− mice to their corresponding treatment-matched C57BL/6 groups, the NOS2−/− animals had consistently greater arginase1 and 2 content in the airway compartment. Interestingly, this phenomenon of significant (P<0.05) up-regulation of arginase1 in OVA-exposed NOS2−/− mice was specific for the knockout of the NOS2 isoform; when arginase1 expression levels were determined in NOS1−/− and NOS3−/− mice exposed to OVA, we found no significant differences between the level of expression of arginase1 in the NOS knockout strains and in the C57BL/6 wild type animals (Figure 7).
Figure 7.
Arginase1 expression levels in C57BL/6, NOS1−/−, NOS2−/−, and NOS3−/− mice exposed to OVA as quantified by Western blot analysis of isolated airways. N, the number of animals per group, is indicated in parentheses. *p<0.05 compared to the expression levels in the NOS1−/− and NOS3−/− strains.
Effect of arginase inhibition on lung compliance and airway resistance in mice administered methacholine aerosol
We examined dynamic lung compliance and airway resistance in mice (Figures 8 and 9) before and after methacholine (MCh) challenge. Using the initial baseline values and the final MCh dose response for each experimental group of mice we calculated the percentage change in both compliance (%ΔCdyn) and resistance (%ΔRst) in response to bronchoprovocation. Mice of both strains exposed to ovalbumin aerosol had lower average baseline lung compliance and greater overall decrease in lung compliance in response to MCh bronchoprovocation compared to their corresponding FA-exposed group (data not shown).
Figure 8.
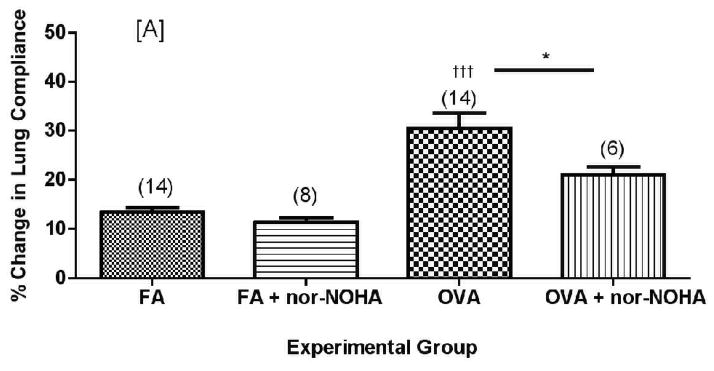
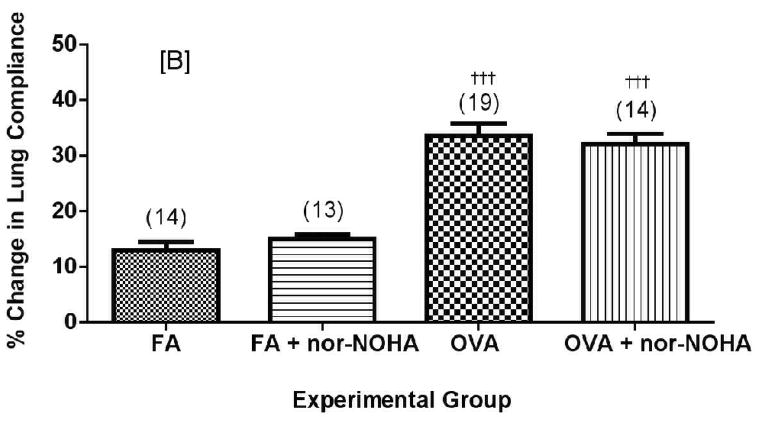
Effect of arginase inhibition on dynamic lung compliance in C57BL/6 (A) or NOS2−/− (B) mice exposed to filtered air or to OVA aerosol. Percent change compliance (represented as an absolute value) was calculated from the mean baseline compliance and maximum compliance after MCh challenge (See Materials and Methods for calculation). Data are presented as mean ± SEM with (n) indicated above column in parentheses. †††p<0.001 compared to FA, *p<0.05 compared to OVA.
Figure 9.
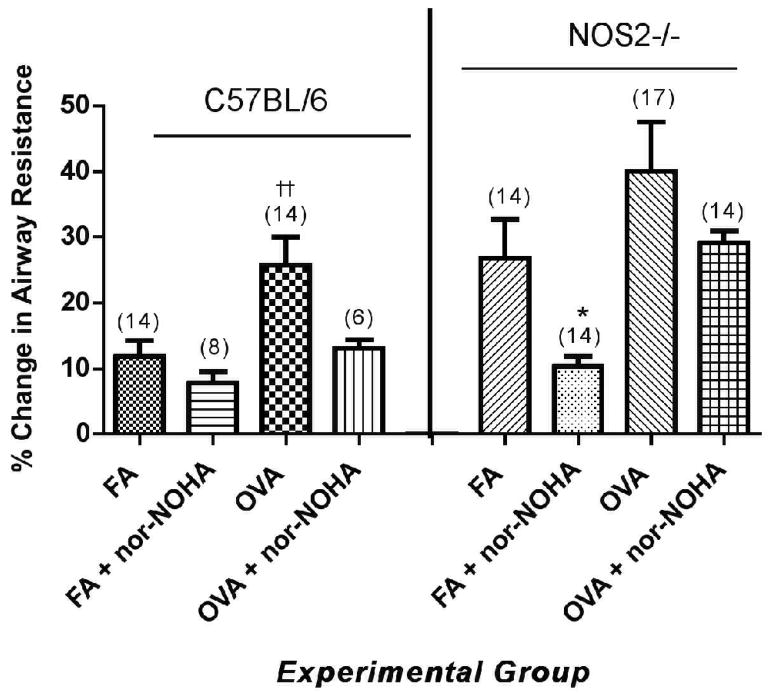
Effect of arginase inhibition on airway resistance in C57BL/6 or NOS2−/− mice exposed to filtered air or to OVA aerosol. The percent change in resistance was measured as the percent change from the baseline average and after the 2.0 mg/ml MCh dose. Percent change in resistance is presented as group mean ± SEM in with (n) indicated above the column in parentheses. ††p<0.01 compared to treatment-matched FA control and *p<0.05 compared to FA.
Nor-NOHA administration significantly decreased the effects of MCh-induced bronchoprovocation on dynamic lung compliance in C57BL/6 mice (Figure 8A, p<0.05) but had no significant effect on airway resistance in the same mice (Figure 9, p=0.074 by two-tailed t-test). Administration of nor-NOHA to the NOS2−/− mice exposed to ovalbumin aerosol did not significantly change lung compliance (Figure 8B) or airway resistance (Figure 9). Interestingly, nor-NOHA administration significantly (P<0.05) lowered airway resistance in NOS2−/− mice exposed only to filtered air (Figure 9).
Arginase1 expression correlates with increased AHR in C57BL/6 but not NOS2−/− mice
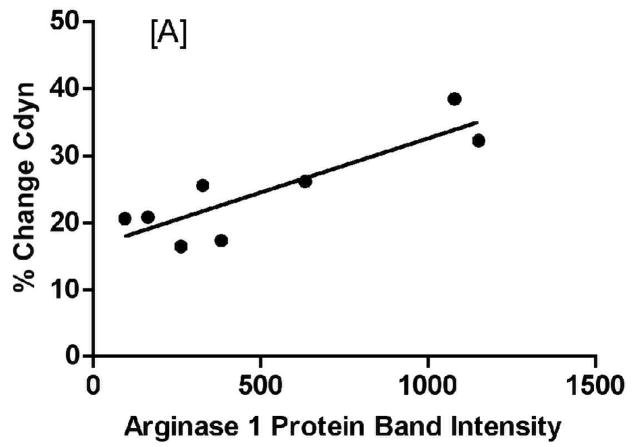
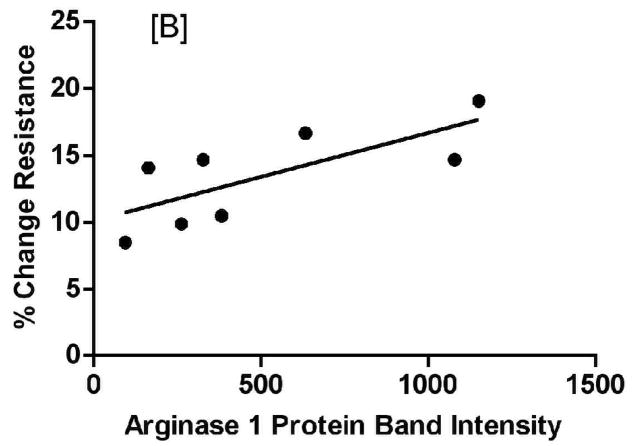
Analysis of lung function measurements showed a significant correlation between arginase1 expression levels and both dynamic lung compliance (Figure 10A: %ΔCdyn slope= −0.0162 ± 0.00379, F= 18.28, p-value= 0.0052) and airway resistance (Figure 10B: %ΔRst slope= 0.00658 ± 0.00246, F= 7.17, p-value= 0.037). On the other hand, NOS2−/− mice did not display a correlation between levels of arginase1 expression and lung function measurements (data not shown). Comparable linear regression analyses showed no correlation with increased inflammatory cell counts in BAL from C57BL/6 or NOS2−/− mice (data not shown), suggesting that the inflammatory cells are not significantly contributing to the observed increase in arginase1 expression in these mice upon exposure to ovalbumin.
Figure 10.
Arginase1 expression correlates with parameters of AHR as measured by percent change in dynamic lung compliance (A) or airway resistance (B) (calculated as % change from baseline to the final dose of the MCh dose response). Points indicate individual animal measurements (including all OVA-exposed groups); the solid line indicates the best fit to the data by linear regression analysis.
Effect of arginase inhibition on the concentration of NO in exhaled breath
Inhibition of arginase activity by nor-NOHA should in theory result in arginine being shunted from the degradation pathway of ornithine and subsequent metabolites to the nitric oxide synthase pathway, allowing for the synthesis of greater amounts of NO. To test whether this shunting was actually occurring in our model of ovalbumin-induced lung inflammation, we collected a 5 minute sample of the expired breath from representative animals from each of the various experimental groups described above and measured the concentration of NO exhaled by the mice (N = 8–22 animals per group tested in these experiments). Neither C57BL/6 nor NOS2 −/− mice showed any significant differences in FENO between any of the experimental groups studied (filtered air +/− nor-NOHA, OVA +/− nor-NOHA), nor were there any significant differences between the two strains (data not shown).
There were no significant differences in exhaled NO between the C57BL/6 mice and the NOS2−/− strain, which had respectively 9.62±1.59, and 7.44±1.00 ppb NO in their exhaled air while they were breathing filtered air, Nor did we observe any significant differences between the C5BL/6 and NOS2−/− strains of mice in the levels of NOx we observed in the supernatant from lung lavage: 3.32±0.48 versus 4.16±0.74 umol/ml of NOx, respectively. These findings suggest that there is a compensatory mechanism operative in the NOS2−/− mice that sustained their production of NO under ambient conditions, presumably by means of a pathway not dependent upon NOS2 and therefore operating through enhanced NOS1, NOS3, or both.
Apparent crosstalk between NOS expression and arginase1 expression in mice exposed to OVA
Western blot analysis for arginase levels in NOS1, NOS2, or NOS3 knockouts or C57BL/ 6 (wild type) mice demonstrated similar levels of arginase1 expression in the isolated airways from the C57BL/6, NOS1−/−, or NOS3−/− strains (Figure 7). Interestingly, however, arginase1 expression was significantly increased (by about 2.5-fold) in the NOS2−/− mice (see also Figure 5A, in which different animals were analyzed). These results suggest that NOS2 expression (or the NO specifically produced by NOS2) directly or indirectly helps to maintain the levels of arginase1 in the airways at “normal levels”. There were no apparent changes in either NOS1 or NOS3 expression evaluated by western blots in mice treated with nor-NOHA (data not shown).
Discussion
We first verified that we could alter L-arginine content in the conducting airways of C57BL/6 mice by administration of nor-NOHA, a competitive inhibitor of arginase (Figure 2). The in vivo half-life of nor-NOHA in the lung is not known, but given that the compound does not act as a substrate for either arginase or NOS, we would expect a relatively slow clearance rate from the lung. We selected nor-NOHA as the arginase inhibitor of choice for these experiments because nor-NOHA is not a substrate for NOS, so as to avoid the possibility of increasing NO production independently of the arginase inhibition. Despite the efficacy of the administered dose of nor-NOHA, as shown by increased L-arginine content in the airways, we were not able to demonstrate significant differences in exhaled NO levels between C57BL/6 and NOS2−/− mice treated with ovalbumin with or without nor-NOHA treatment (data not shown).
Nor-NOHA significantly reduced the total inflammatory cell content of lung lavage fluid in C57BL/6 mice exposed to OVA (Figure 3). These results confirm results reported by Maarsingh et al. [2008], who used a guinea pig model of allergen-induced lung inflammation. These investigators observed a decrease in inflammatory cell influx after arginase inhibition using the arginase inhibitor 2(s)-amino-6-boronohexanoic acid (ABH). Our previous studies [Bratt et al., 2009] using the OVA model have indicated that NOS2−/− mice demonstrate more severe airway inflammation than C57BL/6 mice. Data from our current study verify these previous results.
C57BL/6 mice treated with nor-NOHA showed a reduction in eosinophilic influx that did not occur in the NOS2−/− strain (Figure 4), indicating that when L-arginine concentrations in the airway compartment are increased by arginase inhibition, eosinophilic influx is decreased, directly or indirectly, through NOS2. NOS3 over-expression also reduces eosinophilic influx in allergic asthma, with a 46% reduction in eosinophils in the lavage fluid [Ten Broeke et al., 2006]. Thus, an increase of localized NO production by NOS2 by limiting arginase activity may be sufficient to provide the same end result of decreased eosinophilic inflammation.
We observed a significant reduction in arginase1 in OVA-exposed C57BL/6 mice treated with nor-NOHA (Figure 5A) compared to vehicle controls. These results were not observed in NOS2 knockout mice suggesting that the observed reduction in arginase1 is NOS2 dependent. This result is consistent with the observed correlation between arginase1 expression and airway reactivity in the present study.
Modulation of airway reactivity using nor-NOHA has been studied by Meurs and Maarsingh in an ex vivo guinea pig model [Maarsingh et al., 2006; Meuers et al., 2000; 2002], and by Maarsingh et al. [2008] in an in vivo guinea pig model using an inhaled arginase inhibitor, 2(S)-amino-6-boronohexanoic acid (ABH). Both models showed a reduction in airway reactivity with arginase inhibition. In a recent follow-up study using the guinea pig model, Maarsingh et al. [2009] demonstrated that nor-NOHA treatment of guinea pigs with allergen-induced airway inflammation decreased airway hyper-reactivity. Our results confirm and extend these findings into mice with allergen-induced airway inflammation. Maarsingh et al. [2009] suggested that nor-NOHA acted by restoring the production of NO in the lung by shunting arginine to the NOS pathway and consequently increasing the production of peroxynitrite.
Decreases in airway compliance were significantly ameliorated in C57BL/6 animals administered nor-NOHA compared to vehicle controls. Airway resistance measurements followed this trend, though the difference was not statistically significant.
NOS2 knockout mice exposed to OVA and administered nor-NOHA showed no change in airway hyperreactivity compared to vehicle controls suggesting that the effect of arginase inhibition on the development of airway hyperreactivity is NOS2 dependent. Non-specific inhibitors of the three nitric oxide synthase isoforms cause significant increases in airway tone contributing to airway hyperresponsiveness both in vivo and in vitro [Nijkamp et al., 1993]. Previous studies by Eynott et al. [2002] have suggested that the NOS2 isoform contributes to the development of airway hyper-responsiveness, which seemingly conflicts with our data in which NOS2 is necessary for mitigating airway obstruction with OVA exposure. However, differences in the models studied may account for the differences in conclusions. We also note that airway inflammation and airflow obstruction may depend on the function of several NOS isoforms, including NOS2, as inhibition of the arginase enzymes and subsequent increases in local L-arginine concentration may affect numerous enzymes including the NOS isoforms and the synthesis of creatine, agmatine, polyamine, proline, glutamate in the lung.
Arginase1 is expressed in activated macrophages, as well as the airway epithelium and airway fibroblasts of OVA-exposed mice [Takemoto et al., 2007]. We performed linear regression analysis of arginase1 band intensity and lavage fluid inflammatory cell number and found no correlation between the two parameters (data not shown). We concluded that the primary source of arginase1 in the airways of OVA-exposed mice is not the inflammatory cells, but resident lung cells. In contrast, we found a significant correlation with both parameters of airway hyper-responsiveness indicating that increased arginase1 content in the airway cells negatively affected development of airway hyper-responsiveness. A correlation of arginase activity to decreases in FEV1 has been reported in asthmatic patients [Lara et al., 2008] and in a similar mouse model to the one we have studied herein [North et al., 2009].
Despite the strong correlation between airway hyper-responsiveness and arginase1 content in the airways, we cannot conclude that the relationship is causal. Production of nitric oxide by NOS2 may affect arginase1 expression. Arginase and the NOS isozymes are capable of regulating each other’s activity. In addition to reducing the availability of L-arginine substrate for NOS, localized low L-arginine concentration created by increased arginase activity has also been shown to affect NOS2 protein expression [El-Gayar et al., 2003; Lee et al., 2003]. NOS enzyme activity regulates arginase activity through the production of the NG-hydroxy-L-arginine (NOHA) reaction intermediate, which acts as an inhibitor of the arginase enzymes [El-Gayar et al., 2003]. Nitrosation of signal transduction enzymes has also been shown to be a mechanism for regulating gene expression. Ckless et al. [2008] noted increased nitrosated protein content in the bronchiolar epithelium and increased total nitrotyrosince content in the peribronchial regions. Ckless et al. [2007] had previously shown that DNA binding activity of NF-κB is modulated by nitrosation of a cysteine residue in the p50 subunit and administration of the arginase inhibitor, S-(2-boronoethyl)-L-cysteine (BEC), showed increased DNA binding by NF-κB.
Although NOS2 expression, via NF-κB, has been shown to depend on cellular NO concentrations [Ckless et al., 2007; 2008; Hirota et al., 1999; Gobeil et al., 2006], the effects of NO on arginase1 expression have not been examined. The arginase1 gene has numerous upstream regulatory regions that may potentially be affected by nitrosation events upstream of transcription factor binding, in addition to Histone3 (H3) acetylation events. In our study, differences in arginase1 expression data in the C57BL/6 and NOS2 knockout mice exposed to OVA ± nor-NOHA suggest that NOS2 activity is capable of regulating arginase1 protein expression.
The results of our study vary significantly from the results of Ckless et al. [2008], who observed increased inflammation and AHR with BEC-mediated inhibition of arginase in the Balb/ C mouse model. This difference may be due to the difference in mouse strain. We chose to study the C57BL/6 mouse strain because of the availability of the NOS2 knockout strain. These two mouse models differ in their arginase1 response after OVA exposure (Bratt et al., 2009; Kenyon et al., 2008).
We conclude that arginine metabolism plays an important role in the development of airway hyper-responsiveness and eosinophilic inflammatory cell influx. Inhibition of arginase early in the allergic inflammatory response decreases the severity of the chronic inflammatory phenotype. These effects appear to be attributable to NOS2, though the effects of arginase inhibition may also be affecting the turnover of arginine by other NOS isoforms such as NOS1 and NOS3. The increased L-arginine content in the airway compartment may directly or indirectly, through NOS2, control arginase expression both in response to OVA exposure and at a basal level. Interestingly, the balance of arginase and nitric oxide synthase metabolism has also been implicated in the pathogenesis of another chronic lung disease, chronic obstructive pulmonary disease (COPD) [Tadie et al., 2008].
Acknowledgments
We thank Kaitlin Louie and Michelle Rogowsky for their help during an internship in our laboratory over the summer of 2008 and 2009, respectively, and the intellectual and technical input of Teresa Wegesser and Amir Zeki throughout the study.
This work was funded in part by grants from the NHLBI (K08-N.J.K.) and by NCRR, a component of the NIH Roadmap for Medical Research (N.J.K.). Jennifer M. Bratt was supported by an NHLBI and an NIEHS training grant during this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagnost T, Berthelot A, Bouhaddi M, Laurant P, André C, Guillaume Y, Demougeot C. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J Hypertens. 2008;26:1110–1118. doi: 10.1097/HJH.0b013e3282fcc357. [DOI] [PubMed] [Google Scholar]
- Belik J, Shehnaz D, Pan J, Grasemann H. Developmental changes in arginase expression and activity in the lung. Am J Physiol Lung Cell Mol Physiol. 2008;294:L498–504. doi: 10.1152/ajplung.00242.2007. [DOI] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234:273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, Lundblad LK, Norton R, van der Vliet A, Janssen-Heininger YM. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181:4255–4264. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am J Respir Cell Mol Biol. 2007;36:645–653. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs EI, Scheld JS, Sharafi M, Förstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- El-Gayar S, Thuring-Nahler H, Pfeilschifter J, Rollinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol. 2003;171:4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- Escobales N, Rivera-Correa M, Altieri PI, Rodriguez JF. Relationship between NO synthesis, arginine transport, and intracellular arginine levels in vascular smooth muscle cells. Amino Acids. 2000;19:451–468. doi: 10.1007/s007260070023. [DOI] [PubMed] [Google Scholar]
- Eynott PR, Groneberg DA, Caramori G, Adcock IM, Donnelly LE, Kharitonov S, Barnes PJ, Chung KF. Role of nitric oxide in allergic inflammation and bronchial hyperresponsiveness. Eur J Pharmacol. 2002;452:123–133. doi: 10.1016/s0014-2999(02)02237-9. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Jr, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, D'Orléans-Juste P, Goetzl EJ, Chemtob S. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem. 2006;281:16058–16067. doi: 10.1074/jbc.M602219200. [DOI] [PubMed] [Google Scholar]
- Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- Iniesta V, Gómez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxyl-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta V, Gómez-Nieto LC, Molano I, Mohedano A, Carcelén J, Mirón C, Alonso C, Corraliza I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002;24:113–118. doi: 10.1046/j.1365-3024.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- Jeyabalan G, Klune JR, Nakao A, Martik N, Wu G, Tsung A, Geller DA. Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric Oxide. 2008;19:29–35. doi: 10.1016/j.niox.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon NJ, Bratt JM, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol. 2008;230:269–275. doi: 10.1016/j.taap.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon NJ, van der Vliet A, Schock BC, Okamoto T, McGrew GM, Last JA. Susceptibility to ozone-induced acute lung injury in iNOS-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L540–545. doi: 10.1152/ajplung.00297.2001. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Ward RW, Last JA. Airway fibrosis in a mouse model of airway inflammation. Toxicol Appl Pharmacol. 2003;186:90–100. doi: 10.1016/s0041-008x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- King NE, Rothenberg ME, Zimmermann N. Arginine in asthma and lung inflammation. J Nutr. 2004;34:2830S–2836S. doi: 10.1093/jn/134.10.2830S. [DOI] [PubMed] [Google Scholar]
- Lara A, Khatri SB, Wang Z, Comhair SA, Xu W, Dweik RA, Bodine M, Levison BS, Hammel J, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Gaston B, Israel E, Jarjour N, Moore W, Erzurum SC. Alterations of the Arginine Metabolome in Asthma. Am J Respir Crit Care Med. 2008;178:673–681. doi: 10.1164/rccm.200710-1542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginase paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarsingh H, Bossenga BE, Bos IS, Volders HH, Zaagsma J, Meurs H. L-arginine deficiency causes airway hyperresponsiveness after the late asthmatic reaction. Eur Respir J. 2009;34:191–199. doi: 10.1183/09031936.00105408. [DOI] [PubMed] [Google Scholar]
- Maarsingh H, Leusink J, Bos IS, Zaagsma J, Meurs H. Arginase strongly impairs neuronal nitric oxide-mediated airway smooth muscle relaxation in allergic asthma. Respir Res. 2006;7:6–12. doi: 10.1186/1465-9921-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarsingh H, Zuidhof AB, Bos ST, van Duin M, Boucher J, Zaagsma J, Meurs H. Arginase inhibition protects against allergic airway obstruction, hyperresponsiveness & inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- Martín L, Comalada M, Marti L, Closs EI, MacLeod CL, Martín del Río R, Zorzano A, Modolell M, Celada A, Palacín M, Bertran J. Granulocyte-macrophage colony-stimulating factor increases L-arginine transport through the induction of CAT2 in bone marrow-derived macrophages. Am J Physiol Cell Physiol. 2005;290:C1364–1372. doi: 10.1152/ajpcell.00520.2005. [DOI] [PubMed] [Google Scholar]
- Meurs H, Hamer MA, Pethe S, Vadon-Le Goff S, Boucher JL, Zaagsma J. Modulation of cholinergic airway reactivity and nitric oxide production by endogenous arginase activity. Br J Pharmacol. 2000;130:1793–1798. doi: 10.1038/sj.bjp.0703488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs H, McKay S, Maarsingh H, Hamer MA, Macic L, Molendijk N, Zaagsma J. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Br J Pharmacol. 2002;136:391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- Nicholson B, Manner CK, Kleeman J, MacLeod CL. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem. 2001;276:15881–15885. doi: 10.1074/jbc.M010030200. [DOI] [PubMed] [Google Scholar]
- Nijkamp FP, van der Linde HJ, Folkerts G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro. Role of the epithelium. Am Rev Respir Dis. 1993;148:727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L911–920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- Reid KM, Tsung A, Kaizu T, Jeyabalan G, Ikeda A, Shao L, Wu G, Murase N, Geller DA. Liver I/R injury is improved by the arginase inhibitor, N(omega)-hydroxy-nor-L-arginine (nor-NOHA) Am J Physiol Gastrointest Liver Physiol. 2007;292:G512–G517. doi: 10.1152/ajpgi.00227.2006. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL. cNOS–iNOS paradigm and arginase in asthma. Trends Pharmacol Sci. 2003;24:560–561. doi: 10.1016/j.tips.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sarwar G, Botting HG. Rapid analysis of nutritionally important free amino acids in serum and organs (liver, brain, and heart) by liquid chromatography of precolumn phenylisothiocyanate derivatives. J Assoc Off Anal Chem. 1990;73:470–475. [PubMed] [Google Scholar]
- Shen LJ, Beloussow K, Shen WC. Accessibility of endothelial and inducible nitric oxide synthase to the intracellular citrulline-arginine regeneration pathway. Biochem Pharmacol. 2005;69:97–104. doi: 10.1016/j.bcp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Tadié JM, Henno P, Leroy I, Danel C, Naline E, Faisy C, Riquet M, Levy M, Israël-Biet D, Delclaux C. Role of nitric oxide synthase/arginase balance in bronchial reactivity in patients with chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;294:L489–497. doi: 10.1152/ajplung.00109.2007. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Ogino K, Shibamori M, Gondo T, Hitomi Y, Takigawa T, Wang DH, Takaki J, Ichimura H, Fujikura Y, Ishiyama H. Transiently, paralleled upregulation of arginase and nitric oxide synthase and the effect of both enzymes on the pathology of asthma. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1419–1426. doi: 10.1152/ajplung.00418.2006. [DOI] [PubMed] [Google Scholar]
- Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;53:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Broeke R, De Crom R, Van Haperen R, Verweij V, Leusink-Muis T, Van Ark I, De Clerck F, Nijkamp FP, Folkerts G. Overexpression of endothelial nitric oxide synthase suppresses features of allergic asthma in mice. Respir Res. 2006;7:58–70. doi: 10.1186/1465-9921-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther. 2006;318:1368–1374. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: Nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]