Abstract
One proposed mechanism of neuronal death associated with a variety of neurodegenerative diseases is the response of neurons to oxidative stress and consequent cytosolic Ca2+ overload. The predominant hypothesis is that cytosolic Ca2+ overload leads to mitochondrial Ca2+ overload and prolonged opening of the permeability transition pore (PTP), resulting in mitochondrial dysfunction. Elimination of cyclophilin D (CyPD), a key regulator of the PTP, results in neuroprotection in a number of murine models of neurodegeneration in which oxidative stress and high cytosolic Ca2+ have been implicated. However, the effects of oxidative stress on the interplay between cytosolic and mitochondrial Ca2+ in adult neurons and the role of the CyPD-dependent PTP in these dynamic processes have not been examined. Here, using primary cultured cerebral cortical neurons from adult wild type (WT) and mice missing cyclophilin D (CyPD-KO), we directly assess cytosolic and mitochondrial Ca2+, as well as ATP levels, during exposure to oxidative stress. Our data demonstrate that during acute oxidative stress mitochondria contribute to neuronal Ca2+ overload by release of their Ca2+ stores. This result contrasts with the prevailing view of mitochondria as a buffer of cytosolic Ca2+ under stress conditions. In addition, we show that CyPD deficiency reverses the release of mitochondrial Ca2+ leading to lower of cytosolic Ca2+ levels, attenuation of the decrease in cytosolic and mitochondrial ATP, and a significantly higher viability of adult CyPD-KO neurons following exposure of neurons oxidative stress. The study offers a first insight into the mechanism underlying CyPD-dependent neuroprotection during oxidative stress.
Keywords: calcium homeostasis, mitochondria, oxidative stress, permeability transition pore, neuroprotection, cortical neurons, mutant mice
INTRODUCTION
Oxidative and excitotoxic stress are common in a number of neurologic diseases, including ischemic infarction, multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer’s, Huntington’s and Parkinson’s disease (Sayre et al., 2008; Niizuma et al., 2009). While the mechanisms underlying excitotoxic neuronal injury have been widely investigated (Pivovarova et al., 2008; Li et al., 2009; Stanika et al., 2009; Brustovetsky et al., 2010), the response of neurons to oxidative challenges (Reynolds et al., 2007) remains incompletely understood. Both exogenous reactive oxygen species (ROS) and excitotoxic stress lead to increased neuronal cytosolic Ca2+. Elevated cytosolic Ca2+ can then directly result in mitochondrial Ca2+ overload and dysfunction (Vanlangenakker et al., 2008; Krieger and Duchen, 2002). In healthy neurons, mitochondria efficiently buffer cytosolic Ca2+ during normal signaling processes. Given that mitochondrial Ca2+ stimulates several enzymes involved in the generation of ATP, this link serves to regulate energy supply to changing neuronal needs (McCormack et al., 1990; Jouaville et al., 1999; Brookes et al., 2004). However, the critical role of mitochondrial Ca2+ and ATP regulation in neurodegenerative processes associated with oxidative stress remain unknown.
Previously, neuroprotection from exogenous oxidative stress and ensuing high cytosolic Ca2+ has been demonstrated in both cells and mice deficient in the mitochondrial matrix protein cyclophilin D (CyPD), likely through inhibition of the permeability transition pore (PTP) in the absence of CyPD (Schinzel et al., 2005; Bernardi and Forte, 2007; Abramov and Duchen, 2008; Wang et al., 2009). The PTP is a voltage-dependent, cyclosporin A (CsA)-sensitive, high-conductance channel activated by both ROS- and Ca2+ (for reviews see Bernardi, 1999; Bernardi et al., 2006; Rasola and Bernardi, 2007; Baines et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005). CyPD is a key regulator of the PTP and in its absence PTP opening and ensuing mitochondrial failure requires higher levels of individual activators, including mitochondrial Ca2+ and ROS. While CyPD inactivation is neuroprotective in adult mouse models of neurologic diseases, the actual inhibition of the PTP opening via CyPD inactivation, as well as its effects on mitochondrial Ca2+ dynamics and ATP levels, have never been directly established in intact adult neurons during periods of oxidative stress.
Here, using real-time fluorescent imaging and genetically-encoded Ca2+ and ATP indicators targeted to mitochondria, we investigate for the first time mitochondrial Ca2+ and ATP dynamics in intact individual adult cerebral cortical neurons during oxidative stress in the presence and absence of CyPD. The study employs physiologically relevant exogenous ROS levels comparable to those previously found in adult mouse brain in vivo (hydrogen peroxide, H2O2) (Hyslop et al., 1995). In contrast to previous demonstrations of mitochondria as active Ca2+ buffers, our results clearly demonstrate that during elevated intracellular Ca2+ triggered by oxidative stress, mitochondria release their Ca2+ stores and thereby contribute to neuronal Ca2+ overload associated with neuronal death. In CyPD-KO neurons, this process of mitochondrial Ca2+ release is initiated but rapidly reversed. Consequently this study offers a first insight into the mechanism underlying neuroprotection from ROS injury via CyPD inactivation in adult neurons.
MATERIALS AND METHODS
Animals
The generation of CyPD-KO mice in which the nuclear Ppif gene encoding CyPD has been eliminated has been previously described (Basso et al., 2005). The Ppif-null mouse colonies were maintained as homozygotes. All experimental procedures were conducted following NIH guidelines under an Institutional Animal Care and Use Committee (IACUC)-approved protocol from the Oregon Health & Science University.
Constructs
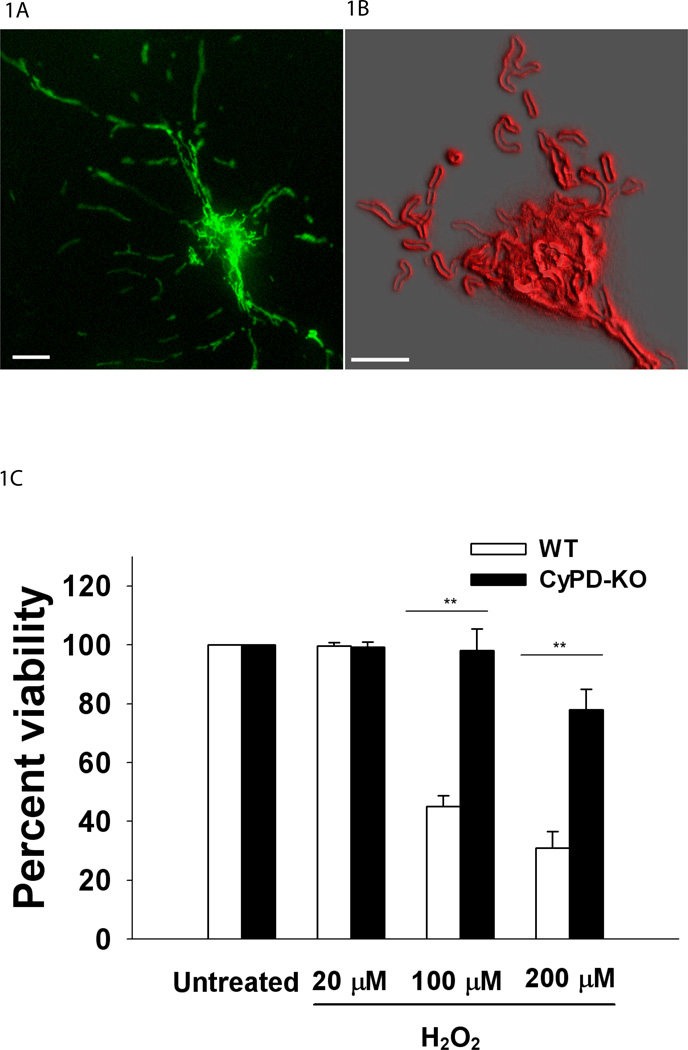
Mitochondrial Ca2+ levels were evaluated following transfection of neuronal cultures with the genetically-encoded, Ca2+ sensor, ratiometric pericam (Fig.1A) (mitoRP; 415 and 494 nm excitation, 515 nm emission) [kindly provided by Dr. Atsushi Miyawaki, Institute of Physical and Chemical Research, Japan;(Nagai et al., 2001)]. This reporter was specifically directed to mitochondria by use of a duplicated mitochondrial targeting sequence (mitoRP) (Filippin et al., 2005) increasing the delivery of mitoRP constructs to the mitochondrial matrix as well as expanding the dynamic range over which mitochondrial Ca2+ levels can be evaluated (Filippin et al., 2003). Notably, the ratiometric nature of this probe minimizes differences in the data reported by mitoRP arising from variations in the levels of expression of this genetically-encoded indicator between individual neurons. To assess changes in mitochondrial matrix pH, neuronal cultures were transfected with EYFP modified as described for mitoRP (mitoYFP; 500 nm excitation, 535 nm emission). Mitochondrial morphology was reported by a fluorescent probe targeted to the outer mitochondrial membrane (Fig.1B). This reporter represents a fusion protein containing the outer membrane peripheral benzodiazapine receptor (PBR) (Papadopoulos et al., 2006) coupled to a GFP derivative (Miesenbock et al., 1998) located at the C-terminus (mitoPBRf; 380 and 460 nm excitation, 515 nm emission). The C-terminus of the PBR, and hence the GFP derivative, faces the cytosol (Joseph-Liauzun et al., 1998). ATP levels were assessed in mitochondria and cytosol using FRET-based reporters, mitAT1.03 and cytAT1.03 plasmids, targeted to mitochondria and cytoplasm respectively (430 nm excitation, 470/525 nm emission). These constructs contain YFP and CFP separated by the ε subunit of a bacterial F0F1 ATPase. The reporters were kindly provided to us by Dr. Hioyuki Noji, Institute for Scientific and Industrial Research, Japan (Imamura et al., 2009).
Figure 1.
A. Visualization of mitochondria in a live adult neuron. Image of a cultured cortical neuron transfected with mitoRP, 60× oil. Scale=10 µm. B. Representative mitochondrial populations analyzed for Ca2+mito dynamics in neuronal soma. Image (magnification 240×, oil) of the soma region in a live neuron transfected with mitoPBRf, targeted to the outer mitochondrial membrane. Red outline represents outer border of mitochondria using deconvolution, 3D reconstruction and 3D edge detection. Scale=5 µm. C. Neuronal viability as assessed by Calcein AM 24 hours after H2O2 treatment. Neurons were treated with for 15 minutes. ** P < 0.01.
Adult neuronal cell culture and transfection
For each experiment two-to-four month-old WT and CyPD-KO mice matched by gender were used. Adult neuronal culture were prepared as outlined in Nathan et al., 2004. The entire cerebral cortex was dissected from the brain and placed in 2 ml B27/Hibernate A medium (B27/HA, Invitrogen) with 0.5 mM glutamine (Sigma) at 4 C°. The cortex was sliced (0.5 mm thickness) and transferred to a 50-ml tube containing 5 ml B27/HA. After warming for 8 min at 30 C°, slices were digested with 6 ml of a 2 mg/ml papain (Sigma) solution in B27/HA for 30 min at 30 C° in a gyrating water bath. The slices were transferred to 2 ml B27/HA. After 2 min at room temperature, the slices were triturated 10 times with a siliconized 9-inch Pasteur pipette, and allowed to settle for 1 min. Approximately 2 ml of the supernatant were transferred to another tube, and the sediment resuspended in 2 ml B27/HA. The above step was repeated twice, and a total of 6 ml collected. The resultant supernatant was subjected to density gradient centrifugation at 800 × g for 15 min. The density gradient was prepared in four 1-ml layers of 35, 25, 20, and 15% Optiprep (Invitrogen) in B27/HA medium (vol/vol). Debris above 7 ml was discarded. The rest of the fractions, excluding the bottom pellet, were collected and diluted in 5 ml of B27/HA. After centrifuging twice at 200 × g for 2 min., the cell pellets were resuspended in 3 ml B27/Neurobasal A medium (Invitrogen) with 0.5 mM glutamine and 0.01 mg/ml gentamicin (Sigma). For transfection, neuronal pellets were re-suspended in 100 µl of nucleofection solution with 3 µg/ml of each plasmid construct and electroporated following Amaxa electroporation system (Amaxa) for neurons, modified by the use of a Ca2+-free buffer immediately after the electroporation, which maintained neuronal viability. A total of 3 × 104 cells were plated in 30-µl aliquots in the center of glass cover slips (25 mm diameter) that were coated overnight with poly-D-lysine (50 mg/ml, Sigma). After 1 h incubation in a humidified incubator at 37 C and 5% CO2, each cover slip was rinsed with B27/HA and transferred to a 6-well plate containing B27/neurobasal A medium. Routinely, 10% of neurons were successfully transfected and the mitoRP signal is detectable 48 hours post transfection.
Use of Fura-2, TMRM and FCCP
For cytosolic Ca2+ measurements, cells were loaded with 5 µM fura-2 (340 and 380 nm excitation, 505 nm emission; Molecular Probes) in imaging buffer (142 mM NaCl, 4 mM NaHCO3, 10 mM Na-Hepes, 2.5 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, and 10 mM glucose, pH 7.4) containing 2% BSA (Sigma) for 15–20 min at 37°C. Cells were washed with imaging buffer containing 0.25% BSA for 10 min at 37°C before recording. For mitochondrial membrane potential measurements, cells were loaded with 10 nM tetramethyl rhodamine methyl ester (TMRM, 535 nm excitation, 575 nm emission; Sigma) and incubated in the presence of 1.6 µM cyclosporin H (Alexis Biochemicals) for 30 min at 37°C exactly as outlined in (Petronilli et al., 2001). Neurons were perfused and imaged in imaging buffer containing 10 nM TMRM (Sigma) and cyclosporin H. Neurons from the same culture preparation but lacking mitoRP were evaluated for cytosolic Ca2+ and mitochondrial membrane potential. To depolarize mitochondria, the protoionophore carbonyl cyanide p-trifluoromethoxy phenyl hydrazone [450 nM; FCCP (Sigma)] was added to cultures.
Fluorescence imaging
Imaging was carried out after 4–5 days in culture. WT and CyPD-KO neurons were perfused (2 ml/min) with 20 µM or 100 µM hydrogen peroxide (H2O2) for 8.5 min starting at 30 sec with imaging buffer containing H2O2 in the presence of 5% CO2 air mixture at 37°C (heated stage, Warner Instruments). Prior to H2O2 treatment, mitochondrial or cytosolic Ca2+ levels among neurons of either genotype were similar (Suppl. Fig. 1A–B). Neurons were also treated with adenosine triphosphate (ATP, Sigma) or potassium chloride (KCl, Sigma) for 30 sec simultaneously with H2O2. Imaging was carried out using an inverted microscope (Olympus IX81, 150×, UPlan, NA 1.45, oil) equipped with a cooled CCD camera (Cascade II; Intelligent Imaging Innovations), a high speed wavelength switcher (Lambda DG-5, Sutter Instruments) controlled by SlideBook software (Intelligent Imaging Innovations), and appropriate Chroma filters. Exposure time was 100–200 msec and frames were taken every 0.5–2 sec. After stimulation and recording, cells were not re-used. Mitochondrial Ca2+ (evaluated in mitoRP-transfected neurons), mitochondrial membrane potential (evaluated by TMRM), mitochondrial pH (evaluated by mitoYFP) and mitochondrial and cytosolic ATP levels (evaluated by mitAT1.03 and cytAT1.03, respectively) were assessed in both clusters of mitochondria and individual mitochondria in the neuronal soma (Fig. 1A–B). Data obtained from mitochondria were averaged to represent the response per cell. Cytosolic Ca2+ was evaluated using fura-2 in the perinuclear region of a cell.
Image analysis
Fluorescence changes in mitochondria or cytosol were evaluated using SlideBook software version 4.2.0. The background fluorescence was taken from fields not containing neurons. For imaging fluorescence changes in mitochondria, individual mitochondria and clusters of mitochondria in the soma of a neuron were chosen by using the Mask feature of this software. Movements of mitochondria were tracked throughout each recording by using the particle tracking function of Slidebook. The response from each cell was normalized to the baseline value before stimulation using Excel software.
Immunocytochemistry and Viability Assays
Immunocytochemistry was carried out with antibodies for neurons (β-tubulin III, Molecular Probes), astrocytes (glial fibrillary acidic protein, Molecular Probes), oligodendrocytes (myelin basic protein, Molecular Probes) and microglia (BS lectin 1, Sigma). Cultures were routinely found to consist of 70% neurons and 30% glia. It should be noted that astrocytes provide neuroprotection in response to H202 (Desagher et al., 1996). Given 4.6:1 ratio of neurons to astrocytes in our model, we believe it is more likely that CyPD deficiency in adult neurons, not glia, provides neuroprotection during oxidative stress but definitive demonstration of this will require specifically inactivating CyPD in neurons. Cortical neurons were routinely grown in a serum-free Neurobasal A medium optimized for neurons and supplemented with a B-27 cocktail. This medium includes components which protect neurons from the oxidative stress. Cultures were transferred to a B27-free medium 12 hours prior to H2O2 treatment. WT and CyPD-KO neurons were treated for 15 minutes with 20, 100 and 200 µM H2O2 and their viability was assessed 24 hours after the treatment using Calcein AM dye. Live neurons were counted in five random fields of view per cover slip using 20× objective. The viability data from H2O2 treatments were normalized to the viability of the untreated culture.
Statistical analysis
Each experiment was replicated three times using neurons obtained from two-three different animals per genotype (n=2–3). Data from each experiment were analyzed by one-way ANOVA with SPSS statistical software (version 15.0 for Windows, IBM Corporation, Route 100 Somers, NY 10589), followed by Bonferroni post-hoc test when needed to analyze data between two or more groups. There was no significant difference in responses within a genotype from animal to animal. Within individual neurons, the mitochondrial responses were remarkably consistent (variation < 5%); as a result, the responses within a neuron could be effectively averaged to generate the response for one neuron. The data from all evaluated cells from all animals per genotype are presented as mean ± standard error of the difference (SED) in the Results section bar graphs.
RESULTS
CyPD Deficiency Increases Adult Neuronal Viability following Oxidative Stress
In order to assess the effect of oxidative stress and CyPD deficiency on neuronal viability following exposure to H202, a classical ROS, primary cortical cultures were prepared from adult isogeneic wild-type (WT) and CyPD-KO (Ppif−/−) mice (Basso et al., 2005). Cultures from adult mice were used since Ca2+ dynamics and neuronal sensitivity to PTP opening are age-dependent (Lalo and Kostyuk, 1998; Wang et al., 2009), and neonatal or embryonic neuronal cultures may not reflect the Ca2+ dynamics in response to oxidative stress in adult mouse models of disease (Schinzel et al., 2005; Bernardi and Forte, 2007; Abramov and Duchen, 2008; Wang et al., 2009). WT and CyPD-KO adult neurons were treated for 15 minutes with 20 µM, 100 µM and 200 µM H2O2 and their viability was assessed after 24 hours using Calcein AM, which stains viable neurons. The results showed that adult CyPD-KO neurons are significantly more resistant to H202 exposure than adult WT neurons in a concentration-dependent manner starting at 100 µM (percent of live neurons compared to the viability of untreated cultures: WT 45%±3.7 vs. CyPD-KO 98%±7.5, F5,74=7.21, P=0.00003, WT n=40 random fields of view, CyPD-KO n=40) (Fig.1C); 15 min exposure to 100 µM H2O2 did not affect the viability of CyPD-KO neurons (Fig. 1C), while almost 60% of WT neurons were lost. Thus, CyPD deficiency provides significant protection to adult neurons during oxidative stress.
Neuronal Mitochondria Release Ca2+ during Oxidative Stress: Reversal via CyPD Deficiency
Mitochondrial Ca2+ levels were evaluated following transfection of neuronal cultures with mitoRP (Nagai et al., 2001) (Fig. 1A). Absence of photo-bleaching of mitoRP is shown in Suppl.Fig.1A. In trace graphs, the baseline recordings (first 0.5 min) demonstrate stable mitochondrial Ca2+ levels before treatment. Neurons were continuously perfused with imaging buffer containing 20 µM or 100 µM H2O2 for 8.5 min starting at 0.5 min. These concentrations of H2O2 are comparable to those previously found in adult murine brain in vivo following global forebrain ischemia and reperfusion (100 µM H2O2) (Hyslop et al., 1995).
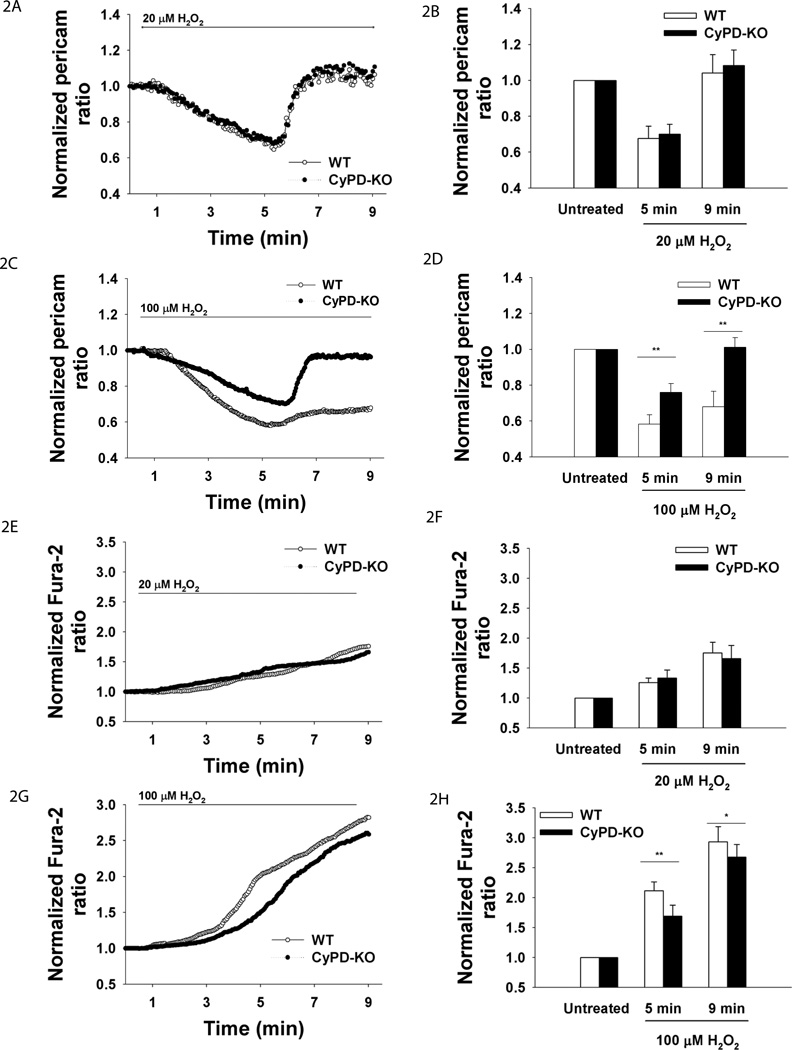
Since it had previously been show that H2O2 treatment increases cytosolic Ca2+ (Pouokam et al., 2009), we anticipated that exposure of adult neurons to H2O2 would lead to an increase in mitochondrial Ca2+ based on previously established mitochondrial Ca2+ uptake following cytosolic Ca2+ rise in sympathetic neurons (Friel, 2000). However, exposure to both 20µM and 100 µM H2O2 led to more than a 30% decrease in mitochondrial Ca2+ in neurons of both genotypes during the first 4.5 minutes of exposure (Fig. 2A–D). Following this initial response, mitochondrial Ca2+ had a steep return to the baseline in neurons of both genotypes treated with 20 µM H2O2 (Fig. 2A). A similar return to baseline mitochondrial Ca2+ levels was observed in CyPD-KO neurons treated with 100 µM H2O2 (Fig. 2C), but not in correspondingly treated WT neurons (Fig. 2C). Therefore, mitochondrial Ca2+ in WT neurons was significantly lower than in CyPD-KO neurons starting at 5 minutes of exposure to 100 µM H2O2 (mitochondrial Ca2+ as reported by mitoRP at 5 min: WT 0.58±0.05 vs. CyPD-KO 0.76±0.04, F5,91=5.62, P=0.0006; at 9 min: WT 0.67±0.08 vs. CyPD-KO 1.01±0.05, F5,91=5.85, P=0.0002, WT n=48 neurons, CyPD-KO, n=49) (Fig. 2C).
Figure 2.
Mitochondrial and cytosolic Ca2+ in adult neurons during oxidative stress. Neurons were perfused with H2O2 for 8.5 min. Ca2+mito was reported by mitoRP. Ca2+cyto was reported by fura-2. A. Mean traces of Ca2+mito in response to 20 µM H2O2. B. Quantification of Ca2+mito at baseline, at 5 and 9 min. **P<0.01. C. Mean traces of Ca2+mito in response to 100 µM H2O2. D. Quantification of Ca2+mito, 100 µM H2O2. **P<0.01. E. Mean traces of Ca2+cyto in response to 20 µM H2O2. F. Quantification of Ca2+cyto, 20 µM H2O2. P>0.05. G. Mean traces of Ca2+cyto in response to 100 µM H2O2. H. Quantification of Ca2+cyto, 100 µM H2O2. *P<0.05. **P<0.01.
Since mitoRP is sensitive to the effects of alkaline pH (Nagai et al., 2001, Filippin et al., 2005), we tested whether changes in mitochondrial pH could be responsible for the mitoRP responses observed. Transfection of mitochondrially-targeted EYFP (mito-EYFP), exhibiting pH-dependent changes in fluorescence (Takahashi et al., 2001), demonstrated an insignificant decrease in pH within first 7 minutes of exposure to H2O2. Furthermore, there was no significant difference in mitochondrial pH between the genotypes during ROS treatment (Suppl. Fig. 2A,B). Indeed, individual traces at each wavelength of mitoRP (e.g., pH-insensitive 405 nm wavelength) clearly demonstrated the differences in fluorescence observed are due to changes in mitochondrial Ca2+ and not in pH (Suppl.Fig. 3C–F).
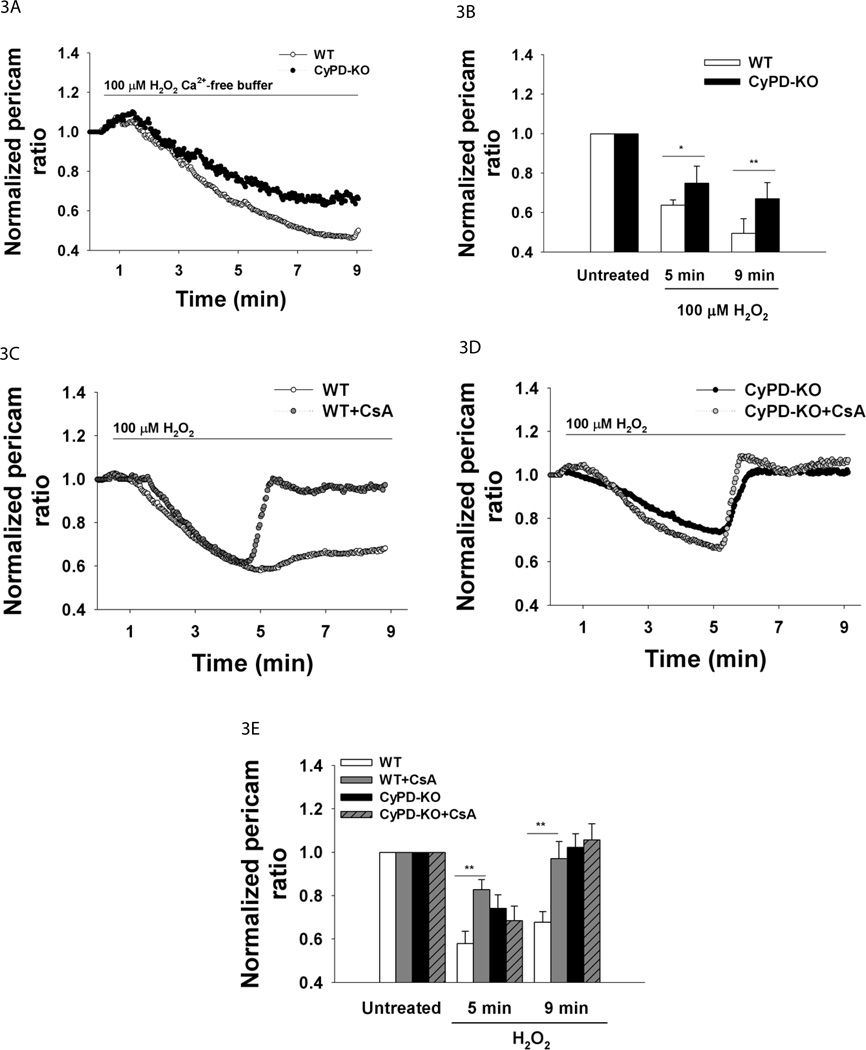
Next, we tested cytosolic Ca2+ under the same treatments by using Fura-2 (Suppl.Fig.1B shows photostability of Fura-2 in our experimental protocol). As expected, dramatic cytosolic Ca2+ increases occurred during 20 µM H2O2 (WT n=47 neurons, CyPD-KO n=51) and 100 µM H2O2 treatments (WT n=30 neurons, CyPD-KO n=31) (Fig. 2E–H, Suppl.Fig. 1C–D traces of individual cells). However, while the changes in cytosolic Ca2+ were not significantly different between the two genotypes following exposure to 20 µM H2O2, significantly higher cytosolic Ca2+ increases occurred in WT neurons than in CyPD-KO neurons treated with 100 µM H2O2 (cytosolic Ca2+ as reported by fura-2 at 5 min: WT 2.11±0.15 vs. CyPD-KO 1.69±0.18, F5,55=3.91, P=0.008; at 9 min: WT 2.93±0.25 vs. CyPD-KO 2.67±0.21, F5,55=3.86, P=0.01 WT n=30 neurons, CyPD-KO n=31) (Fig. 2G). Higher cytosolic Ca2+ level in WT neurons during 100 µM H2O2 treatment starting at 5 min (Fig. 2G–H) corresponded to lower mitochondrial Ca2+ levels in WT neurons starting at 5 min (Fig. 2C–D), demonstrating the lack of efficient buffering by mitochondria in WT neurons when compared to CyPD-KO neurons. Since the differences in adult neuronal viability and mitochondrial and cytosolic Ca2+ levels between genotypes were apparent only at 100 µM H2O2, we used this concentration in our further investigations. Notably, in absence of extracellular Ca2+, mitochondrial Ca2+ failed to reverse in CyPD-KO neurons in response to 100 µM H2O2 (WT n=8 neurons, CyPD-KO n=9) (Fig. 3A–B), indicating that extracellular Ca2+ is the major source of mitochondrial Ca2+ re-uptake during oxidative stress in CyPD-KO neurons.
Figure 3.
Mitochondrial Ca2+ and oxidative stress. A. Mean traces of Ca2+mito in adult neurons in response to 100 µM H2O2 in Ca2+-free buffer. Neurons were treated with H2O2 for 8.5 min. Ca2+mito was reported by mitoRP. B. Quantification of Ca2+mito at baseline, at 5 and 9 min. WT, n=8; CyPD-KO, n=9; **p<0.01. C. Mean traces of Ca2+mito in WT neurons and WT neurons pretreated with CsA in response to 100 µM H2O2. Neurons were pretreated with 10 µM CsA for 30 min and then perfused with H2O2 for 8.5 min. Ca2+mito was reported by mitoRP. D. Mean traces of Ca2+mito in CyPD-KO neurons and CyPD-KO neurons pretreated with CsA in response to 100 µM H2O2. E. Quantification of Ca2+mito in WT and CyPD-KO adult neurons with or without CsA pretreatment at baseline, 5 and 9 min. **p<0.01.
In order to confirm the role of CyPD deficiency in modulating mitochondrial Ca2+ dynamics under exogenous oxidative stress, mitochondrial Ca2+ in WT neurons was assessed following pharmacological inactivation of CyPD by cyclosporin A (CsA). Mitochondrial Ca2+ responses in WT neurons pretreated for 30 min with 10 µM CsA were similar to those in CyPD-KO neurons in response to 100 µM H2O2 (mitochondrial Ca2+ as reported by mitoRP at 5 min: WT 0.57±0.05 vs. WT+CsA 0.82±0.04, F5,72=3.84, P=0.009; at 9 min: WT 0.67±0.04 vs. CyPD-KO 0.97±0.07, F5,72=3.98, P=0.007, WT n=48 neurons, WT+CsA n=30) (Fig. 3C,E compare with 2C,D). As expected, pretreatment of CyPD-KO neurons with CsA had no affect on mitochondrial Ca2+ levels when compared to untreated CyPD-KO neurons (Fig. 3D).
These results demonstrate a novel aspect of mitochondrial function during oxidative stress, release of its Ca2+ stores that contribute to cytosolic Ca2+ increases. Since the PTP serves as an additional Ca2+ efflux pathway and CyPD is an established regulator of the PTP, modulation of mitochondrial Ca2+ release via genetic or pharmacological CyPD inactivation shown here suggests that such release occurs in response to ROS-driven PTP opening. These results are consistent with the notion that CyPD inactivation inhibits the release of mitochondrial Ca2+ stores after 5 min of exposure to high oxidative stress by attenuating the PTP opening. This allows for normalization of mitochondrial Ca2+, which is necessary for normal mitochondrial function, including ATP production.
CyPD Deficiency Modulates Δψ Changes and ATP Levels during Oxidative Stress
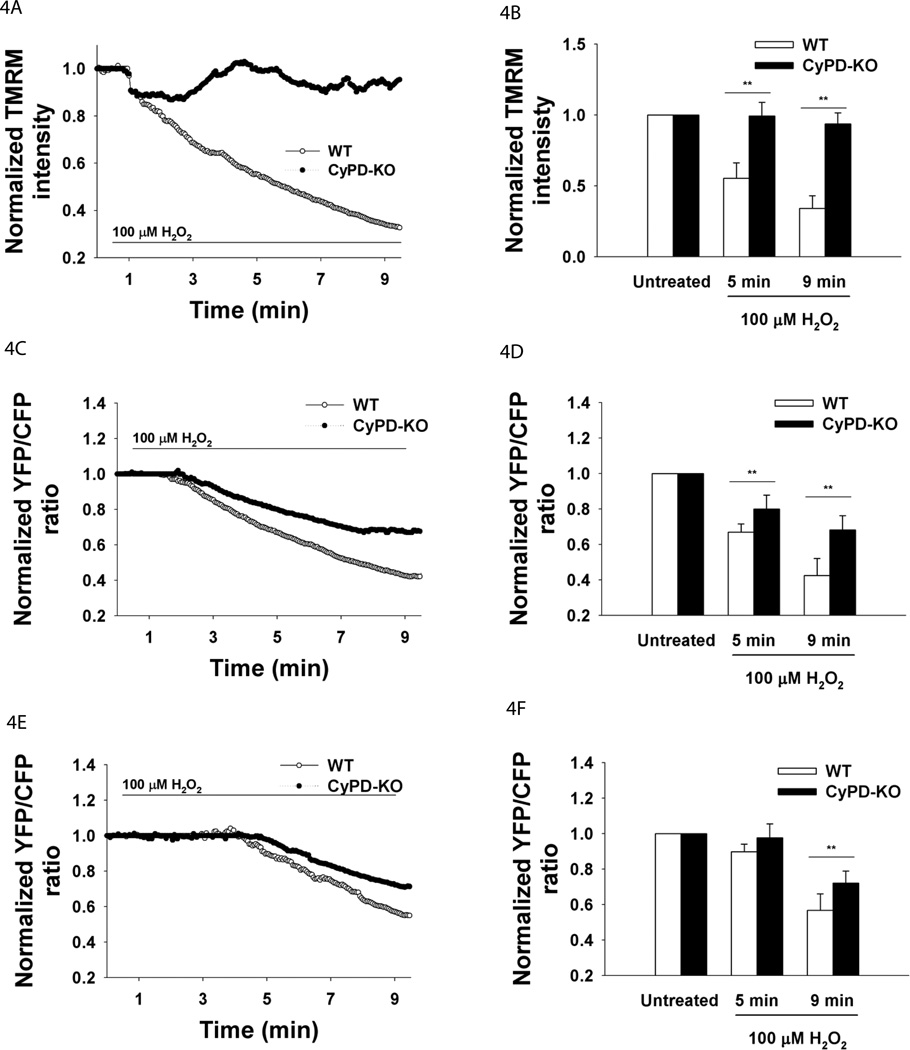
To assess whether PTP opening is transient or persistent in adult neurons under oxidative stress, cultures were loaded with TMRM to measure the changes in the inner mitochondrial membrane (IMM) potential ΔΨ. The IMM potential dissipates following persistent PTP opening (Nicholls and Ward, 2000). A fast initial decrease of 10% in ΔΨ occurred in the first two minutes of exposure to 100 µM H2O2 in both genotypes (Fig. 4A,B). In the next six minutes ΔΨ continued to decrease gradually to 70% of the initial value in WT neurons, whereas in CyPD-KO neurons, ΔΨ recovered to the pretreatment levels (Δψ as reported by TMRM at 5 min: WT 0.55±0.11 vs. CyPD-KO 0.99±0.09, F5,65=5.42, P=0.0007; at 9 min: WT 0.34±0.09 vs. CyPD-KO 0.93±0.07, F5,65=7.21, P=0.0001, WT n=35 neurons, CyPD-KO n=36) (Fig. 4A,B). ΔΨ dissipation in mitochondria of WT neurons is consistent with the previous findings showing ΔΨ decrease in retinal ganglion cells in response to H2O2 (Lieven et al., 2003). These findings suggest that oxidative stress in WT neurons results in persistent opening of the PTP whereas in neurons lacking CyPD the opening of the PTP is transient.
Figure 4.
Δψ, ATP and oxidative stress. Neurons were treated with 100 µM H2O2 for 8.5 min. Δψ was reported by TMRM. ATPmito was reported by mitAT1.03 and ATPcyto was reported by cytAT1.03. A. Mean traces of Δψ. B. Quantification of Δψ at baseline, 5 and 9 min (100 H2O2). **P<0.01. C. Mean traces of ATPmito. D. Quantification of ATPmito. **P<0.01. E. Mean traces of ATPcyto. F. Quantification of ATPcyto. **P<0.01.
Since ATP production by mitochondria is dependent on ΔΨ and mitochondrial Ca2+ levels (Brookes et al., 2004), we next assessed the ATP levels in mitochondria and cytosol of adult neurons during oxidative stress by transfection of FRET-based reporters, YFP-ATP (Imamura et al., 2009). These reporters have been demonstrated to respond to ATP levels in the cytoplasm and mitochondria of transfected cells without interference from related cellular nucleotides (e.g. GTP, ADP, dATP) (Imamura et al., 2009). Consequently, a decrease in YFP/CFP FRET ratio represents a decrease in ATP levels. A decrease in mitochondrial ATP level was observed in both WT and CyPD-KO neurons in response to 100 µM H2O2 (Fig. 4C). However, a significantly smaller rate and extent of decline was observed in CyPD-KO neurons compared to WT neurons (mitochondrial ATP levels as reported by mitAT1.03 at 5 min: WT 0.66±0.04 vs. CyPD-KO 0.80±0.07, F5,37=4.94, P=0.005; at 9 min: WT 0.42±0.09 vs. CyPD-KO 0.68±0.08, F5,37=4.45, P=0.008, WT n=21 neurons, CyPD-KO n=22) (Fig. 4D). At the end of the treatment, mitochondrial ATP in CyPD-KO neurons had decreased by 30% compared to a 50% decline of ATP in mitochondria of WT neurons (Fig. 4D). The decline in cytosolic ATP levels was delayed in both genotypes when compared to the mitochondrial ATP levels and occurred around 5 min of H2O2 exposure (Fig. 4E). A significantly greater decline in cytosolic ATP after 5 minutes was observed in WT neurons than in CyPD-KO neurons (cytosolic ATP levels as reported by cytAT1.03 at 9 min: WT 0.56±0.09 vs. CyPD-KO 0.72±0.06, F5,29=4.82, P=0.009, WT n=18 neurons, CyPD-KO n=17) (Fig. 4F). The lower cytosolic ATP level in WT neurons in response to 100 µM H2O2 at 9 min corresponds to the lower mitochondrial ATP level (Fig. 4C,E). These results demonstrate that CyPD deficiency and attenuation of PTP opening results in a lower decrease in mitochondrial and cytosolic ATP in adult neurons during oxidative stress.
DISCUSSION
This study provides new insights into mitochondrial Ca2+ dynamics in neurons undergoing oxidative stress and further insights into the mechanisms of neuroprotection resulting from CyPD inactivation. By directly assessing mitochondrial Ca2+ dynamics in adult neuronal cultures during oxidative stress, our results demonstrate that mitochondria in WT adult neurons do not buffer cytosolic Ca2+ during oxidative stress. Instead, mitochondria release their Ca2+ stores in response to ROS challenges, thus contributing to the cytosolic Ca2+ rise. This response of neuronal mitochondria to ROS is in contrast to the general view of mitochondria as a buffer of rising cytosolic Ca2+ (Krieger and Duchen, 2002; Vanlangenakker et al., 2008). The effect of oxidative stress on mitochondrial Ca2+ thus differs dramatically from the response to physiological Ca2+ fluctuations and excitotoxic stress, which result in increases in mitochondrial Ca2+ (Friel 2000; Brustovetsky et al., 2009). Demonstrating that mitochondria release Ca2+ in response to oxidative stress provides to new insights into how CyPD inactivation provides neuroprotection. As shown here, CyPD inactivation in adult neurons reverses mitochondrial Ca2+ release resulting from oxidative stress, and thereby lessens cytosolic Ca2+ overload and preserves mitochondrial ΔΨ and ATP production, leading to enhanced neuronal survival.
Mitochondrial Ca2+ During Oxidative Stress: release or uptake?
Mitochondria take up Ca2+ following increases in cytosolic Ca2+ due to physiologic fluxes or excitotoxic stress (Friel 2000; Brustovetsky et al., 2009). It has been assumed that mitochondria serve a similar buffering role following increases of cytosolic Ca2+ resulting from oxidative stress. To test this idea, we directly examined mitochondrial Ca2+ in adult neurons during oxidative stress. As expected, exposure to oxidative stress led to a significant increase in cytosolic Ca2+ levels in adult cortical neurons, consistent with previous findings using WT rat myenteric neurons (Pouokam et al., 2009). However, we demonstrated that mitochondria in adult neurons do not take up Ca2+ in response to the cytosolic Ca2+ increase induced by H202. Rather, mitochondria release Ca2+ during the first 5 minutes of exposure to both 20 µM and 100 µM H2O2. Mitochondria, therefore, do not buffer cytosolic Ca2+ under these conditions but act as second contributor, after ER Ca2+ release (Klohn et al., 2003; Gerich et al., 2009), to the cytosolic Ca2+ increase that occurs following oxidative stress.
Two comments about these results are warranted. First, the differences in Ca2+ dynamics at high ROS between WT and CyPD-KO mitochondria theoretically might be explained by differences between the two genotypes in levels of catalase, an enzyme responsible for conversion of H2O2 to water and molecular oxygen (Zamocky et al., 2008). However, since catalase is not a target of CsA, the data showing modulation of mitochondrial Ca2+ via pharmacological inactivation of CyPD in WT neurons with CsA excludes this possibility. Second, our results differ from the previous findings on mitochondrial Ca2+ in endothelial cells by Jornot et al., 1999. That study demonstrated mitochondrial Ca2+ uptake, not release, in endothelial cells in response to H2O2 at a concentration of 1 mM. The differences between our results and those of Jornot et al. may stem from the 10× higher concentration of H202 used in that study, which is a concentration that has not been demonstrated to occur in vivo, and perhaps differences between neurons and endothelial cells.
The second phase of mitochondrial Ca2+ response to 100 µM H2O2 occurring around 5 minutes may be especially important to neuronal death in response to high oxidative stress and the neuroprotective effects of CyPD deficiency. About 5 minutes after exposure to 20 µM H2O2, in both WT and CyPD-KO neurons, there is a fast switch from mitochondrial Ca2+ release to normalization to the baseline Ca2+. However, in WT neurons exposed to 100 µM H2O2, mitochondrial Ca2+ remains at low level whereas in CyPD-KO neurons exposed to 100 µM H2O2, normalization of mitochondrial Ca2+ occurs after about 5 minutes. The reversal of Ca2+ efflux that occurs in both genotypes at low oxidative stress (20 µM H2O2) and in CyPD deficient neurons at high oxidative stress (100 µM H2O2) is an intriguing result that should be further investigated. While the mechanism of this cessation of Ca2+ efflux is uncertain, our data suggests that the PTP may participate in determining whether mitochondrial Ca2+ efflux continues in neurons undergoing oxidative stress. Our results indicate that opening of the PTP, as assessed by changes in ΔΨ, is transient in CyPD-KO neurons undergoing high oxidative stress whereas in WT neurons it is persistent. We hypothesize that oxidative stress causes PTP opening, which contributes to the excessive mitochondrial Ca2+ efflux exceeding the normal rate of mitochondrial Ca2+ efflux via Na+/Ca2+ mitochondrial exchanger. If PTP opening is persistent, as occurs in WT neurons exposed to high concentrations of H2O2, it results in persistent mitochondrial Ca2+ efflux. If PTP opening is transient, as occurs in WT neurons exposed to low concentrations of H2O2 and in CyPD deficient neurons at low and high H2O2 concentrations, mitochondrial efflux ceases and mitochondrial Ca2+ can normalize. The difference in responses due to H2O2 concentration suggests that the CyPD-dependent PTP opening in adult neurons is sensitive to H2O2 concentration, as has been documented in other cell types (as reviewed in Bernardi et al., 2006). Since ER Ca2+ stores and Na+/Ca2+ mitochondrial exchanger, which regulate mitochondrial and cytosolic Ca2+ levels, are not targets of CyPD, our results are consistent with the hypothesis that PTP opening regulates the mitochondrial Ca2+ efflux and consequently influences cytosolic Ca2+ levels during oxidative stress. Further experimentation will be required to elucidate the mechanisms and regulation of mitochondrial Ca2+ efflux in neurons undergoing oxidative stress.
CyPD Deficiency and Adult Neuronal Viability
A major goal of this study was to investigate the mechanisms of neuroprotection in the setting of oxidative stress resulting from CyPD inactivation. Our results demonstrate that cortical neurons cultured from CyPD-KO adult mice are significantly more resistant to H2O2-induced death than WT neurons. These results are similar to that of cultured WT and CyPD-KO neurons from neonatal mice (Forte et al 2007), although the neonatal WT cultures were more resistant to H2O2-induced death.
Importantly, our observations provide experimental support for the previously formulated hypothesis that CyPD deficiency attenuates PTP opening and thereby results in neuroprotection (Baines et al., 2005; Bernardi and Forte, 2007; Wang et al., 2009). In the current study, attenuation of the PTP opening in adult CyPD-KO neurons is demonstrated by our TMRM data. Mitochondria in CyPD-KO adult neurons exposed to H202 experienced a small decrease in ΔΨ that was reversible in comparison to the more profound and prolonged decrease in ΔΨ in mitochondria of WT adult neurons. Since prolonged opening of the PTP results in collapse of the electrical gradient across the inner mitochondrial membrane, these results are consistent with attenuation of PTP opening in response to H202 in CyPD-KO neurons compared with WT neurons.
While the ability of CyPD inactivation to inhibit the persistent opening of the PTP in response to oxidative stress appears associated with the neuroprotection conferred by CyPD inactivation, how does this occur? Our results point to two mechanisms. First, as discussed above, CyPD deficiency reverses mitochondrial Ca2+ efflux resulting from oxidative stress and thereby lowers cytosolic Ca2+. This helps to inhibit activation of Ca2+ dependent death pathways. Second, CyPD inactivation preserves mitochondrial and cytosolic ATP levels. Our assessment of ATP in adult neurons demonstrated that CyPD deficiency attenuates the decline of mitochondrial ATP exposed to 100 µM H2O2. While the mitochondrial ATP level did not return to the baseline in CyPD-KO neurons, it was significantly higher than in WT neurons and was also reflected in higher cytosolic ATP levels. Since mitochondrial ATP synthesis is ΔΨ-dependent and regulated by mitochondrial Ca2+ (Brookes et al., 2004), the attenuated decline of mitochondrial ATP in CyPD-KO neurons under oxidative stress is consistent with a more stable ΔΨ and a transient decrease in mitochondrial Ca2+. Thus CyPD inactivation may protect neurons undergoing oxidative stress by reducing increases in cytosolic Ca2+ and maintaining ATP production.
Conclusions
Together, our data demonstrate a novel role for mitochondrial Ca2+ in neurodegenerative processes associated with oxidative stress and show that CyPD deficiency leads to preservation of mitochondrial Ca2+ buffering function, lowering of cytosolic Ca2+ and more stable ATP levels during oxidative stress. Our results provide a basis for testing and developing therapeutic approaches allowing for the increased neuronal survival when faced with oxidative stress conditions. In addition, since oxidative and excitotoxic stress most likely coincide during pathological conditions associated with inflammation or ischemia, combined effects of the two stresses should be further investigated.
Supplementary Material
ACKNOWLEGEMENTS
This work was supported by grants from the National Institutes of Health to D.B. and M.F.; National Multiple Sclerosis Society to D.B. and M.F.; the VA Biomedical Laboratory and Clinical Research Service to D.B.; the Laura Fund for Innovation in Multiple Sclerosis Research to M.F.; and the Nancy Davis Center Without Walls to D.B. A.B. was supported in part by National Institutes of Health training grant [grant number T32 NS007381], a Neurobiology of Disease award from the OHSU Brain Institute, a fellowship award from the Tartar Trust, and a gift from Team Eugene. The study was also supported by P30-NS06180. The St. Laurent Foundation of Vancouver, WA graciously provided funds for the microscope used in this study.
ABREVIATIONS
- WT
wild-type
- PTP
permeability transition pore
- CyPD
cylocphilin D
- CyPD-KO
cyclophilin D-knock out (Ppif−/−)
- mitoRP
ratiometric pericam targeted to mitochondria
- mitoYPF
enhanced yellow fluorescent protein targeted to mitochondria
- PBR
peripheral benzodiazepine receptor
- mitoPBRf
PBR modified with GFP derivative
- FCCP
carbonyl cyanide p-trifluoromethoxy phenyl hydrazone
- TMRM
tetramethyl rhodamine methyl ester
REFERENCES
- Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Forte M. The mitochondrial permeability transition pore. Novartis Found Symp. 2007;287:157–164. [PubMed] [Google Scholar]
- Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-dyson E, De Lisa F, Forte M. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Brustovetsky T, Bolshakov A, Brustovetsky N. Calpain activation and Na+/Ca2+ exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res. 2010;88:1317–1328. doi: 10.1002/jnr.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky T, Li V, Brustovetsky N. Stimulation of glutamate receptors in cultured hippocampal neurons causes Ca2+-dependent mitochondrial contraction. Cell Calcium. 2009;46:18–29. doi: 10.1016/j.ceca.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippin L, Abad MC, Gastaldello S, Magalhaes PJ, Sandona D, Pozzan T. Improved strategies for the delivery of GFP-based Ca2+ sensors into the mitochondrial matrix. Cell Calcium. 2005;37:129–136. doi: 10.1016/j.ceca.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu X, Fowlkes J, Rahder M, Stem K, Bernardi P, Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci U S A. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD. Mitochondria as regulators of stimulus-evoked calcium signals in neurons. Cell Calcium. 2000;28:307–316. doi: 10.1054/ceca.2000.0172. [DOI] [PubMed] [Google Scholar]
- Gerich FJ, Funke F, Hildebrandt B, Fasshauer M, Muller M. H(2)O(2)-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch. 2009;458:937–952. doi: 10.1007/s00424-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- Imamura H, Nhat K, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicator. Proc Natl Acad Sci U S A. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornot L, Maechler P, Wollheim CB, Junod AF. Reactive oxygen metabolites increase mitochondrial calcium in endothelial cells: implication of the Ca2+/Na+ exchanger. J Cell Sci. 1999;112:1013–1022. doi: 10.1242/jcs.112.7.1013. [DOI] [PubMed] [Google Scholar]
- Joseph-Liauzun E, Delmas P, Shire D, Ferrara P. Topological analysis of the peripheral benzodiazepine receptor in yeast mitochondrial membranes supports a five-transmembrane structure. J Biol Chem. 1998;273:2146–2152. doi: 10.1074/jbc.273.4.2146. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klohn PC, Soriano ME, Irwin W, Penzo D, Scorrano L, Bitsch A, Neumann HG, Bernardi P. Early resistance to cell death and to onset of the mitochondrial permeability transition during hepatocarcinogenesis with 2-acetylaminofluorene. Proc Natl Acad Sci U S A. 2003;100:10014–10019. doi: 10.1073/pnas.1633614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger C, Duchen MR. Mitochondria, Ca2+ and neurodegenerative disease. Eur J Pharmacol. 2002;447:177–188. doi: 10.1016/s0014-2999(02)01842-3. [DOI] [PubMed] [Google Scholar]
- Lalo U, Kostyuk P. Developmental changes in purinergic calcium signalling in rat neocortical neurones. Brain Res Dev Brain Res. 1998;111:43–50. doi: 10.1016/s0165-3806(98)00120-5. [DOI] [PubMed] [Google Scholar]
- Li V, Brustovetsky T, Brustovetsky N. Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp Neurol. 2009;218:171–182. doi: 10.1016/j.expneurol.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieven CJ, Vrabec JP, Levin LA. The effects of oxidative stress on mitochondrial transmembrane potential in retinal ganglion cells. Antioxid Redox Signal. 2003;5:641–646. doi: 10.1089/152308603770310310. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proc Natl Acad Sci U S A. 2001;98:3197–3202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Barsukova AG, Shen F, McAsey M, Struble RG. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145:3065–3073. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–174. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109:133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Stanika RI, Watts CA, Brantner CA, Smith CL, Andrews SB. Reduced calcium-dependent mitochondrial damage underlies the reduced vulnerability of excitotoxicity-tolerant hippocampal neurons. J Neurochem. 2008;104:1686–1699. doi: 10.1111/j.1471-4159.2007.05080.x. [DOI] [PubMed] [Google Scholar]
- Pouokam E, Rehn M, Diener M. Effects of H2O2 at rat myenteric neurones in culture. Eur J Pharmacol. 2009;615:40–49. doi: 10.1016/j.ejphar.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Zhang Y, Centonze E, Herman B. Measurement of mitochondrial pH in situ. BioTechniques. 2001;30:804–808. doi: 10.2144/01304rv01. 810, 812 passim. [DOI] [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2009;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- Wang X, Carlsson Y, Basso E, Zhu C, Rousset CI, Rasola A, Johansson BR, Blomgren K, Mallard C, Bernardi P, Forte MA, Hagberg H. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. J Neurosci. 2009;29:2588–2596. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamocky M, Furtmuller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2008;10:1527–1548. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.