Abstract
Of those individuals who are infected with M. tuberculosis, 90% do not develop active disease and represents a large reservoir of M. tuberculosis with the potential for reactivation of infection. Sustained TNF expression is required for containment of persistent infection and TNF neutralization leads to tuberculosis reactivation. In this study, we investigated the contribution of soluble TNF (solTNF) and transmembrane TNF (Tm-TNF) in immune responses generated against reactivating tuberculosis. In a chemotherapy induced tuberculosis reactivation model, mice were challenged by aerosol inhalation infection with low dose M. tuberculosis for three weeks to establish infection followed chemotherapeutic treatment for six weeks, after which therapy was terminated and tuberculosis reactivation investigated. We demonstrate that complete absence of TNF results in host susceptibility to M. tuberculosis reactivation in the presence of established mycobacteria-specific adaptive immunity with mice displaying unrestricted bacilli growth and diffused granuloma structures compared to WT control mice. Interestingly, bacterial re-emergence is contained in Tm-TNF mice during the initial phases of tuberculosis reactivation, indicating that Tm-TNF sustains immune pressure as in WT mice. However, Tm-TNF mice show susceptibility to long term M. tuberculosis reactivation associated with uncontrolled influx of leukocytes in the lungs and reduced IL-12p70, IFNγ and IL-10, enlarged granuloma structures, and failure to contain mycobacterial replication relative to WT mice. In conclusion, we demonstrate that both solTNF and Tm-TNF are required for maintaining immune pressure to contain reactivating M. tuberculosis bacilli even after mycobacteria-specific immunity has been established.
Introduction
Although a third of the global population has been exposed to tuberculosis the majority harbours a latent form of infection [1]. This global reservoir potential poses significant challenges to therapeutic intervention, made more difficult by poor understanding of the immune mechanisms that exert pressure to maintain bacilli in a state of latency. Real threats are associated with disease reactivation, particularly in disease burden countries in which immune-compromised individuals such as those with HIV/AIDS form a significant part of the population. In low burden, first world countries, reactivation of latent bacilli form the primary cause of active disease as opposed to new infections in developing countries.
Host immune factors that allow for mycobacteria to remain in a persistent state of latency have not been clearly defined although considerable insight has been gained through the application of in vitro models and animal studies that simulate M. tuberculosis reactivation [2], [3], [4], [5]. However, identifying factors responsible for maintaining a latent infectious state and those that are compromised to give rise to reactivation have proven to be complex. Loss of function and neutralization studies has been key to understand the effects of Tumour Necrosis Factor (TNF) in host protection. We and others have shown that while TNF is critical to control acute infection [6], [7], [8], it is similarly important to prevent bacilli replication during chronic infection [9] or during drug induced latent infection [10]. The reemergence of bacilli in the absence of TNF correlated with a lack of proper granuloma structures and the increase of pro-inflammatory cytokines. The importance of TNF for maintaining latent infection was verified in clinical studies in which anti-TNF therapy administered to patients with chronic inflammatory diseases resulted in spontaneous reactivation of tuberculosis [11], [12], [13], [14]. The mechanisms through which TNF mediates control of latent infection is unclear, however studies have reported that administration of TNF inhibitors interferes with TNF mediated phagosome maturation, apoptosis, T cell activation and autophagy [15]. A study by Bruns et al., 2009 showed that anti-TNF neutralizing antibodies reduced the population of effector memory CD8 T cells resulting in reduced antimicrobial activity against M. tuberculosis [16].
TNF is a multifunctional cytokine, initially synthesized as a 26 Kda non-glycosylated type II trans membrane protein (Tm-TNF) which upon cleavage by the metalloprotease TNF-converting enzyme (TACE) forms a soluble 17 KDa protein. Both molecular forms of TNF are biologically functional as homotrimeric proteins that bind and mediate signaling through either TNFRp55 (TNFRSF1A, CD120a,TNFR1) or TNFRp75 (TNFRS1B, CD120b, TNFR2) with Tm-TNF binding strongly TNFRp75 [17]. We and others have previously reported that acute M. tuberculosis infection could be controlled by Tm-TNF but that soluble TNF was required to sustain host immune protection [18], [19], [20], [21]. Moreover, we have demonstrated that rapid and lethal reactivation of M. tuberculosis was associated with lack of proper bactericidal granuloma formation in latently infected complete TNF−/− mice treated with isoniazid and rifampicin [10].
With the current development of new TNF inhibitor biologics which specifically inhibit solTNF and spare Tm-TNF in the treatment of chronic inflammatory disorders [22], [23], [24], [25], we investigated the role of Tm-TNF in controlling reactivation of therapeutically induced latent infection. We show that Tm-TNF mediates control of reactivating bacilli but that soluble TNF is required to sustain long-term growth inhibition. We found that susceptibility in reactivating Tm-TNF mice is associated with unstructured granuloma formation and a defect of protective cytokine synthesis.
Materials and Methods
Mice
C57Bl/6 wild type (WT) control mice, TNF−/− mice [26] and Tm-TNF mice [27] were bred, maintained and housed in individually ventilated cages under specific pathogen free conditions in the animal facility of the University of Cape Town, South Africa. For all the experiments, age matched mice on a C57Bl/6 background were used and genotypes were confirmed by PCR analysis. All the experiments and protocols performed were in accordance with the guidelines of the Research Ethics Committee of the University of Cape Town, South Africa (Approval ID- REF REC: 008/023).
Bacterial infection and chemotherapy
M. tuberculosis H37Rv was grown in Middlesbrook 7H9 broth (Becton, Dickinson and Company, Le Pont de Claix, France) supplemented with 10% Middlebrook OADC enrichment medium (Life Technologies, Gaitherburg, MD), 0.5% glycerol and 0.05% Tween 80 at 37°C until log phase. Prior to usage, mycobacterial aliquots were passed 30× through a 29.5 G needle to minimize bacterial clumping. Pulmonary infection with 100–200 cfu live M. tuberculosis H37Rv bacteria was performed using a Glas-Col Inhalation Exposure System, Model A4224. Inoculum size was confirmed 24 h post-infection by determining the bacterial burden in the lungs of infected mice.
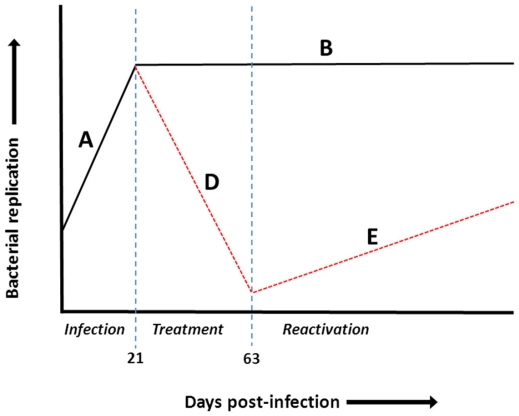
For the M. tuberculosis reactivation model (Fig. 1), we used a modified protocol as previously described [10]. Briefly, groups of mice were infected by aerosol inhalation with 100–200 cfu viable M. tuberculosis H37Rv bacilli. The infection was allowed to progress for 21 days, termed the pre-immune phase (Fig. 1, line A), where unrestricted bacilli replication occurred before commencement of treatment with 25 mg/Kg INH-RIF (Sigma, St. Louis, USA) in drinking water for 6 weeks. In this drug treatment phase (Fig. 1, line D) bacilli numbers were reduced to at least less than 100 CFUs in the lungs after which treatment was withdrawn, allowing reactivation of bacilli (Fig. 1, line E). Alternatively, in control groups that received no therapy after 21 days, bacilli burdens remained constant (steady state phase Fig. 1, line B).
Figure 1. Graphic presentation of drug-based M. tuberculosis reactivation mouse model.
Line A: Preimmune phase, short bacterial replication period post-infection with low dose M. tuberculosis; Line B: Steady state phase: Control of M. tuberculosis growth through host immunity and establishment of chronic infection. Line D: Drug treatment phase: Reduction of bacilli replication through INH-RIF chemotherapy. Line E: Reactivation phase: Reactivation of infection upon cessation of antibiotics.
Colony enumeration assay
Bacterial burdens in the lungs, livers and spleens of infected mice were determined at specific time points after infection with M. tuberculosis H37Rv. Organs were weighed and homogenized in 0.04% Tween 80 saline. Tenfold serial dilution of organ homogenates were plated in duplicates on Middlesbrook 7H10 (Becton, Dickinson and Company) agar plates containing 10% OADC (Life Technologies, Gaitherburg, MD) and incubated at 37°C for 19–21 days. Colonies on plates were enumerated and bacterial burdens determined.
Pulmonary pathology
Mice were euthanized by carbon dioxide inhalation at specific time points. Organs were weighed and fixed in 10% formalin and paraffin-embedded. Two to 3 µm sections were stained with haematoxylin and eosin (H&E) and a modified Ziehl-Nielson (ZN) method as described [28]. For immunostaining, formalin-fixed paraffin-embedded sections were deparaffinised and rehydrated then stained with rabbit anti-mouse specific inducible nitric oxide synthase (iNOS) [29], rat anti-mouse CD11b (clone M1/70) or rat anti-mouse CD3 (clone 145-2c-11) antibodies. Sections were then washed in PBS and incubated for 30 min at room temperature with biotinylated secondary antibody then subsequently incubated with avidin-biotin complexes (Vector Laboratories, CA, USA) for 30 min, washed and incubated with DAB substrate (Dako Corporation, CA, USA).
Lung homogenate preparations
Whole lungs were removed from infected mice at specific time points and were homogenized in 1 ml 0.04% Tween 80 saline containing protease inhibitor (Sigma) and the supernatants were collected after low-speed centrifugation, aliquoted and frozen at −80°C.
Cytokine ELISA
Supernatants from organ homogenates or from cultured cells were harvested and assayed for cytokine concentration using commercially available ELISA reagents for IFNγ, IL-10, and IL-12p70 (R&D Systems, Germany and BD PharMingen, San Diego), according to the manufacturer's instructions.
Statistical Analysis
The data are expressed as the mean ± SEM. Statistical analysis was performed by ANOVA. For mortality studies, analysis was performed using the log-rank test. For all tests, a p-value of <0.05 was considered significant.
Results
Tm-TNF protects mice from severe tuberculosis reactivation
In a study performed by McCune et al., 1966, it was observed that immunizing mice with M. tuberculosis then re-infecting them preceding treatment with anti-tuberculous drugs resulted in mice reactivating with lower M. tuberculosis CFU numbers compared to control groups [30]. This result was interpreted as the influence of the host's acquired immune resistance to mycobacteria. In this study, we investigated the contribution of two molecular forms of TNF, in particular Tm-TNF in host immunity in mice immunized by prior infection with M. tuberculosis. The model used entailed aerosol inhalation infection of WT mice, TNF−/− mice and Tm-TNF mice with 100 viable M. tuberculosis H37Rv bacilli. The infection was allowed to progress for 21 days before commencement of treatment with 25 mg/Kg INH-RIF in drinking water for 6 weeks to reduce bacilli numbers to at least less than 100 CFUs in the lungs after which treatment was withdrawn and tuberculosis reactivation was monitored. Body weights were recorded throughout the infection period and body weight loss was interpreted as severe disease due to reappearing tuberculosis and correlated with susceptibility to infection. Control groups of WT, Tm-TNF and TNF−/− mice that did not receive chemotherapeutic treatment were confirmed to have phenotypes as previously described [18].
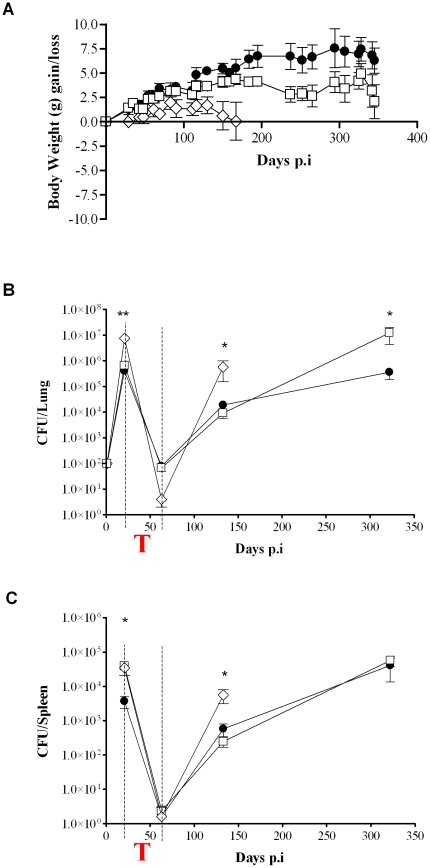
We found that, of the therapeutically treated animals, WT mice showed a steady increase in body weight over the duration of the infection in contrast to TNF−/− mice which displayed significantly lower body weights between 69 and 165 days post-infection (Fig. 2A) coinciding with the period subsequent to the withdrawal of treatment. TNF−/− mice also appeared sick with ruffled fur and hunched backs and eventually became moribund and had to be terminated on day 165. In contrast, Tm-TNF showed an increase in body weight comparable to WT mice for the first 130 days post-infection (Fig. 2A) but significant weight loss was recorded in Tm-TNF mice between 237 days and 265 days post-infection while the body weights in WT mice remained stable (Fig. 2A). For the remainder of the experimental period, Tm-TNF mice maintained slightly lower body weights relative to WT mice with no significant differences observed (Fig. 2A) but exhibited no physical signs of severe disease apparent in TNF−/− mice. These data indicate that complete absence of TNF renders mice susceptible to severe reactivating tuberculosis which is alleviated by the presence of Tm-TNF.
Figure 2. Effect of Tm-TNF on M. tuberculosis replication during reactivation.
WT mice (black circles), TNF−/− mice (white diamonds) and Tm-TNF mice (white squares) were treated for 6 weeks with 25 mg/Kg INH-RIF in drinking water subsequent to 3 weeks aerosol infection with 100–200 CFU's of M. tuberculosis H37Rv. (A) Body weights were recorded throughout the course of the infection period and bacterial burdens in lungs (B) and spleens (C) were enumerated at time points indicated. Data are representative of two experiments and data points are expressed as the mean ± SD of 5 mice/group (for CFUs). The body weight study consisted of between 6–16 mice/group where the data represents weights for remaining mice in each group. The red “T” in the figure corresponds to the drug treatment phase. Significant differences (*p<0.05; **p<0.01) were determined by Student's t test for comparisons between two groups and ANOVA for comparisons between three groups.
Next, bacilli burdens in lungs and spleens of infected mice were determined at specific time points to investigate the effect of the different molecular forms of TNF on mycobacteria recrudescence in the presence pre-existing immunity in WT mice, TNF−/− mice and Tm-TNF mice. Bacilli burdens in WT mice were reduced by 3.5 log10 in the lung (Fig. 2B) and by 2.5 log10 in the spleen (Fig. 2C) after exposure to 6 weeks INH-RIF treatment. Withdrawal of antibiotic treatment resulted in spontaneous M. tuberculosis reactivation with bacilli burdens reaching up to 4 log10 in the lung and 2.5 log10 in the spleen (Fig. 2B & C, respectively). In TNF−/− mice, INH-RIF treatment reduced the high bacilli burden by more than 6 log10 in lung (Fig. 2B) and 4 log10 in spleen (Fig. 2C) after 6 weeks exposure. The more efficacious reduction in bacterial burden in the lung of TNF−/− relative to WT mice after INH-RIF treatment is in agreement with the concept that reduced immune pressure is intimately associated with improved antibiotic-mediated mycobacterial clearance [31]. Within 133 days post-treatment, mycobacteria reappeared in TNF−/− mice and bacilli burden reached at least 6 log10 in lung (Fig. 2B) and 4 log10 in spleen (Fig. 2C) of fully deficient TNF−/− mice. Tm-TNF mice responded to INH-RIF treatment in a manner comparable to WT mice and showed a similar slow kinetic in the rate of reactivation comparable to that of WT mice. However, by 322 days post-treatment, bacterial burdens in the lung of Tm-TNF mice increased significantly by 2 log10 compared to a 1 log10 increase observed in WT mice (Fig. 2B). Bacilli burdens in spleens of Tm-TNF mice were comparable to WT mice at all time points investigated (Fig. 2C). Therefore the data show that Tm-TNF is required to promote early bacterial killing mechanisms even after priming of the host by previous M. tuberculosis infection. However, although Tm-TNF confers early protection against recrudescence M. tuberculosis, Tm-TNF alone does not sustain long term control of the infection, indicating that solTNF is needed for controlling chronic infection.
Abnormal inflammatory response in the absence of TNF during tuberculosis reactivation is delayed in Tm-TNF mice
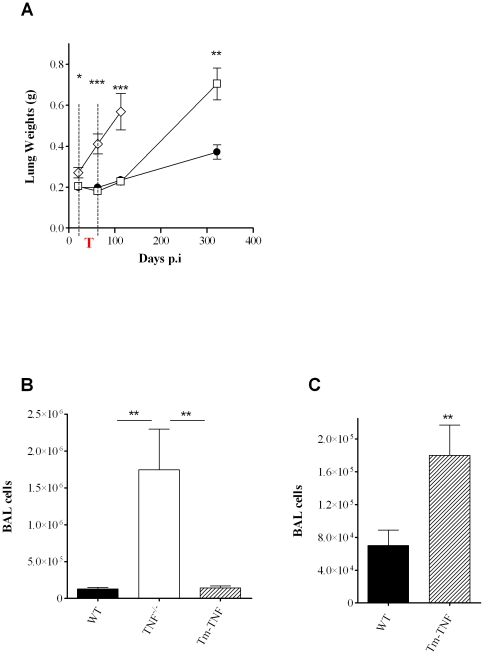
TNF has previously been shown to be at the apex of inflammatory responses [32]. To determine whether Tm-TNF was sufficient in mediating an inflammatory response during tuberculosis reactivation, mouse lung weights were recorded at specific time points during the infection period as a surrogate marker of inflammation. Compared to lung weights determined at day 21 post-infection, WT mice displayed no change in lung weights after exposure to INH-RIF for 6 weeks. However, an increase in lung weights was observed during later stages of disease (322 days post-infection; Fig. 3A) which was consistent with the increase in bacilli burdens at this time point (Fig. 2B). In sharp contrast, by 21 days post-infection, TNF−/− mice already displayed significantly higher lung weights compared to WT mice (Fig. 3A). Paradoxically, 63 days post-infection at the end of the therapy period, the lung weights had increased significantly (p<0.001) compared to WT mice (Fig. 3A) but did not correlate with the decreased number of bacilli at this time point (Fig. 2B). This observation differed from a previous report where similar lung weights were measured in TNF−/− mice in a model using a 3 fold lower infection dose and chemotherapy was initiated earlier [10]. We postulate that here, a delay in onset of chemotherapy until 21 days combined with the potential higher antigenic burden of killed bacilli provided conditions for irreversible proinflammatory stimulation and excessive inflammation in the absence of TNF. Susceptibility of TNF−/− mice was confirmed with a further significant increase (p<0.001) in lung weights noted at 113 days post-infection, 50 days after cessation of therapy. In contrast, lung inflammation in Tm-TNF mice was similar to WT mice during early infection with similar lung weights at 21, 63 and 133 days post-infection (Fig. 3A). However, control of inflammation was not sustained as a significant increase (p<0.01) in lung weights was evident in Tm-TNF mice at day 322 post-infection compared to WT mice (Fig. 2.1B).
Figure 3. Induction of excessive inflammation in the absence of solTNF during reactivation of M. tuberculosis.
WT (black circles), TNF−/− (white diamonds) and Tm-TNF (white squares) mice were exposed by aerosol inhalation infection to 100–200 CFUs/mouse of M. tuberculosis H37Rv for 3 weeks preceding chemotherapy with 25 mg/Kg INH-RIF for 6 weeks in drinking water. (A) Lung weights were measured at specific time points and BAL derived cell numbers were determined 77 days (B) and 378 days (C) post-infection. The red “T” in the figure corresponds to the drug treatment phase. Data are representative of 1 of 2 experiments performed and are expressed as mean ± SD of 5 mice/group. Significant differences (*p<0.05; **p<0.01; ***p<0.001) were determined by Student's t test for comparisons between two groups and ANOVA for comparisons between three groups.
To further analyze airways inflammation in the absence of TNF, or in the presence of Tm-TNF, the number of cells present in the bronchoalveolar lavage (BAL) fluid was determined after infection. The cellularity in the BAL fluid was significantly higher in TNF−/− mice compared to WT mice, whereas Tm-TNF mice had a number of cells comparable to WT mice at day 77 post-infection (Fig. 3B) confirming that the control of inflammation during early infection was membrane TNF dependent. Consistent with chronic lung weight data, Tm-TNF mice displayed significantly increased number of cells in BAL fluid relative to WT mice on day 378 post-infection (Fig. 3C). Together, the data indicates that control of early inflammation is mediated primarily by Tm-TNF but solTNF is required for regulation of inflammation during chronic infection. However, the lack of control of inflammation appears to be strongly associated with the onset of tuberculosis reactivation.
Tm-TNF is inadequate to maintain bactericidal granulomas during reactivating tuberculosis
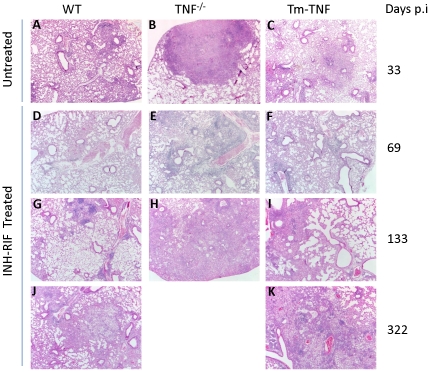
We next asked whether granuloma structures were formed in the presence of Tm-TNF during tuberculosis reactivation. Studies performed by Mohan et al., 2001, illustrated that TNF was required for maintenance of granuloma structure during persistent M. tuberculosis infection whereby upon treatment with TNF-neutralizing antibody, mice displayed severe histopathology marked with excessive inflammation and loss of structured granulomas [33]. Lung sections were obtained from infected WT mice, TNF−/− mice and Tm-TNF mice at the indicated time points post-infection and pulmonary pathology analyzed. Untreated WT mice (Fig. 4A) and Tm-TNF mice (Fig. 4C) displayed small compact lesions with tight lymphocytic wedges and a high degree of clear airway spaces at day 33 post infection. In sharp contrast, TNF−/− mice (Fig. 4B) showed enlarged unstructured lesions with inflammation occupying larger areas of the lung and presenting with evidence of necrosis. At the end of chemotherapeutic treatment (day 63 p.i.), WT (Fig. 4D) and Tm-TNF (Fig. 4F) mice had pulmonary pathology characterized by well-defined granulomas and clear alveoli whereas TNF−/− mice displayed higher inflammation characterized by peri-vascular and peri-bronchiolar inflammation (Fig. 4E). Development of pathology subsequent to withdrawal of chemotherapy (day 133 p.i.) continued to show well-defined granulomas in WT (Fig. 4G) and Tm-TNF (Fig. 4I) mice with clear alveoli, whereas TNF−/− mice now presented with excessive inflammation with no defined granulomas and limited alveolar space (Fig. 4H). The inability of Tm-TNF mice (Fig. 4K) to control development of further pathology was clearly evident at day 322 post-infection where, in contrast to WT mice (Fig. 4J), Tm-TNF mice displayed enlarged unstructured lesions with excess inflammatory responses and interstitial pneumonia.
Figure 4. Tm-TNF contributes to protective bactericidal granuloma formation during M. tuberculosis reactivation but is insufficient to sustain structural integrity and bactericidal efficacy.
WT mice (A,D,G,J), TNF−/− mice (B, E, H) and Tm-TNF mice (C,F,I,K) were infected by aerosol inhalation with 100–200 CFUs/mouse M. tuberculosis H37Rv for 3 weeks preceding chemotherapy with 25 mg/Kg INH-RIF for 6 weeks in drinking water. Lungs were removed at the indicated timepoints and tissue sections stained with haematoxylin and eosin to determine the granulomatous response.
These observations demonstrate that during tuberculosis reactivation, Tm-TNF does not sustain long term maintenance of protective granuloma structure in the absence of soluble TNF resulting in malformed lesions that associate with failure to inhibit M. tuberculosis growth.
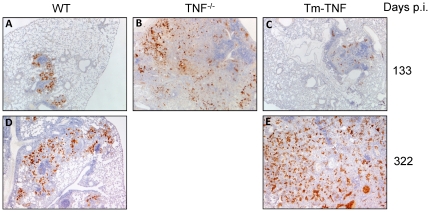
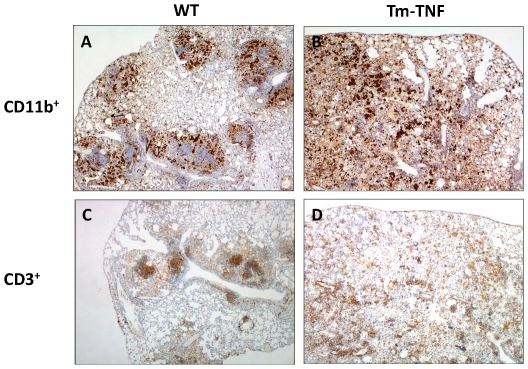
We therefore determined whether effector macrophage anti-mycobacterial function was intact in Tm-TNF mice undergoing M. tuberculosis reactivation. Studies have shown that cell mediated mycobacterial killing function can be achieved through production of toxic reactive intermediates (RNI) via the enzymatic action of macrophage iNOS [34]. Previously, it was demonstrated that inhibition of iNOS in mice chronically infected with M. tuberculosis resulted in reactivation of tuberculosis disease with increased organ bacillary burdens and extensive granulomatous response [10], [35]. In view of these findings we determined iNOS expression immunohistochemically in lung tissue sections of reactivating infected WT mice, TNF−/− mice and Tm-TNF mice after completion of chemotherapy. iNOS expression patterns were largely within the confinement of granuloma lesions in WT mice (Fig. 5A) in contrast to TNF−/− mice where the pattern was dispersed and associated with the diffused granuloma lesions (Fig. 5B) analyzed 133 days post-infection. Tm-TNF mice displayed similar iNOS expression to WT mice at day 133 days post-infection (Fig. 5C). However, during reactivating chronic infection at day 322, in contrast to WT mice (Fig. 5D), increased iNOS expression was evident in Tm-TNF mice (Fig. 5E) with a random distribution and evidence of lung tissue destruction. We further characterized the effect of Tm-TNF signaling on macrophage (CD11b+ cells) and lymphocyte (CD3+ cells) recruitment to investigate whether a correlation existed between macrophage recruitment and iNOS induction, and also to determine Tm-TNF effects on the structural relationship between macrophages and lymphocytes with respect to granuloma formation during reactivation. It is clear that macrophage distribution correlated strongly with iNOS induction where, in WT mice it was localized on the periphery of established granulomas (Fig. 6A) but randomly distributed in Tm-TNF mice (Fig. 6B) at 322 days post-infection. Lymphocytes recruitment was focused and predominantly occupied central areas of granulomas in reactivating WT mice (Fig. 6C) but was unorganized in reactivating Tm-TNF mice (Fig. 6D). Therefore, these observations indicate that, in hosts where tuberculosis reactivation occurs, Tm-TNF on its own cannot sustain the structural integrity of granulomas with respect to the cellular organization of macrophages and lymphocytes, and that iNOS induction by macrophages is insufficient for controlling mycobacterial growth if the granuloma structure is not properly formed.
Figure 5. Extra-granulomatous pulmonary expression of INOS is associated with susceptibility in Tm-TNF reactivating mice.
WT mice (A,D), TNF−/− mice (B) and Tm-TNF mice (C,E) were infected by aerosol inhalation with 100–200 CFUs/mouse M. tuberculosis H37Rv for 3 weeks preceding chemotherapy with 25 mg/Kg INH-RIF for 6 weeks in drinking water. Lungs were removed at 133 and 322 days post infection and tissue sections were stained with polyclonal rabbit anti-mouse antibody (see Materials and methods ). Brown stain represents iNOS expression by activated macrophages. Micrographs represent 4 animals/group and are shown at ×32 magnification.
Figure 6. Macrophage (CD11b+ cells) and lymphocyte (CD3+ cells) recruitment in WT and Tm-TNF mice.
WT mice (A,C) and Tm-TNF mice (B,D) were infected by aerosol inhalation with 100–200 CFUs/mouse M. tuberculosis H37Rv for 3 weeks preceding chemotherapy with 25 mg/Kg INH-RIF for 6 weeks in drinking water. Lungs were removed 322 days post infection and tissue sections were stained with either anti-CD11b anti-mouse (A,B) antibody or anti-CD3 anti-mouse antibody (C,D) (see Materials and methods). Micrographs represent 4 animals/group and are shown at ×32 magnification.
Defective protective cytokine induction in reactivating Tm-TNF mice during late stages of the disease
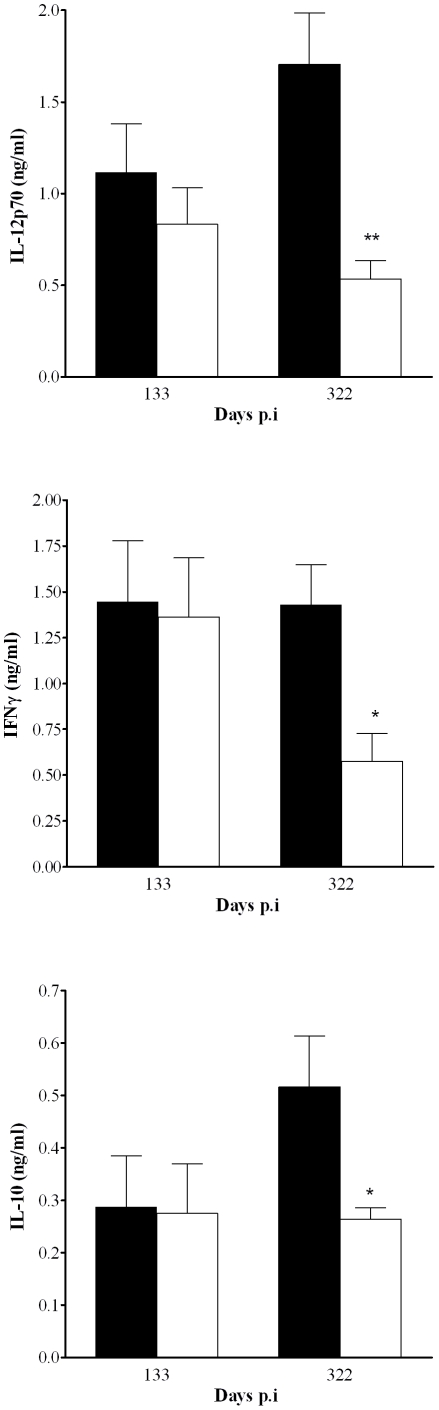
Next we assessed the effect of Tm-TNF mediated immune responses in infected mice subsequent to INH-RIF treatment with particular reference to the quantification of IFNγ and IL-12 levels because of their reported functions in generating and maintaining protective immunity against M. tuberculosis infection [36], [37], [38]. Studies by Feng et al., 2005, demonstrated that continuous IL-12 production is necessary for maintenance of pulmonary IFNγ-producing effector CD4+ T cells and subsequent bacilli control during chronic M. tuberculosis infection suggesting that interruption of IL-12 signal transduction contribute to development of reactivation of tuberculosis [39]. Therefore, we quantified pulmonary IL-12 and IFNγ production during tuberculosis reactivation (Fig. 7). Comparable IL-12p70 and IFNγ concentrations were found in the lung of WT mice and Tm-TNF mice 133 days post-infection. However, there was a significant decrease in IL-12p70 and IFNγ pulmonary concentrations in Tm-TNF compared to WT mice 322 days post-infection associated with susceptibility of Tm-TNF mice to M. tuberculosis reactivation at this time point. The concentration of anti-inflammatory IL-10 was comparable 133 days post-infection but increased in WT mice by day 322 post-infection and remained significantly lower in Tm-TNF mice (Fig. 7). These data suggest that Tm-TNF is not sufficient to sustain protective cytokine induction in post-infection M. tuberculosis chronic immunity and this phenotype is associated with lack of control of bacilli burden in the lung and lethality of these mice.
Figure 7. Reduced pulmonary cytokine expression is associated with increased susceptibility in Tm-TNF mice during M. tuberculosis reactivation.
WT (closed bars) and Tm-TNF mice (open bars) were exposed by aerosol inhalation to 100–200 CFUs/mouse of M. tuberculosis H37Rv for 3 weeks preceding chemotherapy with 25 mg/Kg INH-RIF for 6 weeks in drinking water. Lungs were obtained and homogenized at 133 days and 322 days post-infection and the levels of IL-12p70, IFNγ and IL-10 present in cell supernatants determined by ELISA. Data represent 1 of 2 experiments performed and values are expressed as mean ± SD of 5 animals/group. Significant differences (*p<0.05; **p<0.01) were determined by Student's t test.
Discussion
Estimates indicate that a third of the global population is latently infected with the tuberculosis pathogen, M. tuberculosis [1]. The host mechanisms responsible for maintaining a latent infection remain elusive. Several studies have established that TNF is important in immune responses against mycobacterial infections [6], [28], [40], [41], [42], [43] but it is also a central mediator of pathology in autoimmune diseases [44], [45], [46]. Patients on anti-TNF treatment for chronic inflammatory diseases have an increased incidence of tuberculosis [11], [12], [13], [14] implicating TNF in the preservation from latent tuberculosis. Although highly efficacious, the currently used anti-TNF therapies i.e. etanercept, infliximab, and adalimumab block both Tm-TNF and solTNF [47], [48], [49], [50]. Therefore, further research on TNF biology is required for designing improved therapeutics that will alleviate inflammatory diseases while maintaining protection against mycobacteria.
We and others investigated the contribution made by the two molecular forms of TNF, solTNF and Tm-TNF, in the induction of protective immunity or immunopathology during M. tuberculosis or M. bovis BCG infection [18], [19], [20], [51]. A conclusion derived from these studies revealed that Tm-TNF expressing mice have an intermediary phenotype i.e. they controlled acute infection where gene deficient TNF mice were susceptible. However Tm-TNF mice succumbed to chronic infection with increased bacillary burdens and excessive granulomatous response which was not observed in WT mice. Here, we report that Tm-TNF and solTNF are both required for the maintenance of immune pressure during reactivating tuberculosis. We found in our drug-induced tuberculosis reactivation model that TNF is an absolute requirement for the containment of the re-emergence of tuberculosis reflected by the rapid propagation of mycobacteria after cessation of antibiotic treatment in TNF−/− mice. This observation confirms findings previously reported by Botha and Ryffel, 2003 [10] and support data showing that treatment of chronically infected mice [33], [52] or latently infected humans treated with TNF neutralizing antibodies results in reappearance of tuberculosis [11], [12], [13], [14], [16], [53], [54]. Furthermore, our results show that Tm-TNF contributes to the initial containment of re-emerging tuberculosis but is not sufficient for long-term mycobacteria containment resembling the outcome of chronic infection. Together, these observations demonstrate that Tm-TNF mediates early protection against reactivating M. tuberculosis infection and that solTNF might be required at later stages for sustained protection.
The formation of granulomas in response to mycobacterial challenge leads to killing or alternatively, isolation and confinement of bacilli to local sites of infection. They are dynamic structures that promote cellular interaction to enhance bactericidal efficacy, and the initial establishment and continued maintenance of its structural integrity in murine models is critically dependent on TNF. Nevertheless, the relationship between TNF and granuloma structure as an indicator of protection mediated against infectious mycobacteria remains controversial. More recently, Lin et al, 2010 found that neutralization of TNF in cynomolgus macaques resulted in dissemination of disease without compromising the structural integrity of established granulomas [55]. Previously, we proposed that Tm-TNF alone was sufficient to develop distinct granulomas during respiratory M. tuberculosis infection, but referred to the diminished bactericidal capacity of such granulomatous structures [18]. Moreover, these findings were supported in clinical studies by Lliopoulos et al, 2006 who found typical granulomas in biopsy specimens from patients on anti-TNF therapy who developed tuberculosis [56]. Furthermore, although presence of structurally defined granulomas are widely accepted as the hallmark of protection, Johnson et al, 1998 reported adequate protection in ICAM-1 deficient mice despite lacking structured granulomas during M. tuberculosis infection [57]. These combined observations therefore suggest that factors independent of TNF may determine granuloma structural integrity and that such structures alone cannot be used as a marker to define protection against M. tuberculosis. Here, we show that during tuberculosis reactivation, TNF−/− mice formed larger lesions with inflammation occupying larger areas of the lung with some evidence of necrosis compared to WT mice, an observation that corroborates previous findings by Mohan et al., 2001 and Botha and Ryffel, 2003 where a lack of proper defined granulomas in reactivating TNF−/− mice were described [10], [33]. Furthermore, our results show that Tm-TNF mice were capable of granuloma structure formation during initial tuberculosis reactivation comparable to WT mice but that long term Tm-TNF dependent sustainability was not enough to maintain protective granuloma structures and susceptibility was reflected by formation of larger, more diffuse lesions with excess inflammation and interstitial pneumonia in reactivating mice. These results therefore illustrate a role for both solTNF and Tm-TNF as a requirement for maintaining granuloma structures during tuberculosis reactivation.
We further assessed iNOS induction as a parameter of macrophage activation in situ within granulomas to understand the lack of sustained Tm-TNF mediated protection during M. tuberculosis reactivation. It is clear that Tm-TNF induced activation of macrophages in a focused manner where iNOS expression was localized to structured granulomas unlike in the complete absence of TNF. The sustainability of this initial focused macrophage response was however compromised during latter stages where iNOS synthesis was notably randomized. The failure to sustain focused bactericidal activity within granulomas was accompanied by a reduction in overall cytokine profile that included IFNγ, IL-12p70 and IL-10. Clinically, similar findings were noted in reactivating tuberculosis patients on anti-TNF therapy who presented with a decreased T cell activation profile and reduction in IFNγ and IL-10 synthesis [58]. Lower IFNγ levels, in particular, critically impacts on macrophage responses by suppressing phagosome maturation and promoting mycobacterial survival. We therefore postulate that, in our studies, the dependence of IL-12p70 synthesis on autologous TNF mediated signaling by antigen presenting cells such as macrophages which are mostly located within granulomas may eventually have been compromised in Tm-TNF deficient mice. The inhibition of T cell activation and lower IFNγ production, as a consequence of diminished IL-12 levels, may have resulted in suppressed M. tuberculosis specific phagosome maturation, thereby promoting bacterial survival and replication during long term reactivation. The cytokine profile in reactivating Tm-TNF mice reported here contrasts to that observed in de novo infected Tm-TNF mice [18] with respect to pulmonary IFNγ AND IL-10 concentrations during late stage infection despite similar observed susceptible phenotypes. We postulate that cytokine induction under these very diverse conditions may be driven by distinctive differences in immune responses. Under conditions of de novo M. tuberculosis infection innate cellular responses drive initial protective immunity whereas under conditions of reactivation protective function may primarily be driven by adaptive memory immunity established during infection prior to pathogen resolution after chemotherapeutic treatment.
In conclusion data presented here, illustrates that TNF mediated immunity against M. tuberculosis infection requires both Tm-TNF and solTNF during tuberculosis reactivation. Although Tm-TNF protects mice against acute M. tuberculosis infection, long term protection requires solTNF partly to down regulate inflammatory responses in chronic infection and to sustain immune pressure during recrudescence of M. tuberculosis infection.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the National Research Foundation (South Africa), The Medical Research Council (South Africa), The National Health Laboratory Services (South Africa), The University of Cape Town, the European Union (TB REACT Contract no 028190). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. 2010. GLOBAL TURCULOSIS CONTROL.
- 2.Scanga CA, Mohan VP, Joseph H, Yu K, Chan J, et al. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manabe YC, Kesavan AK, Lopez-Molina J, Hatem CL, Brooks M, et al. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb) 2008;88:187–196. doi: 10.1016/j.tube.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Rhijn I, Godfroid J, Michel A, Rutten V. Bovine tuberculosis as a model for human tuberculosis: advantages over small animal models. Microbes Infect. 2008;10:711–715. doi: 10.1016/j.micinf.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean AG, Roach DR, Briscoe H, France MP, Korner H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 7.Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, et al. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest. 1999;79:379–386. [PubMed] [Google Scholar]
- 8.Smith S, Liggitt D, Jeromsky E, Tan X, Skerrett SJ, et al. Local role for tumor necrosis factor alpha in the pulmonary inflammatory response to Mycobacterium tuberculosis infection. Infect Immun. 2002;70:2082–2089. doi: 10.1128/IAI.70.4.2082-2089.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty SD, Zhu G, Tsai MC, Mohan VP, Marino S, et al. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun. 2008;76:916–926. doi: 10.1128/IAI.01011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol. 2003;171:3110–3118. doi: 10.4049/jimmunol.171.6.3110. [DOI] [PubMed] [Google Scholar]
- 11.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 12.Dimakou K, Papaioannides D, Latsi P, Katsimboula S, Korantzopoulos P, et al. Disseminated tuberculosis complicating anti-TNF-alpha treatment. Int J Clin Pract. 2004;58:1052–1055. doi: 10.1111/j.1742-1241.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- 13.Desai SB, Furst DE. Problems encountered during anti-tumour necrosis factor therapy. Best Pract Res Clin Rheumatol. 2006;20:757–790. doi: 10.1016/j.berh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006;10:1127–1132. [PubMed] [Google Scholar]
- 15.Harris J, Keane J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. 2010;161:1–9. doi: 10.1111/j.1365-2249.2010.04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm. 1995;47:8–17. [PubMed] [Google Scholar]
- 18.Dambuza I, Allie N, Fick L, Johnston N, Fremond C, et al. Efficacy of membrane TNF mediated host resistance is dependent on mycobacterial virulence. Tuberculosis (Edinb) 2008;88:221–234. doi: 10.1016/j.tube.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Fremond C, Allie N, Dambuza I, Grivennikov SI, Yeremeev V, et al. Membrane TNF confers protection to acute mycobacterial infection. Respir Res. 2005;6:136. doi: 10.1186/1465-9921-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders BM, Tran S, Ruuls S, Sedgwick JD, Briscoe H, et al. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J Immunol. 2005;174:4852–4859. doi: 10.4049/jimmunol.174.8.4852. [DOI] [PubMed] [Google Scholar]
- 21.Allie N, Alexopoulou L, Quesniaux VJ, Fick L, Kranidioti K, et al. Protective role of membrane tumour necrosis factor in the host's resistance to mycobacterial infection. Immunology. 2008;125:522–534. doi: 10.1111/j.1365-2567.2008.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- 23.Spohn G, Guler R, Johansen P, Keller I, Jacobs M, et al. A virus-like particle-based vaccine selectively targeting soluble TNF-alpha protects from arthritis without inducing reactivation of latent tuberculosis. J Immunol. 2007;178:7450–7457. doi: 10.4049/jimmunol.178.11.7450. [DOI] [PubMed] [Google Scholar]
- 24.Olleros ML, Vesin D, Lambou AF, Janssens JP, Ryffel B, et al. Dominant-negative tumor necrosis factor protects from Mycobacterium bovis Bacillus Calmette Guerin (BCG) and endotoxin-induced liver injury without compromising host immunity to BCG and Mycobacterium tuberculosis. J Infect Dis. 2009;199:1053–1063. doi: 10.1086/597204. [DOI] [PubMed] [Google Scholar]
- 25.Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, et al. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol. 2007;179:1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- 26.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci U S A. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruuls SR, Hoek RM, Ngo VN, McNeil T, Lucian LA, et al. Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity. 2001;15:533–543. doi: 10.1016/s1074-7613(01)00215-1. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs M, Marino MW, Brown N, Abel B, Bekker LG, et al. Correction of defective host response to Mycobacterium bovis BCG infection in TNF-deficient mice by bone marrow transplantation. Lab Invest. 2000;80:901–914. doi: 10.1038/labinvest.3780094. [DOI] [PubMed] [Google Scholar]
- 29.Garcia I, Guler R, Vesin D, Olleros ML, Vassalli P, et al. Lethal Mycobacterium bovis Bacillus Calmette Guerin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab Invest. 2000;80:1385–1397. doi: 10.1038/labinvest.3780146. [DOI] [PubMed] [Google Scholar]
- 30.McCune RM, Feldmann FM, Lambert HP, McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo MS, Manca C, Yang G, O'Brien P, Sung N, et al. Phosphodiesterase 4 Inhibition Reduces Innate Immunity and Improves Isoniazid Clearance of Mycobacterium tuberculosis in the Lungs of Infected Mice. PLoS One. :e17091. doi: 10.1371/journal.pone.0017091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(Suppl 3):S189–193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 33.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–1855. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn JL, Scanga CA, Tanaka KE, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 36.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, et al. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 39.Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174:4185–4192. doi: 10.4049/jimmunol.174.7.4185. [DOI] [PubMed] [Google Scholar]
- 40.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 41.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 42.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, et al. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 43.Florido M, Appelberg R. Characterization of the deregulated immune activation occurring at late stages of mycobacterial infection in TNF-deficient mice. J Immunol. 2007;179:7702–7708. doi: 10.4049/jimmunol.179.11.7702. [DOI] [PubMed] [Google Scholar]
- 44.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. Embo J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Probert L, Plows D, Kontogeorgos G, Kollias G. The type I interleukin-1 receptor acts in series with tumor necrosis factor (TNF) to induce arthritis in TNF-transgenic mice. Eur J Immunol. 1995;25:1794–1797. doi: 10.1002/eji.1830250647. [DOI] [PubMed] [Google Scholar]
- 46.Schottelius AJ, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, et al. Biology of tumor necrosis factor-alpha- implications for psoriasis. Exp Dermatol. 2004;13:193–222. doi: 10.1111/j.0906-6705.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 47.Agnholt J, Dahlerup JF, Kaltoft K. The effect of etanercept and infliximab on the production of tumour necrosis factor alpha, interferon-gamma and GM-CSF in in vivo activated intestinal T lymphocyte cultures. Cytokine. 2003;23:76–85. doi: 10.1016/s1043-4666(03)00201-1. [DOI] [PubMed] [Google Scholar]
- 48.Mitoma H, Horiuchi T, Tsukamoto H. Binding activities of infliximab and etanercept to transmembrane tumor necrosis factor-alpha. Gastroenterology. 2004;126:934–935; author reply 935–936. doi: 10.1053/j.gastro.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 49.Mitoma H, Horiuchi T, Hatta N, Tsukamoto H, Harashima S, et al. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology. 2005;128:376–392. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 50.Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 51.Olleros ML, Guler R, Vesin D, Parapanov R, Marchal G, et al. Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-guerin and Mycobacterium tuberculosis infections. Am J Pathol. 2005;166:1109–1120. doi: 10.1016/S0002-9440(10)62331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehlers S. Role of tumour necrosis factor (TNF) in host defence against tuberculosis: implications for immunotherapies targeting TNF. Ann Rheum Dis. 2003;62(Suppl 2):ii37–42. doi: 10.1136/ard.62.suppl_2.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anolik JH, Ravikumar R, Barnard J, Owen T, Almudevar A, et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180:688–692. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 54.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 55.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iliopoulos A, Psathakis K, Aslanidis S, Skagias L, Sfikakis PP. Tuberculosis and granuloma formation in patients receiving anti-TNF therapy. Int J Tuberc Lung Dis. 2006;10:588–590. [PubMed] [Google Scholar]
- 57.Johnson CM, Cooper AM, Frank AA, Orme IM. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect Immun. 1998;66:1666–1670. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saliu OY, Sofer C, Stein DS, Schwander SK, Wallis RS. Tumor-necrosis-factor blockers: differential effects on mycobacterial immunity. J Infect Dis. 2006;194:486–492. doi: 10.1086/505430. [DOI] [PubMed] [Google Scholar]