Abstract
This study establishes the role of P5A-type Cta4 ATPase in Ca2+ sequestration in the endoplasmic reticulum by detecting an ATP-dependent, vanadate-sensitive and FCCP insensitive 45Ca2+-transport in fission yeast membranes isolated by cellular fractionation. Specifically, the Ca2+-ATPase transport activity was decreased in ER membranes isolated from cells lacking a cta4+ gene. Furthermore, a disruption of cta4+ resulted in 6-fold increase of intracellular Ca2+ levels, sensitivity towards accumulation of misfolded proteins in ER and ER stress, stimulation of the calcineurin phosphatase activity and vacuolar Ca2+ pumping. These data provide compelling biochemical evidence for a P5A-type Cta4 ATPase as an essential component of Ca2+ transport system and signaling network which regulate, in conjunction with calcineurin, the ER functionality in fission yeast.
Introduction
Calcium plays a key role in signal transduction in eukaryotic cells and modulates a variety of cellular functions. The calcium signaling is initiated by opening the Ca2+ channels located at plasma membrane and membranes of the organelles [1]–[3] which leads to transient and local increase in the concentration of cytosolic calcium ions ([Ca2+]i) ten to hundreds times above the basal level, followed by closing the channels. The calcium signal is terminated when cytosolic-free Ca2+ concentration is reduced to basal levels by Ca2+-ATPases and Ca2+/H+ exchangers that transport calcium out of the cell or sequester it in the organelles [4]. Endoplasmic reticulum (ER) plays a crucial role in calcium sequestering and signaling. High concentrations of Ca2+ ions are required for the activities of numerous enzymes that catalyze the folding, modification, processing and trafficking of secretory proteins [4], [5]. Different stimuli can cause disruption of ER function including calcium depletion from the ER lumen, inhibition of protein glycosylation, reduction of disulfide bonds, which all can affect the efficiency of protein folding and cause accumulation of unfolded proteins in the ER. A first response to this ER stress is the stimulation of a signaling pathway appropriately termed the unfolded protein response that selectively activates transcription of the genes encoding ER-resident molecular chaperones [6]–[8]. Evidences indicate that one of the responses to ER stress is a stimulation of Ca2+ influx at the plasma membrane that serves to replenish the Ca2+-depleted organelle and to trigger calcium signaling pathways in both animal and yeast cells [9], [10]. Ca2+/calmodulin-dependent protein phosphatase or calcineurin which is required for cytokinesis and ion homeostasis [11], [12] was shown to be activated during ER stress [13]–[15].
In animal cells, the calcium transport to endoplasmic/sarcoplasmic reticulum is mediated by SERCA Ca2+-ATPases which belong to the P2A subfamily of P-type ATPases [16], [17]. Yeast cells lack homologs of SERCA pumps. We and others showed by means of fluorescence microscopy that the members of P5A-type ATPases were localized to yeast ER, namely Cta4p ATPase of the fission yeast Schizosaccharomyces pombe [18], and Spf1/Cod1 ATPase from Saccharomyces cerevisiae [19]–[20], which share 49% amino acid sequence identity. In spite of indirect indications that P5A-ATPases are involved in calcium homeostasis [18], [20] the substrate specificity of these pumps remains unassigned. Purified Spf1p/Cod1p was used for determination of ATPase activity in vitro in the presence of several cations including Ca2+, however, they failed to stimulate hydrolytic activity [20]. The authors suggested that the factors coupling Spf1p/Cod1p to a specific ion might be lost during the purification of the enzyme from S. cerevisiae membranes [20]. It appears that the S. cerevisiae Spf1p/Cod1p ATPase forms an oligomeric endomembrane complex [21]. In this case, the biochemical evidence for the P5A ATPase as a Ca2+-ATPase should be obtained from the measurements of ATP-dependent Ca2+ transport activity in native yeast membranes and comparison between wild type and null mutant cells. However, till now, there were no reports on Ca2+-ATPase activity in fission yeast membranes.
Previous data show that the absence of ER located yeast P5A-ATPases leads to sensitivity towards calcium stress [18], [20] and changes in nuclear calcium levels [18]. These data provided impetus for the current study, which addressed the role of P5A-type Cta4 ATPase in controlling ER Ca2+ store and signaling in S. pombe.
Methods
Yeast strains and growth conditions
Strains of Schizosaccharomyces pombe used in this study were the wild type Fy1180 (h+ otr1R(SphI)::ade6+ ura4-D18 leu1-32 ade6-M210), the strain Hu185 expressing GFP-tagged Cta4p (h+ cta4-GFP::kanMX6 otr1R(SphI)::ade6 + ura4-D18 leu1-32 ade6-M210), and the mutant strain Hu285 lacking Cta4p (h− cta4::ura4+ ura4-D18 leu1-32 ade6-M216) [18]. The fission yeast strains were grown in standard YES medium at 30°C containing 0.5% yeast extract and 3% glucose supplemented with adenine, uracil, arginine, histidine and leucine (75 mg/L). For spot assays, yeast cells were serially diluted in five-fold steps, spotted onto YES plates containing DTT (0.4%) or tunicamycin (0.05 µg/mL).
45Ca2+ accumulation measurements in yeast cells
Total cellular accumulation of Ca2+ in yeast cells (cytosol and organelles) was measured as described [22]. Briefly, yeast cells were grown to log phase in YES medium, harvested and resuspended in fresh YES medium supplemented with tracer quantities (110.8 µg/mL; 2.5 mCi/mL, Amersham Pharmacia) of 45CaCl2. After 5 h incubation at 30°C, cells were harvested rapidly by filtration onto GF/F filters (Whatman), washed with ice-cold buffer (10 mM CaCl2, 5 mM MES-NaOH pH 6.5), placed in scintillation vials and processed for liquid scintillation counting on a liquid scintillation counter. The specific activity of the culture medium was determined in each experiment. Cell optical density was determined at 600 nm.
Membrane isolation and fractionation
Yeast membranes were isolated and fractionated according to [23], [24]. Briefly, the middle logarithmic phase cells were transformed to spheroplasts by incubation at 37°C in buffer containing 1.2 M sorbitol, 10 mM Tris-HCl, pH 7.4, 30 mM β-mercaptoethanol and 5 mg of lytic enzymes from Trichoderma (Sigma)/1 g of wet cells. After 50 min the incubation mixture of spheroplasts, old cells and cell walls was rapidly cooled and received EDTA, benzamidine and PMSF at 1.2 M sorbitol and 10 mM Tris–HCl, pH 7.4 to give final concentrations 1 mM of each protease inhibitor. The obtained mixture was added to a solution of 1.4 M sorbitol in 10 mM Tris-HCl, pH 7.4, centrifuged for 5 min at 3,000×g and then resuspended and homogenized in a Potter glass homogenizer using a lysis buffer (12.5% sucrose, 20 mM MOPS-Na pH7.4, 1 mM DTT, 1 mM benzamidine, 1 mM PMSF and a cocktail of the polypeptide protease inhibitors). Total membranes were precipitated for 45 min at 120,000×g, resuspended in lysis buffer and loaded onto a 12-step gradient formed of 56, 52, 48, 45, 42, 39, 36, 33, 30, 25 and 20% sucrose (w/w) prepared in 10 mM MOPS-Na pH 7.2. The cocktail of the polypeptide protease inhibitors was applied to each step of gradient. After centrifugation for 2 h 45 min at 140,000×g membrane fractions were collected from the bottom and frozen. A 3-step sucrose gradient was formed of 50, 38 and 25% sucrose (w/w); membrane fractions enriched with vacuole, Golgi, ER/nuclear envelope were collected from the respective interfaces after centrifugation for 2 h 45 min at 140,000×g [23].
Activities of organellar marker enzymes (NADPH cytochrome c oxidoreductase and GDPase) as well as the protein determination followed already published procedures [23]–[26]). Sucrose concentration was determined using a refractometer. To identify membrane fractions enriched with nuclear membranes, the fission yeast strain with constitutive accumulation of the transcription factor Pap1/Caf3 in the nucleus was used [27] and distribution of the GFP-tagged Pap1/Caf3-89 was analyzed by immunoblot.
45Ca2+ transport in membrane vesicles
45Ca2+ uptake by isolated membranes vesicles was measured by the filtration method [25]. The standard incubation mixture contained 10 mM Tris-HCl pH 7.2; 160 mM KCl, 5 mM MgCl2, 1 mM ATP, 9.8 µM EGTA, 10.4 µM CaCl2, 0.5 µCi 45Ca2+ (Amersham Life Sciences) and 2 µM cyanide p-(trifluoromethoxy)phenyl-hydrazone (FCCP). At concentration of Cl− used in assay (160 mMKCl)) the membrane potential is converted into ΔpH from EM H+ or to ΔpCa2+ from EM Ca2+. The reaction was initiated by adding 10–30 µL of suspension of total membrane vesicles or membrane fractions. After incubation at 30°C, 180 µL aliquots were injected into 10 mL of stop solution (150 mM KCl, 5 mM MgCl2, 10 mM MOPS-KOH, pH 7.2), filtered on 0.45 µm nitrocellulose filters (Millipore) and washed with 10 mL of the same buffer. Radioactivity retained on the filters was measured by scintillation counting. The specific activity of the reaction medium was determined in each experiment. The values were normalized per mg of total membrane protein.
Immunoanalysis
Yeast membranes from the sucrose gradient fractions (10 µL) and total membranes (5 µL, 20 µg/µL and 6 µg/µL of wt and mutant, respectively) were spotted on nitrocellulose membranes and probed with anti-BiP (dilution 1∶5,000) or anti-GFP antibodies (1∶400). Anti-GFP antibodies were purchased from Sigma-Aldrich. Anti-BiP antibodies were provided by Prof. J. Armstrong (University of Sussex, Brighton, UK). The blots were developed with peroxidase-conjugated secondary antibody (GE Healthcare).
Protein extraction and calcineurin phosphatase activity assay
Spheroplasts were obtained and lysed as described above for membrane isolation. The homogenate was passed through a Sephadex G-25 column to remove the free phosphate. The solution eluted from the column was used in the calcineurin phosphatase activity assay. Calcineurin protein phosphatase activity was assayed using the Calcineurin Cellular Activity Assay Kit (Calbiochem) according to the manufacturer's instruction. The assay was carried out in 50 µL volumes in a microtiter plate using RII phosphopeptide, the most efficient and commonly used peptide substrate for calcineurin. The reaction was started by adding the cellular extracts, followed by incubation at 30°C for 30 min, and terminated by adding the Malachite Green reagent according to the manufacturer's instruction. The activity of calcineurin protein phosphatase was determined by the amount of free phosphate released and normalized per mg of protein.
Results
Loss of Cta4 ATPase results in increased intracelullar Ca2+ accumulation
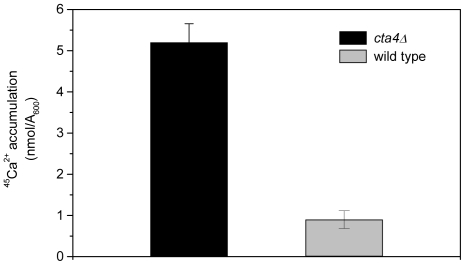
To address the role of Cta4 ATPase in Ca2+ homeostasis, we investigated the effect of cta4+ deletion on cellular calcium levels. We found that mutant cells lacking Cta4 ATPase exhibited 6-fold increase in total calcium accumulation as compared to wild-type cells (Figure 1). This result suggests that loss of Cta4 ATPase leads to enhanced calcium influx which might occur in response to depletion of Ca2+ from secretory organelles.
Figure 1. Loss of cta4+ results in elevated cellular Ca2+ levels.
45Ca2+ accumulation in wild-type cells and mutant cells lacking Cta4 ATPase was measured after incubation for 5 hr in standard medium supplemented with 45Ca2+ as described in Materials and Methods. Values are means (± SE) of three independent experiments.
ATP-dependent Ca2+ transport is reduced in cta4Δ membranes
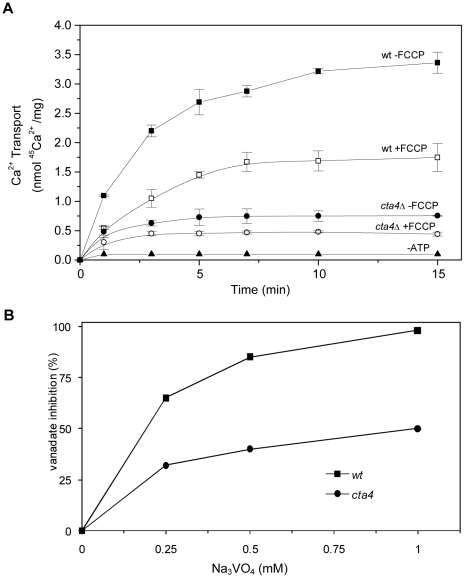
To examine whether Cta4p participates in Ca2+ sequestering along the secretory pathway, we measured at first the ATP-dependent 45Ca2+ transport in total membrane vesicles isolated from wild-type and cta4Δ mutant cells. In order to distinguish between Ca2+ transport mediated by Ca2+-ATPases and Ca2+/H+ exchangers, protonophore FCCP was used to collapse the transmembrane H+ gradient and eliminate the Ca2+/H+ exchanger component of transport. As shown in Figure 2A, Ca2+ transport in total membranes isolated from wild-type cells was decreased by 50% upon FCCP addition (see 15 min point), indicating that remaining 50% of the transport was mediated by Ca2+-ATPases. Indeed, this ATP-dependent FCCP-insensitive 45Ca2+ transport was also sensitive to orthovanadate, the specific inhibitor of P-type ATPases, showing 85% and 98% inhibition at 0.5 mM and 1 mM, respectively (Figure 2B).
Figure 2. 45Ca2+ uptake by total membranes of S. pombe is mediated by Ca2+- ATPases and Ca2+/H+ exchangers.
Total membranes were isolated from wild type (▪,□) and cta4Δ (•,○) cells and subjected to determination of 45Ca2+ transport in the presence of 1 mM ATP and in the presence (□,○) or absence (▪,•) of 2 µM FCCP (A). Inhibition of ATP-dependent FCCP-insensitive 45Ca2+ transport by vanadate (Na3VO4), the inhibitor of P-type ATPases (B). Values are means (± SE) of four independent experiments.
The loss of Cta4p resulted in strong reduction (4.5-fold) of total ATP-dependent 45Ca2+ transport (Figure 2A) of which only 40% was FCCP-sensitive, reflecting lower activity of Ca2+/H+ antiporters in mutant cells. 45Ca2+ transport mediated by Ca2+-ATPase (FCCP-insensitive transport) was 4-fold lower in cta4Δ membranes than in wild type (Figure 2A) and 50% of this activity was vanadate-sensitive with IC50∼0.2 mM (Figure 2B). These data clearly indicate that cta4+ is required for Ca2+-ATPase activity and Ca2+ sequestering in fission yeast membranes.
Cta4 ATPase is required for Ca2+ transport in ER membranes vesicles
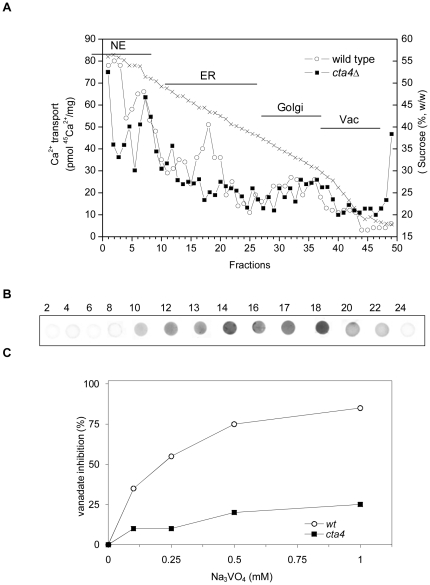
To identify the membrane fractions responsible for Cta4p-dependent Ca2+ uptake, separation of the fission yeast membranes on a 12-step sucrose gradient was performed. Determination of sucrose density in addition to biochemical and immunochemical characterization showed that membrane fractions 1–11 were likely derived from the nucleus according to GFP-tagged Caf3-89 immunodetection (not shown, [27]). Fractions 12–28 and 29–38 were enriched with the ER and Golgi, respectively, as revealed by analysis of BiP and Cta4p∼GFP distribution, and GDPase activity; vacuoles migrated with light membrane vesicles in fractions 39–50 [23].
ATP-dependent FCCP-insensitive 45Ca2+ transport was detected along the sucrose gradient in different membrane fractions indicating the presence of Ca2+ pumps in various compartments of S. pombe (Figure 3A). The highest Ca2+-ATPase activities were detected in ER, nucleus and Golgi membranes. Comparative analysis revealed that the peak of Ca2+-ATPase activity was absent in ER membrane fractions 15–21 of cta4Δ (Figure 3A, closed symbols). In agreement, the corresponding fractions 15–22 isolated from the fission yeast strain expressing GFP-tagged Cta4p were immuno-reactive with anti-GFP antibodies (Figure 3B and [18]), indicating the dependence of Ca2+ transport in these membrane vesicles on cta4+. In addition, Ca2+-ATPase transport activity in ER fractions of wild type was significantly inhibited by vanadate (85%, 1 mM) whereas most of this vanadate-sensitive activity disappeared in ER fraction of cta4Δ (Figure 3C).
Figure 3. cta4+ is required for Ca2+-ATPase activity in ER membrane fractions.
Total membranes were isolated from wt and cta4Δ cells and fractionated on 12-step sucrose density gradient. (A) ATP-dependent FCCP-insensitive 45Ca2+ uptake in the membrane fractions was measured after 10 min of incubation as described in Materials and Methods. Sucrose concentration of each fraction is shown. (B) Dotblot of selected gradient fractions (fraction numbers are indicated) was used for immunolocalization of Cta4-GFP using anti-GFP antibodies. (C) Inhibition of ATP-dependent FCCP-insensitive 45Ca2+ transport in ER membrane fractions by vanadate (Na3VO4), the inhibitor of P-type ATPases. Results shown are representative of three independent experiments. Abbreviation used: NE, nuclear envelope; ER, endoplasmic reticulum; Vac, vacuole.
It is noteworthy that although ATP-dependent FCCP-insensitive 45Ca2+ transport was reduced in ER and membranes heavier than ER (mainly nucleus) of cta4Δ, it was augmented in vacuolar fractions (Figure 3A).
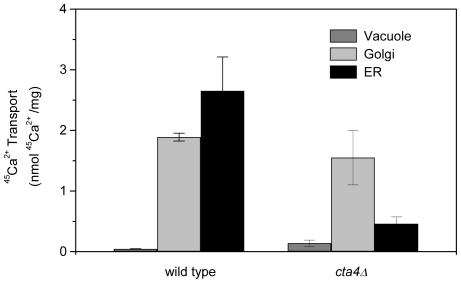
These results were further confirmed using 3-step fractionation yielding three membrane fractions enriched with vacuole, Golgi and ER/nuclear vesicles. ER/N membranes of wild-type cells exhibited 6-fold higher Ca2+-ATPase transport activity as compared to the membranes lacking Cta4p (Figure 4). The deletion of cta4+ resulted in up-regulation of Ca2+ pump activity in light membrane fraction reinforcing a compensatory role of vacuole enzyme.
Figure 4. Loss of Cta4 ATPase results in reduced Ca2+ pumping in endoplasmic reticulum.
Total membranes were isolated from wt and cta4Δ mutant cells and then fractionated on 3-step sucrose density gradient as described in Materials and Methods. Fractions were subjected to determination of the 10 min 45Ca2+ uptake in the presence of 2 µM FCCP and 1 mM ATP. Values are means (± SE) of four independent experiments.
Altogether, these results demonstrate that Cta4p expression is crucial for ATP-dependent Ca2+ transport in endoplasmic reticulum and that cta4+ deletion has a profound effect on Ca2+ homeostasis within membrane network of fission yeast.
ER stress response in cta4 + deletion mutant and activation of calcium influx
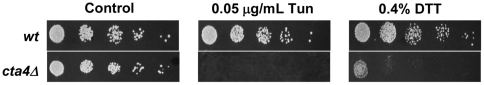
To explore how the cta4+ disruption interferes with the ER function, we tested the sensitivity of yeast cells towards ER stress inducers DTT and tunicamycin which inhibit the protein disulfide bond formation and N-glycosylation, respectively. The mutant cta4Δ cells displayed sensitivity towards these substances while the growth of wild-type cells was not affected (Figure 5). Thus, the absence of Cta4 ATPase results in inability to cope with the accumulation of unfolded proteins.
Figure 5. The growth of cta4Δ is impaired by endoplasmic reticulum stress.
Wild-type and cta4Δ cells were serially diluted in five-fold steps, spotted onto YES plates containing 0.4% DTT and 0.05 µg/mL tunicamycin and incubated for 3 days at 30°C.
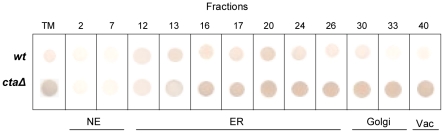
This assumption was further verified by detection of the ER chaperone BiP. In wild-type cells BiP was localized to membrane fractions which correspond to ER (Figure 6, fractions 12–26) and where GFP-tagged Cta4p was also detected (Figure 3A, fractions 8–22). On the other hand, the distribution pattern of BiP in cta4Δ differs from that of wild type occurring not only in ER but also in Golgi and vacuole membranes fractions (Figure 6, fractions 12–38). Also, BiP expression was significantly higher in cta4Δ. Hence, the lack of Cta4 ATPase triggers ER stress response and interferes with secretory pathway.
Figure 6. Cells lacking Cta4p exhibited higher levels of the ER stress indicator, BiP.
Total membranes (TM) were isolated from yeast cells, fractionated on a sucrose density gradient and submitted to immunoblots analysis as described in Materials and Methods. Dot blots of individual fractions (10 µL, numbers are indicated) were used. Abbreviation used: NE, nuclear envelope; ER, endoplasmic reticulum; Vac, vacuole.
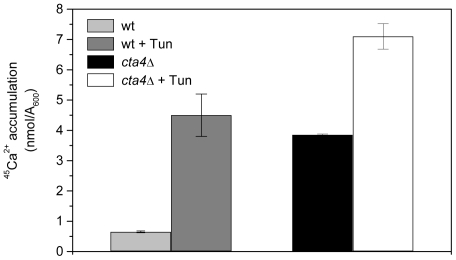
Next, the calcium accumulation in S. pombe cells under ER stress was investigated. As shown in Figure 7, exposure of wild type cells to tunicamycin promoted 5-fold increase in 45Ca2+ accumulation reaching the levels of that of untreated cta4Δ cells, while cta4Δ exhibited only 2-fold stimulation. These findings reinforce a notion that ER function is severely impaired in the absence of Cta4 ATPase and suggest that enhanced calcium accumulation in cta4Δ might be explained as a result of the induction of ER stress response which, in turn, is related to disturbance of calcium transport in ER.
Figure 7. Endoplasmic reticulum stress caused by inhibition of glycosylation stimulates an increase of Ca2+ accumulation.
The accumulation of 45Ca2+ in wild-type and cta4Δ yeast cells was measured after 5 hours of addition of 45Ca2+ to YES medium containing 0.125 µg/mL tunicamycin. Values are means (± SE) of three independent experiments.
Stimulated calcineurin controls calcium influx in cells lacking cta4 +
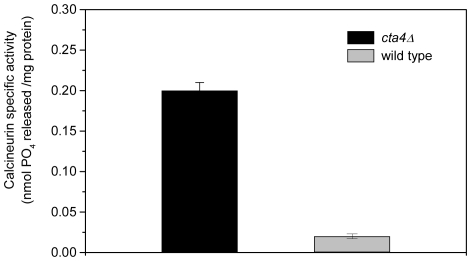
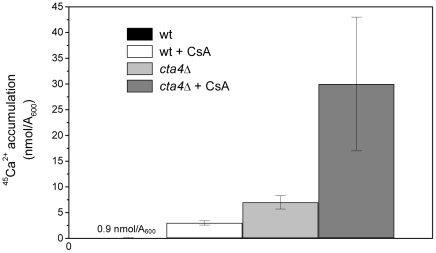
It has been shown previously that cta4Δ is unable to grow in the presence of cyclosporine (CsA) [18], indicating that survival of the mutant cells requires Ca2+/CaM-dependent phosphatase calcineurin. Analysis of calcineurin specific activity in wild type and cta4Δ cells revealed that cta4+ deletion resulted in drastic, 10-fold stimulation in calcineurin activity compared to wild-type cells (Figure 8). Furthermore, CsA treatment induced 45Ca2+ accumulation by 7-fold in cta4Δ mutant cells, reaching nearly 9-fold higher levels than that of wild type cells grown in the presence of calcineurin inhibitor and 30-fold higher than of wild type strain grown in standard conditions (Figure 9). These findings indicate that calcineurin negatively regulates calcium influx into fission yeast cells and performs essential function in cta4Δ preventing lethal elevation of intracellular calcium in response to ER dysfunction.
Figure 8. Mutant cta4Δ displays higher calcineurin phosphatase activity.
The calcineurin protein phosphatase activity was determined by the amount of free phosphate released. The reaction was started by adding the cellular extracts, followed by incubation at 30°C for 30 min with the RII phosphopeptide substrate. Values are means (± SE) of three independent experiments.
Figure 9. Calcineurin inhibition stimulated 45Ca2+ accumulation in wt and cta4Δ mutant cells.
The accumulation of 45Ca2+ in wild-type and cta4Δ yeast cells was measured after 5 hours of addition of 45Ca2+ to YES medium containing 10 µg/mL cyclosporin A (CsA). Values are means (± SE) of three independent experiments.
Discussion
The members of P5-type ATPases are found in all eukaryotic genomes analyzed to date and present a number of striking features in their amino acid sequences and membrane topology that set them apart from the other enzymes of this type [17], [28], [29]. Previous studies based on indirect methods suggested the involvement of ER localized P5A ATPases Cta4 and Spf1/Cod1 from fission and budding yeast, respectively, in calcium homeostasis [18], [20]. Heterologous expression of Arabidopsis MIA protein was shown to restore the growth of spf1/cod1 null mutant on solid medium containing lovastatin [30]. Recent study in S. pombe implies the participation of a second P5A-ATPase in calcium and manganese homeostasis [31]. Unraveling the substrate specificities of P5 ATPases through biochemical means has increasingly been recognized as a major goal [32]. The analysis of Ca2+ transport in isolated fission yeast membranes represents a starting point for biochemical characterization of P5A-ATPase. Apart from Cta4p ATPase, two putative P2-type Ca2+-ATPases were revealed in fission yeast S. pombe, namely Pmc1p (SPAPB2B4.04c), and Pmr1p/Cps5p (SPBC31E1.02c) [28], [33]. Pmr1p localizes predominantly in the endoplasmic reticulum (ER) membrane while Pmc1p is limited to vacuole [33].
Using biochemical and genetic approaches, this study establishes the role of P5A-type Cta4 ATPase in Ca2+ sequestration in the ER. Our conclusion follows from the finding that ATP-dependent, FCCP-insensitive and vanadate-sensitive Ca2+ transport activity was severely diminished in ER membranes isolated from cells lacking Cta4 ATPase. To our knowledge, this is also the first report describing a Ca2+-ATPase transport activity in fission yeast endoplasmic reticulum, Golgi and vacuole membranes. For evolutionary reasons that remain unclear, S. pombe and S. cerevisiae lack homologs of SERCA pumps. In this scenario, another ATPase should assure Ca2+ transport in ER. The present study provides biochemical evidence that the P5A Cta4 ATPase is essential for Ca2+-ATPase activity in ER and is crucial for establishing yeast ER homeostasis by regulating calcium transport. In our previous work only Ca2+/H+ exchange activity was measurable in membranes of S. pombe suggesting that Ca2+-ATPases were not expressed or not detected by unknown reasons [24]. Since both studies differ only by growth medium (peptone lacking YES in present study and YPD in former one), we assume that this factor can determine the effective expression of one or both types of the Ca2+-transporters.
Previous lack of evidence for the substrate specificity of P5A-ATPases led some authors propose that P5A-ATPases could be a phospholipid-ATPase mainly due to its homology with some flippases [34]. However, the available information on the biochemical properties of flippases from S. cerevisiae and other organisms does not support this alternative, since this enzymes shows a very low affinity for ATP (Km∼1.5 mM ATP [35]), in contrast to Km∼15 µM ATP found for Spf1/Cod1p [20] that is in the range of the high affinity for ATP described for most Ca2+-ATPases. In addition, flippases exhibit a very high sensitivity to vanadate with IC50 = 1–5 µM [35], [36] while Ca2+ transport in Cta4p-containing membranes was inhibited by 50% at 200 µM (Figures 2B and 3C), in the same way as Spf1/Cod1 ATPase activity was blocked at 500 µM vanadate [20].
We further showed that cta4+ loss leads to ER stress response and subsequent stimulation of intracellular calcium accumulation. It should be noted that calcium accumulation was recovered to wild-type levels in cta4Δ cells expressing cta4 + (data not shown). Elevated Ca2+ levels promote activation of calcineurin, essential for cta4Δ survival. In animal cells, the inhibitors of SERCA pumps activate the entry of Ca2+ into the cytoplasm through plasma membrane channels, a mechanism known as capacitative calcium entry or CCE [9]. Inhibition of ER Ca2+-ATPases also leads to increase of mRNA levels for the ER stress marker proteins BiP/GRP78 [37]. Elevation of intracellular calcium was demonstrated in budding yeast, in response to deletion of Golgi Ca2+-ATPase Pmr1p [10] and ER stress [14]. In budding yeast, ER stress triggers Ca2+ influx through a plasma membrane HACS channel resulting in activation of calcineurin [14]. Indeed, the activity of HACS channel as judged from detection of Mg2+ insensitive component of 45Ca2+ uptake was significantly increased in cta4Δ (unpublished data).
It has been suggested recently that the activation of HACS is a major response to defects in the secretory, endosomal and vacuolar protein trafficking pathways [38]. Genome-wide approach in S. cerevisiae allowed the identification of group A mutants (HACS regulators) with spontaneous HACS activation and defects in endomembrane trafficking system, and group B mutants which require external stimuli (tunicamycin or α-factor) to activate Ca2+ influx. Importantly, our results place cta4Δ in group A, together with pmr1 mutant. Notably, spf1 and pmc1 mutants belong to group B. It should be emphasized that loss of Spf1/Cod1 ATPase in S. cerevisiae did not result in changes in cellular calcium levels [20], activation of Ca2+ influx and HACS [38]. Thus, although Cta4p and Spf1/Cod1p share amino acid identity, both reside to ER and corresponding mutants display sensitivity towards extracellular calcium, they apparently have distinct functions in endomembrane trafficking system and calcium homeostasis. This study also provides direct biochemical evidence that specific calcineurin phosphatase activity is highly induced upon cta4+ disruption. In this respect, it is noteworthy that mutant lacking Cta4 ATPase was sensitive to inhibition of calcineurin [18] indicating that activated calcineurin is essential for the survival of cta4Δ cells undergoing ER stress. This finding is in agreement with the demonstration of requirement of calcineurin for a survival of mutants with activated HACS [38]. Future study should identify the targets of calcineurin responsible for cell survival under ER stress in S. pombe. However, one of these targets has been already identified. We showed that cta4Δ mutant displays an increase in Ca2+-ATPase activity in vacuole membranes as compared to wild-type. Thus, induced calcium sequestration to vacuoles possibly compensates for the loss of the Cta4p from the ER, and contributes to lowering the cytoplasmic calcium. Also, we found that cta4Δ exhibits a decrease in activity of Ca2+/H+ antiporters. Our findings support the idea that elevated intracellular calcium in cta4Δ leads to activation of the calcineurin which, in turn, differentially regulates other Ca2+ transporters. This is consistent with results obtained for the S. cerevisiae Vcx1, a vacuolar H+/Ca2+ exchanger, which activity is inhibited by calcineurin [21], whereas the expression and activity of vacuolar calcium pump Pmc1p is stimulated by Ca2+ in calcineurin-dependent manner [22], [39]. The S. cerevisiae Golgi-localized Ca2+-ATPase Pmr1p was shown to be dependent on calcineurin [22] but to much less extent [6], [40]. In S. pombe, Pmr1p/Csp5p is localized to ER [33] and is likely responsible for remaining calcium transport in ER of cta4Δ. Our data suggest that vacuolar Ca2+-ATPase Pmc1p assumes a leading role in calcium homeostasis in cta4Δ genetic background. This suggests that vacuolar pump could serve as a backup system when Cta4 ATPase is missing and under calcium overload. Indeed, the S. pombe pmc1 + was required for growth in medium in the presence of high extracellular CaCl2 [41]. It is likely that fission yeast vacuolar Pmc1p expression is activated by calcineurin in cta4Δ. This supposition is consistent with the demonstration of the induction of PMC1 transcription in S. cerevisiae mutants lacking Spf1p/Cod1p P5AATPase using ß-galactosidase reporter plasmid [20].
The data also established a critical role of Cta4p in maintaining the ER folding environment. The findings reported here indicate that cta4Δ mutant is under ER stress as revealed by the increased expression of the ER stress indicator, the chaperone BiP. The distribution of BiP in Golgi and vacuole membrane fractions isolated from cta4Δ mutant deserves special mention. Disruption of cta4+ may affect not only BiP distribution but also that of other proteins of the secretory pathway. In agreement, it was demonstrated that loss of another P5A ATPase, Spf1/Cod1p from S. cerevisiae, resulted in altered distribution of another ER membrane protein, Sec12 [42]. Additionally, a mutation in the P5A ATPase gene MIA from Arabidopsis resulted in major changes in expression of genes involved in protein secretion [30]. Thus, one of physiological roles of P5A ATPases is to control the proper functioning of secretory pathway and protein targeting. This finding is not without precedence, since deletion of S. cerevisiae Pmr1 Ca2+-ATPase also resulted in ER stress [43] and significant redistribution of enzyme activities and total protein in compartments of the secretory pathway [25].
Loss of cta4+ resulted in ER stress and inability to cope with accumulation of unfolded proteins. Although the ER-associated protein degradation constitutes a main mechanism for elimination of unfolded protein, there is growing evidence for a vacuole participation in this process. A recent study in tobacco has shown that BiP is transported to the vacuole and that the ER export of BiP occurs via COPII-dependent transport to the Golgi apparatus [44]. It is likely that in cta4Δ vacuolar disposal of proteins could act in addition to ER-associated protein degradation to improve the efficiency of quality control throughout in the secretory pathway. The stimulation of vacuole Ca2+ transport observed in cta4Δ might be required to maximize vacuole functioning, besides of lowering cytoplasmic calcium levels. Thus, the vacuole shares a role in calcium homeostasis and quality control with ER.
Our results adds biochemical substantiation to the argument that Cta4p might transport Ca2+ or, at least, regulates Ca2+ transport in ER by influencing functional localization of Pmr1p in the ER. Although both alternatives remain to be investigated, the last one should not be the case for Spf1/Cod1p from S. cerevisiae since Pmr1p is localized to Golgi in this yeast [25].
In conclusion, we provide functional evidence of Ca2+ transport mediated by Ca2+-ATPase in fission yeast membranes. Our findings depict a crucial role of P5A-type Cta4 ATPase in Ca2+ transport in ER and regulation of Ca2+ influx system. Finally, our data provide a platform for future studies of the signaling network which encompasses the calcineurin and Ca2+-ATPases activity and controls the calcium homeostasis and the function of the endomembrane system in S. pombe.
Acknowledgments
The authors thank Prof. J. Armstrong for kindly providing anti-BiP antibodies and Prof. M. Sipiczki for providing yeast strain expressing tagged GFP-Pap1/Caf3-89.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by CNPq (Conselho Nacional de Pesquisa e Desenvolvimento) and FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro). ACDML and LMP received a PhD fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal no Nível Superior). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dymamics, homeostasis and remodelling. Nature. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Mol Biol. 1995;46:95–122. [Google Scholar]
- 4.Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- 5.Durr G, Strayle J, Plemper R, Elbs S, Klee SK, et al. The medial-golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum- associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 8.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of misfolded proteins in the endoplasmic reticulum signals the induction of glucose regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 9.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 10.Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol Cell Biol. 2000;20:6686–6694. doi: 10.1128/mcb.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiura R, Sio SO, Shuntoh H, Kuno T. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells. 2002;7:619–627. doi: 10.1046/j.1365-2443.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 12.Cyert MS. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun. 2003;311:1143–1150. doi: 10.1016/s0006-291x(03)01552-3. [DOI] [PubMed] [Google Scholar]
- 13.Groenendyk J, Lynch J, Michalak M. Calreticulin, Ca2+, and calcineurin- signaling from the endoplasmic reticulum. Mol Cells. 2004;17:383–389. [PubMed] [Google Scholar]
- 14.Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonilla M, Cunningham KW. Mitogen-activated protein kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell. 2003;14:4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carafoli E, Brini M. Calcium pumps: structural basis for and mechanism of calcium transmembrane transport. Curr Opin Chem Biol. 2000;4:152–161. doi: 10.1016/s1367-5931(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 17.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- 18.Okorokova-Façanha AL, Appelgren H, Tabish M, Okorokov LA, Ekwall K. The endoplasmic reticulum cation P-type ATPase Cta4p is required for control of cell shape and microtubule dynamics. J Cell Biol. 2002;157:1029–1040. doi: 10.1083/jcb.200111012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki C, Shimma Y. P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol Microbiol. 1999;32:813–823. doi: 10.1046/j.1365-2958.1999.01400.x. [DOI] [PubMed] [Google Scholar]
- 20.Cronin SR, Rao R, Hampton RY. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J Cell Biol. 2002;157:1017–1028. doi: 10.1083/jcb.200203052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thever MD, Saier MH. Bioinformatic characterization of P-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. J Membr Biol. 2009;229:115–130. doi: 10.1007/s00232-009-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samarão SS, Teodoro CE, Silva FE, Ribeiro CC, Granato TM, et al. H+-ATPase along the yeast secretory pathway: Energization of the ER and Golgi membranes. Biochim Biophys Acta. 2009;2:303–313. doi: 10.1016/j.bbamem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Okorokov LA, Silva FE, Okorokova-Façanha AL. Ca2+ and H+ homeostasis in fission yeast: a role of Ca2+/H+ exchange and distinct V-H+ -ATPases of the secretory pathway organelles. FEBS Lett. 2001;505:321–324. doi: 10.1016/s0014-5793(01)02852-6. [DOI] [PubMed] [Google Scholar]
- 25.Okorokov LA, Lehle L. Ca2+-ATPases of Saccharomyces cerevisiae: diversity and possible role in protein sorting. FEMS Microbiol Lett. 1998;162:83–91. doi: 10.1111/j.1574-6968.1998.tb12982.x. [DOI] [PubMed] [Google Scholar]
- 26.Abeijon C, Orlean P, Robbins PW, Hirschberg CB. Topography of glycosylation in yeast: Characterization of GDP mannose transport and luminal guanosine diphosphatase activities in Golgi-like vesicles. Proc Natl Acad Sci USA. 1989;86:6935–6939. doi: 10.1073/pnas.86.18.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benko Z, Fenyvesvolgyi C, Pesti M, Sipiczki M. The transcription factor Pap1/Caf3 plays a central role in the determination of caffeine resistance in Schizosaccharomyces pombe. Mol Genet Genomics. 2004;271:161–170. doi: 10.1007/s00438-003-0967-3. [DOI] [PubMed] [Google Scholar]
- 28.Okorokova-Façanha AL, Okorokov LA, Ekwall K. An inventory of the P-type ATPases in the fission yeast Schizosaccharomyces pombe. Curr Genet. 2003;43:273–280. doi: 10.1007/s00294-003-0395-2. [DOI] [PubMed] [Google Scholar]
- 29.Catty P, Kerchove d'Exaerde A, Goffeau A. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 1997;409:325–332. doi: 10.1016/s0014-5793(97)00446-8. [DOI] [PubMed] [Google Scholar]
- 30.Jakobsen MK, Poulsen LR, Schulz A, Fleurat-Lessard P, Møller A, et al. Pollen development and fertilization in Arabidopsis is dependent on the MALE GAMETOGENESIS IMPAIRED ANTHERS gene encoding a type V P-type ATPase. Genes Dev. 2005;15:2757–2769. doi: 10.1101/gad.357305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furune T, Hashimoto K, Ishiguro J. Characterization of a fission yeast P5-type ATPase homologue that is essential for Ca2+/Mn2+ homeostasis in the absence of P2- type ATPases. Genes Genet Syst. 2008;83:373–381. doi: 10.1266/ggs.83.373. [DOI] [PubMed] [Google Scholar]
- 32.Sørensen DM, Buch-Pedersen MJ, Palmgren MG. Structural divergence between the two subgroups of P5 ATPases. Biochim Biophys Acta. 2010;1797:846–855. doi: 10.1016/j.bbabio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Cortes JC, Katoh-Fukui R, Moto K, Ribas JC, Ishiguro J. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Euk Cell. 2004;3:1124–1135. doi: 10.1128/EC.3.5.1124-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulsen LR, López-Marqués RL, Palmgren MG. Flippases: still more questions than answers. Cell Mol Life Sci. 2008;65:3119–3125. doi: 10.1007/s00018-008-8341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Graham TR. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc Natl Acad Sci USA. 2009;106:16586–16591. doi: 10.1073/pnas.0904293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckford PD, Sharom FJ. The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem J. 2005;389:517–526. doi: 10.1042/BJ20050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price BD, Mannheim-Rodman LA, Calderwood SK. Brefeldin A, thapsigargin and AIF4 stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J Cell Physiol. 1992;152:545–552. doi: 10.1002/jcp.1041520314. [DOI] [PubMed] [Google Scholar]
- 38.Martin DC, Kim H, Mackin NA, Maldonado-Báez L, Evangelista CC, Jr, et al. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J Biol Chem. 2011;286:10744–10754. doi: 10.1074/jbc.M110.177451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimoto H, Saltsman K, Gasch AP, Li HX, Ogawa N, et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:31079–31088. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- 40.Matheos D, Kingsbury TU, Ahsan U, Cunningham KW. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng L, Sugiura R, Takeuchi M, Suzuki M, Ebina H, et al. Real-time monitoring of calcineurin activity in living cells: evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol Biol Cell. 2006;17:4790–4800. doi: 10.1091/mbc.E06-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki C. Immunochemical and mutational analyses of P-type ATPase Spf1p involved in the yeast secretory pathway. Biosci Biotechnol Biochem. 2001;65:2405–2411. doi: 10.1271/bbb.65.2405. [DOI] [PubMed] [Google Scholar]
- 43.Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, et al. Golgi mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell. 2006;18:198–211. doi: 10.1105/tpc.105.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]