Abstract
The enzymatic synthesis of indole-3-acetic acid (IAA) from indole by an in vitro preparation from maize (Zea mays L.) that does not use tryptophan (Trp) as an intermediate is described. Light-grown seedlings of normal maize and the maize mutant orange pericarp were shown to contain the necessary enzymes to convert [14C]indole to IAA. The reaction was not inhibited by unlabeled Trp and neither [14C]Trp nor [14C]serine substituted for [14C]indole in this in vitro system. The reaction had a pH optimum greater than 8.0, required a reducing environment, and had an oxidation potential near that of ascorbate. The results obtained with this in vitro enzyme preparation provide strong, additional evidence for the presence of a Trp-independent IAA biosynthesis pathway in plants.
Until recently, the biosynthesis of IAA was hypothesized to occur via oxidative decarboxylation and deamination of the amino acid Trp (Bandurski et al., 1995). Trp is an important intermediate for IAA biosynthesis in microorganisms and in plants (Patten and Glick, 1996; Normanly, 1997). However, there is now strong genetic and in vivo-labeling evidence for a Trp-independent IAA biosynthesis pathway in several plant species (Baldi et al., 1991; Wright et al., 1991; Michalczuk et al., 1992; Normanly et al., 1993). In some plants and/or developmental stages, the Trp-independent pathway is the primary route for IAA production. It appears to be of particular importance during embryogenesis, when fine control over low levels of IAA are critical to polar development (Michalczuk et al., 1992; Ribnicky, 1996). There have been earlier reports of in vitro IAA biosynthesis with maize (Zea mays L.) using extracts prepared from liquid endosperm tissue (Jensen and Bandurski, 1994; Rekoslavskaya and Bandurski, 1994; Rekoslavskaya, 1995) and coleoptiles (Koshiba and Matsuyama, 1993). The liquid endosperm preparations from maize kernels converted indole to IAA in vitro. This result suggested that the Trp-independent pathway might contribute significantly to the newly synthesized IAA; however, extensive Trp-to-IAA conversion also occurs in such preparations (Rekoslavskaya, 1995; Ilić et al., 1998). This finding is consistent with our observation in carrot that tissues accumulating relatively large quantities of IAA seem to use predominately Trp conversion (Michalczuk et al., 1992) and that maize liquid endosperm is a very active IAA-producing tissue (Jensen and Bandurski, 1994).
In another report, Trp-dependent IAA biosynthesis was detected in an in vitro extract from maize coleoptiles (Koshiba and Matsuyama, 1993). IAA biosynthesis was dependent on indole-3-acetaldehyde oxidase activity in combination with the activity of a cytosolic ascorbate peroxidase. This in vitro system used l- and d-Trp without any discrimination and both l- and d-aminotransferases were present in this tissue extract (Koshiba and Matsuyama, 1993). In vivo-labeling studies using exogenous [14C]Trp labeling of dark-grown coleoptile tips showed metabolism of approximately 10% of the label into [14C]IAA (Koshiba et al., 1995). This tissue is competent to produce IAA from Trp but may do so only if the section is removed from the rest of the plant and/or if a high level (18 μm) of Trp is used exogenously (Koshiba et al., 1995). Overall, these results must be viewed in contrast with the compelling evidence provided by 2H2O and precursor labeling experiments. Such studies have shown that coleoptiles of intact maize seedlings do not produce significant IAA from de novo biosynthesis (Pengelly and Bandurski, 1983; Jensen and Bandurski, 1996), and [15N]Trp tracer studies with another monocotyledonous plant have shown that d-Trp is not an IAA precursor (Baldi et al., 1991).
The maize mutant orange pericarp is a Trp auxotroph as a result of mutations of both genes coding for Trp synthase β (Wright et al., 1992). Light-grown orange pericarp seedlings have higher increased levels of total IAA, indicating that there is a biosynthetic pathway to IAA that does not use Trp as an intermediate (Wright et al., 1991). This was confirmed by experiments with [15N]anthranilic acid or 2H2O labeling of orange pericarp seedlings, which showed incorporation of the label into IAA but not into Trp. In an effort to determine the intermediates and isolate the enzymes involved in the Trp-independent IAA biosynthetic pathway in plants, we have established an in vitro system in which the conversion of indole to IAA can be studied. Unlike the genetic composition of endosperm (which is triploid, the product of triple fusion), biochemical measurements from both normal and orange pericarp genetic materials can be easily compared using preparations from light-grown maize seedlings.
MATERIALS AND METHODS
Reagents
All chemicals were from Sigma unless stated otherwise. [2-14C]Indole (55 mCi/mmol), [carboxyl-14C]IAA (57 mCi/mmol), and l-[side chain-3-14C]Trp (53.8 mCi/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO). l-[U-14C]Ser (120 mCi/mmol) was purchased from Moravek Biochemicals (Brea, CA).
Plant Material
In most experiments, 8- to 9-d-old seedlings of maize (Zea mays L. cv Silver Queen) (The Meyer Seed Co., Baltimore, MD) were used to prepare enzyme extracts. Maize kernels were soaked in running tap water overnight and then planted in moist vermiculite. The plants were grown in a growth room under constant light (cool-white fluorescent lamps, 30 μmol cm−2 s−1) and at 25°C. Some studies used extracts prepared from seedlings grown from kernels of the maize mutant orange pericarp (Wright et al., 1991), and these kernels were a gift from Dr. Allen Wright. Immature maize kernels were obtained from mature Silver Queen plants (from the same seed lot that was used for the seedling experiments) grown in a field plot during the summer of 1996 (Beltsville, MD). Unpollinated ears were obtained by placing a wax-paper bag over the ears before silk emergence to prevent fertilization.
Preparation of the Maize Seedling IAA Biosynthesis in Vitro System
The homogenization buffer consisted of 75 mm POPSO, 0.05 m (NH4)2SO4, 0.25 m ascorbic acid, 10% (v/v) glycerol, 1.5% (w/v) PVP, 1% (w/v) N-decyl-N,N-dimethyl-3-ammonio-1-propanesulfonate, 10 mm EGTA, 1 mm PHMB, 5 mm ε-amino-n-caproic acid, 1 mm PMSF, 1 mm benzamide, 1 mm benzamidine, 50 μg/mL antipain, 1 mg/mL pepstatin, and 50 μg/mL leupeptin in deionized glass-distilled water. The homogenization buffer was prepared from a 2× stock of POPSO, glycerol, (NH4)2SO4, N-decyl-N,N-dimethyl-3-ammonio-1-propanesulfonate, and PVP stored at 4°C. Just before preparation of the enzyme extract, the ascorbic acid was added and the pH adjusted to 8.5 with KOH. Finally, the protease inhibitors EGTA, PHMB, PMSF, ε-amino-n-caproic acid, benzamide, benzamidine, antipain, leupeptin, and pepstatin were added and the solution was diluted to its final concentration. The homogenization buffer was chilled at −20°C to a partially frozen slurry before use. These procedures were adapted with modifications from the general recommendations of Gegenheimer (1990) for preparation of plant enzyme extracts. Two modifications of this basic buffer system were also used. To determine the optimum pH for the complete reaction, an experiment was conducted in which the 75 mm POPSO buffer was changed to a mixed buffer system composed of 40 mm POPSO plus 40 mm Mes. This mixed buffer allowed a pH range of 6.0 to 8.5 to be analyzed without changing the organic buffers present in the reaction. In studies with maize orange pericarp seedlings, 75 mm Tris was substituted for 75 mm POPSO, but all other components remained the same.
Maize seedlings were homogenized in the ice-cold homogenization buffer slurry (10 g of seedlings/50 mL of buffer slurry) using a prechilled mortar on salted ice in a 5°C cold room. The homogenized mixture was then filtered through one layer of cheesecloth plus one layer of Miracloth (Calbiochem). The pH was adjusted, if necessary, to 8.5 with KOH. The extract was then centrifuged at 12,000g for 20 min at 0°C to 5°C. The supernatant was decanted and used as the source of enzymes, endogenous reactants, and cofactors for the in vitro system. The amount of protein was determined by the Bradford dye method (Bio-Rad).
Measurement of Enzyme Activity
One-milliliter aliquots of the plant extract were mixed with 200,000 dpm of [14C]indole or [14C]Trp (1.7 μm) and incubated for 5 h at 25°C or 35°C. In studies with seedling extracts from the maize mutant orange pericarp 600,000 dpm of [14C]indole or [14C]Trp (5.1 μm) was used in each reaction. The reaction was stopped by adjusting the pH to 2.7 with H3PO4 and the solution was partitioned against ethyl acetate. The ethyl acetate was held overnight at −20°C to remove residual water by freezing. The sample was centrifuged at 3000g for 45 s and the water droplet at the bottom of the test tube was removed and discarded. The remaining fraction was dried using a stream of nitrogen gas, resuspended in 20 μL of ethyl acetate, and 50 ng of unlabeled IAA was added as a chromogenic tracer (Ehmann, 1977). The sample was applied to a Silica Gel 60 TLC plate (20 × 20 cm, Merck, Darmstadt, Germany), which was developed using solvent S1tlc (chloroform:methanol:water, 85:14:1 [v/v]). The radioactivity on the plate was detected using a radioimaging system (AMBIS 4000, Scanalytics, Inc., Billerica, MA). The IAA chromogenic standard was visualized using Ehmann's reagent (Ehmann, 1977). The plate was dipped briefly in the reagent, the color was developed by treatment for 5 min at 100°C, and then the plate was washed under running water for 15 min to remove excess reagent. The Ehmann color reaction allowed IAA to be visualized to compensate for changes in RF caused by interfering compounds in the samples. Control samples were boiled for 5 min before addition of radiolabeled indole or Trp.
Please note: (a) this TLC system has been characterized in terms of the RF of a large number of indolic compounds, as described by Sztein et al. (1995); (b) the radioactive compounds other than indole shown in the boiled control were not present in the [14C]indole before incubation with the boiled enzyme sample; and (c) Trp remains in the initial water phase and does not partition into the ethyl acetate phases applied to the TLC plate.
In Vitro System for IAA Production from Maize Liquid-Endosperm Extracts
Maize liquid-endosperm extracts (Ilić et al., 1998) were prepared essentially according to the methods of Rekoslavskaya and Bandurski (1994) and Rekoslavskaya (1995). Experiments were performed using maize liquid endosperm from both fertilized and unfertilized maize ears. To determine if the reaction was dependent on Trp as an intermediate, 50 μg of different metabolites (Gly, IAA, indole, indole-3-butyric acid, Ser, and l-Trp) was added to the reactions and the effects of these compounds on IAA formation were determined.
GC-MS Analysis
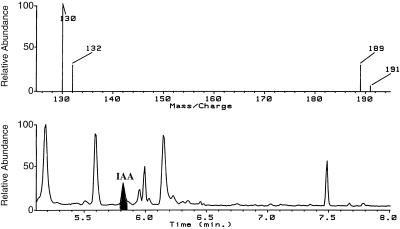
Four standard incubations of seedling extracts with [14C]indole, as described above, were pooled and the IAA was isolated. In these four reactions, unlike those used for TLC analysis, no carrier IAA was added. IAA was purified by ethyl acetate partitioning, C18 HPLC on an UltraCarb (30) column (4.6 × 50 mm, Phenomenex, Torrance, CA) using 27% methanol/water plus 1% acetic acid as the mobile phase, followed by methylation with diazomethane (for additional details, see Ribnicky, 1996). The sample was analyzed by GC-MS monitoring of four ions in the selected-ion mode. Ions at m/z 130 (quinolinium ion) and m/z 189 (molecular ion) were monitored for unlabeled methyl-IAA, and for methyl-[14C]IAA the corresponding ions at m/z 132 and 191 were monitored.
RESULTS
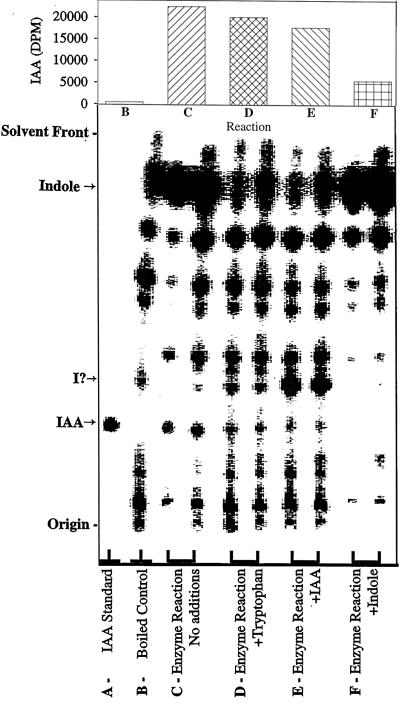
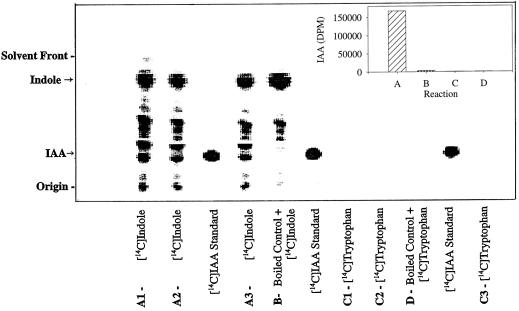
We have achieved the in vitro enzymatic biosynthesis of IAA in a preparation from maize seedlings in which [14C]indole was converted into [14C]IAA (Fig. 1, lanes C). This biosynthesis was independent of Trp because (a) [14C]Trp was not converted to [14C]IAA (Fig. 2); (b) the addition of unlabeled Trp did not inhibit the [14C]indole conversion to [14C]IAA (Fig. 1, lanes D); and (c) Ser did not enhance the same conversion (data not shown). The addition of unlabeled indole decreased the formation of [14C]IAA (Fig. 1, lanes F). The addition of unlabeled IAA reduced the [14C]IAA we observed (Fig. 1, lanes E) and resulted in the accumulation of an unknown 14C-labeled compound. This metabolite was ethyl acetate-soluble and had a RF slightly higher than that of IAA. The formation of IAA in this reaction was verified by GC-MS selected-ion monitoring analysis of its methylated derivative (Fig. 3). The endogenous IAA can be identified by its molecular ion at m/z 189 and its quinolinium ion at m/z 130, whereas the IAA formed from [14C]indole was measured at +2 mass units, e.g. m/z 191 and 132. As an additional confirmation of the Trp independence of the reaction, IAA biosynthesis was measured in the in vitro system from orange pericarp mutant seedlings (Fig. 4). This mutant lacks any functional Trp synthase β and thus cannot make Trp (Wright et al., 1991, 1992). Thus, the results of this experiment provide further evidence excluding the possibility that formation of IAA was via Trp.
Figure 1.
Silica-gel TLC analysis of the radiolabeled reaction products obtained from [14C]indole. The reaction products were partitioned at acidic pH into ethyl acetate and 50 ng of unlabeled IAA was added before concentration and application to the TLC plate. Except for the IAA standard and the boiled control, duplicate reactions were spotted in adjacent lanes, as shown. After development in solvent S1tlc, radiolabeled products were visualized using an AMBIS 4000 radioimaging system. The RF values of IAA and indole (as indicated on the abscissa) were confirmed by visualization of the indole and the added unlabeled IAA using the reagent of Ehmann (1977) and superimposing the two images. A potential intermediate that accumulated with the addition of unlabeled IAA (lanes D) and was not detected in the reactions with added unlabeled indole (lanes F) is indicated on the abscissa (I?). Quantification of radioactivity at the RF of IAA (obtained by software analysis of the scanned image and correction for counting efficiency) is shown in the bar graph at the top of the figure. The data on the graph for C, D, E, and F are the averages of the values from each pair of lanes shown.
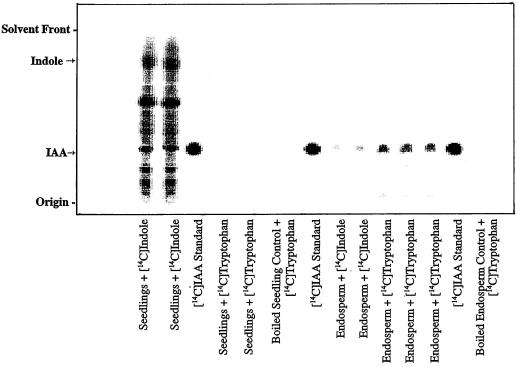
Figure 2.
Results were obtained with the in vitro assay as described for Figure 1, and compared with results obtained from maize endosperm. Note that the endosperm preparations were able to convert [14C]Trp as well as [14C]indole to IAA, whereas only [14C]indole was an effective substrate with the seedling preparations.
Figure 3.
Selected ion chromatogram (bottom) and selected ion spectrum (top) for IAA formed in the enzyme reaction. The sample was purified by C18 HPLC, methylated, and analyzed by GC-MS. Ions at m/z 130 and 189 are characteristic of unlabeled methyl-IAA resulting from unlabeled IAA or indole in the reaction mixture from the plant enzyme preparation. Ions at m/z 132 and 191 are the corresponding ions for the methyl ester from the 14C-labeled IAA formed from the radioactive indole supplied to the reaction.
Figure 4.
Results were obtained from in vitro preparations of orange pericarp seedlings of maize. Conditions are as described for Figure 1. A1 to A3 are three replicated reactions using [14C]indole in the reaction mixture, and for replicates C1 to C3 the radiolabeled substrate was [14C]Trp. Trp does not partition into ethyl acetate from the aqueous phase, so it is not shown on these chromatograms. Quantification of radioactivity at the RF of IAA (obtained by software analysis of the scanned image and correction for counting efficiency) is shown in the bar-graph inset (data from A1–A3 and C1–C3 were averaged).
Because of earlier reports of IAA biosynthesis in a maize liquid-endosperm preparation (Rekoslavskaya and Bandurski, 1994; Rekoslavskaya, 1995), we compared the liquid endosperm in vitro system with our seedling in vitro system (Fig. 2). The liquid endosperm preparation was capable of converting [14C]indole, [14C]Trp (Fig. 2), and [14C]Ser (data not shown) to [14C]IAA. In contrast, the maize seedling in vitro system did not convert any detectable amounts of [14C]Trp to [14C]IAA (Fig. 2). Maize liquid endosperm prepared according to the seedling in vitro protocol did not form any IAA from [14C]indole or [14C]Trp; similarly, maize seedlings prepared according to the endosperm protocol produced no detectable [14C]IAA from [14C]indole or [14C]Trp (data not shown).
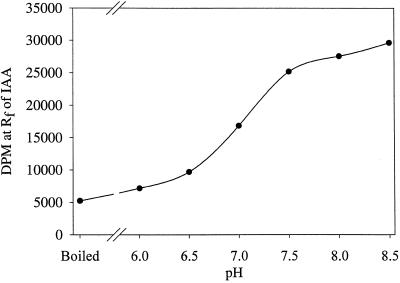
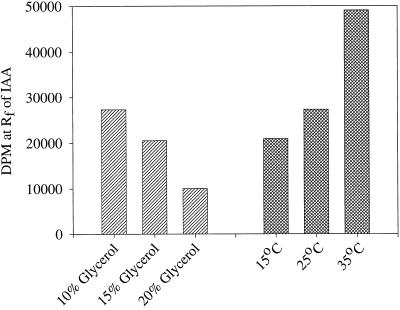
The pH optimum for the reaction was found to be at or above pH 8.0 to 8.5 (Fig. 5). The temperature dependence of the in vitro system showed 35°C to be better than 15°C and 25°C; additionally, although low levels of glycerol (10%) were necessary, higher levels inhibited the reaction (Fig. 6). The addition of 50 μg of Gly, indole-3-butyric acid, or Ser to the reaction mixture had little or no effect on the formation of [14C]IAA and, as expected, [14C]Ser was not an effective substrate for this reaction (data not shown).
Figure 5.
The effect of pH on the formation of IAA from [14C]indole by in vitro preparations from maize seedlings. For this experiment, the standard reaction was changed to a mixed buffer system composed of 40 mm POPSO plus 40 mm Mes. This mixed buffer allowed a pH range of 6.0 to 8.5 to be analyzed without changing the organic buffers present in the reaction.
Figure 6.
The effect of changes in the glycerol concentration and incubation temperature on the amount of [14C]IAA formed from [14C]indole by in vitro preparations from maize seedlings during a 5-h incubation.
DISCUSSION
Trp has long been considered to be the sole or primary precursor of the plant hormone IAA (Normanly et al., 1995), and Trp-dependent IAA biosynthesis pathways have been characterized in several plant systems. At least three pathways have been described from different plants (Cohen and Bialek, 1984). Recently, evidence has accumulated for an alternative pathway for the biosynthesis of IAA that does not use Trp as an intermediate, and this pathway has now been shown to be the primary route for IAA production in several plant species (Wright et al., 1991; Michalczuk et al., 1992; Normanly et al., 1993, 1995; Bartel, 1997). In trying to develop a useful in vitro system to study Trp-independent IAA biosynthesis, our initial interest focused on the maize liquid-endosperm preparations because they were simple to prepare, were stable to freezing, and had high activity for IAA biosynthesis. Evidence that this system probably included both Trp-dependent and -independent IAA biosynthesis has been reported by Rekoslavskaya and Bandurski (1994). However, both our own results (Fig. 2; Ilić et al., 1998) and data presented by Rekoslavskaya (1995) show that the majority of IAA was made from Trp by liquid-endosperm preparations. Specifically, with the liquid-endosperm extracts we observed that the formation of [14C]IAA from [14C]indole was enhanced by the addition of Ser and inhibited by the addition of unlabeled Trp (Ilić et al., 1998). This was true of endosperm preparations from both fertilized (Fig. 2) and unfertilized (data not shown) ears. Because of the high level of Trp conversion to IAA by endosperm preparations, it was not possible to measure significant Trp-independent indole conversion in such extracts. Therefore, we changed our focus to maize seedling extracts, because results from experiments with the orange pericarp mutant (Wright et al., 1991) have shown that this tissue used only the Trp-independent pathway for biosynthesis of IAA.
Plant leaves are known to have an indole-oxidizing system (Nair and Vaidyanathan, 1964; Chauhan et al., 1978), which often makes in vivo use of applied indole unsuccessful. Thus, Wright et al. (1991) were able to label maize seedlings and follow IAA biosynthesis successfully with 2H2O and anthranilate, but not with indole. However, in our in vitro system these oxidizing enzymes are apparently inhibited at pH 8.5 (pH 5.0 is the optimum for the maize indole-oxidizing activity [Chauhan et al., 1978]) and by the use of PHMB, which is also known to inhibit such enzymes (Nair and Vaidyanathan, 1964). The pH optimum for IAA formation (Fig. 5) indicates that the maximal activity for the rate-limiting step would occur in an alkaline cell compartment, such as that found in plastids. However, we cannot exclude the possibility that the pH optima data are in part a reflection of optima of competing reactions for the same substrate. We also found that the redox potential in the reaction mixture had to be carefully controlled. Initial studies using 5 mm DTT or dithioerythritol gave variable results, which were dependent on the individual enzyme preparation. Somewhat better results were obtained when diethyldithiocarbamate was also included. When ascorbate was used as a “redox buffer,” consistently higher yields of IAA were obtained. Thus, the reactions from indole to IAA involve at least one step that is sensitive to the oxidation/reduction potential of the reaction environment.
In vitro IAA biosynthesis in extracts of young maize seedlings involves the direct conversion of [14C]indole to [14C]IAA without Trp as an intermediate. This was verified by using preparations from orange pericarp mutant seedlings. Because orange pericarp seedlings lack a functional Trp synthase β, in these extracts no Trp could be formed from the supplied indole. Furthermore, the addition of unlabeled Trp did not alter the conversion of [14C]indole to [14C]IAA, whereas the addition of unlabeled IAA resulted in the accumulation of a potential intermediate. These results, together with the results from the maize liquid-endosperm preparations, show that there are two different pathways of IAA formation in maize and, as has been shown for carrot (Michalczuk et al., 1992), that the expression of these two pathways is a function of the plant's developmental stage. Further studies on the regulation and activation of these pathways in plants will be important for understanding hormonal, metabolic, and molecular regulation of plant development. Experiments in our laboratories are currently directed toward isolation of the intermediates in this process to understand the metabolic route for Trp-independent IAA biosynthesis.
ACKNOWLEDGMENTS
We thank Dr. Janet P. Slovin (U.S. Department of Agriculture-Agricultural Research Service Climate Stress Laboratory, Beltsville, MD) and Dr. Jennifer Normanly (Department of Biochemistry and Molecular Biology, University of Massachusetts, Amherst) for their comments on the manuscript. Support for A.Ö. by the Swedish Council for Forestry and Agricultural Research is gratefully acknowledged.
Abbreviations:
- PHMB
p-hydroxymercuribenzoic acid
- POPSO
piperazine-N,N′-bis(2-hydroxypropanesulfonic acid)
Footnotes
This study was supported by the U.S. Department of Energy (grant no. DE-AI02-94ER20153) and by the Swedish Council for Forestry and Agricultural Research.
LITERATURE CITED
- Baldi BG, Maher BR, Slovin JP, Cohen JD. Stable isotope labeling in vivo of d- and l-tryptophan pools in Lemna gibba and the low incorporation of label into indole-3-acetic acid. Plant Physiol. 1991;95:1203–1208. doi: 10.1104/pp.95.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski RS, Cohen JD, Slovin JP, Reinecke DM. Auxin biosynthesis and metabolism. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 35–57. [Google Scholar]
- Bartel B. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:51–66. doi: 10.1146/annurev.arplant.48.1.51. [DOI] [PubMed] [Google Scholar]
- Chauhan YS, Rathore VS, Garg GK, Bhargava A. Detection of an indole oxidizing system in maize leaves. Biochem Biophys Res Commun. 1978;83:1237–1245. doi: 10.1016/0006-291x(78)91354-2. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Bialek K. The biosynthesis of indole-3-acetic acid in higher plants. In: Crozier A, Hillman JR, editors. The Biosynthesis and Metabolism of Plant Hormones. Society for Experimental Biology Seminar 23. Cambridge, UK: Cambridge University Press; 1984. pp. 165–181. [Google Scholar]
- Ehmann A. The Van Urk-Salkowski reagent: a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977;132:267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Gegenheimer P. Preparation of extracts from plants. Methods Enzymol. 1990;182:174–193. doi: 10.1016/0076-6879(90)82016-u. [DOI] [PubMed] [Google Scholar]
- Ilić N, Östin A, Cohen JD (1998) Differential inhibition of indole-3-acetic acid and tryptophan biosynthesis by indole analogues. I. Tryptophan dependent IAA biosynthesis. Plant Growth Regul (in press)
- Jensen PJ, Bandurski RS. Metabolism and synthesis of indole-3-acetic acid (IAA) in Zea mays. Levels of IAA during kernel development and the use of in vitro endosperm systems for studying IAA biosynthesis. Plant Physiol. 1994;106:343–351. doi: 10.1104/pp.106.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ, Bandurski RS. Incorporation of deuterium into indole-3-acetic acid and tryptophan in Zea mays seedlings grown on 30% deuterium oxide. J Plant Physiol. 1996;147:679–702. [Google Scholar]
- Koshiba T, Kamiya Y, Iino M. Biosynthesis of indole-3-acetic acid from l-tryptophan in coleoptile tips of maize (Zea mays L.) Plant Cell Physiol. 1995;36:1503–1510. [Google Scholar]
- Koshiba T, Matsuyama H. An in vitro system of indole-3-acetic acid formation from tryptophan in maize (Zea mays) coleoptile extracts. Plant Physiol. 1993;102:1319–1324. doi: 10.1104/pp.102.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD. Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol. 1992;100:1346–1353. doi: 10.1104/pp.100.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair PM, Vaidyanathan CS. An indole oxidase isolated from leaves of Tecoma stans. Biochim Biophys Acta. 1964;81:496–506. doi: 10.1016/0926-6569(64)90134-8. [DOI] [PubMed] [Google Scholar]
- Normanly J. Auxin metabolism. Physiol Plant. 1997;100:431–442. [Google Scholar]
- Normanly J, Cohen JD, Fink GR. Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci USA. 1993;90:10355–10359. doi: 10.1073/pnas.90.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Slovin JP, Cohen JD. Rethinking auxin biosynthesis and metabolism. Plant Physiol. 1995;107:323–329. doi: 10.1104/pp.107.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42:207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- Pengelly WL, Bandurski RS. Analysis of indole-3-acetic acid metabolism using deuterium oxide as a tracer. Plant Physiol. 1983;73:445–449. doi: 10.1104/pp.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekoslavskaya NI. Pathways of indoleacetic acid and tryptophan synthesis in developing maize endosperm: studies in vitro. Russian J Plant Physiol. 1995;42:143–151. [Google Scholar]
- Rekoslavskaya NI, Bandurski RS. Indole as a precursor of indole-3-acetic acid in Zea mays. Phytochemistry. 1994;35:905–909. [Google Scholar]
- Ribnicky DM (1996) The role of auxin in carrot embryogenesis. Ph.D. dissertation. University of Maryland, College Park
- Sztein AE, Cohen JD, Slovin JP, Cooke TJ. Auxin metabolism in representative land plants. Am J Bot. 1995;82:1514–1521. [Google Scholar]
- Wright AD, Moehlenkamp CA, Perrot GH, Neuffer MG, Cone KC. The maize auxotropic mutant orange pericarp is defective in duplicate genes for tryptophan synthase β. Plant Cell. 1992;4:711–719. doi: 10.1105/tpc.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AD, Sampson MB, Neuffer MG, Michalczuk L, Slovin JP, Cohen JD. Indole-3-acetic acid biosynthesis in the mutant maize orange pericarp, a tryptophan auxotroph. Science. 1991;254:998–1000. doi: 10.1126/science.254.5034.998. [DOI] [PubMed] [Google Scholar]