Abstract
The generation of stereochemically-rich benzothiaoxazepine-1,1′-dioxides for enrichment of high-throughput screening collections is reported. Utilizing a microwave-assisted, continuous flow organic synthesis platform (MACOS), scale-out of core benzothiaoxazepine-1,1′-dioxide scaffolds has been achieved on multi-gram scale using an epoxide opening/SNAr cyclization protocol. Diversification of these sultam scaffolds was attained via a microwave-assisted intermolecular SNAr reaction with a variety of amines. Overall, a facile, 2-step protocol generated a collection of benzothiaoxazepine-1,1′-dioxides possessing stereochemical complexity in rapid fashion, where all 8 stereoisomers were accessed from commercially available starting materials.
Keywords: MACOS, Sultam, SNAr, DOS, HTS
1. Introduction
The design, synthesis and production of molecular libraries for high-throughput biological screening (HTS) has been a critical aspect of drug discovery.i A recent survey of current compound collections for HTS has found that on average, they posses a higher sp2 content as well as lower structural and stereochemical complexity when compared to currently available drugs.ii While enrichment of compound collections with natural products and their derivatives is one way to enhance complexity, the aforementioned dichotomy has warranted the development of efficient synthetic methods of increasing stereochemical complexity and sp3 content in screening collections.iii,iv We herein report a facile, 2-step protocol to generate a collection of benzothiaoxazepine-1,1′-dioxides possessing stereochemical complexity in rapid fashion. A microwave-assisted, continuous flow organic synthesis (MACOS) platform is employed to produce all 8 stereoisomers of a benzothiaoxazepine-1,1-dioxide core scaffold via an epoxide opening/SNAr cyclizationv protocol, followed by intermolecular SNAr diversification.
Diversity-oriented synthesis (DOS) has emerged in recent years as a powerful tool to enrich the diversity of molecules for HTS.vi This approach has been led by the development of new methodologies, automated technologies, and collaborations within the area of chemical biology. In addition to structural diversity and complexity, the ability to rapidly access every potential stereoisomer of a structure, while functionalizing a core scaffold at every position, is a key concept at the heart of DOS that has the potential to benefit both early stage HTS and SAR, as well as downstream probe/drug development.iv,vi,vii
It was envisioned that a collection of stereochemically-rich benzofused sultams could be rapidly generated where every stereoisomer is readily accessible from commercially available starting materials. In this regard, utilizing a combination of chiral 2° sulfonamides, epoxides, and amino alcohols, a two-step procedure was designed for the synthesis of core benzothiaoxazepine-1,1-dioxides via an epoxide opening/ SNAr cyclizationv sequence, followed by intermolecular SNAr diversification (Figure 1). Furthermore, the powerful combination of microwave heating and flow chemistry was seen to achieve this goal quickly through shortened reaction times and production at any scale necessary with no process re-optimization.
Figure 1.
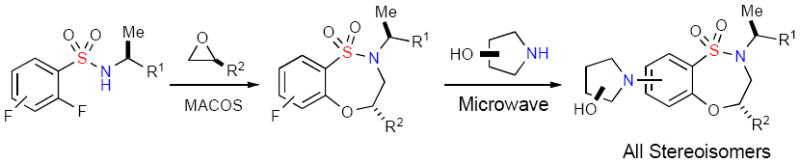
Two-step synthesis of stereochemically-rich benzothiaoxazepine-1,1-dioxides.
2. Results and Discussion
Preliminary investigations focused on addressing the ability to generate multi-gram quantities of core benzothiaoxazepine-1,1-dioxide scaffolds. Initial efforts in batch microwave were limited by reactor size and decreased yields resulting from increased by-product formation during scale-up.viii To address these limitations, the MACOS platform was employed to scale-out, rather than scale-up, the scaffold production runs.ix,x The scale-out approach to microwave transformations has been shown in recent years to be a highly efficient method for accessing large quantities of organic molecules.xi,xii
At the outset, a batch microwave reactor was used to develop reactions times that would be suitable for adaptation to flow (Table 1). With some optimization, it was determined that benzothiaoxazepine-1,1-dioxides 2 could be generated in 70% yield (100% conversion based on 1) utilizing 1.5 equiv. of tBuOK at 180 °C for 1 minute in the microwave (Table 1, entry 7). Such conditions are well tolerated in the MACOS reactor system where flow rates can be set to establish average residence times of approximately one minute for material in the irradiation zone of the microwave (where the sample is actually being irradiated and thus heated).
Table 1.
Optimization studies of epoxide opening/cyclization sequence using batch MW.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Temp (°C) | Time (min) | Base (equiv.) | Epox (equiv.) | % Con. (Yield) | Prod. |
| 1a | 125 | 3 | tBuOK (3) | 1 | Decomp. | 2 |
| 2a | 110 | 3 | tBuOK (2) | 1 | 5 | 2 |
| 3a | 110 | 5 | tBuOK (1.5) | 1 | 30 | 2 |
| 4a | 110 | 20 | tBuOK (1.5) | 1.2 | 54 | 2 |
| 5a | 180 | 1 | tBuOK (1.5) | 1.2 | 61 | 2 |
| 6a | 180 | 1 | tBuOK (1.5) | 2 | 78 | 2 |
| 7a | 180 | 1 | tBuOK (1.5) | 3 | 100 (70) | 2 |
|
| ||||||
| 8b | 125 | 1 | DBU (1.5) | 3 | 25 | 3 |
| 9b | 150 | 1 | DBU (1.5) | 3 | 60 | 3 |
| 10b | 180 | 1 | DBU (1.5) | 3 | 100 (90) | 3 |
| 11b | RT | 72 h | DBU (1.5) | 3 | 26 | 3 |
Compound 2 generated with 2-ethyloxirane;
Compound 3 generated with 2-(butoxymethyl) oxirane.
Taking these preliminary conditions into the MACOS platform, a variety of reactor parameters were investigated, namely flow rate, temperature and power to fine-tune the scale-out protocol (Table 2). When shorter production runs were performed (eg., to produce ~200 mg of 2), the desired sultam was generated in 50% yield (100% conversion of 1) utilizing a flow rate of 150 μL/min at 190 °C and 220 Watts of power (Table 2, entry 7).xiii
Table 2.
Optimization studies for MACOS scale-out of benzothiaoxazepine-1,1-dioxide 2 and 3.
|
| |||||
|---|---|---|---|---|---|
| Entrya | Flow rate (μL/min) | Temp. (°C) | Power (W) | % Con. (Yield) | Prod. |
| 1 | 50 | 145 | 380 | 90 (28) | 2 |
| 2 | 50 | 150 | 240 | 34 | 2 |
| 3 | 50 | 200 | 215 | 100 | 2 |
| 4 | 75 | 195 | 230 | 100 | 2 |
| 5 | 100 | 195 | 230 | 100 | 2 |
| 6 | 125 | 190 | 225 | 100 (44) | 2 |
| 7 | 150 | 190 | 220 | 100 (50) | 2 |
| 8b | 150 | 190 | 220 | 100 (78) | 3 |
1 (1.0 equiv.), Epoxide (3.0 equiv.), tBuOK (1.5 equiv), DMSO (0.4 M);
1 (1.0 equiv.), Epoxide (3.0 equiv.), DBU (1.5 equiv), DMSO (0.4 M).
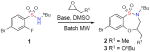
When the MACOS reaction was performed for longer times to increase product output, clogging of reaction capillaries was observed after approximately 3 mL of the reaction solution had been flowed. When isolated, this material was not soluble in an array of solvents, indicating that product decomposition under these conditions may have occurred. Suspecting that this problem might be related to the base, tBuOK was substituted with DBU. Re-optimization of reaction conditions in batch utilizing DBU (Table 1, entries 8–11) was carried out and the best conditions for the generation of 3 were attempted using the MACOS platform leading to a gratifying 78% yield (Table 2, Entry 8). Utilizing 1.5 equiv of DBU at 180 °C with a flow rate of 100–200μL/min, benzothiaoxazepine-1,1′-dioxides 2–5 were synthesised in gram quantities demonstrating the successful application of the MACOS platform for scale-out (Scheme 1). Building on these trial runs, benzothiaoxazepine-1,1-dioxides core scaffolds 6 – 13 , were prepared readily using optically-pure epoxides on the MACOS platform (Scheme 1).xiv
Scheme 1.
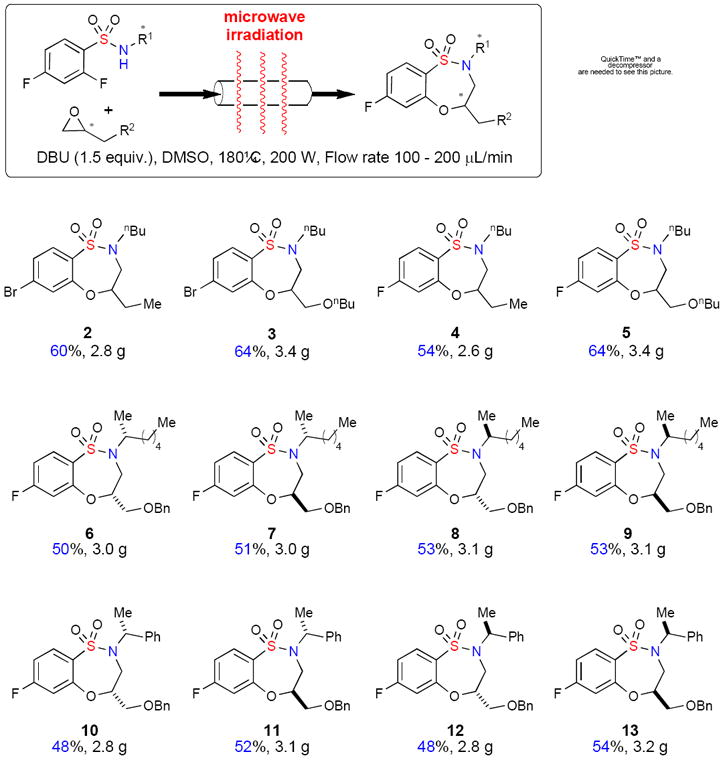
MACOS scale-out of optically-pure benzothiaoxazepine-1,1′-dioxides 6 -13.
With these benzothiaoxazepine-1,1′-dioxide core scaffolds in hand, diversification via microwave-assisted SNAr was investigated.xv Utilizing the Anton Parr Synthos 3000® microwave platform, a collection of eight optically pure, stereoisomeric sultams (14 – 21) was produced demonstrating facile access to stereochemically-rich small-molecule collections for HTS (Scheme 2). Reactions were carried out on 100 mg scale in DMSO at 180 °C for 50 minutes, followed by dilution, filtration, and purification by column chromatography to produce the desired benzothiaoxazepine-1,1-dioxides 14 – 21 in high yield.
Scheme 2.
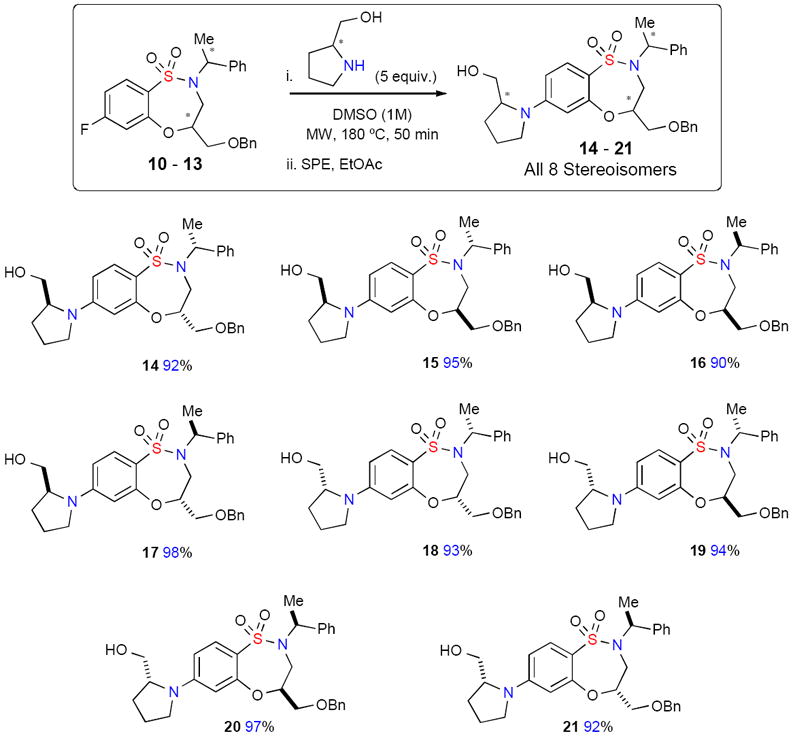
Generation of optically-pure benzosultams 14 – 21 via microwave-assisted SNAr.
3. Conclusions
In conclusion, a method for the rapid access of stereochemically-rich benzothiaoxazepine-1,1′-dioxides utilizing a two-step, microwave-accelerated protocol is reported. A MACOS platform was used for the scale-out production of core benzothiaoxazepine-1,1′-dioxide scaffolds on multi-gram scale using a cascade epoxide opening/cyclization sequence. These core scaffolds were subsequently diversified by a microwave-assisted, intermolecular SNAr reaction with chiral, non-racemic amino alcohols. Overall, this 2-step protocol efficiently generated all 8 stereoisomers of benzothiaoxazepine-1,1′-dioxides in optically-pure form, bearing three stereogenic centers, using readily accessible starting materials. These compounds will be evaluated by HTS for potential biological activity by our collaborators, and these results will be reported in due course.
4. Experimental
Microwave irradiation experiments and Analysis of Compounds 2 – 13
All MACOS experiments were performed in 1700 μ m (ID) borosilicate capillaries, using a single mode Biotage Smith Creator Synthesizer, operating at a frequency of 2.45 GHz with irradiation power from 0 to 350 W. The capillary was fed reactants from Hamilton gastight syringes attached to a Harvard 22 syringe pump preset to the desired flow rate. The system was connected to a sealed collection vial, where a pressurized air line (75 psi) was attached to create backpressure. The temperatures reported were measured off the surface of the capillaries by the IR sensor built into the microwave chamber. All reagents and solvents were purchased from commercial sources and used without additional purification. Column chromatography purifications were carried out using the flash technique on silica gel 60 (200–400 mesh). 1H and 13C NMR spectroscopy was performed on a Bruker Avance 400 MHz instrument (at 400 MHz and 100 MHz respectively) or a Bruker DRX-500 spectrometer (at 500 MHz or 125 MHz respectively). All 1H NMR spectra were calibrated to the signal from the residual proton of the deuterated chloroform solvent (7.26 ppm) while 13C NMR spectra were calibrated to the middle carbon signal of the triplet for deuterated chloroform (77.00 ppm). High-resolution mass spectrometry (HRMS) was recorded on a LCT Premier Spectrometer (Micromass UK Limited) operating the the ESI mode (MeOH). All compounds in this study have been isolated by silica gel/aluminum oxide chromatography for the purpose of spectroscopic identification
General Methods for compounds 14 - 21
All reactions were carried out in 1 dram vials using a reaction heating block in an Anton Paar ® Synthos 3000 synthesizer. Parallel evaporations were performed using a GeneVac EZ-2 Plus evaporator. All reagents and solvents were purchased from commercial sources and used without additional purification. Flash column chromatography was performed on silica gel (30930M-25, Silica Gel 60A, 40-63 um) and thin layer chromatography was performed on silica gel 60F254 plates (EM-5717, Merck). Deuterated solvents were purchased from Cambridge Isotope laboratories. 1H and 13C NMR spectra were recorded in CDCl3 (unless otherwise mentioned) on a Bruker DRX-500 spectrometer operating at 500 MHz, and 125 MHz, respectively, and calibrated to the solvent peak. High-resolution mass spectrometry (HRMS) was recorded on a LCT Premier Spectrometer (Micromass UK Limited) operating in the ESI mode (MeOH). Optical rotations at 589 nm, were measured using AUTOPOL IV Model automatic polarimeter. IR spectra were recorded on a Shimadzu FTIR-8400S instrument.
General procedure A: Scale-out synthesis of benzothiaoxazepine-1,1-dioxide derivatives 2 –13 utilizing MACOS flow-platform
A stock solution containing the sulfonamide (1.0 equiv.), epoxide (3 equiv.) and DBU (1.5 equiv.) in DMSO, (0.3-0.4 M) was prepared and loaded into a 10 mL Hamilton gastight syringe. The tubing was primed with DMSO and the syringe was connected to the reactor system with the aid of Microtight™ fittings. A sealed collection vial was connected to the system, where a pressurized airline (75 psi) was attached to create backpressure. A Harvard 22 syringe pump was set to deliver the reaction solution at a rate of 100-200 μ L/min (see Table 1 and 2 for specific conditions). The single mode microwave was programmed to heat constantly with the power level controlled manually so as to keep the temperature constant at the specified levels (see Scheme 1 and Table 2 for specific conditions). The effluent from the reactor was fed into a sealed vial and analyzed directly by 1H NMR spectroscopy immediately after the reaction. The crude reaction mixture was collected and the product was purified by neutral aluminum oxide column chromatography.
7-Bromo-2-butyl-4-ethyl-1,2- benzoxathiazepine-1,1-dioxide (2)
Utilizing General Procedure A, sultam 2 was isolated in 60% yield (2.8 g, 7.76 mmol) as a yellow solid. FTIR (neat): 2960, 2933, 1573, 1460, 1342, 1164, 1058 cm-1; 1H NMR (300 MHz CDCl3) δ 7.70 (d, J = 8.1 Hz, 1H), 7.37-7.28 (m, 2H), 4.01-3.82 (m, 2H), 3.29-3.17 (m, 2H), 2.85-2.74 (m, 1H), 1.83-1.71 (m, 1H), 1.67-1.53(m, 3H), 1.42-1.27 (m, 2H), 1.17 (t, J =7.2 Hz, 3H), 0.95 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz CDCl3) δ 156.1, 133.5, 130.0, 127.4, 127.2, 123.8 80.9, 53.2, 48.2, 30.8, 26.4, 19.6, 13.7, 10.2 ppm; DEPT 13C NMR (75 MHz CDCl3) δ 130.1, 127.3, 126.5, 80.9, 53.2, 48.2, 30.8, 26.4, 19.6, 13.7, 10.2 ppm; HRMS calculated for C14H21BrNO3S (M+H)+ 362.0426; found 362.0429 (TOF MS ES+).
4-(Butoxymethyl)-7-bromo-2-butyl-1,2-benozoxathiazephine-1,1-dioxide (3)
Utilizing General Procedure A, sultam 3 was isolated in 64% yield (3.45 g, 8.234 mmol) as a colorless viscous oil. FTIR (neat): 2956, 2871, 1577, 1456, 1344, 1164, 1058, 931 cm-1; 1H NMR (300 MHz CDCl3) δ 7.69 (d, J = 9.0 Hz, 1H), 7.37 (m, 2H), 4.22-4.15 (m, 1H), 3.96 (dd, J = 15, 10.5 Hz, 1H), 3.74-3.69 (m, 1H), 3.59-3.46 (m, 3H), 3.43 (d, J = 15 Hz, 1H), 3.26-3.16 (m, 1H), 2.82-2.61 (m, 1H), 1.61-152 (m, 4H), 1.51-1.72 (m, 4H), 0.98-0.83 (m, 6H) ppm; 13C NMR (75 MHz CDCl3) δ 155.5, 133.34, 130.1, 127.4, 126.6, 77.8, 71.7, 70.6, 50.5, 48.0, 31.6, 30.7, 19.6, 19.3, 13.8, 13.6 ppm; DEPT 13C NMR (75 MHz CDCl3) δ 130.1, 127.5, 126.7, 77.8, 71.7, 70.6, 50.5, 48.0, 31.6, 30.7, 19.6, 19.3, 13.9, 13.6 ppm; HRMS calculated for C17H27BrNO4S (M+H)+ 420.0844; found 420.0842 (TOF MS ES+).
2-Butyl-4-ethyl-7-fluoro-1,2- benzoxathiazepine-1,1-dioxide (4)
Utilizing General Procedure A, sultam 4 was isolated in 54% yield (2.6 g, 8.64 mmol) as a colorless viscous oil. FTIR (neat): 2962, 2873, 1598, 1583, 1342, 1141, 1070, 995 cm-1; 1H NMR (300 MHz CDCl3) δ 7.83 (dd, J = 8.7, 6.3 Hz, 1H), 6.94-6.83 (m, 2H), 3.98-3.81 (m, 2H), 3.28-3.18 (m, 2H), 2.81-2.74 (m, 1H), 1.79-1.72 (m, 1H) 1.64-1.54 (m, 3H), 1.38-1.31 (m, 2H), 1.5 (t, J =7.2 Hz, 3H), 0.94 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz CDCl3) δ 167. (d, JC-F = 253.5 Hz), 157.4 (d, JC-F = 12.0 Hz), 130.9 (d, JC-F = 10.5 Hz), 130.6 (d, JC-F =3.7 Hz), 111.4 (d, JC-F = 21.5 Hz), 110.8 (d, JC-F = 23.2 Hz), 80.9, 53.1, 48.1, 30.7, 26.3, 19.6, 13.6, 10.1 ppm; DEPT 13C-NMR (75 MHz CDCl3) δ 130.9 (d, JC-F = 10.5 Hz), 111.4 (d, JC-F = 21.8 Hz), 110.8 (d, JC-F = 22.5 Hz), 80.9, 53.1, 48.2, 30.8, 26.3, 19.6, 13.6, 10.2 ppm; CHN analysis calculated for C14H20FNO3S: C, 55.79; H, 6.69; N, 4.65; S, 10.65: found C, 56.01; H, 6.42; N, 4.53; S, 10.72.
4-(Butoxymethyl)-2-butyl-7-fluoro-1,2-benozoxathiazephine-1,1-dioxide (5)
Utilizing General Procedure A, sultam 5 was isolated in 52% yield (3.0 g, 8.36 mmol) as a colorless viscous oil; FTIR (neat): 2958, 2933, 2871, 1598, 1585, 1423, 1344, 1118, 1070, 989 cm-1; 1H NMR (300 MHz CDCl3) δ 7.82 (dd, J = 8.7, 6.3 Hz, 1H), 6.93 - 6.84 (m, 2H), 4.21 (m, 1H), 3.95 (d, J = 15, 10.5 Hz, 1H), 3.73 - 3.67 (m, 1H), 3.57 - 3.48 (m, 3H), 3.62 - 3.42 (dd, J = 15, 1.3 Hz, 1H), 3.17 (m, 1H), 2.77 – 2.72 (m, 1H), 1.59 – 1.52 (m, 4H), 1.41-1.32 (m, 4H), 0.94 - 0.87 (m, 6H), ppm; 13C NMR (75 MHz CDCl3) δ 167.1 (d, JC-F = 253.5 Hz), 156.9 (d, JC-F = 12.0 Hz), 130.9 (d, JC-F = 10.5 Hz), 130.5 (d, JC-F = 3.0 Hz), 111.6 (d, JC-F = 21.8 Hz), 111.1 (d, JC-F = 23.3 Hz), 77.7, 71.6, 70.6, 50.4, 47.9, 31.6, 30.6, 19.6, 19.2, 13.8, 13.6 ppm; DEPT 13C NMR (75 MHz CDCl3) δ 130.9 (d, JC-F = 10.5 Hz), 111.6 (d, JC-F = 22.5 Hz), 111.1 (d, JC-F = 22.5 Hz), 77.7, 71.6, 70.6, 50.4, 47.9, 31.6, 30.7, 19.6, 19.2, 13.9, 13.6 ppm; CHN analysis calculated for C17H26FNO4S: C, 56.80; H, 7.29; N, 3.90; S, 8.92: found C, 57.18; H, 7.43; N, 3.96; S, 8.85.
((R)-4-(Benzyloxymethyl)-7-fluoro-2-((R)-1-pentylethyl)-1,2-benzoxathiazepine-1,1-dioxide (6)
Utilizing general procedure A, sultam 6 was isolated in 50% yield (3.01 g, 6.92 mmol) as a colorless viscous oil; [α]D20 = + 6.4° (c = 2.7, CHCl3); FTIR (neat): 2929, 2860, 1598, 1471, 1340, 1157, 1116, 1072 cm-1; 1H NMR (400 MHz CDCl3) δ 7.84 (dd, J = 6.4, 8.4 Hz, 1H), 7.42 - 7.35 (m, 5H), 6.92 - 6.85 (m, 2H), 4.65 (s, 2H), 4.33 - 4.31 (m, 1H), 4.29 - 4.14 (m, 1H), 3.84 - 3.77 (m, 2H), 3.67-3.66 (m, 1H), 3.47 (dd, J =14.0, 1.7 Hz, 1H), 1.60 (br s, 1H), 1.50 (m, 1H), 1.29 (br s, 6H), 1.0 (d, J = 6.8 Hz, 3H), 0.89 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz CDCl3) δ 166.2 (d, JC-F = 253.0 Hz), 156.6 (d, JC-F = 12 Hz), 137.4, 133.3 (d, JC-F = 3 Hz), 128.8 (d, J = 11 Hz), 128.4, 127.9, 127.6, 111.1 (d, JC-F = 22 Hz), 110.4 (d, JC-F = 23 Hz), 80.7, 73.6, 70.1, 54.4, 44.8, 34.5, 31.2, 25.7, 22.4, 19.8, 13.9 ppm; HRMS calculated for C23H31FNO4S (M+H)+ 436.1958; found 436.1959 (TOF MS ES+).
((S)-4-(Benzyloxymethyl)-7-fluoro-2-((R)-1-pentylethyl)-1,2-benzoxathiazepine-1,1-dioxide (7)
Utilizing General Procedure A, sultam 7 was isolated in 51% yield (3.05 g, 7.01 mmol) as a colorless viscous oil; [α]D20 = - 10.3° (c = 4.7, CHCl3); FTIR (neat): 2929, 2861, 1561, 1470, 1340, 1156, 1116, 1073 cm-1; 1H NMR (300 MHz CDCl3) δ 7.83 (dd, J = 8.7, 6.3 Hz, 1H), 7.41 - 7.34 (m, 5H), 6.89-6.79 (m, 2H), 4.77 - 4.73 (m, 1H), 4.66 (d, J = 3.6, Hz, 2H), 4.10 (m, 1H), 3.78 - 3.59 (m, 3H), 3.45 (dd, J =14.1, 3.0 Hz, 1H), 1.43 - 1.26 (m, 8H), 1.10 (d, J = 6.6 Hz, 3H), 0.89 (t, J = 6.6 Hz, 3H) ppm; 13C NMR (100 MHz CDCl3) δ 166.0(d, JC-F = 252.0 Hz), 156.5 (d, JC-F = 13 Hz), 137.5, 131.7, 129.1 (d, JC-F = 11 Hz, 1H), 128.4, 127.9, 127.5, 110.6 (d, JC-F = 22 Hz), 109.3 (d, JC-F = 24 Hz), 80.6, 73.5, 69.9, 54.9, 44.6, 35.3, 31.2, 26.1, 22.4, 18.6, 13.9 ppm; CHN analysis calculated for C23H30FNO4S: C, 63.42; H, 6.94; N, 3.22; S, 7.36: found C, 63.74; H, 6.80; N, 3.08; S, 7.18.
((R)-4-(Benzyloxymethyl)-7-fluoro-2-((S)-1-pentylethyl)-1,2-benzoxathiazepine-1,1-dioxide (8)
Utilizing General Procedure A, sultam 8 was isolated in 53% yield (3.15 g, 7.24 mmol) as a colorless viscous oil; [α]D20 = + 7.6° (c = 2.2, CHCl3); FTIR (neat): 2929, 2858, 1600, 1473, 1336, 1157, 1116, 1076 cm-1; 1H NMR (300 MHz CDCl3) δ 7.83 (dd, J = 8.7, 6.3 Hz, 1H), 7.40 - 7.34 (m, 5H), 6.89 - 6.79 (m, 2H), 4.77 - 4.70 (m, 1H), 4.66 (d, J = 3.6 Hz, 2H), 4.10 (m, 1H), 3.78 - 3.59 (m, 3H), 3.45 (dd, J =13.8, 2.8 Hz, 1H), 1.43 - 1.26 (m, 8H), 1.10 (d, J = 6.6 Hz, 3H), 0.89 (t, J = 6.6 Hz, 3H) ppm; 13C NMR (75 MHz CDCl3) δ 166.5(d, JC-F = 252.0 Hz), 156.5 (d, JC-F = 12.8 Hz), 137.5, 131.8 (d, JC-F = 3 Hz), 129.2 (d, JC-F = 10.5 Hz), 128.5, 128.3, 127.9, 127.6, 110.8 (d, JC-F = 22.5 Hz), 109.4 (d, JC-F = 24 Hz), 80.7, 73.6, 69.9, 54.9, 44.6, 35.3, 31.3, 26.1, 22.5, 18.6, 14.0 ppm; DEPT 13C-NMR (75 MHz CDCl3) δ 129.3 (d, JC-F = 10.5 Hz), 128.6, 128.0, 127.7, 110.8, (d, JC-F = 21.8 Hz), 109.4 (d, JC-F = 23.33 Hz), 80.7, 73.6, 70.0, 55.0, 44.7, 35.4, 31.3, 26.2, 22.5, 18.7, 14.0 ppm; CHN analysis calculated for C23H30FNO4S: C, 63.42; H, 6.94; N, 3.22; S, 7.36: found C, 63.84; H, 6.92; N, 3.14; S, 7.14.
((S)-4-(Benzyloxymethyl)-7-fluoro-2-((S)-1-pentylethyl)-1,2-benzoxathiazepine-1,1-dioxide (9)
Utilizing General Procedure A, sultam 9 was isolated in 53% yield (3.15 g, 7.24 mmol) as a colorless viscous oil; [α]D20 = + 26.2° (c = 12.3, CHCl3); FTIR (neat): 2929, 2858, 1596, 1475, 1341, 1157, 1115, 1072 cm-1; 1H NMR (400 MHz CDCl3) δ 7.84 (dd, J = 6.4, 8.4 Hz, 1H), 7.42 - 7.34 (m, 5H), 6.92 - 6.85 (m, 2H), 4.65 (s, 2H), 4.33 - 4.31 (m, 1H), 4.17 - 4.15 (m, 1H), 3.85 - 3.77 (m, 2H), 3.68 (dd, J =10.4, 5.6 Hz, 1H), 3.47 (dd, J = 13.6, 1.7 Hz, 1H), 1.50 - 1.28 (m, 8H), 1.01 (d, J = 6.8 Hz, 3H), 0.89 (t, J = 6.8 Hz, 3H) ppm; 13C NMR (100 MHz CDCl3) δ 166.2(d, JC-F = 253.0 Hz), 156.6 (d, JC-F = 12.0 Hz), 137.5, 133.3 (d, JC-F = 2.0 Hz), 128.8 (d, JC-F = 11.0 Hz), 128.4, 127.9, 127.6, 111.1 (d, JC-F = 22.0 Hz), 110.4 (d, JC-F = 23.0 Hz), 80.7, 73.6, 70.1, 54.4, 44.8, 34.5, 31.2, 25.7, 22.4, 19.8, 1.9 ppm; DEPT 13C-NMR (75 MHz CDCl3) δ 128.8 (d, JC-F = 10.5 Hz), 128.6, 127.9, 127.8, 111.1 (d, JC-F = 21.8 Hz), 110.4 (d, JC-F = 24.0 Hz), 80.8, 73.7, 70.2, 54.4, 44.9, 34.6, 31.2, 25.8, 22.5, 19.9, 13.9 ppm; CHN analysis calculated for C23H30FNO4S: C, 63.42; H, 6.94; N, 3.22; S, 7.36: found C, 63.86; H, 6.81; N, 3.19; S, 7.26.
((R)-4-(Benzyloxymethyl)-2-((S)-1-phenylethyl)-7-fluoro-1,2-benzoxathiazepine-1,1-dioxide (10)
Utilizing General Procedure A, sultam 10 was isolated in 48% yield (2.85 g, 6.463 mmol) as a colorless viscous oil; [α]D20 = + 5.8° (c = 3.7, CHCl3); FTIR (neat): 3029, 2937, 2866, 1598, 1585, 1471, 1338, 1174, 1153, 1070 cm-1; 1H NMR (300 MHz CDCl3) δ 7.93 (dd, J = 8.7, 6.3 Hz, 1H), 7.43 - 7.31 (m, 10H), 6.98 - 6.87 (m, 2H), 5.30 - 5.46 (m, 1H), 4.57 (s, 2H), 4.38 - 4.44 (m, 1H), 3.78 - 3.70 (m, 2H), 3.69 - 3.51 (m, 1H), 3.37 (dd, J = 15.0, 1.8 Hz, 1H), 1.46 (d, J = 6.3 Hz, 3H) ppm; 13C NMR (75 MHz CDCl3) δ 166.9 (d, JC-F = 252.8 Hz), 156.8 (d, JC-F = 12.0 Hz), 139.4, 137.6, 133.3 (d, JC-F = 3.0 Hz),129.1 (d, JC-F = 10.5 Hz), 128.6, 128.5, 128.4, 127.9, 127.8, 127.7, 127.6, 127.5, 111.4 (d, JC-F = 21.8 Hz), 110.9 (d, JC-F = 23.3 Hz), 81.1, 73.5, 70.1, 56.6, 46.1, 17.7 ppm; DEPT 13C-NMR (75 MHz CDCl3) δ 129.1 (d, JC-F = 10.5 Hz), 128.6, 128.5, 128.0, 127.9, 127.6, 127.5, 111.5 (d, JC-F = 21.8 Hz), 111.0 (d, JC-F = 24.0 Hz), 81.1, 73.5, 70.2, 56.6, 46.1, 17.7 ppm; CHN analysis calculated for C24H24FNO4S: C, 65.29; H, 5.48; N, 3.17; S, 7.27: found C, 65.60; H, 5.38; N, 3.05; S, 7.21.
(S)-4-(Benzyloxymethyl)-2-((R)-1-phenylethyl)-7-fluoro-1,2-benzoxathiazepine-1,1-dioxide (11)
Utilizing General Procedure A, sultam 11 was isolated in 52% yield (3.10 g, 7.03 mmol) as a colorless viscous oil; [α]D20 = + 39.2° (c = 6, CHCl3); FTIR (neat): 3020, 2939, 2866, 1596, 1584, 1474, 1338, 1171, 1153, 1070 cm-1; 1H NMR (300 MHz CDCl3) δ 7.94 (dd, J = 8.7, 6.3 Hz, 1H), 7.41 - 7.15 (m, 10H), 6.99 - 6.93 (m,1H), 6.79 (dd, J = 9.6, 2.4 Hz, 1H), 5.41 (d, J = 2.1 Hz, 1H), 4.50 (d, J = 2.1 Hz, 2H), 3.72 (m, 2H), 3.57 - 3.47 (m, 2H), 3.39 (d, J = 12.6, Hz, 1H), 1.60 (d, J = 6.9 Hz, 3H) ppm; 13C NMR (75 MHz CDCl3) δ 166.8(d, JC-F = 253.5 Hz), 156.7 (d, JC-F = 12.0 Hz), 139.0, 137.6, 132.7 (d, JC-F = 3.0 Hz), 129.3 (d, JC-F = 10.5 Hz), 128.6, 128.4, 128.2, 127.8, 127.6, 127.4, 111.2 (d, JC-F = 22.5 Hz), 110.4 (d, JC-F = 23.3 Hz), 79.4, 73.2, 69.9, 56.6, 45.7, 17.5 ppm; DEPT 13C NMR (75 MHz CDCl3) δ 129.3 (d, JC-F = 10.5 Hz), 128.6, 128.4, 128.2, 127.8, 127.6, 127.4, 111.2 (d, JC-F = 21.8 Hz), 110.5 (d, JC-F = 24.0 Hz), 79.5, 73.3, 69.9, 56.6, 45.7, 17.5 ppm; HRMS calculated for C24H25FNO4S (M+H)+ 442.1488; found 442.1490 (TOF MS ES+).
((R)-4-(Benzyloxymethyl)-2-((S)-1-phenylethyl)-7-fluoro-1,2-benzoxathiazepine-1,1-dioxide (12)
Utilizing General Procedure A, sultam 12 was isolated in 48% yield (2.84 g, 6.44 mmol) as a colorless viscous oil; [α]D20 = - 35.0° (c = 4.4, CHCl3); FTIR (neat): 3029, 2935, 2866, 1598, 1585, 1473, 1336, 1155, 1116, 1070 cm-1; 1H NMR (300 MHz CDCl3) δ 7.96 (dd, J = 8.7, 6.3 Hz, 1H), 7.41 - 7.15 (m, 10H), 6.99 - 6.93 (m, 1H), 6.78 (dd, J = 9.3, 2.7 Hz, 1H), 5.42 - 5.38 (m, 1H), 4.57 (d, J = 2.1 Hz, 2H) 3.67 (s, 2H), 3.55 - 3.45 (m, 2H), 3.38 (d, J = 12.6 Hz, 1H), 1.59 (d, J = 6.9 Hz, 3H) ppm; 13C NMR (75 MHz CDCl3) δ 166.8(d, JC-F = 252.7Hz), 156.7 (d, JC-F = 12.0 Hz), 139.0, 137.6, 132.6 (d, JC-F = 3.0 Hz), 129.3 (d, JC-F = 10.5 Hz), 128.6, 128.5, 128.2, 127.9, 127.6, 127.5, 111.2 (d, JC-F = 22.5 Hz), 110.4 (d, JC-F = 23.3 Hz), 79.4, 73.2, 69.8, 56.6, 45.7, 17.5 ppm; CHN analysis calculated for C24H24FNO4S: C, 65.29; H, 5.48; N, 3.17; S, 7.27: found C, 65.58; H, 5.80; N, 3.12; S, 7.22.
((S)-4-(Benzyloxymethyl)-2-((S)-1-phenylethyl)-7-fluoro-1,2-benzoxathiazepine-1,1-dioxide (13)
Utilizing general procedure A, sultam 13 was isolated in 54% yield (3.20 g, 7.26 mmol) as a colorless viscous oil; [α]D20 = - 43.4° (c = 11.3, CHCl3); FTIR (neat): 3028, 2934, 2865, 1597, 1585, 1471, 1339, 1171, 1152, 1070 cm-1; 1H NMR (300 MHz CDCl3) δ 7.92 (m, 1H), 7.42 - 7.30 (m, 10H), 6.98 - 6.85 (m, 2H), 5.52 - 5.45 (m, 1H), 4.57 (s, 2H), 4.38 - 4.33 (m, 1H), 3.77 (m, 2H), 3.56 - 3.51 (m, 1H), 3.40 (dd, J =16.8, 1.8 Hz, 1H), 1.45 (d, J = 6.9 Hz, 3H) ppm; 13C NMR (75 MHz CDCl3) δ 166.9(d, JC-F = 253.5 Hz), 156.7 (d, JC-F = 12 Hz), 139.4, 137.6, 133.2 (d, JC-F = 3 Hz), 129.1 (d, JC-F = 10.5 Hz), 128.6, 128.5, 128.0, 127.9 , 127.6, 127.5, 111.4 (d, JC-F = 22.5 Hz), 110.9 (d, JC-F = 23.3 Hz), 81.1, 73.5, 70.1, 56.6, 46.1, 17.7 ppm; CHN analysis calculated for C24H24FNO4S: C, 65.29; H, 5.48; N, 3.17; S, 7.27: found C, 65.63; H, 5.83; N, 3.15; S, 7.26.
General procedure B: Microwave-assisted diversification of benzothiaoxazepine-1,1-dioxides 2 -13 cores
Into a 1 dram vial was added benzothiaoxazepine-1,1-dioxide 2 - 13 (100 mg, 0.23 mmol, 1 equiv.), dry DMSO (0.23 ml, 1M), DBU (3.4 μL, 10 mol%) and the corresponding amine (5 equiv., 1.15 mmol). The reaction vessel was capped, placed in Anton Paar Synthos 3000 ® microwave and heated at 180 °C for 50 min [Power = 1200 W, 8 minute ramp then 50 min hold]. After such time, the reaction was diluted in EtOAc, filtered through a SiO2 SPE and concentrated. The resulting crude product was purified by flash chromatography (7:3, EtOAc:hexane) to afford the desired benzo-oxathiazepine 1,1-dioxide 14 – 21.
(R)-4-((Benzyloxy)methyl)-7-((S)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((R)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (14)
Utilizing general procedure B, sultam 14 was isolated (92%, 110 mg, 0.21 mmol) as a colorless viscous oil; [α]D20 = - 21.4° (c = 2.3, CHCl3); FTIR (neat): 3352, 2941, 1596, 1494, 1377, 1326, 1132, 1074 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 8.8 Hz, 1H), 7.41 (d, J = 7.3 Hz, 2H), 7.39 – 7.33 (m, 4H), 7.32 (dd, J = 8.0, 2.4 Hz, 2H), 7.32 – 7.27 (m, 2H), 6.44 (dd, J = 8.8, 2.4 Hz, 1H), 6.36 (d, J = 2.3 Hz, 1H), 5.44 (q, J = 7.1 Hz, 1H), 4.56 (q, J = 11.9 Hz, 2H), 4.23 – 4.14 (m, 1H), 3.88 (dd, J = 10.9, 7.1 Hz, 1H), 3.74 (dd, J = 10.2, 5.8 Hz, 1H), 3.72 – 3.62 (m, 2H), 3.54 (dd, J = 12.5, 5.0 Hz, 1H), 3.49 (dt, J = 9.6, 4.8 Hz, 1H), 3.44 (dd, J = 12.1, 5.2 Hz, 1H), 3.21 (ddd, J = 17.2, 11.7, 5.5 Hz, 2H), 2.14 – 2.08 (m, 2H), 2.08 – 2.00 (m, 2H), 1.98 (d, J = 8.4 Hz, 1H), 1.37 (d, J = 7.1 Hz, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 156.4, 151.9, 140.2, 137.8, 128.5, 128.4, 127.8, 127.6, 127.5, 127.4, 123.5, 107.1, 105.4, 80.9, 73.4, 70.6, 62.6, 60.3, 56.1, 48.9, 46.3, 28.4, 23.3, 17.4 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2259 (TOF MS ES+).
(S)-4-((Benzyloxy)methyl)-7-((S)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((R)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (15)
Utilizing general procedure B, sultam 15 was isolated (95%, 114 mg, 0.218 mmol) as a colorless viscous oil; [α]D20 = - 3.0° (c = 0.67, CHCl3); FTIR (neat): 3352, 2939, 1598, 1496, 1377, 1326, 1130, 1074 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 8.8 Hz, 1H), 7.36 – 7.31 (m, 2H), 7.31 – 7.27 (m, 1H), 7.25 (m, 2H), 7.20 (ddd, J = 10.2, 6.9, 3.7 Hz, 5H), 6.41 (dt, J = 10.3, 5.1 Hz, 1H), 6.22 (d, J = 2.4 Hz, 1H), 5.34 (q, J = 7.0 Hz, 1H), 4.45 (q, J = 12.1 Hz, 2H), 3.90 – 3.84 (m, 1H), 3.90 – 3.83 (m, 1H), 3.64 (dd, J = 27.2, 12.0 Hz, 2H), 3.53 (ddd, J = 14.0, 13.2, 7.8 Hz, 2H), 3.45 (dq, J = 10.5, 5.3 Hz, 2H), 3.27 (d, J = 12.5 Hz, 1H), 3.22 – 3.14 (m, 1H), 2.08 (dt, J = 8.4, 4.3 Hz, 2H), 2.06 – 1.98 (m, 2H), 1.64 (d, J = 4.2 Hz, 1H), 1.55 (d, J = 7.0 Hz, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 156.3, 151.6, 139.8, 137.8, 128.6, 128.4, 127.8, 127.7, 127.7, 127.4, 122.7, 107.0, 104.7, 79.0, 73.1, 70.2, 62.7, 59.9, 56.3, 48.9, 45.9, 28.4, 23.3, 17.7 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2258 (TOF MS ES+).
(S)-4-((Benzyloxy)methyl)-7-((S)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((S)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (16)
Utilizing general procedure B, sultam 16 was isolated (90%, 108 mg, 0.207 mmol) as a colorless viscous oil; [α]D20 = - 27.0° (c = 0.51, CHCl3); FTIR (neat): 3353, 2939, 1596, 1496, 1377, 1326, 1130, 1074 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 8.7, 4.5 Hz, 1H), 7.38 (d, J = 7.1 Hz, 2H), 7.36 – 7.31 (m, 4H), 7.31 – 7.25 (m, 4H), 6.40 (dd, J = 8.8, 2.4 Hz, 1H), 6.35 (d, J = 2.4 Hz, 1H), 5.42 (q, J = 7.1 Hz, 1H), 4.57 – 4.50 (m, 2H), 4.17 (ddd, J = 9.4, 4.7, 3.3 Hz, 1H), 3.89 (dt, J = 11.3, 5.6 Hz, 1H), 3.73 – 3.60 (m, 3H), 3.60 – 3.54 (m, 1H), 3.47 (dd, J = 10.1, 6.2 Hz, 2H), 3.22 – 3.16 (m, 2H), 2.09 (tt, J = 10.4, 5.0 Hz, 2H), 2.08 – 2.04 (m, 2H), 1.55 (t, J = 5.2 Hz, 1H), 1.36 (d, J = 7.1 Hz, 3H) ; 13C NMR (126 MHz, CDCl3) δ 156.4, 151.8, 140.1, 137.8, 128.5, 128.4, 127.8, 127.7, 127.6, 127.5, 123.7, 107.1, 105.5, 80.9, 73.4, 70.5, 62.8, 60.0, 56.1, 49.0, 46.3, 28.4, 23.3, 17.4 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2260 (TOF MS ES+).
(R)-4-((Benzyloxy)methyl)-7-((S)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((S)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (17)
Utilizing general procedure B, sultam 17 was isolated (98%, 117 mg, 2.25 mmol) as a colorless viscous oil; [α]D20 = - 36.5° (c = 0.79, CHCl3); FTIR (neat): 3363, 2933, 1598, 1494, 1377, 1325, 1132, 1076 cm-1; 1H NMR (500 MHz CDCl3) δ 7.67 (t, J = 6.6 Hz, 1H), 7.36 – 7.32 (m, 2H), 7.31 – 7.27 (m, 1H), 7.24 (m, 2H), 7.23 – 7.20 (m, 3H), 7.20 – 7.16 (m, 2H), 6.42 (dt, J = 15.3, 7.7 Hz, 1H), 6.21 (d, J = 2.4 Hz, 1H), 5.32 (q, J = 7.0 Hz, 1H), 4.44 (q, J = 12.1 Hz, 2H), 3.90 – 3.83 (m, 1H), 3.68 – 3.57 (m, 3H), 3.53 (dq, J = 10.5, 5.1 Hz, 2H), 3.46 – 3.41 (m, 2H), 3.26 (d, J = 12.5 Hz, 1H), 3.20 – 3.13 (m, 1H), 2.12 – 1.96 (m, 4H), 1.64 (s, 1H), 1.55 (d, J = 7.0 Hz, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 156.3, 151.6, 139.8, 137.9, 128.6, 128.5, 128.4, 127.8, 127.7, 127.4, 122.8, 107.0, 104.6, 78.9, 73.0, 70.3, 62.7, 60.1, 56.3, 48.9, 45.9, 28.4, 23.3, 17.6.ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2261 (TOF MS ES+).
(R)-4-((Benzyloxy)methyl)-7-((R)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((R)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (18)
Utilizing general procedure B, sultam 18 was isolated (93%, 111 mg, 0.214 mmol) as a colorless viscous oil; [α]D20 = + 28.5° (c = 2.4, CHCl3); FTIR (neat): 3348, 2937, 2871, 1596, 1496, 1377, 1326, 1130, 1074 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 8.8 Hz, 1H), 7.38 (d, J = 7.3 Hz, 2H), 7.34 (ddd, J = 7.4, 4.5, 2.0 Hz, 4H), 7.31 – 7.28 (m, 2H), 7.28 – 7.25 (m, 2H), 6.39 (dt, J = 10.3, 5.2 Hz, 1H), 6.35 (d, J = 2.3 Hz, 1H), 5.42 (q, J = 7.1 Hz, 1H), 4.58 – 4.49 (m, 2H), 4.19 – 4.13 (m, 1H), 3.91 – 3.83 (m, 1H), 3.55 – 3.49 (m, 3H), 3.46 (td, J = 9.7, 4.2 Hz, 2H), 3.23 – 3.14 (m, 2H), 2.12 – 1.95 (m, 4H), 1.92 (s, 1H), 1.36 (d, J = 7.1 Hz, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 156.4, 151.8, 140.2, 137.8, 128.5, 128.4, 127.8, 127.6, 127.5, 127.4, 123.5, 107.2, 105.5, 80.8, 73.4, 70.5, 62.6, 60.1, 56.1, 48.9, 46.3, 28.4, 23.3, 17.4 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2257 (TOF MS ES+).
(S)-4-((Benzyloxy)methyl)-7-((R)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((R)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (19)
Utilizing general procedure B, sultam 19 was isolated (94%, 112 mg, 0.216 mmol) as a colorless viscous oil; [α]D20 = + 38.6° (c = 1.95, CHCl3); FTIR (neat): 3369, 2931, 1598, 1494, 1377, 1325, 1132, 1076 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.68 – 7.65 (m, 1H), 7.36 – 7.31 (m, 2H), 7.31 – 7.28 (m, 1H), 7.28 – 7.23 (m, 3H), 7.23 – 7.18 (m, 3H), 7.17 (dd, J = 7.3, 2.1 Hz, 2H), 6.42 (dd, J = 8.9, 2.4 Hz, 1H), 6.21 (d, J = 2.4 Hz, 1H), 5.32 (q, J = 7.0 Hz, 1H), 4.48 – 4.39 (m, 2H), 3.85 (dd, J = 10.5, 6.8 Hz, 1H), 3.67 – 3.58 (m, 2H), 3.52 (dt, J = 14.9, 7.7 Hz, 2H), 3.45 – 3.39 (m, 2H), 3.30 – 3.23 (m, 1H), 3.15 (dt, J = 15.8, 8.0 Hz, 1H), 2.07 (dd, J = 8.0, 5.2 Hz, 2H), 2.04 – 1.96 (m, 2H), 1.91 (s, 1H), 1.55 (d, J = 7.0 Hz, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 156.3, 151.7, 139.7, 137.9, 128.6, 128.5, 128.4, 127.9, 127.8, 127.7, 127.4, 122.6, 107.0, 104.6, 78.8, 73.0, 70.3, 62.6, 60.2, 56.3, 48.8, 45.9, 28.4, 23.3, 17.7 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2262 (TOF MS ES+).
(S)-4-((Benzyloxy)methyl)-7-((R)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((S)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (20)
Utilizing general procedure B, sultam 20 was isolated (97%, 116 mg, 0.223 mmol) as a colorless viscous oil; [α]D20 = + 21.7° (c = 2.6, CHCl3); FTIR (neat): 3352, 2941, 1596, 1494, 1377, 1326, 1132, 1074 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 8.8 Hz, 1H), 7.39 (m, 2H), 7.36 – 7.31 (m, 4H), 7.31 – 7.28 (m, 2H), 7.26 (m, 2H), 6.42 (dd, J = 8.8, 2.4 Hz, 1H), 6.34 (d, J = 2.4 Hz, 1H), 5.42 (q, J = 7.1 Hz, 1H), 4.53 (q, J = 11.9 Hz, 2H), 4.16 (ddd, J = 10.4, 5.9, 4.6 Hz, 1H), 3.86 (td, J = 7.2, 4.1 Hz, 1H), 3.72 (dt, J = 10.4, 5.2 Hz, 1H), 3.69 – 3.61 (m, 1H), 3.55 – 3.49 (m, 1H), 3.47 (dt, J = 10.6, 5.3 Hz, 1H), 3.42 (dd, J = 12.3, 5.2 Hz, 1H), 3.21 – 3.15 (m, 2H), 2.12 – 2.05 (m, 2H), 2.05 – 1.95 (m, 4H), 1.36 (d, J = 7.1 Hz, 3H) ppm; 13C NMR (126 MHz CDCl3) δ 156.4, 151.9, 140.2, 137.8, 128.5, 128.4, 127.8, 127.7, 127.6, 127.5, 123.5, 107.1, 105.4, 80.9, 73.4, 70.6, 62.6, 60.3, 56.1, 48.9, 46.3, 28.4, 23.3, 17.4 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2261 (TOF MS ES+).
(R)-4-((Benzyloxy)methyl)-7-((R)-2-(hydroxymethyl)pyrrolidin-1-yl)-2-((S)-1-phenylethyl)-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine 1,1-dioxide (21)
Utilizing general procedure B, sultam 21 was isolated (92%, 110 mg, 0.211 mmol) as a colorless viscous oil; [α]D20 = + 1.6° (c = 1.84, CHCl3); FTIR (neat): 3390, 2937, 1598, 1496, 1377, 1325, 1130, 1076 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 8.7, 4.3 Hz, 1H), 7.36 – 7.32 (m, 2H), 7.31 – 7.28 (m, 1H), 7.27 – 7.23 (m, 2H), 7.23 – 7.16 (m, 5H), 6.41 (dt, J = 10.7, 5.4 Hz, 1H), 6.21 (d, J = 2.4 Hz, 1H), 5.33 (q, J = 7.0 Hz, 1H), 4.44 (q, J = 12.1 Hz, 2H), 3.88 – 3.83 (m, 1H), 3.69 – 3.57 (m, 3H), 3.56 – 3.49 (m, 2H), 3.47 – 3.41 (m, 2H), 3.28 (d, J = 12.5 Hz, 1H), 3.21 – 3.13 (m, 1H), 2.11 – 1.97 (m, 4H), 1.83 (s, 1H), 1.55 (d, J = 7.0 Hz, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 156.3, 151.6, 139.7, 137.9, 128.6, 128.5, 128.4, 127.9, 127.8, 127.7, 127.4, 122.6, 107.1, 104.7, 78.9, 73.1, 70.3, 62.6, 60.0, 56.3, 48.9, 45.9, 28.4, 23.3, 17.7 ppm; HRMS calculated for C29H35N2O5S (M+H)+ 523.2267; found 523.2263 (TOF MS ES+).
Supplementary Material
Acknowledgments
This work was supported by the National Institute of General Medical Science (Center in Chemical Methodologies and Library Development at the University of Kansas, KU-CMLD, NIH P50 GM069663 and NIH P41-GM076302.
Footnotes
Supporting Information Available: Supplememtary data (1H and 13C spectra) associated with this article can be found in the online version at: doi:xxxxx.
References Cited
- (i).Keseru G, Makara GM. Nature Rev Drug Discov. 2009;8:203–212. doi: 10.1038/nrd2796. [DOI] [PubMed] [Google Scholar]
- (ii).(a) Lovering F, Bikker J, Humblet C. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; (b) Clemons PA, Bodycombe NR, Carrinski HA, Wilson JA, Shamji AF, Wagner BK, Koehler AN, Schreiber SL. Proc Natl Acad Sci. 2010;107:18787–18792. doi: 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (iii).(a) Danishefsky SJ. Nat Prod Rep. 2010;27:1114–1116. doi: 10.1039/c003211p. [DOI] [PubMed] [Google Scholar]; (b) Ganesan A. Curr Opin Chem Biol. 2008;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- (iv).(a) Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; (b) Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613–618. doi: 10.1126/science.1089946. [DOI] [PubMed] [Google Scholar]; (c) Spring DR. Org Biomol Chem. 2003;1:3867–3870. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]; (d) Tan DS. Nat Chem Bio. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]; (e) Schreiber SL. Nature. 2009;457:153–154. doi: 10.1038/457153a. [DOI] [PubMed] [Google Scholar]; (f) Damdapani S, Marcaurelle LA. Curr Opin Chem Biol. 2010;14:362–370. doi: 10.1016/j.cbpa.2010.03.018. [DOI] [PubMed] [Google Scholar]; (g) Marcaurelle LA, Comer E, Dandapani S, Duvall JR, Gerard B, Kesavan S, Lee MD, 4th, Liu H, Lowe JT, Marie J-C, Mulrooney CA, Pandya BA, Rowley A, Ryba TD, Suh B-C, Wie J, Young D, Akellam LB, Ross NT, Zhang Y-L, Fass DM, Reis SA, Zhao W-N, Haggarty SJ, Palmer M, Foley MA. J Am Chem Soc. 2010;132:16962–16976. doi: 10.1021/ja105119r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (v).Rolfe A, Samarakoon TB, Hanson PR. Org Lett. 2010;12:1216–1219. doi: 10.1021/ol100035e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (vi).(a) Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; (b) Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613–618. doi: 10.1126/science.1089946. [DOI] [PubMed] [Google Scholar]; (c) Spring DR. Org Biomol Chem. 2003;1:3867–3870. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]; (d) Tan DS. Nat Chem Bio. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]; (e) Schreiber SL. Nature. 2009;457:153–154. doi: 10.1038/457153a. [DOI] [PubMed] [Google Scholar]; (f) Damdapani S, Marcaurelle LA. Curr Opin Chem Biol. 2010;14:362–370. doi: 10.1016/j.cbpa.2010.03.018. [DOI] [PubMed] [Google Scholar]; (g) Marcaurelle LA, Comer E, Dandapani S, Duvall JR, Gerard B, Kesavan S, Lee MD, 4th, Liu H, Lowe JT, Marie J-C, Mulrooney CA, Pandya BA, Rowley A, Ryba TD, Suh B-C, Wie J, Young D, Akellam LB, Ross NT, Zhang Y-L, Fass DM, Reis SA, Zhao W-N, Haggarty SJ, Palmer M, Foley MA. J Am Chem Soc. 2010;132:16962–16976. doi: 10.1021/ja105119r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (vii).(a) Zhang QS, Lu HJ, Curran DP. J Am Chem Soc. 2004;126:36–37. doi: 10.1021/ja038542e. [DOI] [PubMed] [Google Scholar]; (b) Curran DP, Zhang Q, Richard C, Lu H, Gudipathi V, Wilcox CS. J Am Chem Soc. 2006;128:9561–9573. doi: 10.1021/ja061801q. [DOI] [PubMed] [Google Scholar]
- (viii).Batch scale-up (e.g., 1–2 g) was attempted previously, resulting in yields that were dramatically lower (20–60%, substrate specific), along with poorer percent conversion. In addition, the crude reaction mixtures in batch were far more complex, thus complicating purification. Preliminary investigation into this product loss in batch scale-up suggested a combination of decomposition and potential polymerization of reactive intermediate that did not occur on smaller scale.
- (ix).For a recent review on flow chemistry, see: Razzaq T, Kappe CO. Chem Asian J. 2010;5:1274–1289. doi: 10.1002/asia.201000010. and references therein.
- (x).(a) Achanta S, Liautard V, Paugh R, Organ MG. Chem Eur J. 2010;16:12797–12800. doi: 10.1002/chem.201002102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shore G, Yoo WJ, Li CJ, Organ MG. Chem Eur J. 2010;16:126–133. doi: 10.1002/chem.200902396. [DOI] [PubMed] [Google Scholar]; (c) Shore G, Organ MG. Chem Eur J. 2008;14:9641–9646. doi: 10.1002/chem.200801610. [DOI] [PubMed] [Google Scholar]; (d) Shore G, Organ MG. Chem Commun. 2008:838–840. doi: 10.1039/b715709f. [DOI] [PubMed] [Google Scholar]; (e) Shore G, Morin S, Mallik D, Organ MG. Chem Eur J. 2008;14:1351–1356. doi: 10.1002/chem.200701588. [DOI] [PubMed] [Google Scholar]
- (xi).(a) Moseley JD, Woodman EK. Org Process Res Dev. 2008;12:967–981. [Google Scholar]; (b) Moseley JD, Lenden P, Lockwood M, Ruda K, Sherlock J-P, Thomson AD, Gilday JP. Org Process Res Dev. 2008;12:31–40. [Google Scholar]; (c) Bowman MD, Holcomb JL, Kormos CM, Leadbeater NE, Williams VA. Org Process Res Dev. 2008;12:41–57. [Google Scholar]; (d) Kremsner JM, Stadler A, Kappe CO. Top Curr Chem. 2006;266:233–278. [Google Scholar]
- (xii).Ullah F, Samarakoon TB, Rolfe A, Kurtz RD, Hanson PR, Organ MG. Chem Eur J. 2010:10959–10962. doi: 10.1002/chem.201001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (xiii).Measured temperatures are not exact values due to the problems associated with measuring the temperature inside a thin capillary tube with a standard IR sensor (designed to measure the temperatire of 2–5 mL Pyrex vials).
- (xiv).Due to increased steric hindrance a flow rate of 100 μL/min vs 200 μL/min was utilized to maintain high conversion (100% vs 70–80%) for the synthesis of 6–13 on MACOS platform.
- (xv).Rolfe A, Samarakoon TB, Klimberg SV, Brzozowski M, Neuenswander B, Lushington G, Hanson PR. J Comb Chem. 2010;12:850–854. doi: 10.1021/cc1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


