1. Introduction
Parkinson’s disease (PD) patients are committed to a treatment strategy based on dopaminergic medications to reduce the severity of their motor symptoms. Over time, however, these treatments can lead to, among other complications, motor fluctuations with reemergence of tremor or bradykinesia (slowed movements) and medication-induced dyskinesias (involuntary abnormal movements). The associated worsening of quality of life resulting from motor complications has become better appreciated in recent years (Chapuis et al., 2005). PD motor symptom and motor complications are most commonly appraised during clinic visits through the use of the Unified Parkinson’s Disease Rating Scale (UPDRS) (Goetz et al., 2007). Adjustments in treatment are in part dependent on the outcome of these in-clinic assessments. However, motor fluctuations throughout the day may go unnoticed.
Limited methods are currently available to capture and assess motor complications at home. Patients can manually complete diary entries of medication times and wearing off periods; however, they may have difficulty accurately and objectively evaluating their own symptom severity and distinguishing between symptoms.
Motion sensor technology has previously been leveraged for capturing and quantifying PD motor symptoms (Costa et al., 2010; Giuffrida et al., 2009; Heldman et al., 2011; Manson et al., 2000; Patel et al., 2009; Rigas et al., 2009; Schrag et al., 1999; Weiss et al., 2010). However, home monitoring applications require high patient compliance, a critical element for validation of home testing results and achieving acceptance in the clinical community. While a technology may successfully integrate complex algorithms for automated scoring of motor symptom severity, if patients cannot use the device unsupervised at home or properly follow instructions to complete motor assessments, the technology will have little clinical value.
The objective of this pilot study was to evaluate the feasibility and patient compliance of a home-based PD assessment system (Kinesia™, Cleveland Medical Devices Inc., Cleveland, OH) in which wireless motion sensor technology provided quantitative motor symptom severity scores highly correlated to clinical rating scales throughout the day. Subjects used the system at home to perform video-guided motor tasks while motion sensor data were captured to evaluate tremor and bradykinesia in response to PD medication. We hypothesized the system could be used correctly by PD subjects to undergo automated motor assessments at home.
2. Material and methods
2.1 Subject recruitment
Twenty subjects were enrolled including ten subjects diagnosed with idiopathic PD and prescribed dopaminergic medication (8 male and 2 female; 61.4±7.4 years of age; 1511±933 mg Levodopa Equivalent Daily Dose) and ten non-PD subjects (Control). PD subjects were recruited at the University of Cincinnati while control subjects were recruited at Cleveland Medical Devices Inc. Testing was completed under the purview of each institution’s respective Institutional Review Board. All subjects provided informed consent prior to participation.
2.2 Technology overview
Kinesia™ (Cleveland Medical Devices Inc., Cleveland, OH), a Food and Drug Administration cleared-to-market device, was used to capture and quantify PD motor symptoms (Fig. 1). The finger-worn motion sensor contains three orthogonal accelerometers and three orthogonal gyroscopes to measure linear accelerations (ax, ay, and az) and angular velocities (ωx, ωy, and ωz), respectively. A wrist-worn command module connects to the finger sensor via a thin, flexible cable and houses a battery and 2.4 GHz radio. Kinematic data are sampled at 128 Hz and wirelessly transmitted in real-time to a computer.
Figure 1.
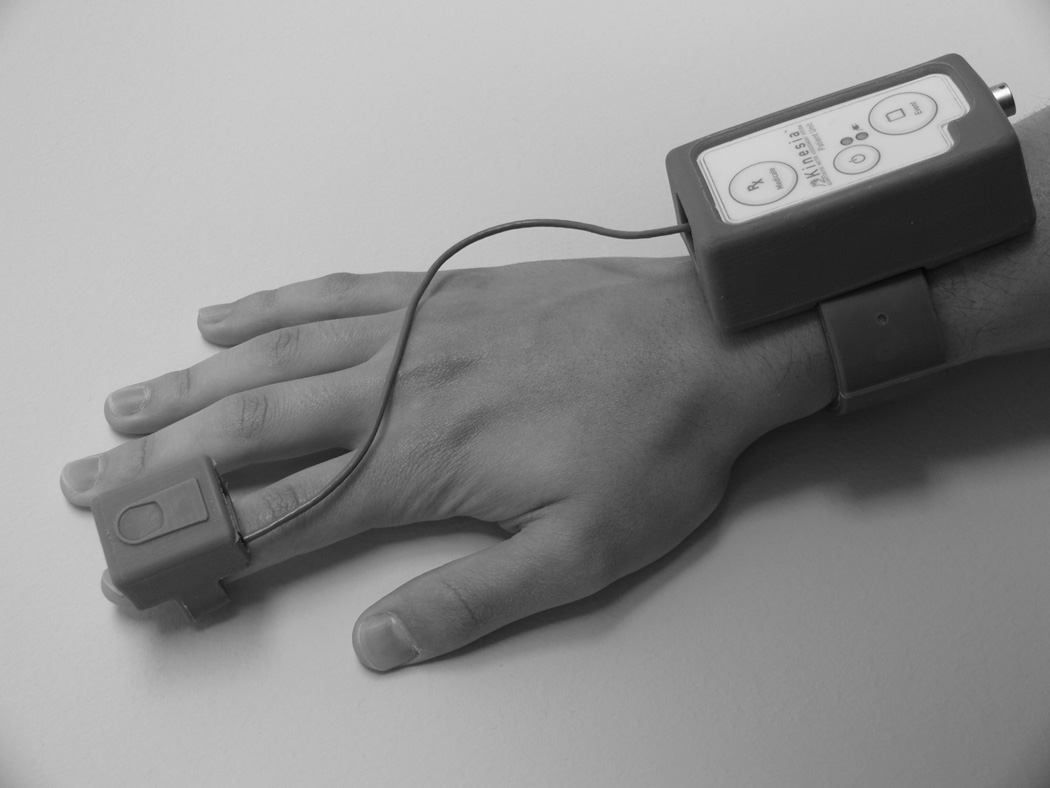
Kinesia motion capture device. The unit consists of a finger-worn motion sensor and wrist-worn command module for data acquisition and wireless data transmission. With the motion sensor positioned above the finger with palm down and fingers extended parallel to the ground, ax is oriented perpendicular to the finger and parallel to the ground with the positive direction to the right, ay is oriented parallel to the finger with the positive direction distal to the hand, and az is perpendicular to the finger and ground with the positive direction up. The ωx, ωy, and ωz gyroscope channels represent the rotation about each linear acceleration axis.
The Kinesia software guides patients through three tremor and three bradykinesia motor tasks based on the UPDRS III Motor Examination: rest, postural, and kinetic tremor, and repetitive finger-tapping, hand-grasping, and pronation-supination for evaluating speed, amplitude, and rhythm. Motion data captured by the finger sensor are processed into 0 (symptom absent) to 4 (severe impairment) severity ratings using previously validated algorithms, which showed high correlations to expert clinician UPDRS tremor (Giuffrida et al., 2009) and Modified Bradykinesia Rating Scale (MBRS) speed, amplitude, and rhythm scores (Heldman et al., 2011).
2.3 Control subjects and compliance criteria
Control subjects were monitored by a trained technician to ensure instructions were properly followed. The motion sensor was placed over the dorsal side of the index finger’s distal phalanx with a thin silicon sleeve. Seated in front a laptop, subjects followed the Kinesia software videos to undergo an automated motor assessment consisting of the six motor tasks, each performed for twenty seconds.
Compliance criteria were developed from the data to ensure subjects were correctly performing the motor tasks during the automated evaluations. Some of the video-guided motor tasks required subjects to remain still in a specific position, while others required them to voluntarily move. Therefore, a voluntary movement threshold (VMT) was calculated. Motion data collected during the rest tremor tasks were used to determine the VMT. The gyroscope angular velocity channels (ωx, ωy, and ωz) were integrated to derive angle and then band-pass filtered from 0.3–3 Hz (2nd order Butterworth) to remove rest tremor frequency components (Rahimi et al., 2009). The peak in the power spectrum was calculated for each angle channel (θx, θy, and θz), and the root mean square (θRMS) of the three values was calculated as a measure independent of the direction of motion. The VMT was defined as the average θRMS plus two standard deviations. A rest or postural tremor task was rejected if the calculated θRMS for that motion data file exceeded the VMT while a kinetic tremor or one of the three bradykinesia tasks was rejected if the θRMS did not exceed the VMT.
In addition to gross motion detection to classify voluntary movement versus involuntary movement tasks, task-specific criteria were developed to determine if the correct tasks were being performed. Linear acceleration channels were used to determine sensor and finger orientation in tasks requiring subjects to maintain a specific posture. Tasks requiring subjects to voluntarily move such as finger tap used the angular velocity channels to determine whether the proper rotational hand movements were performed. Based on these guidelines, a range of quantitative variables were selected and tested. The variable that was rejected the fewest times was selected for each motor task. The resulting six variables were then applied to the tasks completed at home by the PD group.
2.4 PD subjects, home compliance, and medication response
During a clinical visit, PD subjects were given the Kinesia device and laptop computer along with detailed instructions for home use. The clinician instructed subjects when to perform motor task assessments and to document their prescribed PD medication use. Subjects were required to demonstrate to the clinician that they could perform all motor tasks correctly using Kinesia before being sent home.
Each home testing session consisted of performing the six motor tasks while wearing the Kinesia device on the more affected limb. The subjects performed 3–6 motor assessments per day for 3–6 consecutive days depending on their available schedule. All data was stored on the laptop computer. After completing the study at home, subjects returned the Kinesia system and laptop computer to the clinician.
The compliance criteria developed from the Control subjects were used to determine whether the PD subjects performed the motor task assessments correctly at home. If any one criterion failed, the task instance was rejected and excluded from further data analysis.
Additionally, we evaluated the ability of the system to capture motor fluctuations. Algorithms generated scores for six symptoms: 1) rest, 2) postural, and 3) kinetic tremor and 4) average speed, 5) amplitude, and 6) rhythm calculated across the three bradykinesia tasks. For each testing day, motor scores per symptom were grouped into two medication states: 1) baseline: before the first medication dose in the morning when motor symptoms are typically at their worst and 2) treatment: after the first medication dose and for the remainder of the day when symptoms should be well controlled. Severity scores for a particular motor task and day were excluded from analysis if the baseline score was less than one as medication may not improve symptoms with severity scores already so low. Severity scores grouped into the treatment medication state were averaged together as a measure of mean symptom severity throughout the day. A paired t-test was used to compare the baseline and mean treatment scores across all PD subjects, days, and the six motor symptoms.
Motor response across dose cycle was analyzed to evaluate symptom fluctuations. For a given symptom and subject, the baseline score from each day was aligned to time zero of the dose cycle, and treatment scores were binned into one hour intervals based on elapsed time since the previous medication dose. The first, middle, and last two bins were compared using a t-test to determine whether statistically significant differences occurred between bins and motor symptoms.
3. Results
All Control subjects successfully completed their motor assessments in the laboratory setting, while 9 out of 10 PD subjects completed their home assessments as instructed. The PD subject who did not complete the protocol as instructed did not record medication times and only performed a motor assessment session once per day, and therefore was excluded from the analysis. Overall, each of the six motor tasks were performed a total of 210 times by the PD subjects.
Calculating θRMS across all rest tremor motor task data files produced a VMT value of 4.9. No Control group and eleven (5%) PD group rest tremor data files were rejected when sufficient voluntary movement occurred during non-movement tasks as indicated by the VMT. Task-specific criteria were selected based on the lowest Control group data file rejection rate for each motor task. The rest and postural tremor criteria algorithms derived hand orientation relative to gravity by low-pass filtering the accelerometer data at 0.3 Hz (2nd order Butterworth) to remove tremor and checked if the mean of ay was less than zero for rest tremor and if the mean of the az was greater than zero for postural tremor. The kinetic tremor and bradykinesia criteria algorithms band-pass filtered the gyroscope data between 0.3–3 Hz and 0.3–8 Hz (2nd order Butterworth), respectively, calculated angle through integration, calculated the peak in the power spectrum for θx, θy, and θz, and determined which channel yielded the largest value. Based on these criteria, one Control group rest tremor data file (10%), fifteen (7%) postural tremor, and three (1%) kinetic tremor PD group data files were rejected. In summary when including task-specific as well as voluntary movement criteria, approximately 97% (1222/1260) of all motor tasks completed by the PD subjects were accepted.
Tremor and average speed, amplitude, and rhythm motor score trends were analyzed over the course of the day and in response to medication. A paired t-test showed that medication significantly improved rest and postural tremor and average speed and amplitude scores (p<0.02) when comparing the baseline (before the first medication dose in the morning) and mean treatment score (remainder of the day). Kinetic tremor and average rhythm scores did not significantly improve with medication (p>0.4, p>0.1, respectively).
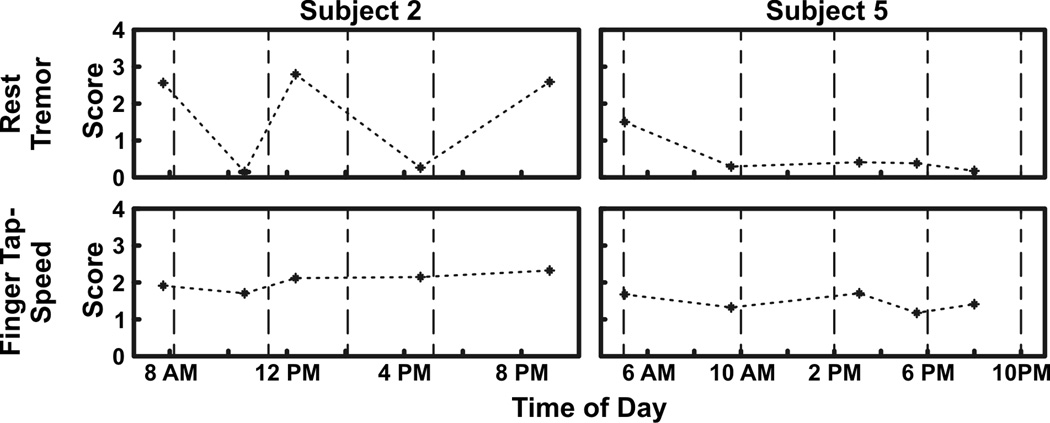
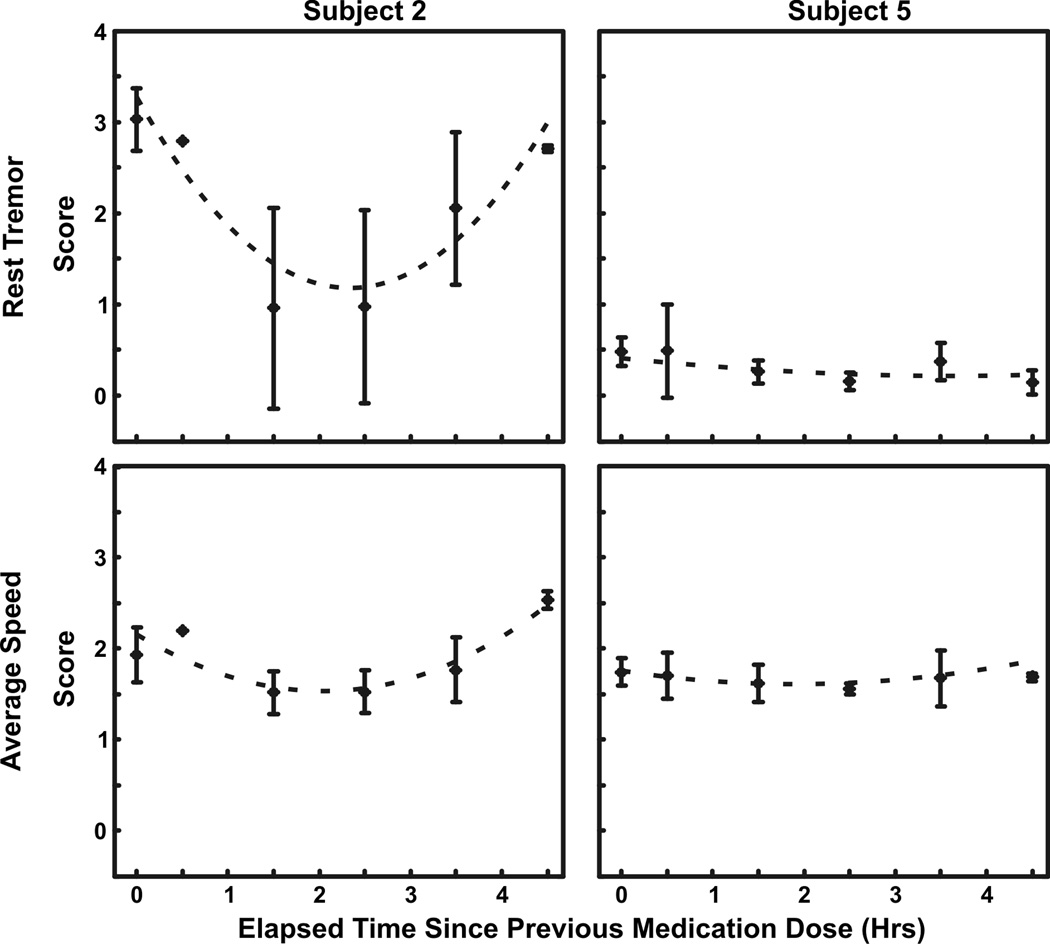
Additionally, symptom scores were plotted to evaluate motor fluctuations and medication effectiveness. Subjects 2 and 5 were used as examples to highlight differences in motor response to medication (Fig. 2). Subject 2’s rest tremor scores greatly fluctuated while subject 5 had a baseline score of 1.5 and a mean treatment score of less than 0.5 for the remainder of the day. Finger tap speed in contrast showed minimal change in both subjects. Figure 3 shows symptom severity versus elapsed time since the previous medication dose compiled over all test days. Subject 2’s rest tremor and average speed improved after 2–3 h (p<0.01, p<0.01, respectively), but worsened before the next medication dose cycle (p<0.05, p<0.01, respectively). Furthermore, rest tremor percent improvement between the first and middle two bins was greater than for average speed (p<0.01). Subject 5’s rest tremor and average speed did not improve after 2–3 h (p>0.1, p>0.3, respectively) and did not worsen before the next medication dose cycle (p>0.4, p>0.4, respectively).
Figure 2.
Symptom severity scores during a single day. Rest tremor and finger tap speed scores are shown for two PD subjects to highlight differences in motor score severity and fluctuations in response to PD medication. Black points indicate motor symptom severity scores while vertical dotted lines indicate the time at which medication was taken. When evaluating rest tremor, Subject 2 exhibited significant fluctuations throughout the day while Subject 5’s severity was well controlled after taking the initial medication dose. Finger tap speed in contrast showed minimal response to medication.
Figure 3.
Averaged symptom severity scores over medication dose cycles. Symptom scores were grouped into one hour intervals based on the elapsed time since the previous medication dose. Baseline scores were aligned with time zero. Error bars represent the standard deviation of the motor scores in each time interval. Second order equations were fitted to the plotted data (dotted curves). Subject 2’s rest tremor and speed averaged across the three bradykinesia tasks improved after 2–3 h (p<0.01, p<0.01, respectively), but worsened before the next medication dose cycle (p<0.05, p<0.01, respectively). Subject 5’s rest tremor and average speed did not improve after 2–3 h (p>0.1, p>0.3, respectively) and did not worsen before the next medication dose cycle (p>0.4, p>0.4, respectively).
4. Discussion
4.1 Patient compliance at home
Patient compliance is essential for acceptance of any home monitoring technology. In general, PD subjects correctly moved voluntarily and remained still as instructed. Upon review of the raw motion data collected during the 5% of rejected rest tremor files, it is likely that these subjects were either voluntarily moving or experienced levodopa-induced dyskinesias. Previous studies have demonstrated that PD dyskinetic movements occur between 1–3 Hz (Lemstra et al., 1999) and, therefore, would cause the θRMS to exceed the VMT even after bandpass filtering to remove rest tremor activity typically occurring between 3–6 Hz (Koller et al., 1989). Task-specific criteria were also established for each motor task. For example, the finger tap task should generate the largest rotational movement about the second metacarpal joint of the index finger. Therefore, in order to not reject a data file, the peak in the power spectrum on θx should be larger than those on θy or θz based on the sensor axis orientation. Although 7% of postural data files were rejected, 13 of the 15 rejected files can be attributed to a single subject. Upon closer review of the raw motion data, mean of az was consistently less than zero, suggesting that the subject either incorrectly performed the task with his palms facing up or with the sensor rotated below the finger.
Motor response to medication was also used to evaluate compliance. Four of six motor symptoms significantly improved, demonstrating that the Kinesia device captured and quantified the expected improvement in symptom severity. Although kinetic tremor and average rhythm did not significantly improve, studies have shown a lack of response from levodopa use for these symptoms (Espay et al., 2011; Raethjen et al., 2005).
Although the compliance criteria results demonstrate that PD subjects were able to complete the home study, additional design considerations should be considered for future system development. Frequency content of tremor, bradykinesia motor tasks, and voluntary motion may overlap. Therefore, tremor activity may be present while a bradykinesia motor task is being performed and may affect the generated motor score. Also the VMT calculation only detects voluntary motion in frequency ranges less than 3 Hz; therefore, this measure may not capture all voluntary activity in order to reject a data file. Ergonomic design and ease of use is equally important as proficient data processing and accurate motor scoring to achieve high compliance. The low rejection results may be in part due to the overall low symptom severity in the recruited PD group. Out of the possible 44 study days across all PD subjects, only ten days resulted in baseline scores greater than one for rest tremor. Therefore, the Kinesia system design may require additional modifications to comply with patients in more advanced stages of PD. This will include reducing the size of the wrist and finger-worn motion sensor and developing an intuitive touch screen interface for simplified computer use.
4.2 Capturing motor fluctuations at home
Many factors exist that can influence PD symptom response to medication. Disease progression can decrease the time duration of symptomatic benefit. Patients in the early stages of PD with mild symptom impairment may experience a 6–8 h therapeutic window whereas advanced patients, who require higher medication doses, may only have a 0.5–2 h window with faster and less predictable wearing-off periods (Ahlskog and Muenter, 2001; Fahn, 2006, 1999). This observation is evident in Figure 3. Subject 2’s scores exhibited a significant improvement into the dose cycle; however, symptoms returned well before the next medication dose. In order to maintain symptom improvement throughout the day, the time between each dose must be optimized to better achieve a more continuous therapeutic window. That is, levodopa dosage and frequency of use can have a major impact on motor fluctuations. Therefore, decreasing the time between doses for Subject 2 may achieve a more continuous therapeutic window throughout the day, similar to the medication response shown for Subject 5. Also, Subject 2’s average speed was less responsive than rest tremor, suggesting that levodopa was more effective at treating rest tremor than bradykinesia. It is plausible that either the prescribed dose may be too low (although just enough for tremor) or an alternative intervention may be necessary for addressing bradykinesia. These observations highlight key advantages of Kinesia home monitoring to optimize treatment outcome.
In this pilot study, we have demonstrated that the PD patients can correctly perform motor tasks based on the UPDRS unsupervised at home to evaluate tremor and hand movement speed, amplitude, and rhythm. Additional outcome measures to consider for future development may include lower extremity function (i.e. gait and balance) and non-motor cognitive symptoms. Whether a patient is newly diagnosed or in advanced stages, the clinician’s role is to utilize the tools available to maximize therapeutic benefit. Unlike the symptom severity snapshot given by the in-clinic UPDRS, Kinesia automated motor scoring and video-guided software technology provides clinicians with a standardized and quantitative monitoring tool to track symptom fluctuations and medication responses throughout the day and across multiple motor symptoms in a patient’s home.
Research Highlights.
PD patients were instructed to perform automated motor symptom tasks at home
Compliance criteria were developed for rejecting motor tasks performed incorrectly
PD patients correctly completed 97% of all motor tasks at home
Acknowledgements
This work was supported by the NIH NINDS, 5R44NS043816.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord. 2005;20:224–230. doi: 10.1002/mds.20279. [DOI] [PubMed] [Google Scholar]
- Costa J, Gonzalez HA, Valldeoriola F, Gaig C, Tolosa E, Valls-Sole J. Nonlinear dynamic analysis of oscillatory repetitive movements in Parkinson's disease and essential tremor. Mov Disord. 2010;25:2577–2586. doi: 10.1002/mds.23334. [DOI] [PubMed] [Google Scholar]
- Espay A, Giuffrida J, Chen R, Payne M, Mazzella F, Dunn E, Vaughan J, Duker A, Sahay A, Kim S, Revilla F, Heldman D. Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson's disease. Mov Disord. 2011 doi: 10.1002/mds.23893. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. A new look at levodopa based on the ELLDOPA study. J Neural Transm Suppl. 2006:419–426. doi: 10.1007/978-3-211-45295-0_63. [DOI] [PubMed] [Google Scholar]
- Fahn S. Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs later L-DOPA. Arch Neurol. 1999;56:529–535. doi: 10.1001/archneur.56.5.529. [DOI] [PubMed] [Google Scholar]
- Giuffrida JP, Riley DE, Maddux BN, Heldman DA. Clinically deployable Kinesia technology for automated tremor assessment. Mov Disord. 2009;24:723–730. doi: 10.1002/mds.22445. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Heldman DA, Giuffrida JP, Chen R, Payne M, Mazzella F, Duker AP, Sahay A, Kim SJ, Revilla FJ, Espay AJ. The modified bradykinesia rating scale for Parkinson's disease: Reliability and comparison with kinematic measures. Mov Disord. 2011;26:1859–1863. doi: 10.1002/mds.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Vetere-Overfield B, Barter R. Tremors in early Parkinson's disease. Clin Neuropharmacol. 1989;12:293–297. doi: 10.1097/00002826-198908000-00006. [DOI] [PubMed] [Google Scholar]
- Lemstra AW, Verhagen Metman L, Lee JI, Dougherty PM, Lenz FA. Tremor-frequency (3–6 Hz) activity in the sensorimotor arm representation of the internal segment of the globus pallidus in patients with Parkinson's disease. Neurosci Lett. 1999;267:129–132. doi: 10.1016/s0304-3940(99)00343-2. [DOI] [PubMed] [Google Scholar]
- Manson AJ, Brown P, O'Sullivan JD, Asselman P, Buckwell D, Lees AJ. An ambulatory dyskinesia monitor. J Neurol Neurosurg Psychiatry. 2000;68:196–201. doi: 10.1136/jnnp.68.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Lorincz K, Hughes R, Huggins N, Growdon J, Standaert D, Akay M, Dy J, Welsh M, Bonato P. Monitoring motor fluctuations in patients with Parkinson's disease using wearable sensors. IEEE Trans Inf Technol Biomed. 2009;13:864–873. doi: 10.1109/TITB.2009.2033471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raethjen J, Pohle S, Govindan RB, Morsnowski A, Wenzelburger R, Deuschl G. Parkinsonian action tremor: interference with object manipulation and lacking levodopa response. Exp Neurol. 2005;194:151–160. doi: 10.1016/j.expneurol.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Rahimi F, Almeida QJ, Wang D, Janabi-Sharifi F. Tremor suppression orthoses for parkinson's patients: a frequency range perspective; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 1565–1568. [DOI] [PubMed] [Google Scholar]
- Rigas G, Tzallas AT, Tsalikakis DG, Konitsiotis S, Fotiadis DI. Real-time quantification of resting tremor in the Parkinson's disease; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 1306–1309. [DOI] [PubMed] [Google Scholar]
- Schrag A, Schelosky L, Scholz U, Poewe W. Reduction of Parkinsonian signs in patients with Parkinson's disease by dopaminergic versus anticholinergic single-dose challenges. Mov Disord. 1999;14:252–255. doi: 10.1002/1531-8257(199903)14:2<252::aid-mds1009>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Weiss A, Herman T, Plotnik M, Brozgol M, Maidan I, Giladi N, Gurevich T, Hausdorff JM. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson's disease? Med Eng Phys. 2010;32:119–125. doi: 10.1016/j.medengphy.2009.10.015. [DOI] [PubMed] [Google Scholar]