Abstract
BCG, the anti- tuberculosis vaccine localizes within immature phagosomes of macrophages and dendritic cells (APCs) and avoids lysosomal degradation. BCG-derived antigenic peptides are thus inefficiently processed by APCs, and we investigated alternate mechanisms of antigen processing. Proteomics identified that BCG phagosomes are enriched for nicastrin, APH and presenilin components of γ-secretase, a multimeric protease. Using an in vitro antigen presentation assay and BCG infected APCs, γ-secretase components were found to cleave BCG-derived antigen-85B to produce a peptide epitope, which in turn, primedIL-2 release from Ag85B-specific T cell hybridoma. siRNA knockdown or chemical inhibition of γ-secretase components using L685458, decreased the ability of BCG or M. tuberculosis infected APCs to present Ag85B. In addition, L685485 inhibition of γ-secretaseled to a decreased ability of BCG-DCs to immunize mice, and induce Ag85B-specific CD4 T cells in vivo. Since BCG and M. tuberculosis sequester within APCs preventing immune recognition, γ-secretase components appear to fortuitously process the immunodominant Ag85B, facilitating immune recognition.
INTRODUCTION
Tuberculosis is the leading cause of death due to an infectious disease and BCG is the only approved vaccine against human tuberculosis. BCG gives variable protection against tuberculosis depending upon geographic region, and this is likely due to the loss of some immunogenic antigensen coded by the RD1 region, strain variation and population immunity factors. Th1 immunity defends against tuberculosis and is dependent upon the ability of antigen presenting cells (APCs) to process peptides from mycobacteria through lysosome or proteasome mediated degradation, and epitope presentation through MHC-II or MHC-I respectively to CD4 and CD8 T cells. However, BCG vaccine sequesters with in immature phagosomes of APCs, which do not fuse with lysosomes(1). Thus, we proposed earlier that BCG infected APCs would be deficient in presenting MHC-II dependent peptides to T cells. Initially, we found that phagosomes of BCG had near neutral pH due to the exclusion of vATPase enzyme, and as a consequence, intra-phagosomal cathepsin-D protease was inactive(2). Using a T cell hybridoma specific for the major secreted antigen 85B (Ag85B), we then reported that BCG infected APCs showed decreased antigen presentation(2). Interestingly, wild type Mycobacterium tuberculosis (Mtb) H37Rv phagosomes that also have near neutral pH, again showed reduced antigen presentation compared to the attenuated mutant, Mtb H37Ra. The wild type Mycobacterium tuberculosis (Mtb) is the first pathogen reported to evade phagosome-lysosome fusion in macrophages(3). It is now evident that Mtb has multiple immune evasion mechanisms and BCG vaccine seems to have inherited similar properties from wild type M. bovis, the pathogen of bovine tuberculosis (4).
Tuberculosis is an important disease for which efficient vaccination strategies are required. We therefore investigated alternative mechanisms of antigen processing within macrophages that could lead to immune recognition of mycobacteria. BCG and Mtb phagosomes purified from macrophages were subjected to proteomic analysis which identified proteins relatively enriched on their phagosomes(5). We found that nicastrin, a component of the multimeric membrane bound γ-secretase, was enriched on the phagosomes of the wild type Mtb, and to a lesser extent on BCG vaccine phagosomes. Since γ-secretase has been implicated in proteolysis and thought to recycle phagosomal proteins, we hypothesized that it could be cleave mycobacterial phagosome-derived proteins. We demonstrate here that nicastrin, presenilin and APH components of γ-secretase are generate a peptide epitope from BCG phagosome-derived Ag85B, which is subsequently presented to the T cells. Since this occurred in naive macrophages, we propose that the APCs have a novel, innate mechanism to produce antigenic peptides, which may fortuitously facilitate recognition of immune-evasive mycobacteria.
MATERIALS AND METHODS
Antigen presentation
C57Bl/6 mouse bone marrow derived primary macrophages and DCs were cultured and infected with BCG (Pasteur) or wild type M. tuberculosis (both from ATCC, USA)as previously described(6, 7). They were overlaid with BB7 T cells (1 APC: 20 T cells) specific for Ag85B (kind gift of Dr. Harding, CWRU) and IL-2 secreted in response to antigen was quantitated using ELISA. Blockade of γ-secretase was carried out using specific siRNA sets (supplemental figure-S1; Santa Cruz Biotechnology and Invitrogen)against nicastrin, APH and presenilin. siRNA knockdown was carried for 4 hrs followed by resting cells for 18 hrs prior to experimentation. siRNAs were a mix of two or three duplexes, and the controls were scrambled siRNA supplied also by the manufacturer (SCB). Transfection was done using the transfectamine kit supplied by SCB (sc-29528). The effect of siRNA or L685458 on the surface expression of MHC-II, CD80 and CD86 among APCs was tested using flow cytometry as described earlier(8)(supplemental figure-S2). Peptide VANNTRLWVYCGNGTP was used to spike treated or untreated APCs, to measure direct presentation by APCs to BB7 T cells(7).
Immunofluorescence (IF) analysis for γ-secretase
APCs were infected with gfp BCG or gfp Mtb(H37Rv), washed, fixed, counterstained and scored for immunofluorescence colocalization with primary antibodies and isotypes for each of the γ-secretase components as per published procedures (2)(9)(10)(supplemental figure-S2). All fluorescent anti–Ig conjugates were further absorbed with killed BCG suspension to avoid non-specific binding.
Phagosome fractionation and antigen presentation
To assess organelle (phagosome) mediated antigen presentation vs. APC mediated presentation after L685458 treatment BCG phagosomes or inert latex beads (3 μM diameter) phagocytosed into APCs were purified using sucrose gradients as per previously standardized methods, and estimated for protein content(8). Pellets of phagosomes estimated to contain 5 μg/mL of protein were gently sonicated to disperse, checked by microscopy, mixed with BB7 T cells (106T cells per 5 μg pellet of phagosomes in triplicate wells per experiment), and supernatants collected after 4 hr were estimated for IL-2.
Membrane or cytosol localization for γ-secretase
Macrophages were treated or not with L685458 in 24 well plates, infected with BCG and washed. Monolayers were washed with PBS, extracted with 0.05%digitonin, 0.5% sucrose in PBS-EDTA (DSPE) buffer for 15 min for cytosol fraction, which was then ultra-filtered and estimated for protein. The monolayer was washed with cold DSPE three times and the membrane fraction solubilized with 0.1%tritonX-100 in PBS for 15 min, estimated for protein, and stored until western blot analysis. Phagosomes localize into the membrane fractions in primary APCs (9).
Mouse experiments
BCG infected DCs were used as vaccines per protocols described before(10). Bone marrow derived cells were cultured in DMEM with GM-CSF (10 ng/mL, Invitrogen) for 7 days and column purified DCs (CD11c beads from Miltenyi Inc. USA) were infected before or after treatment with L685458 (15and 100 μM/106 DCs; 4hr) with BCG. The DCs were washed and 2×106DCs per mouse were injected i.p. into C57Bl/6 mice (4 mice per group; 2 experiments). Untreated DCs injected into mice were negative controls. Viability of DCs before and after treatment was confirmed to be >90% using LDH release assay. After 2 weeks, spleens were removed and IFN γ+ CD4 T cells were detected using flow cytometry for intracellular cytokines, and by ELISPOT analysis with Ag85B re-stimulation(10). All animal procedures were conducted under approved institutional protocols.
RESULTS
γ-secretase components are enriched on mycobacterial phagosomes
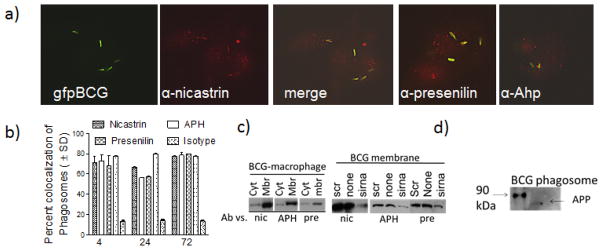
Our proteomic study showed enrichment of nicastrin on wild type Mtb phagosomes while, others showed similar enrichment on BCG phagosomes(5)(11). Using IF analysis, the three γ-secretase components were identified, relative to BCG phagosomes within APCs. Using in situ colocalization with deconvolution microscopy, antibodies to nicastrin, APH and presenilin were found to stain the phagosomes, with minimal speckled stain around the perinuclear zone (Fig. 1a–b). The positive control antibody LAMP1 stained most BCG phagosomes, whereas, the isotype control did not stain a significant number of phagosomes (Fig. 1b). This observation was consistent with the western blot profiles of BCG infected primary macrophages, where, the three components of γ-secretase were enriched on membrane fractions compared to cytosol (Fig. 1c, left). Membrane fractions of siRNA(SCB) treated BCG infected macrophages were similarly tested using western blot. Specific siRNA knock-down reduced the levels of γ-secretase components compared to scrambled control sequence (Fig. 1c, right). In addition, BCG phagosomes purified from macrophages using sucrose gradients, were found to have enzymatically active γ-secretase. They cleaved the amyloid precursor protein into an 87 kDa product (Fig. 1d). Similar studies were performed using DCs with identical results (not shown).
Figure 1. Macrophages infected with mycobacteria colocalize with γ-secretase components.
Antibodies to γ-secretase stain phagosomes with less cytoplasmic localization.(a)C57Bl/6 mouse bone marrow derived macrophages were infected with Mycobacterium bovis BCG vaccine strain tagged with gfp (gfp BCG) and stained with an antibody to nicastrin, APH or presenilin. Panel shows the three color panels for nicastrin and merged images for APH and presenilin, followed by Texas-red conjugates. b) Phagosomes colocalizing with γ-secretase components were scored using antibody stains and deconvolution microscopy (1 of 3 similar experiments, ± SD). Isotype antibody did not stain phagosomes and gfp BCG in dendritic cells showed a similar pattern (not shown). Membrane vs. cytosolic distribution of γ-secretase. (c) Treated or untreated BCG infected macrophages were fractionated into membrane and cytosol fractions and tested using antibodies and western blots. (Left) Untreated fractions:γ-secretase components are enriched in membranes. (Right) Treated fractions: siRNA knock-down reduces the levels of γ-secretase components in the membrane fractions, compared to knock down with scrambled sequence. (d) γ-secretase is active on the phagosomes. BCG phagosomes purified from treated or untreated macrophages were incubated in buffer to determine the cleavage of amyloid precursor protein into an 87 kDa APP product (arrow).
γ-secretase cleaves phagosome derived Ag85Band generates peptide epitopes for antigen presentation
The proteolytic activity of γ-secretase is known, and was found enriched on inert latex bead phagosomes of macrophages. It was proposed that γ-secretase could be involved in recycling and remodeling of phagosome proteins(12). Interestingly, both BCG vaccine and wild type Mtb secrete antigen-85B (Ag85B) and other immunogenic proteins that accumulate within the phagosomes(13, 14)(15). Furthermore, Ag85B has a major T cell epitope and is a powerful immunogen for mice and humans, either vaccinated with BCG or infected with Mtb(14). MHC-II dependent presentation of a peptide epitope of Ag85B from mycobacteria infected APCs has been shown to induce IL-2 secretion from Ag85B specific T cell hybridoma in several studies(6)(2)(7). We therefore hypothesized that, mycobacterial phagosome derived Ag85B could be accessed, and cleaved by γ-secretase components, and this event was measurable in vitro.
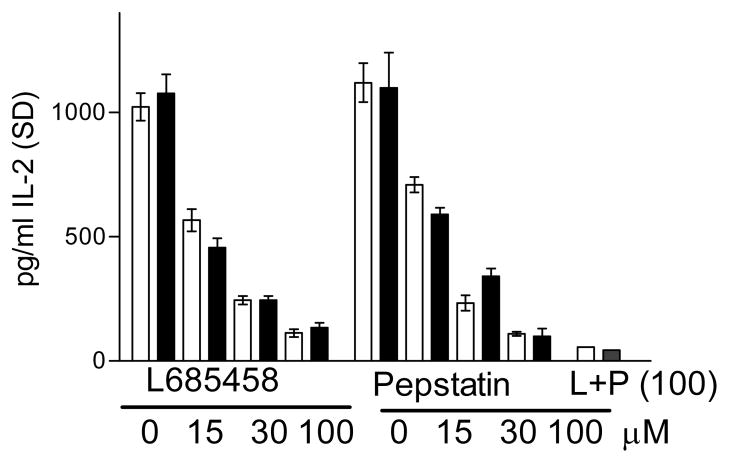
Since a specific chemical inhibitor for γ-secretase(L685458) has been used to block nicastrin activity, we initially tested the effect of L685458 on antigen presentation by APCs infected with the BCG vaccine. Fig. 2 shows that L685458 had a significant, dose-dependent inhibitory effect on antigen presentation by both macrophages and DCs. Flow cytometry showed that L685458 did not affect the surface expression of MHC-II, CD80 or CD86 (supplemental figure-S2). Furthermore, when the Ag985B peptide was added to such cells, direct peptide presentation was comparable.
Figure 2. BCG infected antigen presenting cells (APCs) present mycobacterial Antigen-85B to T cells and the process is inhibited by protease inhibitors.
Macrophages (□)and DCs (■) from C57Bl/6 mouse bone marrow were treated or not with γ-secretase inhibitor L-685485, or Cathepsin-D inhibitor pepstatin, followed by infection with BCG and antigen presentation to BB7 T cell hybridoma, specific for mycobacterial Antigen 85B. T cells were overlaid on macrophages (1:20) for 4 hr and supernatants measured for IL-2 using ELISA. L685485 and pepstatin both show a dose-dependent inhibition of antigen presentation and their combination (L+P) is additive at 100 μM dose (one of 3 similar experiments, ± SD; IL-2 values of all treated groups vs. untreated, p< 0.009, t test).
We previously reported that phagosomal Ag85B production could occur due to the action of Cathepsin-D and pepstatin, an inhibitor of Cathepsin proteases inhibited antigen presentation. To further determine whether both Cat-D and γ-secretase contributed to Ag85B epitope production, APCs were blocked with pepstatin to inhibit Cat-D and L685458to inhibit γ-secretase. Fig. 2 indicates that each agent inhibited peptide presentation and their combination had an additive effect. Pepstatin either alone or in combination did not affect the surface expression of MHC-II, CD80 or CD86 (data not shown). We therefore propose that Cat-D, an intraphagosomal, acid pH-dependent protease and membrane bound, γ-secretase, which works best at neutral pH, both produce Ag85 derived peptide in and around the phagosomes.
siRNA blockade of γ-secretase affects antigen production in macrophages and DCs
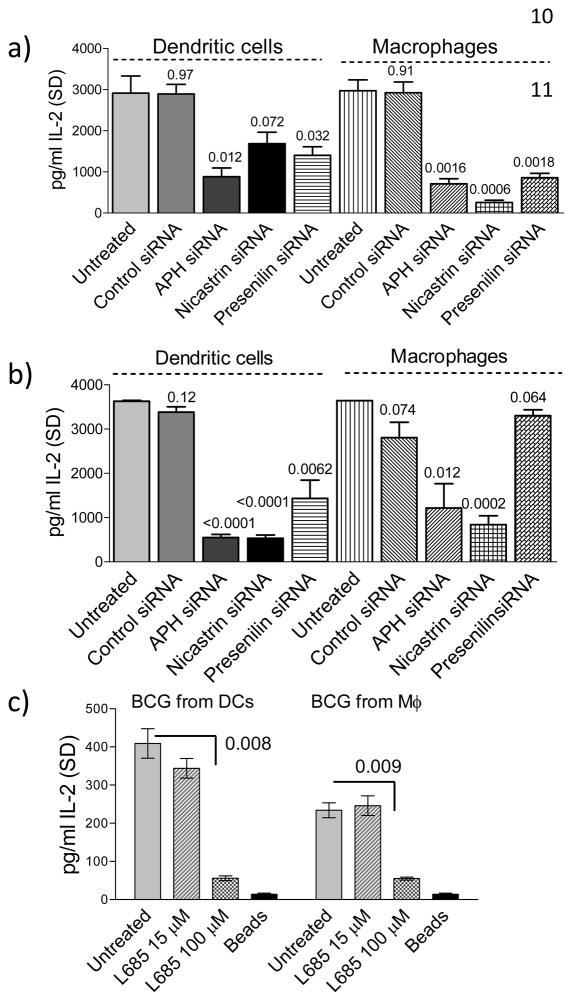
Nicastrin and presenilin have been reported to possess protease activity on their own(16). To further evaluate whether γ-secretase components vary in their protease activity, siRNA knock-down experiments were carried out. APCs were treated or not with siRNA against nicastrin, APH and presenilin followed by BCG or Mtb infection, and antigen presentation. Fig. 3a (BCG) and Fig. 3b (wild type Mtb) show that siRNA knock-down of γ-secretase components affected antigen presentation by APCs infected with BCG and Mtb. Control scrambled siRNA had no significant effect on antigen presentation. siRNA probes from two different sources had comparable effects on antigen presentation (Fig. 3 and supplemental Figure S1). Furthermore, BCG phagosomes but not inert latex beads purified from L685458 treated or untreated macrophages were able to present Ag85B to BB7 T cells. L685458 significantly suppressed antigen presentation (Fig. 3c).
Figure 3. Functional blockade of γ-secretase inhibits antigen presentation in APCs.
APCs were treated or not with siRNA against γ-secretase components, followed by infection with either BCG (a) or Mycobacterium tuberculosis (H37Rv strain) (b), and antigen presentation and IL-2 assay using ELISA. The siRNA knock-down of nicastrin, presenilin and APH affects antigen presentation (one of 3 similar experiments, ± SD, p values shown above bars for comparison with the untreated group; t test).(c) Phagosomes directly present Ag85B, which is inhibited by L685458. BCG phagosomes from L685458 treated or untreated macrophages were purified on sucrose gradients and incubated with BB7 T cells (106 T cells per 5 μg of phagosome protein) followed by ELISA for IL-2 (one of 2 similar experiments, ± SD, p values shown above bars for comparison with the untreated group; t test).
L685458 treatment of DCs affects subsequent in vivo immunogenicity
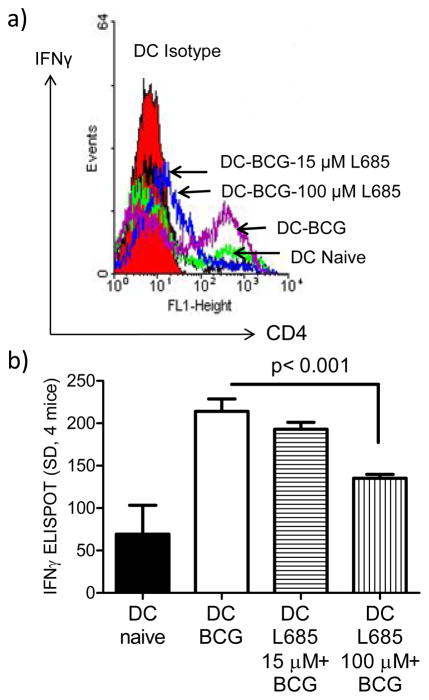
We previously demonstrated that DCs infected with BCG vaccine elicit immune responses in mice following vaccination, which are protective against subsequent challenge with virulent tuberculosis(10). Furthermore, Ag85B protein or its DNA vaccine offers significant protection against tuberculosis in mice. These two concepts provided a model to examine the effect of γ-secretase mediated Ag85B-epitope production on the immunogenicity of BCG infected DCs. DCs were treated or not with L685458, infected with BCG and injected to mice. Two weeks later, splenic T cells were assayed for Ag85B specific responses of T cells secreting IFN γ, using flow cytometric analysis and ELISPOT analysis of spleens. Fig. 4a illustrates that DCs infected with BCG elicited markedly better IFN γ+ CD4T cell response in mouse spleens compared to L685458 treated DCs with BCG. Similarly, ELISPOT analysis of corresponding spleens showed that, L685458 treated DCs induced lesser numbers of Ag85B specific CD4 T cells (Fig. 4b). These data strongly suggest that γ-secretase plays a significant role in generating a peptide epitope from phagosome-associated Ag85B, and this event helps immune recognition of mycobacteria, which are otherwise sequestered within macrophages and DCs.
Figure 4. γ-secretase inhibitor L-685458 decreases the ability of DCs to prime T cells in vivo.
CD11c bead purified DCs from C57Bl/6 mouse bone marrow were left untreated, treated with a low (15 μM per 106 DCs) or high dose (100 μM per 106 DCs) of γ-secretase inhibitor L-685458, followed by infection with BCG. Washed and viable DCs were injected i.p. to naive mice. 2 weeks later, spleen T cells were analyzed for IFN-γ producing CD4 T cells using flow cytometry(a), and Ag85B specific T cells using ELISPOT (b) (p values, t test; 2 experiments).
DISCUSSION
Since wild type Mtb and BCG vaccine sequester within immature phagosomes of APCs avoiding lysosomal degradation, mechanisms of peptide mediated priming of T cells have been thought to be suboptimal during tuberculosis or vaccination. Sequestration enables Mtb to be immune-evasive while, BCG would become less immunogenic (17). Until recently however, the mechanistic basis of immune sequestration remained unclear. Significantly, two studies proposed a new concept that phagosomes themselves are capable of assembling peptide epitopes into MHC-II and MHC-I for eventual presentation to T cells. Harding’s group demonstrated that the immunodominant, secreted, Ag85B is cleaved and presented by mycobacterial phagosomes(6). On the other hand, mycobacterial phagosomes from human macrophages were also found capable of presenting peptides through MHC-I(18, 19).
Since Ag85B is by itself highly immunogenic and used as a vaccine, we addressed the mechanisms of in situ epitope generation using BCG infected macrophages and Ag85B specific T cell hybridoma(20)(21). Although several proteases were found at trace levels, Cat-D was found markedly enriched in BCG phagosomes. Using siRNA knock-down studies, we then demonstrated that Cat-D mediated proteolysis leads to Ag85B-derived peptide epitope, which in turn primes BB7 T cells. However, the efficacy of Cat-D, an acid pH dependent aspartic acid protease was reduced due to the near neutral pH of the BCG phagosome lumen.
We also reported that BCG excluded the acidifying enzyme, vacuolar proton ATPase relative to Mtb H37Ra and the phagosomes of the latter had more active Cat-D. As a consequence, macrophages infected with Mtb H37Ra were better able to activate BB7 T cells to secrete IL-2. We therefore concluded that, intra-phagosome generation of Ag85B epitope is regulated by the pH. This is consistent with the known mechanism of peptide production in lysosomes where, the acidifying enzyme vATPase lowers the pH to activate several proteases. Unexpectedly, the proteomics of BCG phagosomes revealed the presence of γ-secretase, and in this study, we demonstrated that phagosome associated Ag85B is also processed by γ-secretase. Our studies therefore propose a novel observation that mycobacterial phagosomes have at least two mechanisms of peptide generation, one of which is acid pH dependent, and the other is a membrane associated, acid pH independent process.
This model appears consistent with the known cell biology of BCG vaccine and Mtb phagosomes. While BCG and Mtb pathogen have adapted to survive in an intracellular niche avoiding the lysosome, the host seems to have adapted to deliver proteases into phagosomes to facilitate immune recognition. While the characterization of membrane bound γ-secretase has been found difficult, Jutras et al proposed that it may perform a physiological function of recycling phagosome proteins(12)(16). We propose that during this natural recycling process, γ-secretase fortuitously produces peptide epitopes from bacteria enclosed within phagosomes. There is an intriguing possibility that γ-secretase may also produce shorter peptide fragments, and downstream events may channel them towards the CD8 pathway. It is conceivable that it may also cleave proteins from other intra-phagosomal pathogens.
We therefore propose that, APCs have an innate mechanism to generate immune signals from mycobacterial phagosomes that otherwise remain evasive. It is pertinent to note here that, strategies to bypass immune sequestration have generally enhanced the immunogenicity of BCG vaccine. Thus, expression of a pH independent, active Cat-S in BCG led to increased Ag85B presentation by macrophages infected with recombinant BCG-Cat-S (22). Expression of the recombinant pore-forming toxin listeriolysin in BCG enabled antigens to leak into cytosol across phagosome membrane, and enhanced the ability of recombinant BCG-LLO to induce CD8 T cells in mice(23). Finally, enhanced localization of BCG into autophago-lysosomes through rapamycin treatment of APCs, led to increased Ag85B presentation as well as enhanced immunogenicity in mice (23)(24)(10).
The enrichment of nicastrin on mycobacterial phagosomes was initially identified using proteomics of phagosomes(5)(11). These studies highlighted that the mycobacterial phagosomes are complex structures containing several hundred proteins and the daunting task of functional characterization. Current study illustrates the use of a system biology approach to dissect the function of a phagosome-associated protein. We anticipate that a similar dissection of other novel phagosome proteins will lead to a better understanding of mycobacterial immunogenicity.
Supplementary Material
Acknowledgments
This study was supported by NIH AI78420 (CJ). Authors are thankful for Dr. C. Harding (CWRU) for BB7 T cells and Dr. Dhandayuthapani, UTHSC- San Antonio for gfp BCG strain. CRS was supported from CRS from T32-AI055449 (S.J.Norris).
References
- 1.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 2.Singh CR, Moulton RA, Armitige LY, Bidani A, Snuggs M, Dhandayuthapani S, Hunter RL, Jagannath C. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol. 2006;177:3250–3259. doi: 10.4049/jimmunol.177.5.3250. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 5.Rao PK, Singh CR, Jagannath C, Li Q. A systems biology approach to study the phagosomal proteome modulated by mycobacterial infections. Int J Clin Exp Med. 2009;2:233–247. [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandra L, Noss E, Boom WH, Harding CV. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J Exp Med. 2001;194:1421–1432. doi: 10.1084/jem.194.10.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramachandra L, Smialek JL, Shank SS, Convery M, Boom WH, Harding CV. Phagosomal processing of Mycobacterium tuberculosis antigen 85B is modulated independently of mycobacterial viability and phagosome maturation. Infect Immun. 2005;73:1097–1105. doi: 10.1128/IAI.73.2.1097-1105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katti MK, Dai G, Armitige LY, Rivera Marrero C, Daniel S, Singh CR, Lindsey DR, Dhandayuthapani S, Hunter RL, Jagannath C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel DS, Dai G, Singh CR, Lindsey DR, Smith AK, Dhandayuthapani S, Hunter RL, Jr, Jagannath C. The reduced bactericidal function of complement C5-deficient murine macrophages is associated with defects in the synthesis and delivery of reactive oxygen radicals to mycobacterial phagosomes. J Immunol. 2006;177:4688–4698. doi: 10.4049/jimmunol.177.7.4688. [DOI] [PubMed] [Google Scholar]
- 10.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 11.Lee BY, Jethwaney D, Schilling B, Clemens DL, Gibson BW, Horwitz MA. The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome. Mol Cell Proteomics. 9:32–53. doi: 10.1074/mcp.M900396-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutras I, Laplante A, Boulais J, Brunet S, Thinakaran G, Desjardins M. Gamma-secretase is a functional component of phagosomes. J Biol Chem. 2005;280:36310–36317. doi: 10.1074/jbc.M504069200. [DOI] [PubMed] [Google Scholar]
- 13.Mustafa Abu S, Al-Attiyah R. Tuberculosis: looking beyond BCG vaccines. J Postgrad Med. 2003;49:134–140. [PubMed] [Google Scholar]
- 14.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harth G, Lee BY, Wang J, Clemens DL, Horwitz MA. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin-gamma -secretase complex reveals nicastrin and a gamma substrate. Proc Natl Acad Sci U S A. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pancholi P, Mirza A, Bhardwaj N, Steinman RM. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 18.Grotzke JE, Harriff MJ, Siler AC, Nolt D, Delepine J, Lewinsohn DA, Lewinsohn DM. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewinsohn DM, Grotzke JE, Heinzel AS, Zhu L, Ovendale PJ, Johnson M, Alderson MR. Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J Immunol. 2006;177:437–442. doi: 10.4049/jimmunol.177.1.437. [DOI] [PubMed] [Google Scholar]
- 20.Vordermeier HM. T-cell recognition of mycobacterial antigens. Eur Respir J Suppl. 1995;20:657s–667s. [PubMed] [Google Scholar]
- 21.Ganguly N, Siddiqui I, Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis (Edinb) 2008;88:510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Soualhine H, Deghmane AE, Sun J, Mak K, Talal A, Av-Gay Y, Hmama Z. Mycobacterium bovis bacillus Calmette-Guerin secreting active cathepsin S stimulates expression of mature MHC class II molecules and antigen presentation in human macrophages. J Immunol. 2007;179:5137–5145. doi: 10.4049/jimmunol.179.8.5137. [DOI] [PubMed] [Google Scholar]
- 23.Hess J, Miko D, Catic A, Lehmensiek V, Russell DG, Kaufmann SH. Mycobacterium bovis Bacille Calmette-Guerin strains secreting listeriolysin of Listeria monocytogenes. Proc Natl Acad Sci U S A. 1998;95:5299–5304. doi: 10.1073/pnas.95.9.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008;198:1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.