Abstract
Introduction
Pulmonary endarterectomy (PEA) is the most effective treatment for chronic thromboembolic pulmonary hypertension (CTEPH). The aim of this study is to evaluate long-term survival and freedom from clinical worsening after PEA.
Methods
All patients who underwent PEA in our hospital between May 2000 and August 2009 were included. Follow-up parameters were all-cause mortality and time to clinical worsening, defined as a combination of death, need for pulmonary hypertension-specific medication or 15% decrease in six-minute walk distance without improvement in functional class. The Cox proportional hazard regression was used to identify predictors.
Results
Seventy-four consecutive patients (mean age 55.9 ± 13.8 years, 51% female) underwent PEA. Prior to surgery, 55 patients were in NYHA functional class III or higher. The mean pulmonary artery pressure was 41.3 ± 11.9 mmHg with a mean pulmonary vascular resistance of 521 ± 264 dyn·s·cm−5 (range 279–1331 dyn·s·cm−5). Five patients (6.8%) died in-hospital. Out of hospital, 5 out of 69 patients (7.2%) died during a median follow-up of 3.7 ± 2.2 years [range 0.1–8.5 years]). The one- and five-year survival rates were 93% and 89%, respectively. During follow-up, clinical worsening occurred in 13 out of 69 patients (18.8%). The one- and five-year rates of freedom from clinical worsening were 94% and 72%, respectively. The baseline NT-pro BNP level tended to be a predictor for occurrence of clinical worsening.
Conclusion
Pulmonary endarterectomy is associated with good long-term survival in patients with CTEPH. However, clinical worsening occurred in a substantial number of patients at long-term follow-up.
Keywords: Pulmonary embolism, Pulmonary hypertension, Endarterectomy, Clinical worsening
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) has been estimated to occur in up to 3.8% of patients 2 years following an episode of confirmed pulmonary embolism [1, 2]. It is characterised by intraluminal thrombus organisation which causes stenosis or complete obliteration of the pulmonary arteries [3], resulting in an increased pulmonary vascular resistance (PVR), pulmonary hypertension and progressive right heart failure. Although obstruction of pulmonary arteries is recognised as the inciting event, small-vessel arteriopathy is believed to appear in the course of the disease and to contribute to its progression [4].
Pulmonary endarterectomy (PEA) is the treatment of choice, offering immediate haemodynamic benefits and providing a potential cure for many patients [5–7]. However, 10–50% of CTEPH patients are inoperable, due to either distal pulmonary vascular obstruction that is surgically inaccessible or significant comorbidities thought to be associated with unacceptable high risk [8]. The direct postoperative mortality rates of PEA range from 5% in most experienced centres to 10% [5, 6, 9].
However, limited data are available concerning clinical worsening after PEA. The aim of this study is to describe clinical worsening during long-term follow-up in CTEPH patients who underwent PEA.
Methods
Patient selection
The present study was conducted at the Pulmonary Hypertension Unit of the St Antonius Hospital in Nieuwegein. This centre is one of the two centres for surgical treatment of CTEPH in the Netherlands. The diagnosis of CTEPH was based on a standardised assessment of all patients including ventilation-perfusion scanning, CT angio of the chest, chest radiography, transthoracic echocardiography, pulmonary angiography and right-sided heart catheterisation, and arterial blood gas analysis at rest and exercise. Other aetiologies for pulmonary hypertension were excluded. A multidisciplinary panel including pulmonologists, radiologists, cardiologists, and cardiothoracic surgeons reviewed each case.
Patients were considered suitable for surgery when they were symptomatic, had an elevated pulmonary vascular resistance (PVR) (>250 dyn·s·cm−5), segmental or more proximal lesions and no severe comorbidity. Severity of haemodynamic disease was not a reason to decline a patient for surgery. At the beginning of our PEA program severely compromised haemodynamic patients were preoperatively treated with prostacyclin. After 2003, high-risk patients with PVR >1000 dyn·s·cm−5 were treated with oral disease-modifying therapy consisting of phosphodieesterase-5 inhibitors (PDE-5 inhibitors) and/or endothelin receptor antagonist (ERA).
Study design
This is a retrospective observational cohort study of patients with operable CTEPH. All consecutive patients were enrolled between May 2000 and August 2009. Cohort entry was defined as the date of the PEA. All patients were consistently operated by two surgeons using a standardised technique discussed elsewhere [10, 11]. The study design was approved by the local ethics committee.
Follow-up
Follow-up of the patients was performed 8 weeks after discharge from the hospital followed by visits to the outpatient clinic after 3 months and every 6 months thereafter. Investigations consisted of clinical examination by means of assessing the New York Heart Association (NYHA) functional class, transthoracic echocardiography if appropriate, six-minute walk distance (6-MWD), assessing the NT-pro BNP value, and right-sided heart catheterisation was performed when pulmonary hypertension was suspected on echocardiography. Residual or recurrent CTEPH was defined as a mean pulmonary artery pressure (mPAP) > 25 mmHg with a pulmonary wedge pressure < 15 mmHg and a PVR > 250 dyn·s·cm−5
Endpoints
The primary endpoint was time to clinical worsening, defined as the combination of death, need for pulmonary hypertension medication initiated after PEA or 15% decrease in 6-MWD without improvement of functional class during follow-up.
Statistical analysis
The data are expressed as mean and standard deviation if normal distribution was present and as median with range in the absence of normal distribution. Changes from baseline were evaluated with a paired t test for continuous variables and with a Wilcoxon rank-sum test or χ2 test for ordinal variables if appropriate. Time to event data are presented as Kaplan–Meier curves and Cox proportional hazard regression. Significance was determined at p < 0.01; all reported p values were two tailed. All statistical analyses were performed by using SPSS software (SPSS Inc., version 17.0 for Windows; Chicago, IL).
Results
Patient characteristics
Seventy-four consecutive patients (mean age 55.9 ± 13.8 years, 51% female) were enrolled in this study. The patient baseline characteristics are summarised in Table 1. At baseline, 55 patients (74%) were in NYHA functional class III or higher. The mPAP was 41.3 ± 11.9 mmHg with a mean PVR of 521 ± 264 dyn·s·cm−5 (range 279–1331 dyn·s·cm−5). The mean 6-MWD at baseline was 389 ± 130 m.
Table 1.
Baseline characteristics
| Number of patients | 74 |
| Age (years) | 55.9 ± 13.8 |
| Female (n (%)) | 35 (50.7) |
| BMI (kg/m2) | 27.8 ± 4.7 |
| Systolic blood pressure (mmHg) | 129.9 ± 21.2 |
| Diastolic blood pressure (mmHg) | 80.1 ± 13.7 |
| NYHA functional class(I/II/III/IV) | 2/12/48/7 |
| 6-MWD (m) | 389 ± 130 |
| NT-pro BNP (pg/mL) | 1265 ± 1346 |
| Creatinine (μmol/L) | 96.7 ± 63.8 |
| Right-sided heart catheterisation | |
| PAP mean (mmHg) | 41.3 ± 11.9 |
| PVR (dyn·s·cm−5) | 521 ± 264 |
| CO (L/min) | 4.7 ± 1.2 |
| VC (l) | 3.7 ± 1.1 |
| FEV1 (%) | 88.4 ± 18.1 |
| pO2 (kPa) | 9.1 ± 2.1 |
| Saturation (%) | 93.6 ± 4.5 |
| Follow-up (years) | 3.7 ± 2.2 (range 0.1–8.5) |
BMI = body mass index; NYHA FC = New York Heart Association Functional Class; 6-MWD = six minute walk distance; NT-pro BNP = N-terminal pro brain natriuretic peptide; PAP mean = mean pulmonary artery pressure; PVR = pulmonary vascular resistance; CO = cardiac output; VC = vital capacity; FEV1 = forced expiratory volume in 1 s; pO2 = O2 pressure
In hospital
Within 3 days after the PEA, the mPAP decreased to 25.2 ± 11.2 mmHg (p = 0.001). During the postoperative period, five patients out of 74 (6.8%) died in the hospital. Three patients died as a consequence of reperfusion pulmonary oedema, one of whom was treated with extracorporeal membrane oxygenation. The fourth patient died as a consequence of respiratory failure after septic pneumonia. The fifth patient died after multi-organ failure.
Follow-up
The mean follow-up was 3.7 ± 2.2 (range 0.1–8.5). One year after the PEA, the mean 6-MWD increased to 480 ± 141 m (p < 0.001). There was also an improvement in NYHA functional class, 91.4% of the patients were in NYHA class I or II (p < 0.001). During long-term follow-up, another five patients (out of 69) died (7.2%). Of these patients, only one patient died as the consequence of progressive residual pulmonary hypertension. The overall one-, three- and five-year survival rates, with exclusion of the patients who died in-hospital, were 93%, 91% and 89%, respectively.
Clinical worsening
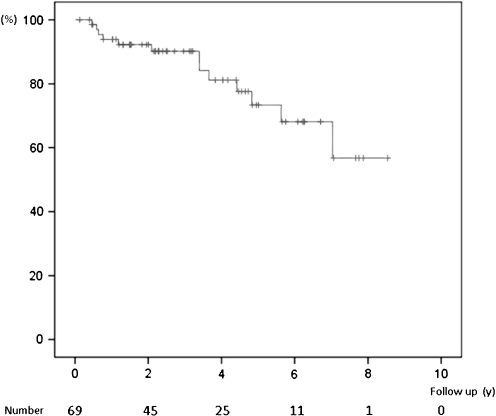
After the initial hospitalisation, clinical worsening occurred in 13 out of 69 patients (18.8%) during follow-up. In 8 patients initiation of pulmonary hypertension-specific medication was needed during follow-up (Table 2). The one-, two-, four-, six- and eight-year rates of freedom from clinical worsening were 94%, 90%, 81%, 68% and 57%, respectively. Figure 1 shows the Kaplan-Meier curve for freedom of clinical worsening. A comparison of the baseline characteristics of patients with and without clinical worsening is outlined in Table 3. There were no significant differences between the two groups. The baseline NT-pro BNP level tended to be the only predictor for the occurrence of clinical worsening (Table 4).
Table 2.
Follow-up
| Treatment during FU | |
| -ERA only | 3 (4.3) |
| -PDE-5 inhibitor only | 3 (4.3) |
| -Combination of ERA and PDE-5 inhibitor | 2 (2.9) |
| -None | 61 (88.4) |
| Endpoints | |
| -Death | 5 (7.2) |
| -PH medication | 8 (11.6)a |
| -6-MWD worsening with no improvement in NYHA FC | 3 (4.3)b |
| -Combination | 13 (18.8) |
FU = follow-up; ERA = endothelin receptor antagonist; PDE-5 inhibitor = phosphodiesterase type-5 inhibitor; PH medication = pulmonary hypertension medication; 6-MWD = six minute walk distance; NYHA FC = New York Heart Association Functional Class
a2 of these patients died during FU
bPH medication was initiated in 1 patient
Fig. 1.
Free from clinical worsening
Table 3.
Baseline characteristics of patients with or without clinical worsening
| No CW | CW | p | |
|---|---|---|---|
| Number in follow-up | 56 | 13 | |
| Age (years) | 55.1 ± 14.3 | 59.6 ± 11.3 | 0.29 |
| Female (n (%)) | 27 (51.8) | 8 (61.5) | 0.39 |
| BMI (kg/m2) | 27.9 ± 4.9 | 27.4 ± 3.7 | 0.78 |
| Systolic blood pressure (mmHg) | 130.4 ± 21.5 | 127.7 ± 20.7 | 0.69 |
| Diastolic blood pressure (mmHg) | 80.3 ± 14.3 | 79.6 ± 11.2 | 0.88 |
| NYHA functional class (I/II/III/IV) | 2/9/39/6 | 0/3/9/1 | 0.88 |
| 6-MWD (m) | 393 ± 138 | 374 ± 89 | 0.68 |
| NT-pro BNP (pg/mL) | 1092 ± 1132 | 2648 ± 2177 | 0.19 |
| Right-sided heart catheterisation | |||
| PAP mean (mmHg) | 41.9 ± 12.1 | 38.5 ± 11.0 | 0.36 |
| PVR (dyn·s·cm−5) | 534 ± 264 | 467 ± 269 | 0.48 |
| CO (L/min) | 4.7 ± 1.2 | 5.0 ± 1.2 | 0.51 |
| VC (l) | 3.7 ± 1.0 | 3.7 ± 1.4 | 0.83 |
| FEV1 (%) | 87.8 ± 17.0 | 90.4 ± 22.3 | 0.66 |
| pO2 (kPa) | 9.2 ± 2.3 | 9.0 ± 1.2 | 0.77 |
| Saturation (%) | 93.4 ± 4.9 | 94.1 ± 1.6 | 0.70 |
| Follow up (years) | 3.4 ± 2.2 | 5.1 ± 1.8 | 0.02 |
CW = clinical worsening; BMI = body mass index; NYHA FC = New York Heart Association Functional Class; 6-MWD = six minute walk distance; NT-pro BNP = N-terminal pro brain natriuretic peptide; Creat = creatinine; PAP mean = mean pulmonary artery pressure; PVR = pulmonary vascular resistance; CO = cardiac output; VC = vital capacity; FEV1 = forced expiratory volume in 1 s; pO2 = O2-pressure
Table 4.
Predictors for clinical worsening during follow-up using Cox proportional hazards
| HR (univariate) | P | |
|---|---|---|
| Male | 0.54 (0.18–1.68) | 0.29 |
| BMI | 1.02 (0.89–1.16) | 0.80 |
| Age | 1.03 (0.98–1.08) | 0.23 |
| NYHA FC | 0.73 (0.32–1.65) | 0.45 |
| 6-MWD | 1.00 (1.00–1.00) | 0.79 |
| PAP mean | 0.98 (0.94–1.03) | 0.47 |
| PVR | 1.00 (1.00–1.00) | 0.36 |
| CO | 1.69 (0.89–3.32) | 0.13 |
| NT-pro BNP | 1.00 (1.00–1.00) | 0.04 |
| VC | 0.92 (0.58–1.47) | 0.73 |
| FEV1 | 1.01 (0.98–1.05) | 0.53 |
| pO2 | 0.95 (0.72–1.26) | 0.73 |
| Saturation | 1.04 (0.88–1.23) | 0.64 |
CW = clinical worsening; HR = hazard ratio; BMI = body mass index; NYHA FC = New York Heart Association Functional Class; 6-MWD = six minute walk distance; PAP mean = mean pulmonary artery pressure; PVR = pulmonary vascular resistance; CO = cardiac output; NT-pro BNP = N-terminal pro brain natriuretic peptide; Creat = creatinine; VC = vital capacity; FEV1 = forced expiratory volume in 1 s; pO2 = O2 pressure
Discussion
A PEA is thought to provide a potential cure for patients with CTEPH. However, the development of clinical worsening had never been described in patients who underwent a PEA. We found that clinical worsening occurred in almost 19% of the patients within four years after the surgical treatment. With increasing institutional and surgical experience, most centres report improved in-hospital survival as case volume increases. Jamieson et al. described the decline in perioperative mortality over the years from 17 to 4.4% after changes in the operative technique [5]. In 2006, Matsuda and colleagues reported an in-hospital mortality rate of 7.8% after PEA (n = 102) and a three- and five-year survival rate of 91% and 84%, respectively [12].
We found an in-hospital mortality rate after PEA of 6.8%. The overall one-, three- and five-year survival rates were 93%, 91% and 89%, respectively. These results are comparable with the results mentioned in the studies above.
Residual pulmonary hypertension after PEA has been described in several reports. The reported rates vary from 5 to 35%, depending on the definition [13–16]. It is believed that a substantial component of persistent postoperative pulmonary hypertension is related to distal pulmonary vasculopathy in small precapillary vessels both in the occluded and non-occluded pulmonary vascular bed [17, 18]. The extent and type of microvascular disease in CTEPH have a strong influence on the likelihood of a successful outcome in PEA [19]. Freed et al. described the survival of a study cohort of 314 CTEPH patients who underwent PEA and suffered from residual pulmonary hypertension. Surprisingly, in this study residual pulmonary hypertension after PEA did not have a significant effect on survival. In 8% of the patients, pulmonary hypertension-specific medication was started after PEA [13]. A report by Reesink et al. described initiation of pulmonary hypertension medication in 3 of 38 patients (8%) who underwent PEA and of whom follow-up data were available [21]. In our study, initiation of pulmonary hypertension medication after PEA was needed in 8 (in two patients it was started immediately after surgery) of 69 patients (11.5%) within 1.8 years (range 0–5.6 years) after PEA, which is in agreement with the rates mentioned above.
Another frequently used endpoint in clinical trials is NYHA functional class. Freed et al. reported an improvement in NYHA functional class among 229 patients with CTEPH who underwent PEA. At baseline 88% of the patients were in NYHA class III or IV. Three months after PEA, 87% of the patients were in NYHA class I or II. One year after PEA 91% of the patients were in NYHA functional class I or II [20]. Corsico and associates described the long-term outcome after PEA in 157 patients [22]. At baseline 97% of the patients were in NYHA functional class III or IV. One year after PEA only 12% of the patients were in NYHA class III or IV. Postoperative NYHA class III or IV appeared to be associated with the highest risk of late events (i.e. death related to CTEPH or PEA, lung transplant or PEA redo between 3 months and 5 years after PEA). In our present study 55 patients (74%) were in NYHA functional class III or IV at baseline. One year after PEA, 91% of the patients were in NYHA class I or II, conform to the results of other reports.
The six-minute walk distance is another frequently used parameter to evaluate the effect of PEA. Reesink et al. demonstrated that the 6-MWD correlated with parameters reflecting clinical and haemodynamic severity of disease in CTEPH. A PEA performed in 42 patients resulted in a significant increase in the 6-MWD, one year after surgery [21]. In our study, at baseline the mean 6-MWD was 389 m and increased to 480 m at one-year follow-up. This is comparable with the results from other reports [20, 21].
Recent pivotal trials in pulmonary arterial hypertension have used time to clinical worsening as a composite endpoint as described by McLaughlin and associates [23]. It is a combination of mortality and different parameters which described morbidity after the initiation of specific pulmonary hypertension therapy. The definition most frequently used is a combination of all-cause mortality, non-elective hospital stay for pulmonary hypertension to initiate intravenous prostanoid or lung transplantation, and disease progression defined as a reduction from baseline in 6-MWD distance by 15% [23]. In our CTEPH patients we defined the composite endpoint time to clinical worsening as the combination of death after discharge from the hospital after PEA, initiation of pulmonary hypertension-specific medication, or a decrease in 6-MWD by 15% with no improvement in functional class. Because CTEPH is potentially a curable disease, in almost all patients the pulmonary hypertension medication is discontinued shortly after PEA. The need to initiate pulmonary hypertension medication after surgery reflects deterioration of the clinical situation in this specific group of patients.
In our study, although postoperative values regarding mortality, residual pulmonary hypertension, NYHA class, and 6-MWD were comparable with other studies, clinical worsening occurred in 13 patients (19%) during long-term follow-up, with a five-year rate of freedom from clinical worsening of 72%. The initiation of pulmonary hypertension-specific medication was the main reason for clinical worsening, whereas in 12% pulmonary hypertension medication was started because of residual pulmonary hypertension.
In our study we could not find a significant predictor at baseline for clinical worsening during long-term follow-up. This might be due to the small number of patients included in our study. Data from other studies to compare our results are lacking.
The main limitation of this study is its retrospective observational design. Another limitation is the small study population and small number of events; therefore the results should be interpreted with caution.
Conclusion
Pulmonary endarterectomy is associated with a good long-term survival in patients with CTEPH. However, clinical worsening occurred in a substantial number of patients after long-term follow-up.
References
- 1.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130:172–5. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 2.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 3.Klepetko W, Mayer E, Sandoval J, et al. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:73S–80. doi: 10.1016/j.jacc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011–20. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1500 cases. Ann Thorac Surg. 2003;76:1457–62. doi: 10.1016/S0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 6.Saouti N, Morshuis WJ, Heijmen RH, Snijder RJ. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: a single institution experience. Eur J Cardiothorac Surg. 2009;35:947–52. doi: 10.1016/j.ejcts.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–7. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 8.Kim NH. Assessment of operability in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:584–8. doi: 10.1513/pats.200605-106LR. [DOI] [PubMed] [Google Scholar]
- 9.Masuda M, Nakajima N. Our experience of surgical treatment for chronic pulmonary thromboembolism. Ann Thorac Cardiovasc Surg. 2001;7:261–5. [PubMed] [Google Scholar]
- 10.Daily PO, Dembitsky WP, Iversen S. Technique of pulmonary thromboendarterectomy for chronic pulmonary embolism. J Card Surg. 1989;4:10–24. doi: 10.1111/j.1540-8191.1989.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson SW, Kapelanski DP. Pulmonary endarterectomy. Curr Probl Surg. 2000;37:165–252. doi: 10.1016/S0011-3840(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda H, Ogino H, Minatoya K, et al. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82:1338–43. doi: 10.1016/j.athoracsur.2006.03.105. [DOI] [PubMed] [Google Scholar]
- 13.Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: Effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2010 May 12. [Epub ahead of print]. [DOI] [PubMed]
- 14.Bonderman D, Skoro-Sajer N, Jakowitsch J, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007;115:2153–8. doi: 10.1161/CIRCULATIONAHA.106.661041. [DOI] [PubMed] [Google Scholar]
- 15.Auger WR, Kerr KM, Kim NH, Ben-Yehuda O, Knowlton KU, Fedullo PF. Chronic thromboembolic pulmonary hypertension. Cardiol Clin. 2004;22:453–66. doi: 10.1016/j.ccl.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Kim NH, Fesler P, Channick RN, et al. Preoperative partitioning of pulmonary vascular resistance correlates with early outcome after thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Circulation. 2004;109:18–22. doi: 10.1161/01.CIR.0000111841.28126.D4. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N, Kim NH. Pulmonary microvascular disease in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:571–6. doi: 10.1513/pats.200605-113LR. [DOI] [PubMed] [Google Scholar]
- 18.Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637–48. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]
- 19.Kim NH. Assessment of operability in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:584–8. doi: 10.1513/pats.200605-106LR. [DOI] [PubMed] [Google Scholar]
- 20.Freed DH, Thomson BM, Tsui SS, et al. Functional and haemodynamic outcome 1 year after pulmonary thromboendarterectomy. Eur J Cardiothorac Surg. 2008;34:525–9. doi: 10.1016/j.ejcts.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Reesink HJ, Plas MN, Verhey NE, Steenwijk RP, Kloek JJ, Bresser P. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;133:510–6. doi: 10.1016/j.jtcvs.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Corsico AG, D'Armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med. 2008;178:419–24. doi: 10.1164/rccm.200801-101OC. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S97–107. doi: 10.1016/j.jacc.2009.04.007. [DOI] [PubMed] [Google Scholar]