Abstract
Both congenital and acquired orthopaedic deformities are common in patients with spina bifida. Examples of congenital deformities, which are present at birth, include clubfoot and vertical talus. Acquired developmental deformities are related to the level of neurologic involvement and include calcaneus and cavovarus. Orthopaedic deformities may also result from postoperative tethered cord syndrome. The previously published Part I reviewed the overall orthopaedic care of a patient with spina bifida, with a focused review of hip, knee, and rotational deformities. This paper will cover foot and ankle deformities associated with spina bifida, including clubfoot, equinus, vertical talus, calcaneus and calcaneovalgus, ankle and hindfoot valgus, and cavovarus. In addition, this paper will address the issues surrounding skin breakdown in patients with spina bifida.
Keywords: Spina bifida, Foot deformity, Ankle deformity
Introduction
In the previously published Part I, the incidence, etiology, classification, and prognosis of patients with spina bifida were covered [1]. The use of gait analysis and orthoses were also addressed. This paper will focus on the orthopaedic care of foot and ankle deformities seen in patients with spina bifida.
Similar to other orthopaedic deformities, foot and ankle deformity in spina bifida may result from congenital, developmental, or iatrogenic causes. Congenital deformities are those present at birth and include clubfoot and vertical talus. Acquired developmental deformities are related to the level of neurologic involvement [2]. These deformities, such as calcaneus and cavovarus, are caused by paralysis, decreased sensation in the lower extremities, and muscle imbalance [3]. Foot and ankle deformity may also result from iatrogenic injury, such as postoperative tethered cord syndrome. Because of this, the orthopaedic surgeon must remain vigilant in assisting with monitoring the neurologic status of each patient.
Foot and ankle deformity
Almost all patients with spina bifida will experience problems with foot deformity [3, 4], which may present as calcaneus, equinus, varus, valgus, or a combination of deformities. Clubfoot and vertical talus are also seen quite commonly. Foot deformities not only affect the cosmetic appearance of the foot, but also lead to problems with efficient ambulation by causing difficulty with bracing and shoe wear. Foot deformity can also cause skin irritation, which may lead to breakdown and pressure sores.
In a patient with the potential for ambulation, the goal of the treatment of foot deformity in spina bifida is a plantigrade, supple, and braceable foot with maximally preserved range of motion. Even in non-ambulatory patients, treatment may be necessary if foot deformity prevents shoe wear or positioning in a wheelchair. As part of the routine annual examination, serial manual muscle testing performed by a skilled physical therapist plays an important role in the early detection of subtle muscle imbalance, which can lead to significant deformity if left untreated. Early intervention with casting, bracing, or surgical treatment may prevent fixed bony deformities.
When deformities become fixed and unbraceable, surgical treatment includes the use of tendon excisions, which are more reliable for achieving lasting correction and preventing recurrence compared to tendon transfer or lengthening. For bony deformities, osteotomies are the treatment of choice, as correction is achieved while preserving joint motion and articular surfaces. Surgical arthrodesis in the foot should be strictly avoided. The stiffness resulting from fusion in combination with an insensate foot can result in the development of neuropathic skin changes [5, 6]. Postoperatively, a solid ankle–foot orthosis (AFO) should be used during the daytime, supplemented with a splint at night in order to maintain correction and prevent recurrence.
The long-term consequences of foot deformity are being examined as more and more adults with spina bifida are evaluated. A recent review of 84 adults with spina bifida found that surgical procedures aimed at maintaining a plantigrade foot were helpful [7]. The authors noted that adults with significant deformity of the feet had difficulty with ambulation, even if their neurologic level of involvement was low and they demonstrated good muscle strength.
Clubfoot
Idiopathic clubfoot is a common, complex congenital foot deformity which occurs in 11 out of 10,000 live births [8]. Non-idiopathic clubfoot is seen in patients with genetic syndromes, chromosomal abnormalities, or neurological disorders, such as spina bifida [9]. The non-idiopathic clubfoot seen in patients with spina bifida differs from the idiopathic clubfoot in that the foot is severely rigid (Fig. 1) and recalcitrant to treatment, similar to that seen in a patient with arthrogryposis.
Fig. 1.
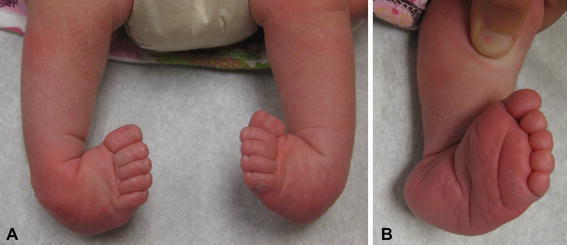
a Rigid bilateral clubfoot in a patient with spina bifida. b Note the severity of the deformity and deep medial and posterior creases
In patients with spina bifida, clubfoot is the most common foot deformity and has been reported to occur in 30–50% of patients [3, 10–12]. Many factors may contribute to the development of clubfoot in patients with spina bifida, including spasticity, intrauterine positioning, contractures, and muscle imbalance. For instance, in a patient with low-lumbar level of involvement, clubfoot may develop due to either retained activity or contracture of the tibialis muscles in combination with the functional absence of the peroneal muscles [12]. Additionally, the incidence of clubfoot varies with the neurologic level of involvement of the patient. Clubfoot occurs in approximately 90% of patients with thoracic or lumbar levels of involvement and 50% of patients with sacral level involvement [10].
Since the clubfoot deformity in spina bifida is rigid and severe, traditionally, these patients have been treated primarily with extensive soft-tissue release surgery. It was believed and reported that non-surgical management using methods such as splinting, serial casting, and stretching was rarely successful and fraught with complications, including skin breakdown and recurrence [13]. However, multiple recently published studies have reported early results using the Ponseti method of serial manipulation and casting in clubfeet associated with spina bifida [9, 14, 15]. Long-term follow-up studies for this method are still lacking.
The Ponseti method [16] consists of serial manipulation and long-leg casting to gradually correct the clubfoot deformity. A percutaneous tenotomy of the Achilles tendon is done to treat residual equinus. After this, foot abduction bracing is used for several years to maintain the correction. This method has been used successfully for the treatment of idiopathic clubfeet. Both short-term and long-term studies have demonstrated the ability to achieve and maintain correction with excellent results [16–18].
With regards to the use of the Ponseti method in the non-idiopathic clubfoot associated with spina bifida, Gerlach et al. reported that initial correction was achieved in 27 of 28 clubfeet. Relapses occurred in 68% of the clubfeet, but were treated successfully without extensive soft-tissue release surgery in all but four feet [9]. Similarly, Janicki et al. reported initial correction with the Ponseti method in 9 out of 9 clubfeet [14]. Five feet had recurrences and three of these required extensive soft-tissue release. They noted skin breakdown in two of the clubfeet. In another series, Gurnett et al. compared outcomes using the Ponseti method in idiopathic and non-idiopathic clubfeet, but did not separate patients with spina bifida [15]. Overall, they noted that the non-idiopathic patients required significantly more casts for correction compared to idiopathic patients. They also noted a higher recurrence rate of 15% in the non-idiopathic group compared to 4% in the idiopathic group.
The authors of this review also have experience in using the Ponseti method for the treatment of clubfoot in patients with spina bifida with good initial success and short-term follow-up. Based on the authors’ experience, consideration for open rather than a percutaneous approach is recommended for the tendo-Achilles to allow for the excision of at least 1 cm of tendon to prevent the recurrence of equinus. In addition, the authors recommend the use of an AFO brace postoperatively to decrease the incidence of skin breakdown and fracture. The authors believe that the Ponseti method can be useful in the treatment of clubfoot to decrease the need for extensive soft-tissue surgery, but families should be counseled about the high risk of recurrence, potential need for further treatment, and risk of skin breakdown and fractures. Because of the elevated risks, especially skin complications in insensate feet, the authors believe that the casts should be applied by the treating orthopaedic surgeon.
In the authors’ experience, the forefoot deformity corrects well with the Ponseti method. However, residual or recurrent hindfoot equinus often requires a posterior release. This is done using the posterior aspect of the Cincinnati incision, from the medial malleolus to the lateral malleolus. The medial and lateral neurovascular bundles are identified and protected. A 1-cm portion of the tendo-Achilles is excised, as well as all of the other posterior tendons. Using the calcaneofibular ligament as a landmark, the subtalar and tibiotalar joints are then opened from the lateral to the medial malleolus. A Kirschner wire (K-wire) is placed in the subtalar joint to maintain correction.
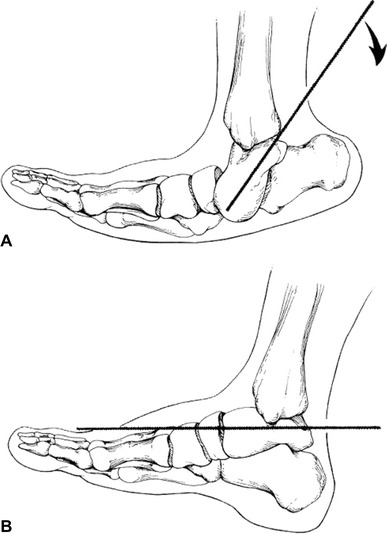
In patients requiring extensive soft-tissue release, the optimum age for surgical treatment is 10–12 months. When surgery is done at this age, patients can be started in a standing program to help decrease recurrence as soon as the postoperative casting is completed. Surgical treatment consists of a radical posteromedial-lateral release (PMLR) using a Cincinnati incision. All tendons are excised rather than lengthened. The anterior tibialis tendon should be included as well. A complete release is performed of the subtalar, calcaneocuboid, and talonavicular joints. If needed, a plantar release can be performed through a separate plantar incision. Improved results have been shown with the use of a temporary K-wire to derotate the talus in the ankle mortise (Fig. 2) [11]. The K-wire is placed into the posterolateral aspect of the talus to rotate the talus medially and the navicular is reduced on the talar head. A second K-wire is driven through the body of the talus into the navicular to hold the reduction and the temporary K-wire is then removed. Another K-wire is used to maintain the proper alignment of the talocalcaneal joint. The incision should be closed with interrupted, non-absorbable sutures. Postoperatively, a long leg posterior mold splint is used with the foot in slight equinus to decrease tension on the suture line. After 2 weeks, the patient is changed to a long leg cast with the foot held in the corrected position for an additional 6 weeks. After casting, day- and night-time AFOs are used to maintain correction. A standing A-frame is prescribed as soon as the casting is completed in order to initiate a standing program of at least 3 h a day to decrease recurrence (Fig. 3).
Fig. 2.
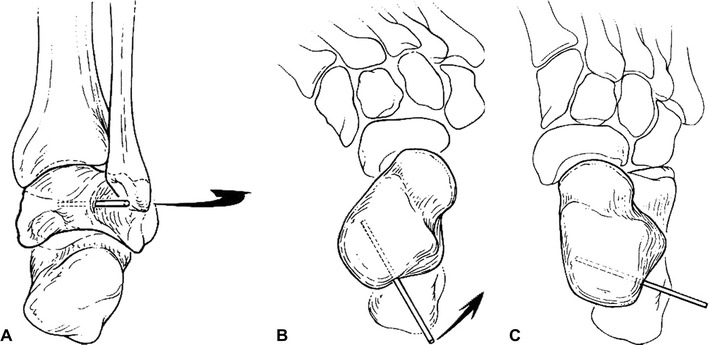
a Temporary Kirschner wire (K-wire) inserted into the posterolateral aspect of the talus to derotate the talus medially in the ankle mortise. b Malrotation of the talus before correction. c After derotation of the talus, showing corrected alignment. (Reprinted from de Carvalho et al. [11] with permission; reprint permission license #:2724320846255)
Fig. 3.
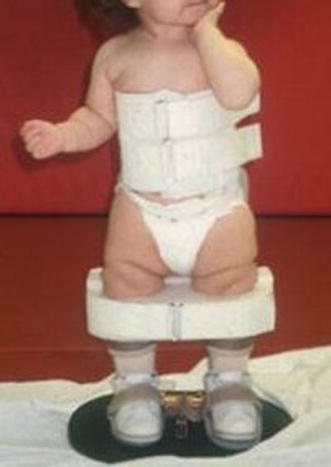
Patient with spina bifida in a standing frame
Although the outcome seems to vary with the motor level of involvement, overall, good results have been reported in 61–83% of patients after surgical release [10–12]. de Carvalho et al. reported 50% poor results in patients with thoracic and high-lumbar level of involvement compared to only 11% poor results in patients with low-lumbar and sacral levels of involvement [11]. Flynn et al. reported 87.5% of 72 feet with good or fair results after surgical release. Poor results, defined as hindfoot and forefoot deformities that require a second posteromedial release or talectomy, occurred in 12.5% of feet [12]. The recurrence rate after surgical treatment is higher than in patients with idiopathic clubfoot and may be due, in part, to the lack of normal muscles around the ankle joint and the lack of weight-bearing [11].
Partial or complete recurrence occurs in 20–50% of patients after primary surgical correction [10, 11]. Patients with partial recurrence often develop adduction deformity, which may result from growth imbalance between an elongated lateral column and a shortened medial column. If the deformity is somewhat flexible, bracing may be attempted. However, if the foot is rigid or if bracing is not successful, the surgical correction of adduction deformity consists of a combination of lateral column shortening and medial column lengthening. This is done with the “double osteotomy”, which consists of a closing wedge osteotomy of the cuboid with an opening wedge osteotomy of the medial cuneiform (Fig. 4) [19]. Good results have been shown using this technique in children older than 4 years of age [19].
Fig. 4.
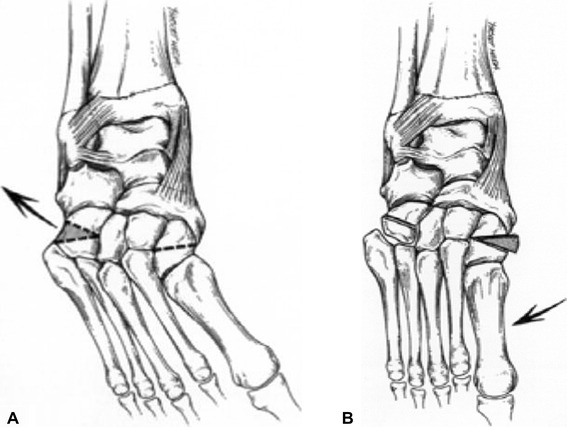
a “Double osteotomy” to correct forefoot adduction: closing wedge osteotomy of the cuboid, b with an opening wedge osteotomy of the medial cuneiform. (Reprinted from Lourenco et al. [19] with permission; reprint permission license #:2724320665380)
In an older patient, or in the case of complete recurrence after PMLR, the best procedure for achieving a plantigrade foot is talectomy [20, 21]. The talectomy is a salvage surgery used for a severe, non-braceable, rigid equinovarus foot. Using an Ollier incision, the tibiotalar, subtalar, and talonavicular joints are identified and opened widely. If contracture and scar make dissection difficult, needles can be used with intraoperative imaging to confirm the location of the joints. An attempt is made to remove the talus as one piece (Fig. 5a), and, to avoid recurrence, it is important not to leave any fragments of the talus remaining. Once the talus is removed, the calcaneus is thrust posteriorly in the ankle mortise and held in position with a K-wire placed across the joint into the tibia (Fig. 5b). A short leg cast is then applied for a total of 6 weeks, with one cast change performed at 3 weeks to remove the K-wire. Postoperatively, correction is maintained with an AFO used during the day and at night. Dias and Stern reported good results in 82% of feet treated with talectomy. The authors noted that severe forefoot deformities are not corrected by the talectomy, hence, any residual adduction deformity must be treated separately with concomitant closing wedge osteotomy of the cuboid [20].
Fig. 5.
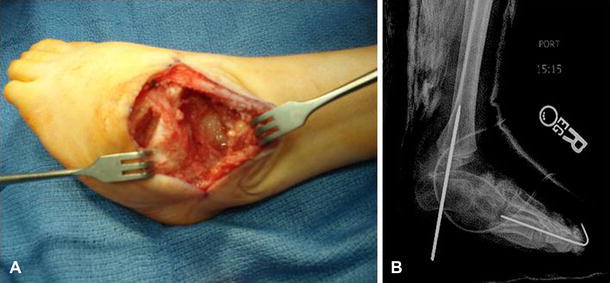
a Intra-operative clinical photograph demonstrating the talus removed en bloc. b Radiograph demonstrating postoperative alignment and K-wire fixation
Equinus
Although equinus deformity has been reported in patients with all levels of motor involvement, it occurs more commonly in patients with thoracic and high-lumbar levels of involvement [22]. In fact, in patients with thoracic level lesions, the most frequent foot deformity in one series was equinus, noted in 55% of patients [23]. The etiology of equinus in the high-level patient is not completely understood. Muscle imbalance does not play a role, since there is no voluntary motor activity at the level of the feet in patients with thoracic level involvement. Spasticity may play a role. One series of 126 feet with equinus found that 22 (17%) had spasticity of the gastrocnemius [24].
A regular routine of passive stretching combined with a night-time AFO can be used to attempt to prevent equinus contracture in a flail foot [13]. Surgical treatment is indicated for an unbraceable foot if skin breakdown or positioning is a problem, or to achieve a plantigrade, braceable foot in a patient with the potential for ambulation. The type of surgical procedure selected depends on the severity of deformity. Mild deformities respond to open Achilles tendon excision. More severe contractures require a radical posterior release including the posterior tibiotalar and talocalcaneal joints. The authors prefer to use a limited Cincinnati incision and excise all tendons. The calcaneofibular ligament must be divided in order to achieve full correction. A K-wire may be used in the talocalcaneal joint to maintain neutral hindfoot alignment. A short leg cast is used for at least 6 weeks postoperatively, followed by an AFO during the day and night.
Vertical talus
Vertical talus deformity occurs in approximately 10% of patients with spina bifida [3]. This deformity is characterized by a rigid rocker-bottom flatfoot (Fig. 6a) with malalignment of the hindfoot and midfoot. The hindfoot demonstrates equinovalgus, with the calcaneus everted on the talus. The talus is nearly vertical (Fig. 6b) and the talonavicular joint is subluxated or dislocated, with the navicular positioned dorsally and laterally on the talus.
Fig. 6.
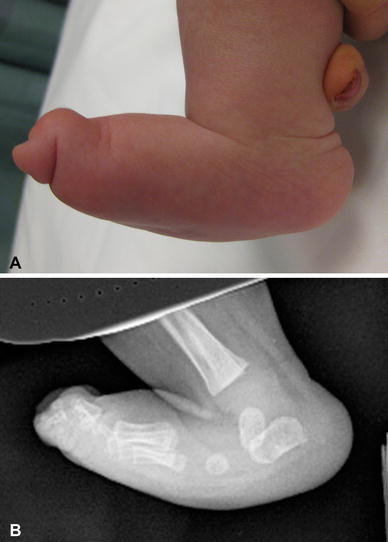
a Vertical talus deformity in an infant with spina bifida. Clinical photograph demonstrating rigid rocker-bottom deformity. b Radiograph demonstrating dislocation of the talonavicular joint with vertical position of the talus
Vertical talus occurs in two forms in patients with spina bifida, either congenital, which is more common, or developmental. The etiology of the congenital form is not clear. Sharrard and Grosfield noted that vertical talus was often associated with L5 or S1 level of involvement [13]. He proposed that the deformity resulted from muscle imbalance between the preserved action of the foot dorsiflexors and evertors, with no opposing functional activity from the long toe flexors and intrinsic foot muscles.
The goals of treatment are to restore the normal anatomic relationships between the talus, navicular, and calcaneus, and provide a plantigrade weight-bearing surface [25, 26]. Traditional treatment has been with complete posteromedial-lateral and dorsal release when the patient is between 10 and 12 months of age. However, a new technique of serial manipulation and cast immobilization followed by open talonavicular pin fixation and percutaneous tenotomy of the Achilles tendon has been reported in idiopathic congenital vertical talus with excellent short-term results [27]. We have begun using this method for the correction of vertical talus in newborns with spina bifida with good initial success (Fig. 7). Similar to serial casting for the clubfoot in this population, correction should proceed slowly, with meticulous attention given to padding adequately in order to prevent skin breakdown.
Fig. 7.
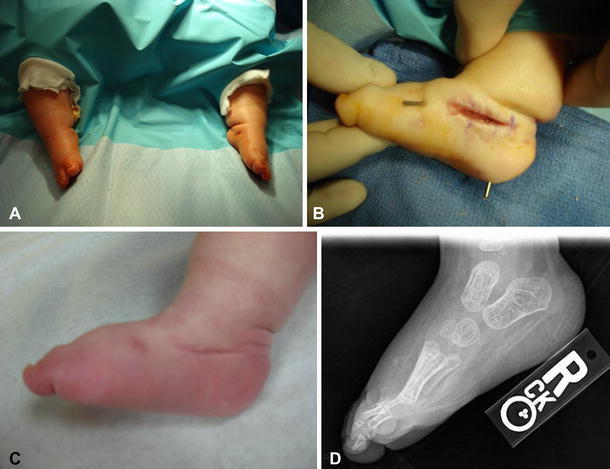
a Appearance of the feet (same patient as in Fig. 6) after serial casting in plantar flexion and inversion. b Photograph demonstrating an open approach to the talonavicular joint with K-wire fixation of the talonavicular joint and subtalar joint in reduced alignment. c Clinical photograph taken 4 months postoperatively. d Radiograph taken 17 months postoperatively. Note the restoration of normal talonavicular alignment
When extensive soft-tissue release is necessary, good results have been reported with a single-stage surgical correction addressing both the hindfoot and the forefoot [25]. Most surgeons now prefer this approach over the two-stage procedure, which has been associated with complications such as avascular necrosis of the talus [28]. Using a Cincinnati incision, the Achilles tendon is z-lengthened and capsulotomy is performed to the posterior aspects of the tibiotalar and subtalar joints. The posterior and anterior tibial tendons are detached from their insertions and tagged for later repair. After this, the medial and dorsal aspects of the talonavicular joint and the medial and lateral aspects of the subtalar joint are released. If necessary, the calcaneocuboid joint is released as well. Next, a small K-wire is placed into the posterolateral aspect of the talus and used as a joystick to elevate the talus into a reduced position while plantarflexing the navicular and forefoot (Fig. 8). Both the talonavicular and subtalar joints are then pinned in a reduced position, and, if needed, the extensor and peroneal tendons can be lengthened.
Fig. 8.
K-wire placed into the posterolateral aspect of talus and used as a joystick to elevate the talus into the reduced position while plantarflexing the navicular and forefoot. (Reprinted from Kodros and Dias [25] with permission; reprint permission license #:2724320267614)
Using this method, Kodros and Dias reported 100% of 42 feet with good or fair results at the final follow-up [25]. They noted no incidence of wound complications or avascular necrosis of the talus. Although mild pain was reported in three feet, all patients and their families were satisfied with the results and appearance of their feet.
Calcaneus/calcaneovalgus
Calcaneus deformity occurs in approximately 17–35% of patients with myelomeningocele [10, 29, 30]. It is most common in patients with L4 or L5 level of involvement, due to the strength or spasticity of the ankle dorsiflexors combined with weak or absent plantar flexors [3, 10, 29, 31]. When the ankle evertors and lateral extensors of the foot predominate over the anterior and posterior tibialis, a calcaneovalgus deformity develops [29]. Calcaneus deformity can also be seen in the high-level patient when spasticity of the ankle dorsiflexors and evertors is present.
The calcaneus deformity causes difficulty with ambulation due to the loss of normal toe-off, crouch gait, and the development of foot ulcerations [10, 31]. In addition, continued weight-bearing with a calcaneus deformity leads to a bulbous heel prone to pressure sores complicated by secondary osteomyelitis [10]. While an AFO may be useful in the flexible calcaneus foot to maintain a neutral position, the deformity is often progressive. Most authors recommend early surgical correction, since a rigid deformity can be very difficult to treat either conservatively or surgically [29–31]. Another reason to intervene early is to prevent the development of secondary osseous deformities, such as external tibial torsion, which frequently develops in association with calcaneovalgus but can be avoided by the early correction of the muscle imbalance [32].
Multiple surgical procedures to treat calcaneus have been discussed in the literature, with varying results. Transfer of the anterior tibialis tendon to the calcaneus yields somewhat unpredictable results. Bliss and Menelaus reported on a series of 46 such transfers followed for at least 12 years [30]. Only four feet remained plantigrade with a functioning transferred muscle. Eight feet required a release of the transferred tendon due to progressive equinus after the transfer. Another option for surgical treatment is complete anterolateral release, including tenotomy of all ankle dorsiflexors and toe extensors, as well as the peroneus brevis and longus. Rodrigues and Dias reported a series of 76 patients treated with anterolateral release and achieved a good result with a plantigrade, braceable foot in 82% [29]. The authors found the anterolateral release to be a simpler procedure with shorter postoperative recovery compared to the anterior tibial tendon transfer to the calcaneus, with similar results.
More recently however, Park et al. reported on a series of 31 calcaneus feet treated with anterior tibialis tendon transfer with concomitant osseous surgeries in 12 feet [31]. At a mean follow-up of 47 months, they noted no recurrence or worsening of the deformity in any patient and no other type of foot deformity developed after the surgery. Despite this, the current authors maintain that similar or better results can be achieved by the anterolateral release. The anterior tibial tendon transfer may correct the muscle imbalance, but it is not enough to produce functional plantarflexion power, hence, all patients still need to wear an AFO brace.
In older patients who have developed rigid osseous deformity, surgical treatment requires not only the release of all of the extensor tendons and peroneals, but also bony correction. A posterior closing wedge osteotomy of the calcaneus with a plantar release can improve hindfoot alignment [33]. If calcaneal valgus is present, a lateral opening wedge osteotomy of the cuboid may be necessary in order to achieve complete correction.
Ankle valgus
In contrast to foot deformities such as clubfoot and vertical talus, which are generally present at birth, valgus deformity of the hindfoot and/or ankle tends to become more prevalent as a child matures, begins ambulation, and gains weight [6]. The pathogenesis of ankle valgus in patients with spina bifida has been previously described [34, 35]. During normal ambulation, the fibula moves distally during weight-bearing due to the action of the calf muscles with an origin on the fibula, including the soleus, flexor hallucis longus, tibialis posterior, peroneus longus, and peroneus brevis [34]. In a patient with L5 level of involvement or higher, the significant soleus weakness leads to a decrease in the downward pull of the fibula. This, in turn, causes less stimulation of the fibular growth plate, with the development of relative fibular shortening. The abnormal shortening of the fibula leads to the altered distribution of forces across the distal tibia articular surface. Increased compression forces occur at the lateral portion of the tibial epiphysis, inhibiting growth, while decreased compression on the medial portion of the tibial epiphysis accelerates growth [34]. This produces lateral wedging of the distal tibial epiphysis, leading to valgus inclination of the talus (Fig. 9). The amount of lateral wedging correlates with the degree of fibular shortening.
Fig. 9.
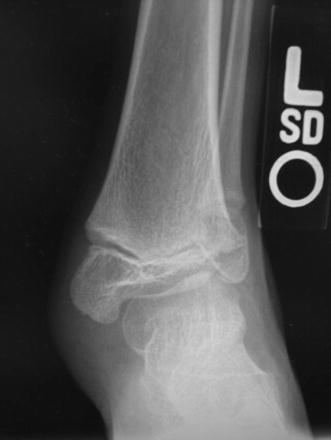
Radiograph of an ankle with valgus deformity. Note the extreme relative shortening of the fibula and the triangular shape of the distal tibial epiphysis with lateral wedging causing valgus inclination of the talus
If the valgus deformity is flexible, a well-made rigid AFO can be used to provide correction and stability. Often, the deformity progresses, causing increased prominence of the medial malleolus and talar head, which leads to skin irritation and breakdown from excessive pressure against the brace (Fig. 10a, b). Surgery is indicated when the deformity is severe, rigid, and associated with pain, difficulty with brace wear, and ulceration. The goal of surgical treatment is to correct the deformity, prevent further deformity, and preserve the best potential for ambulation.
Fig. 10.
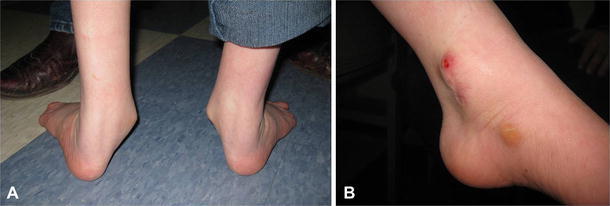
a Severe hindfoot and ankle valgus in an adolescent patient with spina bifida. b Medial view of the left ankle in the same patient demonstrating skin breakdown over the medial malleolus and callous over the talar head
An important point regarding the successful treatment of valgus deformity in patients with spina bifida is that careful attention must be paid to determine the precise anatomical location of the deformity. Valgus can result from deformity in the hindfoot, distal tibia, or both. Failure to treat all contributing locations of deformity will lead to an unsuccessful result. If needed, standing radiographs should be obtained of the ankle and the hindfoot for confirmation.
When valgus is present in the distal tibia, options for treatment include hemiepiphysiodesis of the distal tibia or distal tibia corrective osteotomy. Hemiepiphysiodesis is a good option for a patient with a mild deformity and enough potential for growth remaining to achieve correction. It is important to note that, in this case, correction proceeds slowly and, hence, is not appropriate for a patient with significant skin problems requiring acute correction [36]. Temporary growth arrest of the medial physis with continued growth of the lateral physis allows gradual correction of the valgus tilt (Fig. 11). The use of a single cannulated screw has been reported in a series of 50 feet with satisfactory improvement of ankle valgus, low morbidity, and no incidence of permanent physeal closure [37]. To avoid permanent closure of the physis, the screw should be removed within 2 years of its insertion.
Fig. 11.
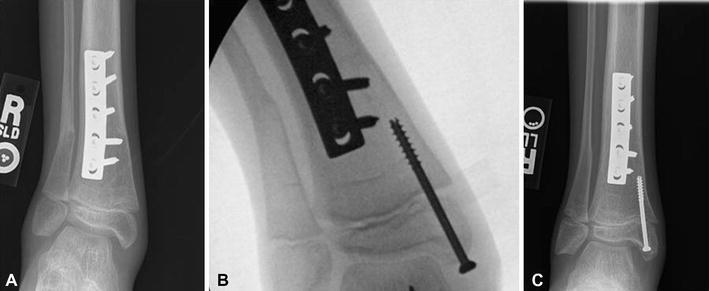
a Radiograph demonstrating distal tibia valgus deformity in a 9-year-old patient with spina bifida. b Intra-operative radiograph demonstrating the placement of a cannulated screw in the distal medial tibia. c Radiograph 19 months postoperatively demonstrating correction of the valgus alignment
In an older patient with less growth potential or in the case of a more severe deformity, a distal tibia osteotomy is indicated. In general, osteotomies of the distal tibia are associated with a high incidence of complications, such as delayed union, non-union, wound infection, and loss of correction. However, the authors have had good success with the transphyseal medially based closing wedge osteotomy of the distal tibia described by Lubicky and Altiok [36]. To preserve the healing potential of the bone, care should be taken to create the osteotomy with multiple drill holes connected by an osteotome rather than with power instruments. If concomitant external tibial torsion is present, as is often the case, internal rotation of the distal fragment should be done at the same time.
Hindfoot valgus
When the deformity is located in the hindfoot, good results have been reported with a medial sliding osteotomy of the calcaneus, as described for the idiopathic flatfoot by Koutsogiannis [38]. The advantage of this procedure is the preservation of subtalar motion while correcting the deformity. If any concomitant forefoot abduction is present, an opening wedge osteotomy of the cuboid is indicated as well. Torosian and Dias reported on the medial sliding osteotomy of the calcaneus for hindfoot valgus in a series of 38 feet in patients with spina bifida. Using a lateral L-shaped incision to provide adequate exposure, full-thickness flaps are elevated to allow extraperiosteal dissection of the calcaneus. An oblique osteotomy is made, and the amount of displacement of the distal fragment required for correction is usually 50% of the width of the fragment [6]. In their series, good results were obtained in 82% of the feet, and three of the poor results were due to unrecognized concomitant distal tibia valgus deformity.
Various types of arthrodesis procedures have been reported in patients with spina bifida and valgus deformity [39, 40]. However, these should be avoided at all costs in this patient population with impaired sensation. The resulting inflexibility in an insensate foot has been shown to be related to the development of neuropathic skin changes, such as ulceration and breakdown [5]. In addition, arthrodesis leads to inhibition in growth, the production of excessive stress in other joints, and loss of heel height [6]. Surgical fusion should be avoided in spina bifida patients in whom the goal of treatment is a supple, plantigrade, braceable foot.
Cavus/varus/cavovarus
Varus deformity of the hindfoot, often with co-existing cavus, has been reported in 8–17% of patients with spina bifida [13, 22]. Cavovarus (Fig. 12a, b) occurs most commonly in patients with sacral level of involvement and in patients with lipomeningocele. An elevated longitudinal arch, or cavus, is the primary deformity. Varus then results from the muscle imbalance between the posterior tibialis and the peroneal muscles, as well as intrinsic muscle weakness.
Fig. 12.
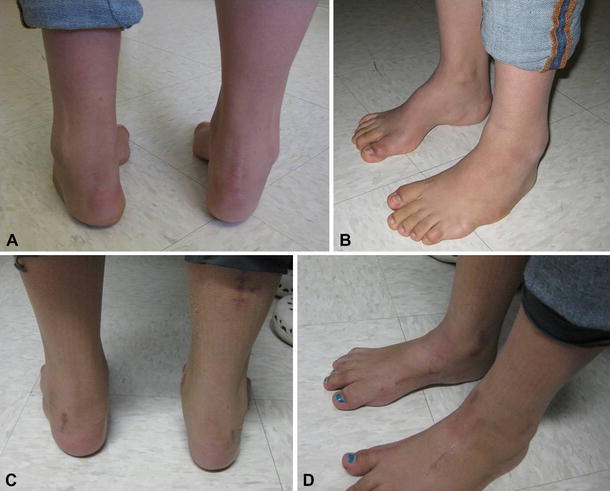
a Cavovarus deformity in a 14-year-old patient with spina bifida. Preoperative posterior view. b Preoperative side view. c Postoperative posterior view after bilateral calcaneus, medial cuneiform, first metatarsal osteotomies with soft-tissue releases. d Postoperative side view
Treatment is based on the flexibility of the deformity. A mild, flexible deformity can be treated with a rigid AFO. With progressive deformity, the weight-bearing axis deviates laterally, causing callosity and possible skin breakdown over the lateral border of the foot. When surgical treatment is being considered, the precise location of deformity must be noted. The extent of surgical treatment will depend on whether or not the hindfoot deformity is flexible. The Coleman block test is one method that can be used to determine whether the hindfoot deformity is flexible or fixed [41]. When the hindfoot varus is flexible, treatment is focused on the forefoot and consists of a radical plantar release. When the hindfoot varus is rigid, correction involves both the forefoot and the hindfoot [42]. Muscle imbalance must be corrected at the same time. In our experience, with weakness of the peroneal muscles, an anterior tibialis transfer to the midfoot may be required to balance the foot. If clawing of the toes is present, a modified Jones transfer may be necessary to transfer the extensor hallucis longus to the first metatarsal. If this is done, an intraphalangeal joint fusion of the great toe should be performed as well in order to prevent flexion deformity. For the lesser toes, we perform a transfer of the long toe flexors to the midfoot as well.
Mubarak and Van Valin have described the use of selective, joint-sparing osteotomies to address deformity correction [43]. They recommend a closing wedge osteotomy of the first metatarsal, opening plantar wedge osteotomy of the medial cuneiform, closing wedge osteotomy of the cuboid, and, if necessary, a Dwyer closing wedge osteotomy of the calcaneus and osteotomies of the second and third metatarsals. They also performed plantar release and peroneus longus-to-brevis tendon transfer when needed. In a series of 20 feet in patients with varying underlying etiologies, 95% had good or very good outcomes with this protocol [43]. We have also had success with this technique (Fig. 12c, d). As discussed in the section on valgus deformity, triple arthrodesis should be avoided in this patient population with impaired sensation due to the risk of skin breakdown and complications [42]. In very severe cases, gradual correction can be obtained using a circular external fixator as an attempt to avoid amputation.
Skin breakdown/amputation
Since patients with spina bifida have impaired or absent protective sensation, care of the skin must be undertaken with extreme vigilance. From a young age, patients should be counseled about the risk of skin breakdown and the development of pressure sores. The role of the physician is to foster the patient’s independent ability to effectively monitor his or her skin health.
The reported incidence of pressure sores varies in the literature from 17 to 82% of patients [5, 7, 10, 44–46]. The most common locations are over the sacrum, ischial tuberosity, greater trochanter, or on the feet [10]. Harris and Banta followed a cohort of 75 patients with spina bifida treated for pressure sores over a 13-year study period. They determined that over two million dollars were spent on their treatment [47]. In another study, Akbar et al. reported 415 admissions solely for the treatment of pressure ulcers at their institution over a 17-year period [10].
A major complication of skin breakdown and pressure sores is osteomyelitis of the underlying bone, such as that seen in heel pressure sores with concomitant osteomyelitis of the calcaneus. When pressure sores do not heal with appropriate care of the soft tissue, underlying infection must be suspected. Laboratory and radiographic work-up can help to confirm the diagnosis. If the infection cannot be cleared with surgical debridements and appropriate antibiotic therapy, amputation may become necessary. One series reported that four sacral-level adult patients (7% of the examined patients with spina bifida) had required amputations of or within the foot as a result of pressure sores [7]. In these cases, the amputations, one Symes and three ray resections, were performed during debridement for osteomyelitis, and an attempt was made to preserve as much length of the limb as possible.
All patients should be instructed from a young age on the proper care of the skin and the prevention of pressure sores. Barefoot walking should be avoided, especially on rough or hot surfaces. Water shoes should be worn to protect the feet while swimming. Orthoses should be inspected by the physician on a regular basis, at least annually, to ensure proper fit and absence of pressure points or sharp edges. In addition, patients using orthoses should be taught to perform daily skin checks. A long-handled mirror can be employed if a patient’s mobility prevents adequate visualization of the skin on the feet. When breaking in new orthoses, skin checks may be required more often than once a day.
During serial or postoperative casting, ample padding must be used and applied in a smooth fashion. Self-adhering foam pads can be used to supplement padding over pressure points such as the anterior knee, heel, or ankle malleoli. In addition, surgical arthrodesis within the foot should be strictly avoided, since the resulting inflexibility in an insensate foot has been shown to be related to the development of neuropathic skin changes. Maynard et al. reported on 72 feet in ambulatory patients with spina bifida [5]. They found a 38% risk of skin breakdown in plantigrade arthrodesed feet and a 100% risk if the arthrodesis failed to achieve a plantigrade position. In contrast, there was no skin breakdown in patients with plantigrade, non-arthrodesed feet, and 62% in those with non-plantigrade, non-arthrodesed feet.
Acknowledgments
Conflict of interest
None.
Contributor Information
Vineeta T. Swaroop, Phone: +1-312-2382235, FAX: +1-312-2382230, Email: vswaroop@ric.org
Luciano Dias, Phone: +1-312-2382231, FAX: +1-312-2382230, Email: ldias@ric.org.
References
- 1.Swaroop VT, Dias L. Orthopedic management of spina bifida. Part I: hip, knee, and rotational deformities. J Child Orthop. 2009;3:441–449. doi: 10.1007/s11832-009-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guille JT, Sarwark JF, Sherk HH, Kumar SJ. Congenital and developmental deformities of the spine in children with myelomeningocele. J Am Acad Orthop Surg. 2006;14:294–302. doi: 10.5435/00124635-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Westcott MA, Dynes MC, Remer EM, Donaldson JS, Dias LS. Congenital and acquired orthopedic abnormalities in patients with myelomeningocele. Radiographics. 1992;12:1155–1173. doi: 10.1148/radiographics.12.6.1439018. [DOI] [PubMed] [Google Scholar]
- 4.Noonan KJ, Didelot WP, Lindseth RE. Care of the pediatric foot in myelodysplasia. Foot Ankle Clin. 2000;5(2):281–304. [PubMed] [Google Scholar]
- 5.Maynard MJ, Weiner LS, Burke SW. Neuropathic foot ulceration in patients with myelodysplasia. J Pediatr Orthop. 1992;12:786–788. doi: 10.1097/01241398-199211000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Torosian CM, Dias LS. Surgical treatment of severe hindfoot valgus by medial displacement osteotomy of the os calcis in children with myelomeningocele. J Pediatr Orthop. 2000;20(2):226–229. [PubMed] [Google Scholar]
- 7.Roach JW, Short BF, Saltzman HM. Adult consequences of spina bifida: a cohort study. Clin Orthop Relat Res. 2011;469:1246–1252. doi: 10.1007/s11999-010-1594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kancherla V, Romitti PA, Caspers KM, Puzhankara S, Morcuende JA. Epidemiology of congenital idiopathic talipes equinovarus in Iowa, 1997–2005. Am J Med Genet A. 2010;152A(7):1695–1700. doi: 10.1002/ajmg.a.33481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerlach DJ, Gurnett CA, Limpaphayom N, Alaee F, Zhang Z, Porter K, Kirchhofer M, Smyth MD, Dobbs MB. Early results of the Ponseti method for the treatment of clubfoot associated with myelomeningocele. J Bone Joint Surg Am. 2009;91(6):1350–1359. doi: 10.2106/JBJS.H.00837. [DOI] [PubMed] [Google Scholar]
- 10.Akbar M, Bresch B, Seyler TM, Wenz W, Bruckner T, Abel R, Carstens C. Management of orthopaedic sequelae of congenital spinal disorders. J Bone Joint Surg Am. 2009;91:87–100. doi: 10.2106/JBJS.I.00613. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho Neto J, Dias LS, Gabrieli AP. Congenital talipes equinovarus in spina bifida: treatment and results. J Pediatr Orthop. 1996;16(6):782–785. doi: 10.1097/01241398-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Flynn JM, Herrera-Soto JA, Ramirez NF, Fernandez-Feliberti R, Vilella F, Guzman J. Clubfoot release in myelodysplasia. J Pediatr Orthop B. 2004;13:259–262. doi: 10.1097/01.bpb.0000124491.13918.b7. [DOI] [PubMed] [Google Scholar]
- 13.Sharrard WJ, Grosfield I. The management of deformity and paralysis of the foot in myelomeningocele. J Bone Joint Surg Br. 1968;50:456–465. [PubMed] [Google Scholar]
- 14.Janicki JA, Narayanan UG, Harvey B, Roy A, Ramseier LE, Wright JG. Treatment of neuromuscular and syndrome-associated (nonidiopathic) clubfeet using the Ponseti method. J Pediatr Orthop. 2009;29(4):393–397. doi: 10.1097/BPO.0b013e3181a6bf77. [DOI] [PubMed] [Google Scholar]
- 15.Gurnett CA, Boehm S, Connolly A, Reimschisel T, Dobbs MB. Impact of congenital talipes equinovarus etiology on treatment outcomes. Dev Med Child Neurol. 2008;50:498–502. doi: 10.1111/j.1469-8749.2008.03016.x. [DOI] [PubMed] [Google Scholar]
- 16.Ponseti IV, Smoley EN. Congenital club foot: the results of treatment. J Bone Joint Surg Am. 1963;45:261–344. [Google Scholar]
- 17.Laaveg SJ, Ponseti IV. Long-term results of treatment of congenital club foot. J Bone Joint Surg Am. 1980;62:23–31. [PubMed] [Google Scholar]
- 18.Cooper DM, Dietz FR. Treatment of idiopathic clubfoot. A thirty-year follow-up note. J Bone Joint Surg Am. 1995;77:1477–1489. doi: 10.2106/00004623-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Lourenco AF, Dias LS, Zoellick DM, Sodre H. Treatment of residual adduction deformity in clubfoot: the double osteotomy. J Pediatr Orthop. 2001;21:713–718. [PubMed] [Google Scholar]
- 20.Dias LS, Stern LS. Talectomy in the treatment of resistant talipes equinovarus deformity in myelomeningocele and arthrogryposis. J Pediatr Orthop. 1987;7:39–41. doi: 10.1097/01241398-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sherk HH, Ames MD. Talectomy in the treatment of the myelomeningocele patient. Clin Orthop Relat Res. 1975;110:218–222. doi: 10.1097/00003086-197507000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Frawley PA, Broughton NS, Menelaus MB. Incidence and type of hindfoot deformities in patients with low-level spina bifida. J Pediatr Orthop. 1998;18:312–313. doi: 10.1097/01241398-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Frischhut B, Stöckl B, Landauer F, Krismer M, Menardi G. Foot deformities in adolescents and young adults with spina bifida. J Pediatr Orthop B. 2000;9:161–969. doi: 10.1097/01202412-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Broughton NS, Graham G, Menelaus MB. The high incidence of foot deformity in patients with high-level spina bifida. J Bone Joint Surg Br. 1994;76:548–550. [PubMed] [Google Scholar]
- 25.Kodros SA, Dias LS. Single-stage surgical correction of congenital vertical talus. J Pediatr Orthop. 1999;19(1):42–48. [PubMed] [Google Scholar]
- 26.Alaee F, Boehm S, Dobbs MB. A new approach to the treatment of congenital vertical talus. J Child Orthop. 2007;1:165–174. doi: 10.1007/s11832-007-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobbs MB, Purcell DB, Nunley R, Morcuende JA. Early results of a new method of treatment for idiopathic congenital vertical talus. J Bone Joint Surg Am. 2006;88:1192–1200. doi: 10.2106/JBJS.E.00402. [DOI] [PubMed] [Google Scholar]
- 28.Ogata K, Schoenecker PL, Sheridan J. Congenital vertical talus and its familial occurrence: an analysis of 36 patients. Clin Orthop Relat Res. 1979;139:128–132. [PubMed] [Google Scholar]
- 29.Rodrigues RC, Dias LS. Calcaneus deformity in spina bifida: results of anterolateral release. J Pediatr Orthop. 1992;12(4):461–464. doi: 10.1097/01241398-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Bliss DG, Menelaus MB. The results of transfer of the tibialis anterior to the heel in patients who have a myelomeningocele. J Bone Joint Surg Am. 1986;68:1258–1264. [PubMed] [Google Scholar]
- 31.Park KB, Park HW, Joo SY, Kim HW. Surgical treatment of calcaneal deformity in a select group of patients with myelomeningocele. J Bone Joint Surg Am. 2008;90(10):2149–2159. doi: 10.2106/JBJS.G.00729. [DOI] [PubMed] [Google Scholar]
- 32.Dias L. Myelomeningocele and intraspinal lipoma. In: Sponseller PD, editor. Orthopaedic knowledge update: pediatrics. 2. Newton, MA: American Academy of Orthopaedic Surgeons; 2002. pp. 249–259. [Google Scholar]
- 33.Mitchell GP. Posterior displacement osteotomy of the calcaneus. J Bone Joint Surg Br. 1977;59:233–235. doi: 10.1302/0301-620X.59B2.873985. [DOI] [PubMed] [Google Scholar]
- 34.Dias LS. Valgus deformity of the ankle joint: pathogenesis of fibular shortening. J Pediatr Orthop. 1985;5:176–180. doi: 10.1097/01241398-198505020-00011. [DOI] [PubMed] [Google Scholar]
- 35.Dias LS. Ankle valgus in children with myelomeningocele. Dev Med Child Neurol. 1978;20:627–633. doi: 10.1111/j.1469-8749.1978.tb15281.x. [DOI] [PubMed] [Google Scholar]
- 36.Lubicky JP, Altiok H. Transphyseal osteotomy of the distal tibia for correction of valgus/varus deformities of the ankle. J Pediatr Orthop. 2001;21(1):80–88. doi: 10.1097/01241398-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Stevens PM, Belle RM. Screw epiphysiodesis for ankle valgus. J Pediatr Orthop. 1997;17(1):9–12. [PubMed] [Google Scholar]
- 38.Koutsogiannis E. Treatment of mobile flat foot by displacement osteotomy of the calcaneus. J Bone Joint Surg Br. 1971;53:96–100. [PubMed] [Google Scholar]
- 39.Levitt RL, Canale ST, Gartland JJ. Surgical correction of foot deformity in the older patient with myelomeningocele. Orthop Clin North Am. 1974;5:19–29. [PubMed] [Google Scholar]
- 40.Gallien R, Morin F, Marquis F. Subtalar arthrodesis in children. J Pediatr Orthop. 1989;9:59–63. doi: 10.1097/01241398-198901000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Coleman SS, Chesnut WJ. A simple test for hindfoot flexibility in the cavovarus foot. Clin Orthop Relat Res. 1977;123:60–62. [PubMed] [Google Scholar]
- 42.Schwend RM, Drennan JC. Cavus foot deformity in children. J Am Acad Orthop Surg. 2003;11:201–211. doi: 10.5435/00124635-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Mubarak SJ, Van Valin SE. Osteotomies of the foot for cavus deformities in children. J Pediatr Orthop. 2009;29(3):294–299. doi: 10.1097/BPO.0b013e31819aad20. [DOI] [PubMed] [Google Scholar]
- 44.Bartonek A, Saraste H, Samuelsson L, Skoog M. Ambulation in patients with myelomeningocele: a 12-year follow-up. J Pediatr Orthop. 1999;19(2):202–206. doi: 10.1097/01241398-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Díaz Llopis I, Bea Muñoz M, Martinez Agulló E, López Martinez A, García Aymerich V, Forner Valero JV. Ambulation in patients with myelomeningocele: a study of 1500 patients. Paraplegia. 1993;31(1):28–32. doi: 10.1038/sc.1993.5. [DOI] [PubMed] [Google Scholar]
- 46.Plaum PE, Riemer G, Frøslie KF. Risk factors for pressure sores in adult patients with myelomeningocele—a questionnaire-based study. Cerebrospinal Fluid Res. 2006;3:14–15. doi: 10.1186/1743-8454-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris MB, Banta JV. Cost of skin care in the myelomeningocele population. J Pediatr Orthop. 1990;10(3):355–361. doi: 10.1097/01241398-199005000-00012. [DOI] [PubMed] [Google Scholar]


