Abstract
Purpose/Background/Introduction
The aim of this study was to retrospectively evaluate the impact of neonatal sonographic hip screening using Graf’s method for the management and outcome of orthopaedic treatment of decentered hip joints with developmental dysplasia of the hip (DDH), using three decades (1978–2007) of clinical information compiled in a medical database.
Methods
Three representative cohorts of consecutive cases of decentered hip joints were selected according to different search criteria and inclusion and exclusion parameters: (1) cohort 1 (1978–1982; n = 80), without sonographic screening; (2) cohort 2.1 (1994–1996; n = 91), with nationwide established general sonographic screening according to the Graf-method; (3) cohort 2.2 (2003–2005; n = 91), with sonographic screening including referred cases for open reduction from non-screened populations. These three cohorts were compared for the following parameters: age at initial treatment, successful closed reduction, necessary overhead traction, necessary adductor-tenotomy, rate of open reduction, rate of avascular necrosis (AVN) and rate of secondary acetabuloplasty.
Results
The age at initial treatment was reduced from 5.5 months in the first cohort to 2 months in the two subsequent two cohorts and the rate of successful closed reduction increased from 88.7 to 98.9 and 95.6%, respectively. There was a statistically significant improvement in six out of seven parameters with sonographic hip screening; only the rate of secondary acetabuloplasty did not improve significantly.
Conclusion
Compared to the era before the institution of a sonographic hip screening programme according to the Graf-method in Austria in 1992, ultrasound screening based-treatment of decentered hip joints has become safer, shorter and simpler: “safer” means lower rate of AVN, “shorter” means less treatment time due to earlier onset and “simpler” means that the devices are now less invasive and highly standardized.
Keywords: Developmental dysplasia of the hip, Decentered hip joints, Sonographic hip screening, Outcome of treatment, Retrospective comparative cohort study
Introduction
Our institution has always been a paediatric orthopaedic tertiary referral centre for the treatment of decentered hip joints. Its clinical database contains the clinical histories of babies who have been treated for decentered hip joints during a period of more than 30 years. Thus, a retrospective comparative cohort study could clarify the impact of sonographic hip screening on the management and outcome of treatment measures in our department. With the support of the German scientific non-profit association “Deutsche Arthrosehilfe e.V.” we designed a study that focused primarily on the management and outcome of the initial biomechanical treatment of decentered hip joints, especially those cases with and without population-based sonographic hip screening for developmental dysplasia of the hip (DDH). The diagnostic tool of sonographic imaging was developed and methodically standardized by Graf in the early 1980s [1–3]. A general sonographic hip screening based on Graf’s method (referred to herein as the Graf-method) within the first 6 weeks of life was implemented in 1992 in Austria and in 1996 in Germany. Although the Graf-method of sonographic hip screening has been in clinical use for almost 30 years and several outcome studies have been published, the scientific discussion about “to screen or not to screen” [4] remains controversial [5–40].
Historical background
Developmental dysplasia of the hip with its potential for early osteoarthritis is the most common congenital dysplasia in newborns, especially in Caucasians [41]. Depending on the pathoanatomical definition and geographical region, an extremely wide range of incidence of DDH can be found in the literature: for Austria and Central Europe, a mean incidence of about 2.5% can be expected [42]. In Austria, about 9% of all total hip replacements (THR) in young patients are implanted due to early osteoarthritis in late DDH [19, 43].
One parameter that potentially impacts significantly the quality and effectiveness of screening programs in different countries may be the rate of open reductions (numbers per 1,000 live births) due to late presenting cases of irreducible hip luxations. The following short overview of the three basic categories of screening programmes provides an indication of the published numbers of open reductions per 1,000 live births and represents the basis for scientific discussions and improvements:
Countries with clinical screening without sonography (such as Ireland, New Zealand, Northern Ireland, UK) report open reduction rates of between 0.78 and 1.30 per 1,000 live births [44–47] and represent the baseline for comparison and possible improvements.
Countries (for example England and Norway) with a “selective” sonographic screening (based on anamnestic risk factors and/or suspect clinical findings) report open reduction rates of between 0.57 and 0.70 [20, 27, 48, 49]. The authors of these studies state that selective ultrasound screening does not significantly reduce the overall rate of surgery compared with the best conventional clinical screening programmes and cannot be fully recommended.
In countries with established general songraphic hip screening programs (Austria, Czech Republic, Germany) rates of open reduction of between 0.07 and 0.26 are reported [15, 19, 22, 50].
Given this historical background and combined with a review of literature, our single centre retrospective comparative cohort study may be a valuable contribution as it is based on a distinct clinical database: our data are not population based (rate of open reductions per 1,000 live births), as presented above, but are related to the number of clinical procedures (rate of open reductions per total number of treated decentered hip joints) performed in our paediatric orthopaedic tertiary referral center before and after the nationwide institution of a sonographic hip screening programme based on the Graf-method [1–3] in Austria in 1992 [19].
Materials and methods
Algorithm of data acquisition
The clinical histories and the medical imaging data (especially ultrasonographies and X-rays) of all babies treated for decentered hip joints in DDH between 1978 and 2007 were scanned. The primary data source in our hospital has always been our “surgery registry”: all babies treated conservatively or surgically for decentered hip joints are recorded in the surgery registry. The surgery registry is a book which is kept in the operating theatre and which contains handwritten entries of all patients treated in the operating theatre. Each procedure is numbered consecutively, and pertinent information, such as the date of procedure, patient’s name and first name and age, type and side of procedure and surgical and anaesthesiology team, is recorded.
The first step of this survey was to manually scan (performed by F.F.) the surgery registry from 1978 to 2007 according to several search criteria and key words, such as “congenital hip dislocation”, “subluxation”, “hip dysplasia”, “avascular necrosis”, “closed reduction”, “open reduction”, “adductor tenotomy”, “overhead traction”, “acetabuloplasty”, “intertrochanteric osteotomy”, “Salter-osteotomy”, “Chiari-osteotomy”, “triple pelvic osteotomy”, “periacetabular osteotomy”, “Fettweis-cast”, “hip spica” and “Hilgenreiner-splint”. For this primary search of the surgery registry we defined a wide range of search criteria in order to select all potential cases, particularly those cases treated more than once. Using this widespread searching strategy, all possible re-interventions in the same patient are “automatically” scanned. The result of this primary search were 4,122 entries matching the applied search criteria (Fig. 1). A subsequent manual search of the surgery registry resulted in a long list of names that had to be compared with the corresponding clinical records.
Fig. 1.
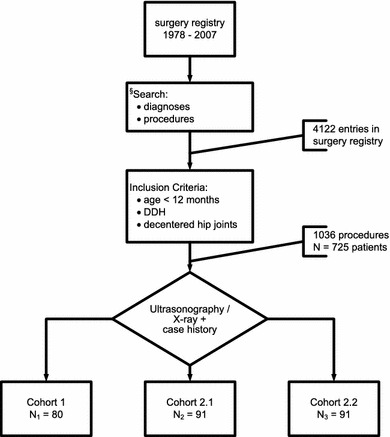
Flowchart of data collection and case selection (details in the text). DDH Developmental dysplasia of the hip
In the second step, we applied the following inclusion criteria:
DDH
Decentered hip joints with radiographic gradings according to Tönnis [51] and sonographic classification according to Graf et al. [52–54]
Diagnosis/initial treatment before the end of the first year of life
All neurogenic and teratologic and all syndromic cases were excluded. All children older than 1 year at onset of treatment were excluded. All sonographically stable hip joints with maturational deficit (Graf-types: 2c-stable, 2b, 2a-minus) were excluded; these stable but immature hip joints are routinely treated by functional bracing in an out-patient regime.
Only cases of DDH with already decentered unstable hip joints (Graf-types: 2c-unstable, D, 3, 4) with initial treatment before the end of the first year are included. The primary search list of names and clinical records was then checked with the list of procedures to ensure there was a match with the defined inclusion criteria. Ultimately, during the time span 1978–2007, 1,036 hip joint procedures in 725 patients matched these inclusion criteria.
In a third step we defined three clinically representative periods and the consecutive number of cases that were sufficiently documented for this comparative evaluation (Table 1). The aim was to be able to directly compare cases before the initiation of sonographic screening and those following the implementation of sonographic screening under comparable circumstances: same population, same treatment centre, same pathomorphologic diagnosis, same treatment principles and standards. Three cohorts eventually were determined, with the main difference between them being the time and method of definitive pathomorphological diagnosis provided by ultrasound screening in cohorts 2.1 and 2.2 (Table 1).
Table 1.
Characteristics of the three representative cohorts (1, 2.1, 2.2)
| Cohort | Period | Number of consecutive cases | Clinical significance |
|---|---|---|---|
| 1 | 1978–1982 | 80 | Diagnosis without sonography (“pre-sono-cohort”): primary clinical screening with secondary (“late”) radiological diagnosis |
| 2.1 | 1994–1996 | 91 | Diagnosis with sonography, including first effects of a nationwide sono-screening in Austria instituted in 1992 (“sono-screening-cohort”): routine (“early”) sonographic diagnosis within the first 6 weeks of life |
| 2.2 | 2003–2005 | 91 | Diagnosis with established nationwide sono-screening, but including the referral of external non-screened “immigration” cases for open reduction (“mixed-sono-screening-cohort”) |
Sufficiently documented cases match the inclusion criteria (DDH, decentered, <12 months) and provide a standardized minimum set of clinical records and imaging (before and after initial treatment, early follow-up after the end of treatment at the age of 12–18 months). If this minimal clinical documentation was not met, the cases were excluded due to lack of data:
In cohort 1 21 of originally 101 cases (20.8%) excluded (N = 80 included),
In cohort 2.1 5 of originally 96 cases (5.2%) excluded (N = 91 included),
In cohort 2.2 4 of originally 95 cases (4.2%) excluded (N = 91 included).
In cohort 2.1, all cases were detected by the nationwide sonographic screening programme according to the Graf-method and organized by the Austrian Ministry of Health. In cohort 2.2, a minority of cases referred for open reduction derived from non-screened populations was included in the majority of screened Austrian babies (=“mixed”-sono-screening-cohort 2.2; Table 1). All open reductions in cohort 2.2 derive from these referred cases!
Our routinely used treatment algorithm throughout the whole first year of life is based on Graf’s sonographic classification [52, 53] and has always been applied in a highly standardized way, which is summarized in Table 2.
Table 2.
Treatment algorithm with biomechanical treatment phases
| Pathobiomechanical phase | Sonographic Graf-types | Treatment-phase 1 “reduction” | Treatment-phase 2 “retention” | Treatment-phase 3 “maturation” |
|---|---|---|---|---|
| Decentered | 4, 3, D | x | x | x |
| Sonographically unstable | 2c-unstable | – | x | x |
| Sonographically stable but with maturational deficit | 2c-stable, 2b, 2a-minus | – | – | x |
| Physiologically immature | 2a-plus ➙ follow-up | – | – | – |
| Mature | 1 ➙ finished | – | – | – |
Our treatment measures (Table 3) for the different treatment phases [54] are manual closed reduction, instantly followed by retention in a modified plaster cast according to Fettweis [55] for 4 weeks in a physiological “squatting” position of 100° flexion and 50° abduction. Subsequent maturation is provided by removable functional bracing until a Graf hip type 1 (mature hip joint) is sonographically documented.
Table 3.
Treatment measures in the different treatment phases
| Phase of treatment | Reduction | Retention | Maturation |
|---|---|---|---|
| Measure of treatment | Manual reduction by flexion >100° and abduction <50°: “squatting” position (=“safe zone” of SALTER’s “human position”) | Non-removable plaster cast in this “squatting” position for 4 weeks | Removable “functional” bracing in this position until a Graf-type-1 is documented |
| Pathobiomechanical phase | Decentered | Unstable | Maturational deficit |
| Sonographic Graf-types | 4, 3, D | 2c-unstable | 2c-stable, 2b, 2a-minus |
Throughout the whole first year of life this therapeutic sequence (closed reduction → cast immobilization → functional bracing) is applied in all cases of decentered hip joints in a standardized way.
Indication for open reduction
A basic rule for indicating open reduction was applied throughout the evaluated 30-year period: if an originally decentered hip joint was not reduced and stable after two 4-week-periods of a correct “squatting” cast, a pathoanatomical obstacle against deep closed reduction is present and has to be removed surgically. In the clinical routine currently used this is the case only in Graf-type-4 hips which have been missed in the first weeks after birth; all other types of decentered hip joints (Graf-types D and 3) can routinely be managed by closed reduction, as already described (closed reduction → cast immobilization → functional bracing).
Parameters of outcome
To compare the management and outcome between the three representative cohorts, we defined the age at diagnosis and initial treatment and the rate of successful conservative treatment as primary outcome parameters. We additionally looked at the rate of necessary preparatory measures of closed reduction (overhead traction), the rate of necessary surgical support of closed reduction (adductor tenotomy), the rate of open reduction, the rate of avascular necrosis at the end of primary treatment and the rate of persistent or secondary sloping of the ossified acetabular roof with the need for secondary surgical correction (acetabuloplasty).
Statistical analysis
The data of the three cohorts were first analysed using descriptive statistical methods. Continuous data were described by the mean and standard deviation (SD) or median, minimum and maximum, as appropriate. For categorical data, absolute and relative frequencies were used. To analyze differences between the three cohorts, analysis of variance (ANOVA) methods or the Kruskal–Wallis test were applied. The chi-square test was used to compare the groups for categorical data. All analyses were performed with the statistical software package PASW18. A P value <5% was considered to be significant.
Declaration of ethical standards
This retrospective study has been approved by the ethics committee of the Medical University Graz.
Results
Between 1978 and 2007, 725 babies were treated for decentered hip joints in DDH within the first year of life. Three cohorts of consecutive cases, including 262 procedures, were included in the survey for analysis (cohort 1, n = 80; cohort 2.1, n = 91; cohort 2.2, n = 91).
Primary outcome parameters
Mean age at diagnosis/initial treatment (Table 4; Fig. 2)
Table 4.
Mean age (months) at diagnosis/initial treatment
| Parameter | Cohort 1 | Cohort 2.1 | Cohort 2.2 | P value |
|---|---|---|---|---|
| Mean age (months) | 5.5 (2.09) | 2.0 (1.70) | 2.1 (1.97) | <0.001 |
See text for definition of each cohort
Standard deviation (SD) is given in parentheses
Fig. 2.
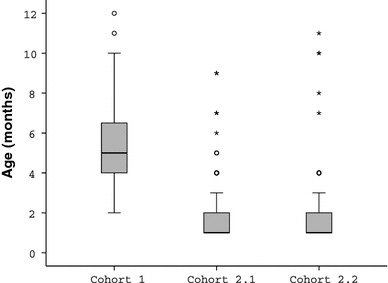
Boxplot of age (months) at initial treatment (details given in the text)
According to Tönnis [51], the age at definitive pathomorphological diagnosis can be rated as the most important outcome parameter and therefore defined as a “primary” outcome parameter. In the pre-sono cohort (cohort 1), initial treatment started at a mean age of 5.5 months, while in the sono-screening cohorts (2.1 and 2.2), treatment started statistically significantly earlier, at a mean age of 2 months (P = 0.0001). The reason for this improvement seems to be related to the nationwide implementation of sonographic screening in Austria within the first 6 weeks of life.
Rate of successful conservative treatment by closed reduction (Table 5)
Table 5.
Successful conservative treatment by closed reduction
| Parameter | Cohort 1 | Cohort 2.1 | Cohort 2.2 | P value |
|---|---|---|---|---|
| Successfully conservative treatments, n | 71/80 (88.7) | 90/91 (98.9) | 87/91 (95.6) | 0.012 |
The percentage of cohort is given in parentheses
Successful closed reduction without preparatory traction and without surgical soft tissue release seems to be an equally important outcome goal and therefore was defined as a “primary” outcome parameter. With earlier initial treatment in the sono-screening cohorts 2.1 and 2.2, a significantly higher percentage of hips could be treated by simple closed reduction and a surgical intervention could be avoided (P = 0.012).
Secondary outcome parameters (Table 6)
Table 6.
Synoptic table of secondary outcome parameters
| Secondary outcomes | Cohort 1 | Cohort 2.1 | Cohort 2.2 | Significance (P) |
|---|---|---|---|---|
| Overhead traction (%) | 75 | 8.8 | 4.4 | <0.001 |
| Adductor tenotomy (%) | 66.3 | 0 | 0 | <0.001 |
| Open reduction (%) | 11.3 | 1.1 | 4.4 | 0.012 |
| AVN (%) according to TÖNNIS scaling | ||||
| Grade 0 | 56.9 | 81.8 | 96.2 | <0.001 |
| Grade 1 | 33.9 | 18.2 | 3.8 | |
| Grade 2 | 9.2 | 0 | 0 | |
| Acetabuloplasty (%) | 7.5 | 7.7 | 3.3 | 0.382 |
AVN, Avascular necrosis (Tönnis grading)
Rate of necessary preparatory measures (overhead traction) before closed reduction
Over time, untreated decentered hip joints may develop soft tissue contractures. Overhead traction may be a preparatory measure to enable closed reduction in these cases. At initial treatment in the pre-sono-screening cohort (cohort 1), considered in this study to comprise the “old” babies, 60 of 80 hips (75%) had already developed secondary soft tissue contractures and needed preparatory overhead traction. The sono-screened cohorts (2.1 and 2.2) comprised mainly “young” babies of whom a small minority (2.1: <10%; 2.2: <5%) needed preparatory overhead traction.
Rate of necessary surgical support (adductor tenotomy) at closed reduction
Despite preparatory overhead traction older children may present tight and contract adductor muscles and may need percutaneous adductor tenotomy to enable closed reduction. At initial treatment in the pre-sono-screening cohort (cohort 1), i.e., the “old” babies (see above), 53 of 80 hips (66.25%) presented with persisting adductor contractures and needed additional percutaneous adductor tenotomies at the time of closed reduction in order to enable a tension-free closed reduction. In the sono-screening cohorts (2.1 and 2.2), comprising mainly “young” babies without adductor contractures, none (0%) of the babies needed preparatory adductor tenotomy to manage a tension-free closed reduction.
Rate of open reductions
The pathomorphology in untreated decentered hip joints will progress over time, and the risk of the development of secondary pathoanatomic obstacles against closed reduction might increase. This situation is reflected by our rates of open reductions. At initial treatment, in the pre-sono-screening cohort (cohort 1), i.e., the “old” babies, nine of 80 hips (11.25%) needed open reductions. With earlier onset of treatment at a less developed pathomorphological stage, such as in the sono-screening cohorts (2.1 and 2.2), significantly fewer open reductions had to be performed (P = 0.012). In the “mixed” sono-screening cohort (2.2) all included open reductions were referred cases from non-screened populations!
Rate of AVN at the end of primary treatment (Table 6; Fig. 3)
Fig. 3.
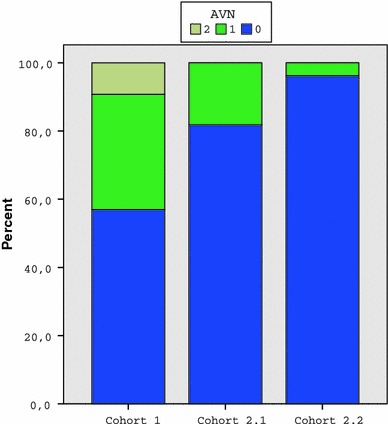
Distribution of avascular necrosis (AVN; TÖNNIS grading) in the three cohorts
As a reference, the grading according to Tönnis [51] of the X-ray at the end of primary treatment at about 12–18 months of age was evaluated. At initial treatment, in the pre-sono-screening cohort (cohort 1), i.e. “old” babies, 9.2 and 33.9% presented with grade 2 and grade 1 AVN, respectively, according to Tönnis. In the sono-screening cohorts (2.1 and 2.2), with mainly “young” babies, treatment started earlier and was less invasive and produced significantly less AVN, with complete disappearance of grade 2 AVN (P < 0.001).
Rate of persistent or secondary sloping of the ossified acetabular roof with the need for secondary surgical correction by acetabuloplasty
The P values are listed in Table 6: There was no statistical significance between the three cohorts (1, 2.1, 2.2) in terms of the rate of acetabuloplasties (P = 0.382).
Summary of results
Summarizing the results based on six out of seven parameters, the clinically important improvement and—if present—the statistical significance results between the cohorts 1 (without) and 2.1 and 2.2 (with sonographic screening); the cohorts 2.1 and 2.2 are different only in minor details and the differences are statistically not significant. But it should be mentioned again, that in the mixed-sono-screening-cohort 2.2 all included open reductions are referred cases from non-screened populations. The rate of acetabuloplasty is the only parameter statistically not significant in all three compared cohorts.
Discussion
In our retrospective comparative cohort study the clinically most important feature is a “young” age at diagnosis and initial treatment in the sonographic-screening cohorts (2.1 and 2.2) at a mean age of 2 months. Hip sonography according to the Graf-method [1–3, 52–54] provides a methodically standardized, safe, reliable and reproducible morphological diagnosis of the current pathomorphological state of the hip joint of the individual within the first 6 weeks of life and has been incorporated into the national sonographic screening programs of the public health authorities in Austria and Germany [15, 19, 22, 36]. If diagnosis is safe, reliable and reproducible, the stage of pathomorphology at early diagnosis is still “contained”. Thus, if biomechanical treatment follows in a quick and adequate fashion, the path back to a normo-morphology is comparatively short because secondary soft tissue contractures are still absent and the risk of AVN is lower. These facts explain the significant reduction in the number of preparatory measures, such as overhead traction, and supporting surgical procedures, such as adductor tenotomy, required to provide successful closed reduction and the absence of persisting stage 2 AVN [51] in the sono-screening cohorts (2.1, 2.2). Because of definitive early sonographic diagnosis, the formerly undiagnosed (“hidden”) pathomorphology cannot proceed to extremely severe stages of luxation with accompanying pathoanatomic obstacles against a successful closed reduction: thus, the chance for a successful closed reduction increases and the risk of requiring an open reduction decreases. Therefore, the most important message of our results is: age matters!
On the other hand, reproducible sonographic imaging of hip pathomorphology avoids overtreatment because the treatment is not based on subjective clinical suspicion but on documented pathomorphological findings [15, 19, 41].
In two studies, Grill et al. [15, 19] focused on the impact of sonographic hip screening on the rate of necessary surgical interventions, especially on the rate of open reductions. Using official population data compiled by the Austrian Ministry of Public Health, these authors were able to show that the overall mean rate of open reductions between 1993 and 2008 (1993 was the first year following the national implementation of general sonographic hip screening in Austria) was 0.23/1,000 (=2.3 of 10,000 live births), with a lowest single year value of 0.14/1,000 in 1998. These values, also given similar data from Germany [22, 36], are the lowest rates found in the literature, although an “adverse” effect was already considered by these authors based on the inclusion of non-screened “immigrants” from the neighbouring countries of the former Yugoslavia.
The data on our cohort 2.2 reflect the present situation in Austria, namely, babies routinely are scanned in Austria, and there are still some non-screened or late-diagnosed “immigrant” babies referred for open reduction. Thus, our cohort 2.2 reflects the clinical reality with a “mixed” group of decentered hip joints. If we had excluded the referred babies from this cohort, a homogeneous group of exclusively sonographically screened decentered hip joints would have provided a zero rate of open reductions.
Although we did not focus on the economic side of the Austrian national screening programme, the cost issues of various screening policies have been discussed in the literature.
To the best of our knowledge, there has been no published study documenting that sonographic screening would increase the overall costs of the public health system.
The authors of a number of studies [20, 22, 27, 38, 48, 49] were unsure as to whether sonographic screening reduces total healthcare costs. Gray et al. [18] calculated that in the sonography group the overall costs per patient were $190 less than those in the non-sonography group, but could not prove statistical significance. Clegg et al. [8] calculated the surgical costs per 1,000 live births based on a comparison of three screening policies in the city of Coventry: clinical screening (₤UK 5,110), selective sonographic screening (₤UK 3,811), general sonographic screening (₤UK 468).
Finally, there are three papers from Austria [15, 19, 41] that document a significant reduction of treatment costs with a moderate increase of diagnostic expenses. The calculations on the overall financial burden of the Austrian Public Health System seem to decrease over the time. Two special features in Austria support the financial containment for the public health system: the moderate costs for one ultrasonography (approx. 30 €) and the already documented decreased burden of THR in young patients with dysplastic osteoarthritis [15, 41].
In an as yet unpublished analysis, Thallinger and Grill (personal communication) focus on the cost-effectiveness of the Austrian sonographic hip screening programme: based on the official population data complied by the Austrian Ministry of Public Health, they can show that the Austrian approach to sonographic screening is not only effective in terms of orthopaedic outcome but also highly cost-efficient for the public health-system.
Basically, “to screen or not to screen” [4] is not a scientific decision but a political choice: do public health authorities spend our money for prevention (sono-screening) or for treatment (surgery)? In 1992, the Austrian Government voted for a general sonographic hip screening program according to the Graf-method for all infants within the first 6 weeks of life. Our data suggest that the vote for sono-screening has been a good decision on many fronts. According to information compiled by the Austrian Federal Ministry of Public Health, the coverage of the sonographic screening is about 96% [41].
The limitations of our single-center retrospective comparative cohort study are basically design-dependent. As it is a retrospective study, there is no directly corresponding control group and—as in all retrospective studies—there might have been some selection bias. However, we feel that we have avoided significant selection bias through out very careful case selection as described in the Materials and methods and in Fig. 1.
The merits of our study are that it reflects 30 years of experience of a tertiary referral center with a powerful clinical database: this study is not based on the official population data of the Austrian Ministry of Public Health, but evaluates and compares the development and outcome of treatment of the same pathology in the same institution under the same circumstances before and after the implementation of a nationwide sonographic hip screening in Austria in 1992. The comparison between our cohort 1 (without sono-screening) and cohorts 2.1 and 2.2 (with sono-screening) reveals overall clinically relevant and statistically significant improvements in the conservative treatment of decentered hip joints in DDH enabled by early diagnosis with sono-screening.
Our results suggest that early sonography-based standardized conservative treatment has become safer, shorter and simpler: “safer” because of a lower rate of AVN, “shorter” because of a shorter treatment time due to earlier onset and “simpler” due to fewer invasive treatment measures (Fig. 4). The key feature of all these improvements is a very early diagnosis of the pathomorphology and pathobiomechanics by a highly standardized diagnostic tool, which is made possible by hip sonography according to the Graf-method [1–3, 52–54]. A safe and reproducible diagnosis before the 6th week of life has a double positive impact on the outcome of treatment: a developing pathology will be detected at a very early stage of decentering, and the optimum potential of remodelling and maturation according to the sonographic “maturation curve” of Tschauner et al. 1994 [56] will be respected after an instant onset of an adequate conservative treatment.
Fig. 4.
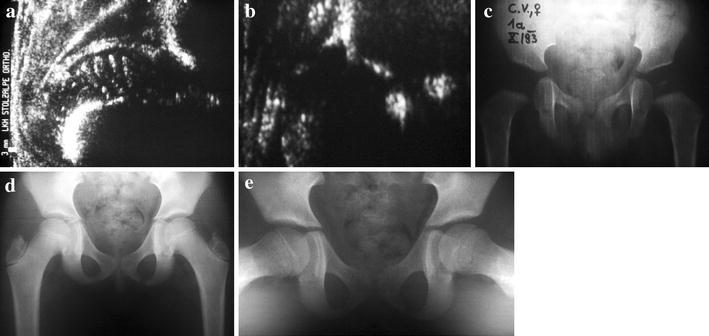
Typical clinical follow-up after early sonographic diagnosis. a Diagnosis of a highly decentered sonographic type 4 hip at the age of 3 weeks; treatment by closed reduction and squatting cast. b Well (re-)centered sonographic type 2b hip at the age of 3 months, still under functional biomechanical treatment using a removable brace in squatting position. c X-ray at the age of 1 year: sufficient bony maturation and free of AVN. d, e X-ray at the age of 9 years in anterior-posterior (d) and axial (e) views: symmetric bony development in both projections is shown
There was no statistical significance between the three cohorts in terms of rate of secondary surgical corrections by acetabuloplasty. We are currently unable to explain this surprising finding. One possible hypothesis might be the concept of “endogenous” dysplasia by Matthiessen [57], with a supposed “endogenous” growth disturbance factor that predominates after the end of primary biomechanical treatment. Another possible explanation might be the inclusion of non-screened referred cases in cohort 2.2. On the other hand, in our department, the frequency of late joint-preserving surgical corrections in young adults [58], such as multiplanar reorienting osteotomies of Ganz et al. [59] or Tönnis et al. [60], has significantly decreased since 2006: these are the adolescents who were born in the late 1980s and early 1990s, following implementation of the Austrian screening programme. A similar tendency is reported by Thaler et al. [41].
Conclusion
The national public health-based sonographic hip screening programme according to the Graf-technique within the first 6 weeks after birth, implemented in Austria in 1992, seems to be highly effective and cost efficient in enabling an adequate early conservative treatment with an extremely low rate of open reductions. The results of our single-center, retrospective, comparative cohort study support a statement of Tönnis, which is published in his “classic” study [51]: “Today the advantages of early treatment are well known: better results in less time with fewer complications”.
Acknowledgments
The authors would like to thank Prof. Graf for his pioneering work of a standardized and reproducible ultrasonographic technique to visualize all stages of DDH. This study was made possible by a grant of “Deutsche Arthrosehilfe e.V.” (a scientific non-profit organization with the aim of preventing osteoarthritis).
References
- 1.Graf R. The diagnosis of congenital hip joint dislocation by the ultrasonic compound treatment. Arch Orthop Traumat. 1980;97:117–133. doi: 10.1007/BF00450934. [DOI] [PubMed] [Google Scholar]
- 2.Graf R. The ultrasonic image of the acetabular rim in infants. An experimental and clinical investigation. Arch Orthop Traumat. 1981;99:35–41. doi: 10.1007/BF00400907. [DOI] [PubMed] [Google Scholar]
- 3.Graf R. New possibilities for the diagnosis of congenital hip joint dislocation by the ultrasonic compound treatment. J Pediatr Orthop. 1983;3:354–359. doi: 10.1097/01241398-198307000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Mahan ST, Katz JN, Kim YJ. To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Jt Surg (Am) 2009;91(7):1705–1719. doi: 10.2106/JBJS.H.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeree NR, Clarke NMP. Ultrasound imaging and secondary screening for congenital dislocation of the hip. J Bone Jt Surg (Br) 1994;76-B:525–533. [PubMed] [Google Scholar]
- 6.Castelein RM, Sauter AIM. Ultrasound screening for congenital dysplasia of the hip in newborns; its value. J Pediatr Orthop. 1988;8:666–670. doi: 10.1097/01241398-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Clarke NMP, Clegg J, Al-Chalabi AN. Ultrasound screening for hips at risk for CDH: failure to reduce the incidence of late cases. J Bone Jt Surg (Br) 1989;71-B:9–12. doi: 10.1302/0301-620X.71B1.2644290. [DOI] [PubMed] [Google Scholar]
- 8.Clegg J, Bache CE, Raut VV. Financial justification for routine ultrasound screening of the neonatal hip. J Bone Jt Surg (Br) 1999;81-B:852–857. doi: 10.1302/0301-620X.81B5.9746. [DOI] [PubMed] [Google Scholar]
- 9.Dorn U, Neumann D. Ultrasound for screening developmental dysplasia of the hip: a European perspective. Curr Opin Pediatr. 2005;17:30–33. doi: 10.1097/01.mop.0000151554.10176.34. [DOI] [PubMed] [Google Scholar]
- 10.Düppe H, Danielsson LG. Screening of neonatal instability and of developmental dislocation of the hip—a survey of 132 601 living newborn infants between 1956 and 1999. J Bone Jt Surg (Br) 2002;84-B:878–885. doi: 10.1302/0301-620X.84B6.12326. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood DM. Neonatal hip screening. Lancet. 2003;361:595–597. doi: 10.1016/S0140-6736(03)12519-6. [DOI] [PubMed] [Google Scholar]
- 12.Eggl H, Krismer M, Klestil T, Frischhut B. Auswirkungen des Hüftsonographiescreenings. Eine epidemiologische Studie (in German) Orthopäde. 1993;22:277–279. [PubMed] [Google Scholar]
- 13.Exner GU. Ultrasound screening for hip dysplasia in neonates. J Pediatr Orthop. 1988;8:656–660. doi: 10.1097/01241398-198811000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Falliner A, Hassenpflug J. Der Einfluß der Sonographie auf Diagnose und Behandlung der sog. angeborenen Hüftgelenksluxation (in German) Z Orthop. 1994;132(1994):505–512. doi: 10.1055/s-2008-1039478. [DOI] [PubMed] [Google Scholar]
- 15.Farr S, Grill F, Müller D. Wann ist der optimale Zeitpunkt für ein sonographisches Hüftscreening? When is the optimal time for hip ultrasound screening? (in German) Orthopäde. 2008;37(6):532–540. doi: 10.1007/s00132-008-1236-2. [DOI] [PubMed] [Google Scholar]
- 16.Graf R, Tschauner Chr, Klapsch W. Progress in prevention of late developmental dislocation of the hip by sonographic newborn hip “screening”: results of a comparative follow-up study. J Pediatr Orthop (Part B) 1993;2:115–121. doi: 10.1097/01202412-199302020-00005. [DOI] [Google Scholar]
- 17.Graf R, Tschauner Chr. Ultrasound screening in the neonatal period. Baillière's Clin Orthop. 1996;1(1):117–133. [Google Scholar]
- 18.Gray A, Elbourne D, Dezateux C, King A, Quinn A, Gardner F. Economic evaluation of ultrasonography in the diagnosis, management of developmental hip dysplasia in the United Kingdom, Ireland. J Bone Jt Surg (Am) 2005;87(11):2472–2479. doi: 10.2106/JBJS.D.01997. [DOI] [PubMed] [Google Scholar]
- 19.Grill F, Müller D. Results of hip ultrasonographic screening in Austria (in German) Orthopäde. 1997;26(1):25–32. doi: 10.1007/s001320050066. [DOI] [PubMed] [Google Scholar]
- 20.Holen KJ, Tegnander A, Bredland T, Johansen OJ, Saether OD, Eik-Nes SH, Terjesen T. Universal or selective screening of the neonatal hip using ultrasound—a prospective, randomised trial of 15 529 newborn infants. J Bone Jt Surg (Br) 2002;84-B:886–890. doi: 10.1302/0301-620X.84B6.12093. [DOI] [PubMed] [Google Scholar]
- 21.Homer CJ, Baltz RD, Hickson GB, Miles PV, Newman TB, Shook JE, Zurhellen WM. Clinical practice guideline: early detection of developmental dysplasia of the hip committee on quality improvement of the American academy of pediatrics. Pediatrics. 2000;105(4):896–905. doi: 10.1542/peds.105.4.896. [DOI] [PubMed] [Google Scholar]
- 22.Ihme N, Altenhofen L, von Kries R, Niethard FU. Sonographisches Hüftscreening in Deutschland:Ergebnisse und Vergleich mit anderen Screeningverfahren. Hip ultrasound screening in Germany. Results and comparison with other screening procedures (German) Orthopäde. 2008;37(6):541–549. doi: 10.1007/s00132-008-1237-1. [DOI] [PubMed] [Google Scholar]
- 23.Jellicoe P, Aitken A, Wright K. Ultrasound screening in developmental hip dysplasia: do all scanned hips need to be followed up? J Pediatr Orthop B. 2007;16(3):192–195. doi: 10.1097/BPB.0b013e328014058d. [DOI] [PubMed] [Google Scholar]
- 24.Jones D, Dezateux CA, Danielsson LG, Paton RW, Clegg J. Topic for debate: at the crossroads—neonatal detection of developmental dysplasia of the hip. J Bone Jt Surg (Br) 2000;82-B:160–164. doi: 10.1302/0301-620X.82B2.10761. [DOI] [PubMed] [Google Scholar]
- 25.Marks DS, Clegg J, Al-Chalabi AN. Routine ultrasound screening for neonatal hip instability. J Bone Jt Surg (Br) 1994;76-B:534–538. [PubMed] [Google Scholar]
- 26.Patel H, Canadian Task Force on Preventive Health Care Preventive health care, 2001 update: screening and management of developmental dysplasia of the hip in newborns. Can Med Assoc J. 2001;164(12):1669–1677. [PMC free article] [PubMed] [Google Scholar]
- 27.Paton RW, Hossain S, Eccles K. Eight-year prospective targeted ultrasound screening program for instability and at-risk hip joints in developmental dysplasia of the hip. J Pediatr Orthop. 2002;22:338–341. [PubMed] [Google Scholar]
- 28.Roovers EA, Boere-Boonekamp MM, Mostert AK, et al. The natural history of developmental dysplasia of the hip: sonographic findings in infants of 1–3 months of age. J Pediatr Orthop B. 2005;14:325–330. doi: 10.1097/01202412-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Rosendahl K, Toma P. Ultrasound in the diagnosis of developmental dysplasia of the hip in newborns: the European approach. A review of methods, accuracy and clinical validity. Eur Radiol. 2007;17(8):1960–1967. doi: 10.1007/s00330-006-0557-y. [DOI] [PubMed] [Google Scholar]
- 30.Rosendahl K, Dezateux C, Fosse KR, Aase H, Aukland SM, Reigstad H, Alsaker T, Moster D, Lie RT, Markestad T. Immediate treatment versus sonographic surveillance for mild hip dysplasia in newborns. Pediatrics. 2010;125:e9–e16. doi: 10.1542/peds.2009-0357. [DOI] [PubMed] [Google Scholar]
- 31.Schwend RM, Schoenecker P, Richards BS, et al. Pediatric orthopaedic society of North America: screening the newborn for developmental dysplasia of the hip: now what do we do? J Pediatr Orthop. 2007;27(6):607–610. doi: 10.1097/BPO.0b013e318142551e. [DOI] [PubMed] [Google Scholar]
- 32.Shipman SA, Helfand M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117(3):557–576. doi: 10.1542/peds.2005-1597. [DOI] [PubMed] [Google Scholar]
- 33.Terjesen T. Ultrasonography for evaluation of hip dysplasia. Methods and policy in neonates, infants, and older children. Acta Orthop Scand. 1998;69:653–662. doi: 10.3109/17453679808999277. [DOI] [PubMed] [Google Scholar]
- 34.Tomà P, Valle M, Rossi U, Brunenghi GM. Paediatric hip—ultrasound screening for developmental dysplasia of the hip: a review. Eur J Ultrasound. 2001;14:45–55. doi: 10.1016/S0929-8266(01)00145-8. [DOI] [PubMed] [Google Scholar]
- 35.Tschauner Ch, Klapsch W, Graf R. Einfluß der sonographischen Neugeborenenhüftvorsorge auf die Hüftkopfnekroserate und die Rate an operativen Interventionen (in German) Orthopäde. 1993;22:268–276. [PubMed] [Google Scholar]
- 36.von Kries R, Ihme N, Oberle D, Lorani A, Stark R, Altenhofen L, Niethard F. Effect of ultrasound screening on the rate of first operative procedures for developmental hip dysplasia in Germany. Lancet. 2003;362:1883–1887. doi: 10.1016/S0140-6736(03)14957-4. [DOI] [PubMed] [Google Scholar]
- 37.Wientroub S, Grill F. Ultrasonography in developmental dysplasia of the hip current concepts review. J Bone Jt Surg. 2000;82-A(7):1004–1018. doi: 10.2106/00004623-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Wirth T, Stratmann L, Hinrichs F. Evolution of late presenting developmental dysplasia of the hip and associated surgical procedures after 14 years of neonatal ultrasound screening. J Bone Jt Surg (Br) 2004;86-B:585–589. [PubMed] [Google Scholar]
- 39.Woolacott NF, Puhan MA, Steurer J, Kleijnen J. Ultrasonography in screening for developmental dysplasia of the hip in newborns: systematic review. Br Med J. 2005;330:1413. doi: 10.1136/bmj.38450.646088.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zenios M, Wilson B, Galasko CSB. The effect of selective ultrasound screening on late presenting DDH. J Pediatr Orthop B. 2000;9:244–247. doi: 10.1097/01202412-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Thaler M, Biedermann R, Lair J, Krismer M, Landauer F. Cost-effectiveness of universal ultrasound screening compared with clinical examination alone in the diagnosis and treatment of neonatal hip dysplasia in Austria. J Bone Jt Surg (Br) 2011;93-B:1126–1130. doi: 10.1302/0301-620X.93B8.25935. [DOI] [PubMed] [Google Scholar]
- 42.Dorn U. Hip screening in newborn infants. Clinical and ultrasound results (German) Wiener Klin Wochenschr (Suppl) 1990;181:3–22. [PubMed] [Google Scholar]
- 43.Austrian Federal Ministry of Public Health (2003) Einkauf von Hüftendoprothesen. Tätigkeitsbericht des Rechnungshofes (German) Wien 2003
- 44.Godward S, Dezateux C. Surgery for congenital dislocation of the hip in the UK as a measure of outcome of screening. Lancet. 1998;351:1149–1152. doi: 10.1016/S0140-6736(97)10466-4. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell SL, Ruiz AL, Lappin KJ, Cosgrove AP. Quality improvement report: clinical screening for developmental dysplasia of the hip in Northern Ireland. Br Med J. 2002;324(7344):1031–1033. doi: 10.1136/bmj.324.7344.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Grady MJ, Mujtaba G, Hanaghan J, Gallagher D. Screening for developmental dysplasia of the hip: current practices in Ireland. Ir J Med Sci. 2010;179(2):279–283. doi: 10.1007/s11845-009-0339-z. [DOI] [PubMed] [Google Scholar]
- 47.Vane AGS, Gwynne Jones DP, Dunbar JD, Theis JC. The diagnosis and management of neonatal hip instability. Results of a clinical and targeted ultrasound screening program. J Pediatr Orthop. 2005;25(3):292–295. doi: 10.1097/01.bpo.0000152944.02864.d4. [DOI] [PubMed] [Google Scholar]
- 48.Kamath S, Mehdi A, Wilson N, Duncan R. The lack of evidence of the effect of selective ultrasound screening on the incidence of late developmental dysplasia of the hip in the Greater Glasgow Region. J Pediatr Orthop B. 2007;16(3):189–191. doi: 10.1097/01.bpb.0000236229.44819.43. [DOI] [PubMed] [Google Scholar]
- 49.Rosendahl K, Markestad T, Lie RT. Ultrasound screening for developmental dysplasia of the hip in the neonate: the effect on treatment rate and prevalence of late cases. Pediatrics. 1994;94:47–52. [PubMed] [Google Scholar]
- 50.Dungl P, Grill F, Cechová I. Results of surgical repositioning of congenital hip dislocation. Acta Chir Orthop Traumatol Czech. 1993;60(6):324–333. [PubMed] [Google Scholar]
- 51.Tönnis D. Congenital dysplasia and dislocation of the hip in children and adults. Berlin: Springer; 1987. [Google Scholar]
- 52.Graf R, Scott S, Lercher K, Baumgartner F, Benaroya A (2006) Hip sonography: diagnosis and management of infant hip dysplasia, 2nd edn. Springer, Berlin
- 53.Graf R. DDH: diagnosis and treatment strategies. In: Bentley G, editor. European instructional lectures. Berlin: Springer; 2009. pp. 41–46. [Google Scholar]
- 54.Graf R, Lercher K, Tschauner C, Baumgartner F, Plattner F (2010) Sonographie der Säuglingshüfte und therapeutische Konsequenzen Ein Kompendium; 6. vollständig überarbeitete. Auflage Thieme, Stuttgart
- 55.Fettweis E. Sitz-Hock-Stellungsgips bei Hüftgelenksdysplasien (German) Arch Orthop Trauma Surg. 1968;63:38–51. doi: 10.1007/BF00418889. [DOI] [PubMed] [Google Scholar]
- 56.Tschauner C, Klapsch W, Baumgartner A, Graf R. „Reifungskurve“des sonographischen Alpha-Winkels nach Graf unbehandelter Hüftgelenke im ersten Lebensjahr (in German) Z Orthop. 1994;132:502–504. doi: 10.1055/s-2008-1039477. [DOI] [PubMed] [Google Scholar]
- 57.Matthiessen HD (1997) Das Problem der „endogenen“Dysplasie (in German). In: Tschauner C (Hrsg) Die Hüfte. S. Enke, Stuttgart, pp 45–57
- 58.Millis MB, Kim YJ. Rationale of osteotomy and related procedures for hip preservation: a review. Clin Orthop Relat Res. 2002;405:108–121. doi: 10.1097/00003086-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Ganz R, Klaue K, Vinh TS, Mast JW, et al. A new periacetabular osteotomy for the treatment of hip dysplasia. Clin Orthop. 1988;232(1988):26–36. [PubMed] [Google Scholar]
- 60.Tönnis D, Arning A, Bloch M, Heinecke A, Kalchschmidt K. Triple pelvic osteotomy. J Pediatr Orthop B. 1994;3:54–67. doi: 10.1097/01202412-199403010-00011. [DOI] [Google Scholar]


