Abstract
mRNAs of vesicular stomatitis virus (VSV), a prototype of nonsegmented negative strand (NNS) RNA viruses (e.g., rabies, measles, mumps, Ebola, and Borna disease viruses), possess the 5′-terminal cap structure identical to that of eukaryotic mRNAs, but the mechanism of mRNA cap formation is distinctly different from the latter. The elucidation of the unconventional capping of VSV mRNA remained elusive for three decades since the discovery of the cap structure in some viral and eukaryotic mRNAs in 1975. Only recently our biochemical studies revealed an unexpected strategy employed by vesiculoviruses (VSV and Chandipura virus, an emerging arbovirus) to generate the cap structure. This article summarizes the historical and current research that led to the discovery of the novel vesiculoviral mRNA capping reaction.
Keywords: vesicular stomatitis virus, vesiculovirus, mRNA capping, cap structure, polyribonucleotidyltransferase, guanylyltransferase
1. Introduction
The Mononegavirales order consists of four families, Rhabdoviridae, Paramyxoviridae, iloviridae, and Bornaviridae, and comprises a large number of non-segmented negative strand (NNS) RNA viruses including life-threatening human pathogens (e.g., rabies (Rhabdoviridae), measles (Paramyxoviridae), Ebola (Filoviridae)). Over the past forty years, vesicular stomatitis virus (VSV, an animal vesiculovirus belonging to the Rhabdoviridae family) has served as a paradigm for studying the molecular mechanisms of mRNA biogenesis by these NNS RNA viruses. These studies include pioneering discoveries of the presence of the virion-associated RNA-dependent RNA polymerase (RdRp) (Baltimore et al., 1970), mRNA capping enzyme (CE) (Abraham et al., 1975a; Abraham et al., 1975b), cap methyltransferases (MTases) (Abraham et al., 1975a; Testa and Banerjee, 1977), and poly(A) polymerase (Banerjee and Rhodes, 1973) activities. Consequently, VSV became a prototype virus for biochemical studies on mRNA synthesis of NNS RNA viruses primarily due to the following reasons: VSV shows only negligible pathogenicity in humans, multiplies rapidly in a variety of cultured cells, and exhibits the highest RdRp activity in vitro compared to other NNS RNA viruses (e.g., Sendai virus (Paramyxoviridae), human respiratory syncytial virus (Paramyxoviridae), measles virus (Paramyxoviridae)); their transcription activities in vitro are significantly lower than that of VSV. Thus, the inability to develop a robust in vitro transcription system for other NNS RNA viruses stymied the progress in our understanding of the molecular mechanisms of their mRNA biogenesis. Thus, VSV historically served as an excellent model to gain information of its gene expression that could be directly extended to other NNS RNA viruses belonging to different families.
Among NNS RNA viruses, VSV has the simplest RNA genome (approximately 11 kilobases), which is composed of five genes encoding nucleocapsid (N), phospho- (P), matrix (M), glyco- (G), and large (L) proteins (Fig. 1) (reviewed in Lyles and Rupprecht, 2007). The genomic RNA and the N, P, and L proteins are assembled into a viral ribonucleoprotein (RNP) complex that serves as a basal transcription apparatus (Emerson and Yu, 1975). The RNP complex is encased by an envelope composed of a cellular lipid bilayer studded by the G protein, which is essential for receptor binding and cell entry (reviewed in Roche et al., 2008). The M protein is located underneath the lipid bilayer to ensure the structural integrity of the virus particle (reviewed in Jayakar et al., 2004). Recently, the supermolecular structure of the bullet-shaped virus particle has been determined by cryo-electron microscopy (Ge et al., 2010). The genomic RNA is encapsidated with the N proteins to form the helical N-RNA complex (Green et al., 2006), which functions as a template for transcription as well as replication. The multifunctional L protein is associated with its co-factor P protein to form the RdRp complex (Emerson and Yu, 1975). The RdRp complex interacts with the N-RNA complex and transcribes the genomic RNA into five monocistronic mRNAs with a 5′-cap structure and 3′-poly(A) tail (Fig. 2).
Fig. 1. Schematic representations of the VSV virion and genome.
The relative locations of the viral proteins and genome in the VSV virion are indicated. The gene organization of the VSV genome is shown in the 3′ to 5′ order. Le and Tr indicate the terminal leader and trailer regions, respectively.
Fig. 2. Synthesis of the capped and polyadenylated mRNA from the VSV genome.
The VSV RdRp complex transcribes the negative strand genome in the N-RNA complex into the uncapped leader RNA and five monocistronic mRNAs with the 5′-cap 1 (m7GpppAm-) structure and 3′-poly(A) tail. Each viral gene possesses the conserved gene-start and gene-end sequences, which act as transcription initiation and terminination/polyadenylation signals, respectively.
Recently, we have made some seminal findings, which have a far-reaching implication for understanding the basis of a common strategy for “mRNA capping”; one of the major viral mRNA processing events, used by NNS RNA viruses. A structural hallmark of eukaryotic mRNA is the presence of the 5′-terminal cap structure, which is required for various aspects of mRNA metabolism including mRNA splicing, transport, translation, and stability (reviewed in Banerjee, 1980; Cougot et al., 2004; Furuichi and Shatkin, 2000). Although VSV mRNAs were found to contain the cap structure, the precise mechanism of VSV mRNA capping remained elusive for a long time. By using our new in vitro RNA capping system, we have recently discovered that the mechanism of mRNA capping mediated by the VSV L protein is fundamentally different from that of eukaryotic host cells (Ogino and Banerjee, 2007; Ogino and Banerjee, 2008; Ogino et al., 2010). In this review, we briefly trace the history of research on viral and eukaryotic mRNA capping and then describe the newly discovered mechanism of mRNA capping by VSV and another vesiculovirus.
2. mRNA cap structure
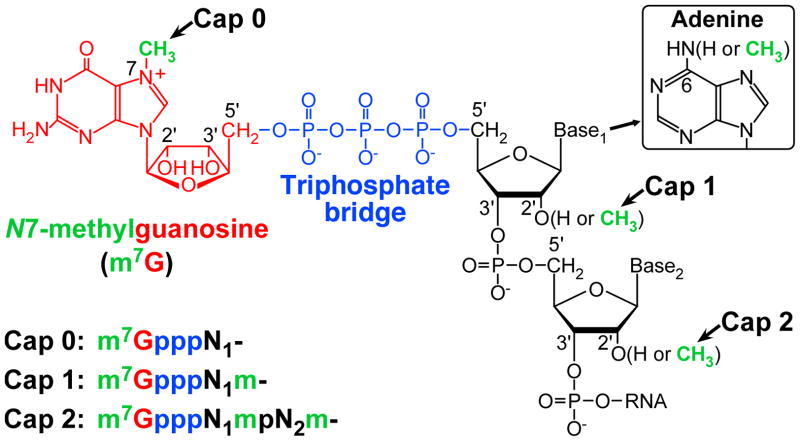
Eukaryotic mRNAs have the 5′-terminal cap structure, m7G(5′)ppp(5′)N1(m)pN2(m)-, in which N7-methylguanosine (m7G) blocks the first nucleoside (N1) of mRNA through a 5′-5′ triphosphate linkage (Fig. 3). The blocking guanosine is universally methylated at the N7 position in all eukaryotes. In higher eukaryotes, the N1 and N2 nucleosides are further methylated at the 2′-O positions to various degrees, thereby generating different types of the cap structure: m7GpppN- (cap 0), m7GpppNm- (cap 1), and m7GpppNmpNm- (cap 2) (reviewed in Banerjee, 1980; Furuichi and Shatkin, 2000). In addition, when N1 is adenosine, the cap structure is known to be methylated at the adenine-N6 position to form m7Gpppm6Am- (m6Am denotes N6,2′-O-dimethyladenosine) (Wei et al., 1975a).
Fig. 3. The 5′-terminal cap structure of eukaryotic mRNA.
The cap structure is composed of N7-methylguanosine (m7G) linked to the first nucleoside (N1) of mRNA through an inverted 5′-5′ triphosphate bridge. The blocking guanosine and triphosphate bridge are shown in red and blue, respectively. Methyl groups are shown in green. In higher eukaryotes, the cap 0 structure (m7GpppN-) is further methylated at the 2′-O positions of N1 and N2 to generate the cap 1 and cap 2 structures. When Base1 is adenine, its N6 position is frequently methylated.
The unique cap structure was originally identified in mRNAs of dsRNA viruses (cytoplasmic polyhedrosis virus (Furuichi and Miura, 1975) and reovirus (Furuichi et al., 1975a)) and a DNA virus (vaccinia virus (Wei and Moss, 1975)), and was subsequently found in eukaryotic mRNAs (Adams and Cory, 1975; Desrosiers et al., 1975; Furuichi et al., 1975b; Perry and Kelley, 1975; Wei et al., 1975b). Shortly after the discovery of the cap structure, the same blocking structure was also found in VSV mRNAs (Abraham et al., 1975a).
3. Conventional mRNA cap formation
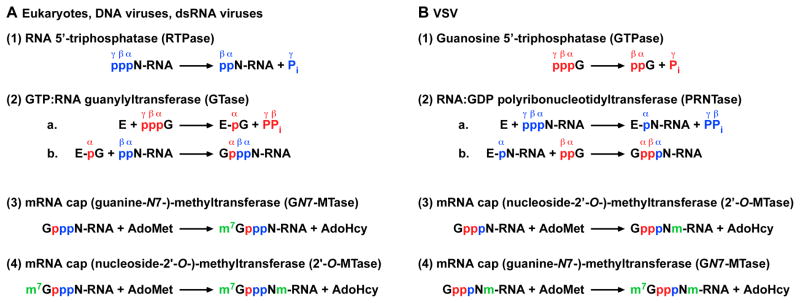
In eukaryotic cells, the cap core structure (GpppN-) is co-transcriptionally formed on the 5′-end of pre-mRNA by the conventional CE having two enzymatic activities, as follows (see Fig. 4A) (reviewed in Banerjee, 1980; Furuichi and Shatkin, 2000; Ghosh and Lima, 2010; Mizumoto and Kaziro, 1987; Shuman, 2001): (1) RNA 5′-triphosphatase (RTPase) removes the γ-phosphate from 5′-triphosphorylated RNA (pppN-RNA) to generate 5′-diphosphorylated RNA (ppN-RNA), (2a) GTP:RNA guanylyltransferase (GTase) reacts with GTP to form a covalent enzyme-GMP (E-pG) intermediate, and (2b) the E-pG intermediate transfers GMP to ppN-RNA to yield Gp-ppN-RNA. After the cap core formation on pre-mRNA, (3) mRNA cap (guanine-N7)-methyltransferase (GN7-MTase) transfers a methyl group from S-adenosyl-L-methionine (AdoMet) to GpppN-RNA to produce m7GpppN (cap 0)-RNA. In higher eukaryotes, (4) m7GpppN-RNA is further methylated at the ribose-2′-O position by mRNA cap (nucleoside-2′-O-)-methyltransferase (2′-O-MTase) to form m7GpppNm (cap 1)-RNA. These sequential reactions for the cap 1 formation were initially proposed for reovirus (Furuichi et al., 1976) and vaccinia virus (Ensinger et al., 1975; Shuman and Hurwitz, 1981; Venkatesan et al., 1980). Currently, all eukaryotes, nucleocytoplasmic large DNA viruses (e.g., vaccinia virus, baculovirus), and dsRNA viruses (e.g., reovirus, bluetongue virus) are thought to follow the same pathway of mRNA cap formation (Furuichi and Shatkin, 2000; Mizumoto and Kaziro, 1987; Shuman, 2001).
Fig. 4. Conventional and unconventional pathways of mRNA cap formation.
The mechanisms of mRNA capping and cap methylation for eukaryotes, DNA viruses, and dsRNA viruses (A) are compared with those for VSV (B). The 5′-phosphate groups of GTP and RNA are shown in red and blue, respectively. Pi and PPi denote inorganic phosphate and pyrophosphate, respectively. E shows enzyme. AdoMet and AdoHcy indicate S-adenosyl-L-methionine and S-adenosyl-L-homocysteine, respectively. Methyl groups formed on the cap core structure (GpppN-) are shown in green.
4. VSV-associated cap-forming activities
As reported for other viruses (Furuichi and Miura, 1975; Furuichi et al., 1975a; Wei and Moss, 1975), mRNA capping and cap methylation activities of purified VSV were demonstrated by using an in vitro transcription system (Abraham et al., 1975a; Abraham et al., 1975b). When in vitro transcription was performed with detergent-disrupted VSV in the absence of AdoMet, the unmethylated cap core (GpppA-) structure with a -3 net charge was exclusively formed on the 5′-termini of in vitro mRNAs (Abraham et al., 1975b). The cap core structure was found to be quantitatively methylated at the guanine-N7 and ribose-2′-O positions during in vitro transcription in the presence of AdoMet to generate the cap 1 structure (m7GpppAm-) with a -2.5 net charge (Abraham et al., 1975a). These in vitro transcripts (12 to 18 S) including N, P, M and G mRNAs were shown to start with a common 5′-terminal sequence, (m7)GpppA(m)pApApCpApG- (Rhodes and Banerjee, 1976). Importantly, VSV mRNAs isolated from infected cells were demonstrated to possess more extensively methylated cap structures as m7Gppp(m6)A1mp(m6)A2(m)-, where A1 is predominantly m6Am and A2 is A, Am, or m6Am (Moyer et al., 1975; Moyer and Banerjee, 1976). Since the N6-methylation of A1 and A2 and 2′-O-methylation of A2 were not observed in in vitro VSV mRNAs (Abraham et al., 1975a), these reactions were suggested to be catalyzed by host MTases. Properties of the VSV-associated CE were significantly different from those of eukaryotic and other viral capping systems. In vitro VSV mRNA synthesis experiments with GTP or ATP labeled with 32P at different positions demonstrated that the α and β phosphates of GTP and the α phosphate of ATP are incorporated into the nuclease P1 and alkaline phosphatase-resistant cap core structure as (m7)Gpp-pA(m)- (Abraham et al., 1975a; Abraham et al., 1975b). In contrast, dsRNA viral, DNA viral, and eukaryotic capping systems are known to incorporate the α phosphate, but not the β phosphate, of GTP into the cap core structure as Gp-ppN- (Ensinger et al., 1975; Furuichi and Miura, 1975; Wei and Moss, 1977). Furthermore, unlike eukaryotic and other viral CEs, the VSV-associated CE cannot use exogenously added ppA-RNAs as substrates. Based on these observations, the VSV CE was suggested to co-transcriptionally transfer the GDP moiety of GTP to 5′-monophosphorylated RNA (pRNA), which, presumably, is produced by internal cleavage of a long precursor RNA (Banerjee et al., 1977; Colonno et al., 1976) or removal of 5′-β,γ-phosphates from pppRNA (Testa et al., 1980). However, unlike eukaryotic and other viral GTases, no VSV protein formed a covalent intermediate (e.g., enzyme-GDP complex) for the putative GDP transfer reaction when incubated with [α-32P]GTP. Due primarily to the recalcitrant nature of the VSV capping system, a thought-provoking model for unique VSV mRNA capping was envisaged and proposed based on some presumptive biochemical considerations (Shuman, 1997). Nevertheless, the precise mechanism of cap formation in the VSV system remained enigmatic for decades.
With regard to the MTase activity, a striking difference was also observed between VSV and other viral and eukaryotic systems. When in vitro transcription was performed in the presence of limited concentrations (<0.1 μM) of AdoMet, a unique cap structure singly methylated at the ribose-2′-O position (GpppAm-) was generated on mRNAs (Testa and Banerjee, 1977). In the presence of higher concentrations (>5 μM) of AdoMet, the cap structure was methylated at both the guanine-N7 and ribose-2′-O positions to form the cap 1 structure (m7GpppAm-) (Testa and Banerjee, 1977). Furthermore, pulse-chase experiments showed that GpppAm-RNA acted as a precursor for m7GpppAm-RNA (Testa and Banerjee, 1977), indicating that ribose-2′-O methylation of the VSV mRNA cap precedes guanine-N7 methylation. This sequence of cap methylation is distinctly different from higher eukaryotes (Langberg and Moss, 1981; Mizumoto and Lipmann, 1979; Wei and Moss, 1977), vaccinia virus (Barbosa and Moss, 1978; Ensinger et al., 1975; Martin and Moss, 1975; Martin and Moss, 1976), reovirus (Furuichi et al., 1976), and cytoplasmic polyhedrosis virus (Furuichi, 1981). Again, in contrast to eukaryotes, vaccinia virus, and reovirus, the VSV MTases could not methylate exogenously added GpppA (a dinucleotide cap analogue) or full-length VSV mRNAs starting with GpppApApCpApG-, suggesting that these methylation reactions are tightly coupled to mRNA synthesis (Banerjee, 1980).
5. Unconventional mRNA capping by the VSV L protein
It became apparent that the major obstacle in investigating the VSV capping reaction was the lack of an appropriate and suitable in vitro assay system to measure the capping activity independent of transcription. The recent technical breakthrough is the development of a new VSV capping system with an exogenously added RNA substrate that has greatly facilitated our understanding of the molecular mechanism of VSV mRNA capping (Ogino and Banerjee, 2007).
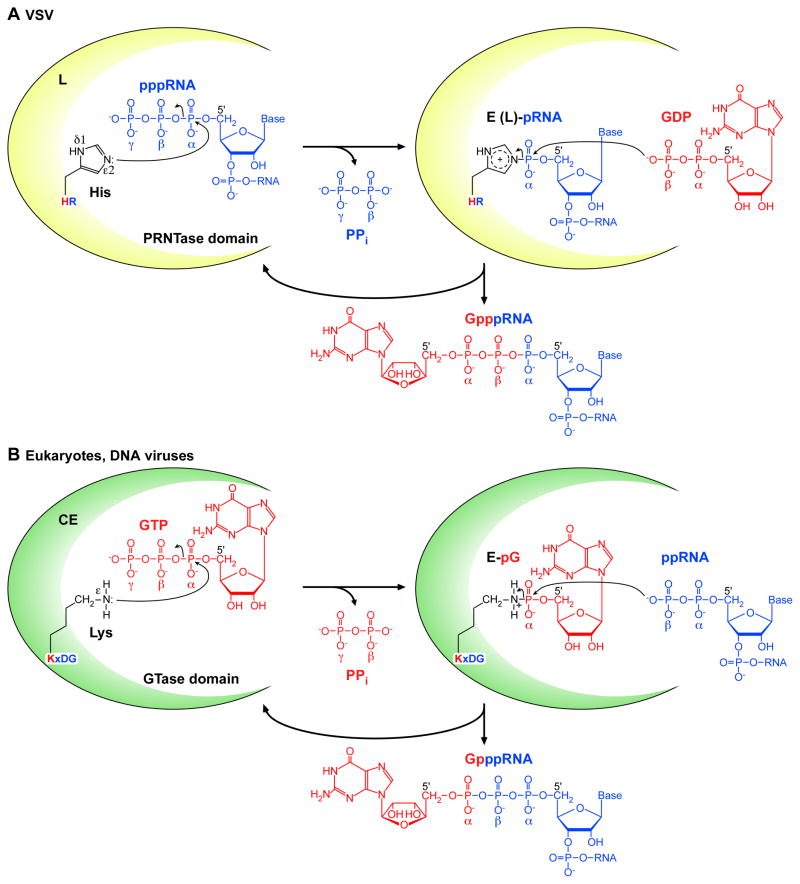
As shown in Fig. 2, all capped VSV mRNAs start with the common mRNA-start sequence (GpppAACGA-) (Rhodes and Banerjee, 1976), while the uncapped leader RNA synthesized from the 3′-end of the genomic RNA starts with a different sequence ((p)ppAACAG-) (Colonno and Banerjee, 1976; Colonno and Banerjee, 1978). Furthermore, several earlier studies have shown that small amounts of uncapped (ppp- or pp-) (11–42 nt) and capped (23–41 nt) short RNAs starting with the AACAG sequence are abortively synthesized during in vitro transcription (Lazzarini et al., 1982; Pinney and Emerson, 1982; Piwnica-Worms and Keene, 1983; Testa et al., 1980). These observations suggest that the VSV CE may specifically cap the 5′-triphosphate end of pre-mRNA starting with the AACAG sequence at an early stage of transcription. To prepare RNA substrates for the VSV CE, oligo-RNAs representing the VSV mRNA-start (AACAG) and leader RNA-start (ACGAA) sequences were synthesized by T7 RNA polymerase from unique oligo-DNA templates (Ogino and Banerjee, 2007). Using these oligo-RNAs, it was shown that highly purified VSV RNP specifically caps the AACAG mRNA-start sequence, but not the ACGAA leader RNA-start sequence (Ogino and Banerjee, 2007). By using GTP labeled with 32P at different positions ([α-32P]GTP, [β-32P]GTP, and [γ-32P]GTP) as substrates, it was revealed that the VSV RNP incorporates the GDP moiety of GTP into the 5′-cap core structure as Gpp-pA- (Ogino and Banerjee, 2007). Thus, the development of this new capping assay conclusively demonstrated that a specific oligo-RNA could faithfully reproduce the unique VSV mRNA capping reaction that usually occurs co-transcriptionally in vitro (Abraham et al., 1975b). Subsequently, by using the AACAG RNA with a varying number of 5′-phosphate groups, it was shown that the VSV CE could use pppRNA, but not ppRNA or pRNA, as the substrate (Ogino and Banerjee, 2007). In contrast, the conventional CEs are known to use ppRNA, generated from pppRNA, as the guanylyl acceptor (Furuichi et al., 1976; Venkatesan et al., 1980; Venkatesan and Moss, 1980). Importantly, the fact that the VSV CE can use only pppRNA as the substrate explicitly ruled out the previous capping models, in which pRNA, produced from pppRNA or a long precursor RNA, was presumed to serve as the guanylyl acceptor (Banerjee et al., 1977; Colonno et al., 1976; Testa et al., 1980). Furthermore, the VSV CE was found to use GDP, instead of GTP, as a substrate (Ogino and Banerjee, 2007). In contrast, GTases of the conventional CEs utilize GTP, but not GDP, as the guanylyl donor (Furuichi et al., 1976; Venkatesan and Moss, 1980). Consistent with the finding that GDP can replace GTP in the capping reaction, the VSV RNP was found to exhibit a guanosine 5′-triphosphatase (GTPase) activity that converts GTP to GDP, which in turn acts as a substrate for the capping reaction (Ogino and Banerjee, 2007; Ogino and Banerjee, 2008). Finally, all these enzymatic activities required for the unique RNA capping reaction were found to be catalyzed solely by the recombinant VSV L protein (Ogino and Banerjee, 2007).
Another important feature of the VSV capping reaction is that the VSV L protein specifically caps RNAs with the ARCNG (R = A/G) sequence, in which the first A and third pyrimidine residues were critical (Ogino and Banerjee, 2007; Ogino and Banerjee, 2008). An earlier study showed that mutations of the first three nucleotides in the conserved gene-start sequence (3′-UUGUCNNUAG-) in a model VSV genome resulted in generation of 5′-uncapped (or unmethylated) short transcripts instead of 5′-capped full-length mRNAs (Stillman and Whitt, 1999). These observations suggested that the 5′-terminal ARC sequence of mRNA synthesized from the 3′-UYG (Y = U/C) gene-start sequence in the genomic RNA is essential for capping (and/or cap methylation) of nascent mRNA and subsequent mRNA chain elongation. Recently, a similar mutagenesis study also suggested the critical role of the 3′-UYG gene-start sequence in co-transcriptional mRNA capping (Wang et al., 2007). The mRNA-start sequences (5′-AACA[G/C/U]-) are conserved in mRNAs of rhabdoviruses belonging to the Vesiculovirus (e.g., VSV, SVCV, Chandipura virus, and Isfahan virus) (Hoffmann et al., 2002; Marriott, 2005), Ephemerovirus (e.g., bovine ephemeral fever virus and Adelaide River virus) (McWilliam et al., 1997), and Lyssavirus (e.g., rabies virus, Lagos bat virus, and Mokola virus) (Bourhy et al., 1993) genera in the Rhabdoviridae family. Therefore, these rhabdoviral L proteins may specifically recognize their mRNA-start sequences for mRNA capping, as observed for the VSV L protein. In contrast, other NNS RNA viruses have unique sets of mRNA start-sequence that are different from those of above rhabdoviruses (Kolakofsky et al., 1998; Muhlberger et al., 1996; Schneemann et al., 1994), suggesting that CEs of other NNS RNA viruses may exhibit distinct RNA sequence specificities.
As shown in Fig. 4B (1), the GTPase activity associated with the VSV L protein is required for removal of the γ-phosphate group of GTP to generate GDP at the first step of the unique capping reaction. At the second step, the VSV L protein was found to catalyze a novel RNA transfer reaction to GDP (Fig. 4B (2)) (Ogino and Banerjee, 2007; Ogino et al., 2010). When the VSV RNP complex or the recombinant L protein was incubated with various poly- and mono-nucleotides (pppAACAG, ppAACAG, pppACGAA, ATP, GTP, and GDP), the L protein was found to specifically react with pppAACAG (VSV mRNA start-sequence) to form an SDS-resistant complex with pRNA (designated as L-pRNA). Further analyses of the L-pRNA complex uncovered that the 5′-end phosphate of the RNA is covalently linked to the L protein through a phosphoamide bond (Ogino and Banerjee, 2007). Based on these findings and coupled with the analogy to the enzyme-GMP complex formation of GTases (Mizumoto et al., 1982; Shuman and Hurwitz, 1981; Venkatesan and Moss, 1982), it was suggested that the L-pRNA complex is a covalent intermediate in the unconventional capping reaction catalyzed by a new RNA transfer enzyme, referred to as RNA:GDP polyribonucleotidyltransferase (PRNTase) (Fig. 4B (2)) (Ogino and Banerjee, 2007). Recently, the purified L-pRNA complex was shown to transfer pRNA to GDP, but not to other NDPs, to form Gpp-pRNA (Ogino et al., 2010). Thus, PRNTase represents a new class of viral CEs that transfers pRNA from pppRNA to GDP (an RNA acceptor) through a covalent enzyme-pRNA (E-pRNA or L-pRNA) intermediate. In addition to GDP, dGDP was found to act as an efficient RNA acceptor to form 2′-deoxyguanosine(5′)triphospho(5′)adenosine (dGpppA) cap structure (Ogino et al., 2010). An earlier study has also showed that detergent-disrupted VSV can synthesize dGpppA-capped mRNAs containing internal dGMP residues in the presence of dGTP and three other NTPs (Schubert and Lazzarini, 1982). Therefore, the 2′-OH group of GDP does not contribute to its binding to the putative PRNTase domain in the VSV L protein. In contrast, for human GTase, dGTP does not serve as a guanylyl donor to generate the cap structure (Venkatesan and Moss, 1980), even though the enzyme inefficiently forms a covalent enzyme-dGMP complex (Venkatesan and Moss, 1982). Interestingly, vaccinia virus GTase can use dGTP as a guanylyl donor although to a lesser extent (Martin and Moss, 1976). The VSV L protein can also transfer pRNA to GTP to generate a cap-like structure with a −4 net charge, guanosine(5′)tetraphospho(5′)adenosine (Gppp-pA-), although the transfer efficiency is very low (Ogino and Banerjee, 2008). Similarly, some viral and eukaryotic GTases can form tetraphosphate-containing cap-like structures, but these enzymes transfer GMP from GTP to NTP or pppRNA to generate Gp-pppN or Gp-pppRNA (Cleveland et al., 1986; Smith and Furuichi, 1982; Wang and Shatkin, 1984; Yu and Shuman, 1996).
It is also important to note that the recombinant L protein alone was found to methylate the cap core structure on an oligo-RNA having the 10-nucleotide VSV mRNA-start sequence at the ribose-2′-O position followed by the guanine-N7 position (Fig. 4B (3) and (4)) (Rahmeh et al., 2009).
6. Unconventional capping enzyme domain in the VSV L protein
Although NNS RNA viruses show different morphological and biological properties, the structures of their L proteins are similar (Fig. 5) (Briese et al., 1994; Poch et al., 1990; Volchkov et al., 1999). Amino acid sequence analyses of some rhabdoviral, paramyxoviral, and filoviral L proteins suggested that they contain six conserved amino acid sequence blocks (I–VI) that are interrupted by variable sequences (Poch et al., 1990; Volchkov et al., 1999). The N-terminal region including the block III and the C-terminal region including the block VI were predicted to fold into RdRp (Poch et al., 1990) and AdoMet-dependent MTase (Bujnicki and Rychlewski, 2002; Ferron et al., 2002) domains, respectively, on the basis of their amino acid sequence similarities to known enzymes. The conserved GDN motif in the block III was predicted to be a counterpart of the divalent metal ion coordination motif (GDD) of positive strand RNA viral RdRp (Poch et al., 1990), and was shown to be essential for transcriptase activities of several L proteins (Chattopadhyay et al., 2004; Malur et al., 2002; Schnell and Conzelmann, 1995; Sleat and Banerjee, 1993). The C-terminal region including the block VI contains an AdoMet-binding glycine-rich motif ([G/A][D/E]GxG, x represents any amino acid) and a ribose-2′-O-MTase specific motif (K–D–K–E tetrad) (Bujnicki and Rychlewski, 2002; Ferron et al., 2002), and was identified as the cap MTase domain that may catalyze both the guanine-N7 and ribose-2′-O methylation reactions (Galloway et al., 2008; Grdzelishvili et al., 2005; Li et al., 2005; Li et al., 2006; Murphy and Grdzelishvili, 2009; Ogino et al., 2005; Rahmeh et al., 2009). However, other regions do not have any sequence similarity to known cellular and other viral proteins.
Fig. 5. Schematic structures of NNS RNA viral L proteins.
The L proteins of VSV (Rhabdoviridae), Sendai virus (SeV, Paramyxoviridae), Zaire ebolavirus (ZEBOV, Filoviridae), and Borna disease virus (BDV, Bornaviridae) are depicted as schematics. The positions of the six conserved amino acid sequence blocks (I–VI) and the putative RNA-dependent RNA polymerase (RdRp), polyribonucleotidyltransferase (PRNTase), and cap methyltransferase (MTase) domains are shown. The positions of some conserved amino acid sequence motifs are indicated.
The putative PRNTase domain in the VSV L protein was shown to form the covalent enzyme-pRNA (L-pRNA) intermediate for the unique capping reaction (Ogino and Banerjee, 2007; Ogino et al., 2010). The chemical nature of the covalent linkage between the L protein and pRNA is similar to that of a phosphoamide bond formed on histidine (Ogino and Banerjee, 2007). Recently, to identify the active site of the PRNTase domain in the VSV L protein, the covalent RNA attachment site was directly mapped by biochemical and mass spectrometric analyses (Ogino et al., 2010). These analyses indicated that the histidine residue at position 1,227 (H1227) in the VSV L protein is covalently linked to the 5′-monophosphate end of the RNA. H1227 was found as part of the histidine-arginine (HR) motif (H1227-R1228) in the blocks V of the L proteins. The HR motif is remarkably conserved in the L proteins of most NNS RNA viruses (more than 100 species, not shown) belonging to different families (see Fig. 6) (Koonin and Moss, 2010; Ogino et al., 2010), while the L proteins of a few fish rhabdoviruses (e.g., infectious haematopoietic necrosis virus, viral hemorrhagic septicemia virus) belonging to the Novirhabdovirus genus possess a similar histidine-lysine sequence instead of the HR motif. However, it remains unknown whether the HK sequence in the novirhabdoviral L proteins is a counterpart of the HR motif, because amino acid sequences surrounding the HK sequence show little similarities to those surrounding the HR motif (Koonin and Moss, 2010). Mutational analyses of the VSV L protein further showed that the HR motif and a basic amino acid residue (R1221) close to the HR motif are critical for the PRNTase activity at the step of the covalent L-pRNA intermediate formation (Ogino et al., 2010). Similarly, for the L protein of Chandipura virus (CHPV, a vesiculovirus closely related to VSV), the R1211, H1217, and R1218 residues (the counterparts of the R1221, H1227, and R1228 residues of the VSV L protein, respectively) were shown to be essential for the PRNTase activity at the step of the L-pRNA intermediate formation (Ogino and Banerjee, 2010). Since the arginine residue (VSV, R1221; CHPV, R1211) close to the HR motif is conserved only in the L proteins of rhabdoviruses belonging to the Vesiculovirus, Lyssavirus (e.g., rabies virus), and Ephemerovirus (e.g., bovine ephemeral fever virus) genera (Ogino et al., 2010), it appears to be specifically required for the rhabdoviral PRNTase reaction. Li et al. (Li et al., 2008) have identified the G1154, T1157, H1227, and R1228 residues of the VSV L protein that are required for cap formation by alanine scanning mutagenesis. The G1154 and T1157 residues are found within the [Y/W]xG[S/T/A]xT motif that is located ~75 residues upstream of the HR motif and conserved in L proteins of all known NNS RNA viruses including novirhabdoviruses (see Fig. 6). However, it remains unknown which step(s) of capping is impaired by the G1154A and T1157A mutations and whether other conserved amino acid residues in the block V have any roles in the capping reaction. The recent electron microscopic analysis (Rahmeh et al., 2010) suggested that the recombinant VSV L protein is composed of a ring-like N-terminal RdRp domain and three C-terminal globular domains, which may be responsible for mRNA modifications. However, the precise location of the putative PRNTase domain in the L protein remains unknown.
Fig. 6. Amino acid sequence alignment of the blocks V of NNS RNA viral L proteins.
The amino acid sequence of the block V of the VSV L protein (GenBank accession no.: K02378) is aligned with those of other representative NNS RNA viral L proteins using the PSI-Coffee program (Di Tommaso et al., 2011). Virus names (virus genera, virus families, and GenBank accession nos. in brackets) are as follows: RABV, rabies virus (Lyssavirus, Rhabdoviridae, M13215); BEFV, bovine ephemeral fever virus (Ephemerovirus, Rhabdoviridae, AF234533); SYNV, sonchus yellow net virus (Nucleorhabdovirus, Rhabdoviridae, L32603); NCMV, northern cereal mosaic virus (Cytorhabdovirus, Rhabdoviridae, AB030277); SeV, Sendai virus (Respirovirus, Paramyxoviridae, X03614); MeV, measles virus (Morbillivirus, Paramyxoviridae, M20865); MuV, mumps virus (Rubulavirus, Paramyxoviridae, D10575); NDV, Newcastle disease virus (Avulavirus, Paramyxoviridae, AY262106); NiV, Nipah virus (Henipavirus, Paramyxoviridae, AF212302); HRSV, human respiratory syncytial virus (Pneumovirus, Paramyxoviridae, M75730); HMPV, human metapneumovirus (Metapneumovirus, Paramyxoviridae, AF371337); ZEBOV, Zaire ebolavirus (Ebolavirus, Filoviridae, AF086833); MBGV, Marburg virus (Marburgvirus, Filoviridae, Z29337); BDV, Borna disease virus (Bornavirus, Bornaviridae, U04608); ABV, avian bornavirus (unclassified, Bornaviridae, GU249596); VHSV, viral hemorrhagic septicemia virus (Novirhabdovirus, Rhabdoviridae, Y18263); IHNV, infectious haematopoietic necrosis virus (Novirhabdovirus, Rhabdoviridae, X89213). The numbers indicate the amino acid positions in these L proteins. The conserved H, R (or K in the VHSV and IHNV L proteins), and other amino acid residues are shown in red, blue, and green, respectively. Identical (*), conserved (:), and semi-conserved (.) amino acids are indicated. The G1154, T1157, R1221, H1227 (the covalent RNA attachment site), and R1228 residues required for VSV mRNA capping are highlighted in yellow. Secondary structures of the blocks V of the VSV (upper) and IHNV (bottom) L proteins were predicted using the I-TASSER program (Roy et al., 2010). H (orange) and S (magenta) indicate predicted α-helices and β-strands, respectively.
7. Two distinct catalytic mechanisms of mRNA capping
Based on our biochemical analyses (Ogino and Banerjee, 2007; Ogino and Banerjee, 2008; Ogino and Banerjee, 2010; Ogino et al., 2010), a plausible chemical mechanism for the unconventional mRNA capping reaction by the vesiculoviral L proteins was proposed (Fig. 7A). At the first step, an electron lone pair on the ε2-nitrogen of the catalytic histidine residue (VSV, H1227; CHPV, H1217) in the HR motif probably attacks the αphosphorus in the 5′-tiphosphate group of pppRNA to form the enzyme-(histidyl-Nε2)-pRNA (E-pRNA or L-pRNA) intermediate with the concomitant release of inorganic pyrophosphate (PPi) (Ogino et al., 2010). Then, the β-phosphoryl group of GDP may nucleophilically attack the 5′-terminal α-phosphorus of the RNA in the L-pRNA intermediate to liberate Gpp-pRNA from the L protein (see Fig. 7A). Therefore, this novel polyribonucleotidyl transfer reaction engages the following chemical bond transformations: phosphoanhydride (pp-pRNA) → phosphoamide (L-pRNA) → phosphoanhydride (Gpp-pRNA). Although this bond transformation pathway and the final capped RNA product are identical to those for the mRNA cap formation by GTase of the conventional CE, the donor and acceptor substrates for PRNTase are completely opposite to those for GTase. The eukaryotic and DNA viral GTases have a conserved active site motif KxDG, in which the lysine (K) residue acts as the covalent GMP attachment site (reviewed in Ghosh and Lima, 2010; Shuman and Lima, 2004). As shown in Fig. 7B, an electron lone pair formed on the ε-nitrogen of the active site lysine residue in GTase nucleophilically attacks the α-phosphorus of GTP to form the covalent enzyme-(lysyl-Nε)-GMP (E-pG) intermediate with the release of PPi. Then, the β-phosphoryl group of ppRNA nucleophilically attacks the α-phosphorus of GMP in the E-pG intermediate, resulting in the release of Gp-ppRNA from the enzyme. These widely disparate mechanisms of mRNA capping by PRNTase and GTase conclusively account for the difference in the origins of phosphate groups forming the 5′-5′ triphosphate bridge in the cap structure generated by VSV and eukaryotes.
Fig. 7. Proposed catalytic mechanisms for the unconventional and conventional mRNA capping reactions.
(A) The putative PRNTase domain in the vesiculoviral L protein transfers pRNA from pppRNA (blue) to GDP (red) through the covalent enzyme-(histidyl-Nε2)-pRNA (E-pRNA or L-pRNA) intermediate. The histidine residue in the conserved HR motif acts as a nucleophile for the intermediate formation. (B) The GTase domain in the conventional CE transfers GMP from GTP (red) to ppRNA (blue) through the covalent enzyme-(lysyl-Nε)-GMP (E-pG) intermediate. The lysine residue in the conserved KxDG motif acts as a nucleophile for the intermediate formation. For detail, see text.
8. Conclusions and future perspectives
In recent years, there has been significant progress in understanding the unconventional mechanism of mRNA capping by vesiculoviruses. It is now apparent that the vesiculoviral L proteins employ a novel “RNA transfer mechanism” rather than “GDP transfer mechanism” to produce the cap structure. The RNA transfer reaction to GDP by a novel enzymatic (PRNTase) domain in the vesiculoviral L proteins proceeds via a covalent enzyme-(histidyl-)-pRNA intermediate. The fact that the covalent polyribonucleotidyl site (HR motif) is highly conserved in most NNS RNA viral L proteins strongly suggests that the PRNTase domains in these L proteins have evolved from a common ancestor (Ogino et al., 2010). This hypothesis is further supported by extensive amino acid sequence analyses of the putative PRNTase domains in NNS RNA viral L proteins (Koonin and Moss, 2010). If the implied generality of the unconventional mechanism of NNS RNA viral mRNA capping is verified by using other L proteins, these findings will contribute enormously to NNS RNA virus biology. Furthermore, detailed characterization of NNS RNA viral PRNTase domains will provide new insight into their evolutionary origin and molecular diversification.
Although several amino acid residues in the blocks V of the VSV and CHPV L proteins were reported to be required for the PRNTase activity (Ogino and Banerjee, 2010; Ogino et al., 2010) or some step(s) of RNA capping (Li et al., 2008), the precise location of the PRNTase domain in the L protein remains unknown. Mapping of the putative PRNTase domain followed eventually by X-ray crystallographic analyses of its three-dimensional structures would certainly provide deeper insight into the mode of the unique capping reaction. In contrast to abrogation of the VSV L PRNTase and RdRp activities upon mutations in the HR and GDN motifs, respectively, these mutations did not abolish the GTPase activity (Ogino et al., 2010). These results suggest that the GTPase active site is located separately from the PRNTase and RdRp active sites. However, there are no significant amino acid sequence similarity between NNS RNA viral L proteins and known NTPases, suggesting that the L protein may have a novel GTPase domain. Thus, it is important to identify the active site and domain of GTPase in the L protein. Several studies suggest that failure in co-transcriptional mRNA capping in VSV and HRSV possibly causes premature-termination of transcription (Li et al., 2009; Li et al., 2008; Liuzzi et al., 2005; Stillman and Whitt, 1999). Since mRNA capping occurs on nascent pre-mRNA at an early stage of transcription (Lazzarini et al., 1982; Pinney and Emerson, 1982; Piwnica-Worms and Keene, 1983; Tekes et al., 2011; Testa et al., 1980), it is interesting to reveal the precise mechanisms underlying the cooperative regulation of mRNA capping and elongation by a transcribing L protein. Finally, we propose that PRNTase is an ideal target to develop anti-NNS RNA viral agents for the following reasons: (1) the capping reaction mediated by the PRNTase activity is completely different from that by the mammalian CE (Ogino and Banerjee, 2007; Ogino and Banerjee, 2010; Ogino et al., 2010), (2) there appears to be no PRNTase homologue in mammals (Koonin and Moss, 2010), and (3) the PRNTase active site is essential for viral growth (unpublished data). Hence, finding specific PRNTase inhibitors will be a challenge for the future development of anti-NNS RNA viral agents.
Acknowledgments
We thank Satya P. Yadav for his contribution to our work. The work was supported by a grant from the National Institutes of Health (AI26585).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tomoaki Ogino, Email: oginot@ccf.org.
Amiya K. Banerjee, Email: banerja@ccf.org.
References
- Abraham G, Rhodes DP, Banerjee AK. The 5′ terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975a;5:51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Abraham G, Rhodes DP, Banerjee AK. Novel initiation of RNA synthesis in vitro by vesicular stomatitis virus. Nature. 1975b;255:37–40. doi: 10.1038/255037a0. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. Modified nucleosides and bizarre 5′-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D, Huang AS, Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci USA. 1970;66:572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AK. 5′-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980;44:175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AK, Abraham G, Colonno RJ. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977;34:1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Rhodes DP. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci USA. 1973;70:3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa E, Moss B. mRNA(nucleoside-2′-)-methyltransferase from vaccinia virus. Characteristics and substrate specificity. J Biol Chem. 1978;253:7698–7702. [PubMed] [Google Scholar]
- Bourhy H, Kissi B, Tordo N. Molecular diversity of the Lyssavirus genus. Virology. 1993;194:70–81. doi: 10.1006/viro.1993.1236. [DOI] [PubMed] [Google Scholar]
- Briese T, Schneemann A, Lewis AJ, Park YS, Kim S, Ludwig H, Lipkin WI. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnicki JM, Rychlewski L. In silico identification, structure prediction and phylogenetic analysis of the 2′-O-ribose (cap 1) methyltransferase domain in the large structural protein of ssRNA negative-strand viruses. Protein Eng. 2002;15:101–108. doi: 10.1093/protein/15.2.101. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Raha T, Shaila MS. Effect of single amino acid mutations in the conserved GDNQ motif of L protein of Rinderpest virus on RNA synthesis in vitro and in vivo. Virus Res. 2004;99:139–145. doi: 10.1016/j.virusres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Cleveland DR, Zarbl H, Millward S. Reovirus guanylyltransferase is L2 gene product lambda 2. J Virol. 1986;60:307–311. doi: 10.1128/jvi.60.1.307-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno RJ, Abraham G, Banerjee AK. Blocked and unblocked 5′termini in vesicular stomatitis virus product RNA in vitro: their possible role in mRNA biosynthesis. Prog Nucleic Acid Res Mol Biol. 1976;19:83–87. doi: 10.1016/s0079-6603(08)60909-5. [DOI] [PubMed] [Google Scholar]
- Colonno RJ, Banerjee AK. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976;8:197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno RJ, Banerjee AK. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978;15:93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Cougot N, van Dijk E, Babajko S, Seraphin B. Cap-tabolism. Trends Biochem Sci. 2004;29:436–444. doi: 10.1016/j.tibs.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC, Friderici KH, Rottman FM. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5′ terminus. Biochemistry. 1975;14:4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011;39:W13–17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SU, Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975;15:1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger MJ, Martin SA, Paoletti E, Moss B. Modification of the 5′-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci USA. 1975;72:2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron F, Longhi S, Henrissat B, Canard B. Viral RNA-polymerases -- a predicted 2′-O-ribose methyltransferase domain shared by all Mononegavirales. Trends Biochem Sci. 2002;27:222–224. doi: 10.1016/s0968-0004(02)02091-1. [DOI] [PubMed] [Google Scholar]
- Furuichi Y. Allosteric stimulatory effect of S-adenosylmethionine on the RNA polymerase in cytoplasmic polyhedrosis virus. A model for the positive control of eukaryotic transcription. J Biol Chem. 1981;256:483–493. [PubMed] [Google Scholar]
- Furuichi Y, Miura K. A blocked structure at the 5′ terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975;253:374–375. doi: 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y, Morgan M, Muthukrishnan S, Shatkin AJ. Reovirus messenger RNA contains a methylated, blocked 5′-terminal structure: m-7G(5′)ppp(5′)G-MpCp. Proc Natl Acad Sci USA. 1975a;72:362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y, Morgan M, Shatkin AJ, Jelinek W, Salditt-Georgieff M, Darnell JE. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci USA. 1975b;72:1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y, Muthukrishnan S, Tomasz J, Shatkin AJ. Mechanism of formation of reovirus mRNA 5′-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976;251:5043–5053. [PubMed] [Google Scholar]
- Furuichi Y, Shatkin AJ. Viral and cellular mRNA capping: past and prospects. Adv Virus Res. 2000;55:135–184. doi: 10.1016/S0065-3527(00)55003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SE, Richardson PE, Wertz GW. Analysis of a structural homology model of the 2′-O-ribose methyltransferase domain within the vesicular stomatitis virus L protein. Virology. 2008;382:69–82. doi: 10.1016/j.virol.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Tsao J, Schein S, Green TJ, Luo M, Zhou ZH. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science. 2010;327:689–693. doi: 10.1126/science.1181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Lima CD. Enzymology of RNA cap synthesis. Wiley Interdiscip Rev RNA. 2010;1:152–172. doi: 10.1002/wrna.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grdzelishvili VZ, Smallwood S, Tower D, Hall RL, Hunt DM, Moyer SA. A single amino acid change in the L-polymerase protein of vesicular stomatitis virus completely abolishes viral mRNA cap methylation. J Virol. 2005;79:7327–7337. doi: 10.1128/JVI.79.12.7327-7337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313:357–360. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Schutze H, Mettenleiter TC. Determination of the complete genomic sequence and analysis of the gene products of the virus of Spring Viremia of Carp, a fish rhabdovirus. Virus Res. 2002;84:89–100. doi: 10.1016/s0168-1702(01)00441-5. [DOI] [PubMed] [Google Scholar]
- Jayakar HR, Jeetendra E, Whitt MA. Rhabdovirus assembly and budding. Virus Res. 2004;106:117–132. doi: 10.1016/j.virusres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Moss B. Viruses know more than one way to don a cap. Proc Natl Acad Sci USA. 2010;107:3283–3284. doi: 10.1073/pnas.0915061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg SR, Moss B. Post-transcriptional modifications of mRNA. Purification and characterization of cap I and cap II RNA (nucleoside-2′-)-methyltransferases from HeLa cells. J Biol Chem. 1981;256:10054–10060. [PubMed] [Google Scholar]
- Lazzarini RA, Chien I, Yang F, Keene JD. The metabolic fate of independently initiated VSV mRNA transcripts. J Gen Virol. 1982;58:429–441. doi: 10.1099/0022-1317-58-2-429. [DOI] [PubMed] [Google Scholar]
- Li J, Fontaine-Rodriguez EC, Whelan SP. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J Virol. 2005;79:13373–13384. doi: 10.1128/JVI.79.21.13373-13384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Rahmeh A, Brusic V, Whelan SP. Opposing effects of inhibiting cap addition and cap methylation on polyadenylation during vesicular stomatitis virus mRNA synthesis. J Virol. 2009;83:1930–1940. doi: 10.1128/JVI.02162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Rahmeh A, Morelli M, Whelan SP. A conserved motif in region v of the large polymerase proteins of nonsegmented negative-sense RNA viruses that is essential for mRNA capping. J Virol. 2008;82:775–784. doi: 10.1128/JVI.02107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JT, Whelan SP. A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc Natl Acad Sci USA. 2006;103:8493–8498. doi: 10.1073/pnas.0509821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi M, Mason SW, Cartier M, Lawetz C, McCollum RS, Dansereau N, Bolger G, Lapeyre N, Gaudette Y, Lagace L, Massariol MJ, Do F, Whitehead P, Lamarre L, Scouten E, Bordeleau J, Landry S, Rancourt J, Fazal G, Simoneau B. Inhibitors of respiratory syncytial virus replication target cotranscriptional mRNA guanylylation by viral RNA-dependent RNA polymerase. J Virol. 2005;79:13105–13115. doi: 10.1128/JVI.79.20.13105-13115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles DS, Rupprecht CE. Rhabdoviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1363–1408. [Google Scholar]
- Malur AG, Gupta NK, De Bishnu P, Banerjee AK. Analysis of the mutations in the active site of the RNA-dependent RNA polymerase of human parainfluenza virus type 3 (HPIV3) Gene Expr. 2002;10:93–100. [PMC free article] [PubMed] [Google Scholar]
- Marriott AC. Complete genome sequences of Chandipura and Isfahan vesiculoviruses. Arch Virol. 2005;150:671–680. doi: 10.1007/s00705-004-0452-2. [DOI] [PubMed] [Google Scholar]
- Martin SA, Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975;250:9330–9335. [PubMed] [Google Scholar]
- Martin SA, Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976;251:7313–7321. [PubMed] [Google Scholar]
- McWilliam SM, Kongsuwan K, Cowley JA, Byrne KA, Walker PJ. Genome organization and transcription strategy in the complex GNS-L intergenic region of bovine ephemeral fever rhabdovirus. J Gen Virol. 1997;78:1309–1317. doi: 10.1099/0022-1317-78-6-1309. [DOI] [PubMed] [Google Scholar]
- Mizumoto K, Kaziro Y. Messenger RNA capping enzymes from eukaryotic cells. Prog Nucleic Acid Res Mol Biol. 1987;34:1–28. doi: 10.1016/s0079-6603(08)60491-2. [DOI] [PubMed] [Google Scholar]
- Mizumoto K, Kaziro Y, Lipmann F. Reaction mechanism of mRNA guanylyltransferase from rat liver: isolation and characterization of a guanylyl-enzyme intermediate. Proc Natl Acad Sci USA. 1982;79:1693–1697. doi: 10.1073/pnas.79.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K, Lipmann F. Transmethylation and transguanylylation in 5′-RNA capping system isolated from rat liver nuclei. Proc Natl Acad Sci USA. 1979;76:4961–4965. doi: 10.1073/pnas.76.10.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer SA, Abraham G, Adler R, Banerjee AK. Methylated and blocked 5′ termini in vesicular stomatitis virus in vivo mRNAs. Cell. 1975;5:59–67. doi: 10.1016/0092-8674(75)90092-6. [DOI] [PubMed] [Google Scholar]
- Moyer SA, Banerjee AK. In vivo methylation of vesicular stomatitis virus and its host-cell messenger RNA species. Virology. 1976;70:339–351. doi: 10.1016/0042-6822(76)90276-2. [DOI] [PubMed] [Google Scholar]
- Muhlberger E, Trommer S, Funke C, Volchkov V, Klenk HD, Becker S. Termini of all mRNA species of Marburg virus: sequence and secondary structure. Virology. 1996;223:376–380. doi: 10.1006/viro.1996.0490. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Grdzelishvili VZ. Identification of sendai virus L protein amino acid residues affecting viral mRNA cap methylation. J Virol. 2009;83:1669–1681. doi: 10.1128/JVI.01438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Banerjee AK. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol Cell. 2007;25:85–97. doi: 10.1016/j.molcel.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ogino T, Banerjee AK. Formation of guanosine(5′)tetraphospho(5′)adenosine cap structure by an unconventional mRNA capping enzyme of vesicular stomatitis virus. J Virol. 2008;82:7729–7734. doi: 10.1128/JVI.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Banerjee AK. The HR motif in the RNA-dependent RNA polymerase L protein of Chandipura virus is required for unconventional mRNA-capping activity. J Gen Virol. 2010;91:1311–1314. doi: 10.1099/vir.0.019307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Kobayashi M, Iwama M, Mizumoto K. Sendai virus RNA-dependent RNA polymerase L protein catalyzes cap methylation of virus-specific mRNA. J Biol Chem. 2005;280:4429–4435. doi: 10.1074/jbc.M411167200. [DOI] [PubMed] [Google Scholar]
- Ogino T, Yadav SP, Banerjee AK. Histidine-mediated RNA transfer to GDP for unique mRNA capping by vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci USA. 2010;107:3463–3468. doi: 10.1073/pnas.0913083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RP, Kelley DE. Methylated constituents of heterogeneous nuclear RNA: presence in blocked 5′ terminal structures. Cell. 1975;6:13–19. doi: 10.1016/0092-8674(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Pinney DF, Emerson SU. Identification and characterization of a group of discrete initiated oligonucleotides transcribed in vitro from the 3′ terminus of the N-gene of vesicular stomatitis virus. J Virol. 1982;42:889–896. doi: 10.1128/jvi.42.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H, Keene JD. Sequential synthesis of small capped RNA transcripts in vitro by vesicular stomatitis virus. Virology. 1983;125:206–218. doi: 10.1016/0042-6822(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Poch O, Blumberg BM, Bougueleret L, Tordo N. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol. 1990;71:1153–1162. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]
- Rahmeh AA, Li J, Kranzusch PJ, Whelan SP. Ribose 2′-O methylation of the vesicular stomatitis virus mRNA cap precedes and facilitates subsequent guanine-N-7 methylation by the large polymerase protein. J Virol. 2009;83:11043–11050. doi: 10.1128/JVI.01426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmeh AA, Schenk AD, Danek EI, Kranzusch PJ, Liang B, Walz T, Whelan SP. Molecular architecture of the vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci USA. 2010;107:20075–20080. doi: 10.1073/pnas.1013559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DP, Banerjee AK. 5′-terminal sequence of vesicular stomatitis virus mRNA’s synthesized in vitro. J Virol. 1976;17:33–42. doi: 10.1128/jvi.17.1.33-42.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S, Albertini AA, Lepault J, Bressanelli S, Gaudin Y. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell Mol Life Sci. 2008;65:1716–1728. doi: 10.1007/s00018-008-7534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann A, Schneider PA, Kim S, Lipkin WI. Identification of signal sequences that control transcription of borna disease virus, a nonsegmented, negative-strand RNA virus. J Virol. 1994;68:6514–6522. doi: 10.1128/jvi.68.10.6514-6522.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Conzelmann KK. Polymerase activity of in vitro mutated rabies virus L protein. Virology. 1995;214:522–530. doi: 10.1006/viro.1995.0063. [DOI] [PubMed] [Google Scholar]
- Schubert M, Lazzarini RA. In vitro transcription of vesicular stomatitis virus. Incorporation of deoxyguanosine and deoxycytidine, and formation of deoxyguanosine caps. J Biol Chem. 1982;257:2968–2973. [PubMed] [Google Scholar]
- Shuman S. A proposed mechanism of mRNA synthesis and capping by vesicular stomatitis virus. Virology. 1997;227:1–6. doi: 10.1006/viro.1996.8305. [DOI] [PubMed] [Google Scholar]
- Shuman S. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol. 2001;66:1–40. doi: 10.1016/s0079-6603(00)66025-7. [DOI] [PubMed] [Google Scholar]
- Shuman S, Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci USA. 1981;78:187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S, Lima CD. The polynucleotide ligase and RNA capping enzyme superfamily of covalent nucleotidyltransferases. Curr Opin Struct Biol. 2004;14:757–764. doi: 10.1016/j.sbi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Banerjee AK. Transcriptional activity and mutational analysis of recombinant vesicular stomatitis virus RNA polymerase. J Virol. 1993;67:1334–1339. doi: 10.1128/jvi.67.3.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Furuichi Y. A unique class of compound, guanosine-nucleoside tetraphosphate G(5′)pppp(5′)N, synthesized during the in vitro transcription of cytoplasmic polyhedrosis virus of Bombyx mori. Structural determination and mechanism of formation. J Biol Chem. 1982;257:485–494. [PubMed] [Google Scholar]
- Stillman EA, Whitt MA. Transcript initiation and 5′-end modifications are separable events during vesicular stomatitis virus transcription. J Virol. 1999;73:7199–7209. doi: 10.1128/jvi.73.9.7199-7209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekes G, Rahmeh AA, Whelan SP. A freeze frame view of vesicular stomatitis virus transcription defines a minimal length of RNA for 5′ processing. PLoS Pathog. 2011;7:e1002073. doi: 10.1371/journal.ppat.1002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D, Banerjee AK. Two methyltransferase activities in the purified virions of vesicular stomatitis virus. J Virol. 1977;24:786–793. doi: 10.1128/jvi.24.3.786-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D, Chanda PK, Banerjee AK. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980;21:267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Venkatesan S, Gershowitz A, Moss B. Modification of the 5′ end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980;255:903–908. [PubMed] [Google Scholar]
- Venkatesan S, Moss B. Donor and acceptor specificities of HeLa cell mRNA guanylyltransferase. J Biol Chem. 1980;255:2835–2842. [PubMed] [Google Scholar]
- Venkatesan S, Moss B. Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proc Natl Acad Sci USA. 1982;79:340–344. doi: 10.1073/pnas.79.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchkov VE, Volchkova VA, Chepurnov AA, Blinov VM, Dolnik O, Netesov SV, Feldmann H. Characterization of the L gene and 5′ trailer region of Ebola virus. J Gen Virol. 1999;80:355–362. doi: 10.1099/0022-1317-80-2-355. [DOI] [PubMed] [Google Scholar]
- Wang D, Shatkin AJ. Synthesis of Gp4N and Gp3N compounds by guanylyltransferase purified from yeast. Nucleic Acids Res. 1984;12:2303–2315. doi: 10.1093/nar/12.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, McElvain LE, Whelan SP. Vesicular stomatitis virus mRNA capping machinery requires specific cis-acting signals in the RNA. J Virol. 2007;81:11499–11506. doi: 10.1128/JVI.01057-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Gershowitz A, Moss B. N6, O2′-dimethyladenosine a novel methylated ribonucleoside next to the 5′ terminal of animal cell and virus mRNAs. Nature. 1975a;257:251–253. doi: 10.1038/257251a0. [DOI] [PubMed] [Google Scholar]
- Wei C, Moss B. 5′-Terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci USA. 1977;74:3758–3761. doi: 10.1073/pnas.74.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975b;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- Wei CM, Moss B. Methylated nucleotides block 5′-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci USA. 1975;72:318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Shuman S. Mutational analysis of the RNA triphosphatase component of vaccinia virus mRNA capping enzyme. J Virol. 1996;70:6162–6168. doi: 10.1128/jvi.70.9.6162-6168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]