Abstract
Purpose
To study the differences of functional connectivity and anatomical connectivity of the cerebellum between schizophrenic patients and normal controls by combining resting-state functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) method.
Materials and Methods
Both fMRI during rest and DTI were performed on 10 patients and 10 healthy subjects at a GE 3.0 T Signa scanner. Resting-state functional connectivities of the bilateral cerebellum were separately analyzed by selecting seed regions in cerebellum. The integrity of white matter fiber in bilateral middle cerebellar peduncles (MCP) and superior cerebellar peduncles (SCP) was evaluated using fractional anisotropy (FA). Two sample t-test was used to detect differences between patients and normal controls, and correlation test was taken to analyze the correlation between the strength of functional connectivity and anatomical connectivity.
Results
In patients with schizophrenia, the bilateral cerebellum showed reduced functional connectivities to some regions compared to controls, such as left middle temporal gyrus, bilateral middle cingulate cortex, right paracentral lobule, right thalamus, and bilateral cerebellum, and the FA of the left SCP was significantly reduced in patients. Meanwhile, There was significantly positive correlation between the connective strength of both the left cerebellum-right paracentral lobule connectivities and right cerebellum-right thalamus connectivities and the FA of the MCP within the control group.
Conslusion
The multimodal imaging approaches presently used provide a new avenue to understand the role of cerebellum in the pathophysiology of schizophrenia. Meanwhile, the current findings of the functional disconnectivity and damaged anatomical connectivity between the cerrebullum and other regions in schizophrenia suggest that the functional–anatomical relationship need to be further investigated.
Keywords: Schizophrenia, Cerebellum, Resting-state fMRI, Diffusion tensor imaging, Functional connectivity, Anatomical connectivity
Introduction
The cerebellum has traditionally been accorded a primarily motor role in coordination, gait and balance, but more recently, its involvement in higher cognitive and affective function has been demonstrated. Neuropathological and neuroimaging studies have linked cerebellar abnormalities with the widespread neuropsychological deficits in schizophrenia (1), so it is important to investigate the interaction between the cerebellum and other brain regions.
In recent years, resting-state functional connectivity networks have been intensively investigated to study the brains of patients with schizophrenia because such studies do not require a complicated task design (2). This approach also makes the resting-state approach readily accepted by schizophrenic patients (3). Previous task-related results demonstrate that schizophrenics might suffer from dysfunctional integration between some specific brain regions (4–7); however, the functional connectivity of the cerebellum is often neglected. Therefore, we attempted to assess the possibility of widespread disconnectivity of the cerebellum in schizophrenia using resting-state datasets.
Diffusion tensor imaging (DTI), a relatively new magnetic resonance imaging (MRI) technique, is capable of investigating water diffusivity and providing non-invasive information about white matter tissue structure, and it enables the anatomical connectivity of the human brain to be explored in vivo. The degree of diffusion anisotropy might be influenced by many factors, such as the dense packing of axons, relative membrane permeability to water, internal axonal structure, tissue water content, and the degree of myelination (8). The middle cerebellar peduncle (MCP) and the superior cerebellar peduncle (SCP) are major pathways linking the cerebellum with other brain regions. Therefore, the two cerebellar peduncles were regions of interest (ROI) in the current study. Although previous research has assessed the integrity of the MCP (9–11) and SCP (12,13) in schizophrenia using DTI, the results are inconsistent. We aim to investigate in further detail the anatomical connectivity of the bilateral cerebellum, mainly through the MCP and SCP.
We hypothesized that in schizophrenic patients, the resting-state functional connectivity of the cerebellum would be decreased and that the integrity of the MCP or SCP, as measured by DTI, would be damaged. By combining DTI and functional connectivity analyses, the current study provides an original viewpoint to understand the abnormality of the functional and anatomical connectivities of the cerebellum in schizophrenia. This investigation is expected to improve our understanding of the role of the cerebellum in the pathophysiology of schizophrenia.
Materials and methods
Subjects
Patients with schizophrenia were recruited between May 2009 and November 2009 from outpatient departments and inpatient units at the Department of Psychiatry, First Hospital of China Medical University, and paid normal controls were recruited by advertisements. Subjects met the following criteria: right-handed, no history of neurological or significant physical disorders, no history of alcohol or drug dependence, no history of receiving electroconvulsive therapy, and completing both blood oxygen level-dependent (BOLD) functional MRI (fMRI) and DTI scans. Finally, 10 patients (5 males and 5 females, mean age = 25.6±2.1 years, 11.9±1.8 years of education) and 10 normal subjects (5 males and 5 females, mean age = 24.9±2.3 years, 10.2±2.2 years of education) were accepted into this study. And the groups did not significantly differ in age and education. Confirmation of the diagnosis was made by clinical psychiatrists for all patients, using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994) criteria.
Written informed consent was obtained from all part participants. This study was approved by the Medical Research Ethics Committee of the First Hospital of China Medical University.
Data acquisition
MRI was performed on 3.0-T GE Signa System (GE Signa, Milwaukee, Wisconsin, USA). Foam pads were used to reduce head motion and scanner noise. Three-dimensional T1-weighted images were acquired in a sagittal orientation employing a 3D-SPGR sequence: repetition time (TR)=7.1 ms, echo time (TE)=3.2 ms, field of view (FOV)=24 cm×24 cm, flip angle=15°, matrix=256×256, slice thickness=1.8 mm, no gap.
The fMRI scanning was performed in darkness, and the participants were explicitly instructed to keep their eyes closed, relax, and move as little as possible. Functional images were collected using a gradient echo planar imaging (EPI) sequence sensitive to BOLD contrast: TR=2000 ms, TE=30 ms, FOV=24 cm×24 cm, flip angle=90°, matrix=64×64, slice thickness=3 mm, gap=1 mm, slices=40. For each participant, the fMRI scanning lasted 8 min.
Diffusion tensor imaging was acquired with single-shot echo planar imaging sequence in alignment with the anterior–posterior commissural plane. The diffusion sensitizing gradients were applied along 25 non-collinear directions (b=1000 s/mm2), together with an acquisition without diffusion weighting (b=0). Contiguous axial slices were acquired with a slice thickness of 2 mm and no gap. The acquisition parameters were as follows: TR=17 000 ms, TE=85.7 ms, matrix=120×120, FOV=24 cm×24 cm, NEX=2.
fMRI procedure
Data preprocessing
For each participant, the first 10 volumes of scanned data were discarded because of the magnetic saturation effects, and the remaining images were preprocessed using the following steps: slice timing, motion correction, spatial normalization to the standard Montreal Neurological Institute (MNI) space and resampling to 3×3×3 mm3, followed by spatial smoothing with 4-mm full-width at half-maximum (FWHM) Gaussian kernel. All of these processes were conducted by the statistical parametric mapping software package (SPM5, Wellcome Department of Imaging Neuroscience, London, UK).
According to the record of head motions within each fMRI run, all participants had less than 1 mm maximum displacement in the x, y or z plane and less than 1° of angular rotation about each axis. To further reduce the effects of confounding factors, six motion parameters, linear drift and the mean time series of all voxels within the entire brain were removed from the smoothed data through linear regression. Then, the fMRI data were temporally band-pass–filtered (0.01–0.08 Hz) using AFNI (http://afni.nimh.nih.gov/) (14,15).
To create a mask, first the T1 anatomical images of all subjects were skull stripped using the software MRIcro (http://www.sph.sc.edu/comd/rorden/mricro.html). The images were then normalized to the standard MNI space. Finally, the mask was obtained by taking the intersections of the normalized T1 anatomical images of all subjects. Only the voxels within the mask were further processed. By averaging the normalized high-resolution 3D T1-weighted images across all subjects, a mean anatomical image was obtained to visualize the statistical results.
Definition of seed regions
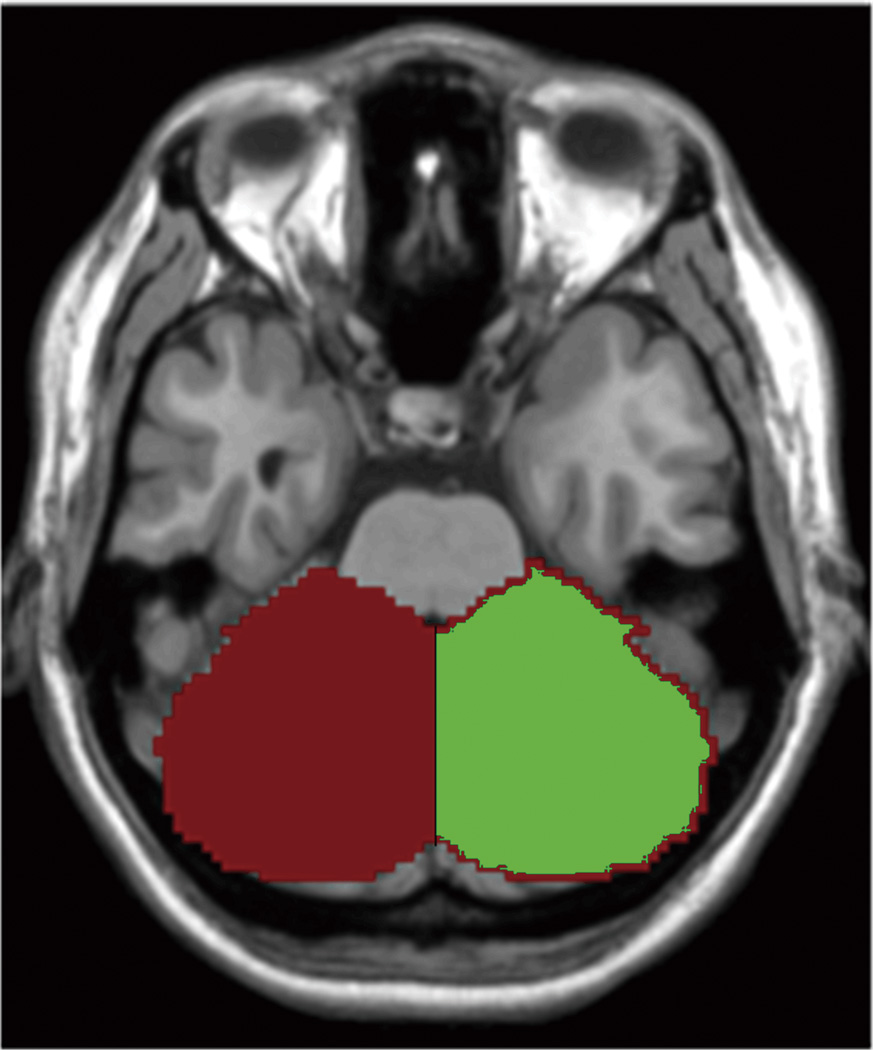
The left and right cerebellum seed regions were defined by WFU_PickAtlas (http://www.ansir.wfubmc.edu) (16) and then were resliced into the MNI space. The left and right seed regions are both 3568 voxels and cover the entire cerebellum (Fig. 1). For each seed region, the BOLD time series of the voxels within the seed region were averaged to generate the reference time series for this seed region.
Fig. 1.
The generated left (red) and right (green) seed regions in a representative subject.
Functional connectivity and statistical analysis
A voxel-wise functional connectivity analysis of ROI was used. Correlation coefficients were calculated between the seed regions and the entire brain in a voxel-wise manner (17, 18). Then, the correlation coefficients were transformed to z-values using the Fisher r-to-z transformation to improve normality. This transformation produced spatial maps in which the values of voxels represented the strengths of the correlations with the ROIs.
Within each group, individual z-values were entered into a one-sample t-test in a voxel-wise manner to determine the brain regions showing significantly positive or negative connectivity to the left or right cerebellum. A combined threshold of contrast maps was set at p<0.001 for each voxel and a cluster size of at least 162 mm3, which was equal to the corrected threshold of p<0.05, determined by AlphaSim (see program AlphaSim by B.D. Ward in AFNI software. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). To compare the functional connectivity of the left cerebellum between the control and patient groups, the z-values were entered into a two-sample t-test in a voxel-wise manner to determine the brain regions that showed significant differences in positive or negative connectivity to the left cerebellum. A combined threshold of contrast maps was set at p<0.001 for each voxel and a cluster size of at least 162 mm3, which was equal to the corrected threshold of p<0.05 determined by AlphaSim. An identical statistical analysis was performed for the right cerebellum.
DTI procedure
Data preprocessing
The data were transferred to a dedicated computer workstation (Sun Microsystems, AW4.0_02), where the DTI data were post-processed using Functool2 (GE Medical Systems, Milwaukee, WI). EPI distortion was corrected automatically. The minimal set of six diffusion tensor components was calculated from the data set acquired with a diffusion-sensitizing gradient along 25 directions in each voxel. The diffusion eigenvectors (λ1, λ2, λ3) and eigenvalues, which correspond to the main diffusion direction and associated diffusivity, were then calculated from the diffusion tensor components (19). Fractional anisotropy (FA) reveals information about the degree of diffusion anisotropy in white matter. FA was calculated according to the following formula:
Regions of interest
The MCP contains numerous neural fibers that project from the cerebral cortex and subsequently cross in the pons, and the cerebellar nuclei also send neural fibers to the cerebral cortex and thalamus via the SCP, thus completing the circuit between the cerebral cortex and the cerebellum. Several case reports demonstrate that impairments of these fibers are associated with cognitive dysfunctions similar to schizophrenia (20). Thus, the two cerebellar peduncles became ROIs in the current study.
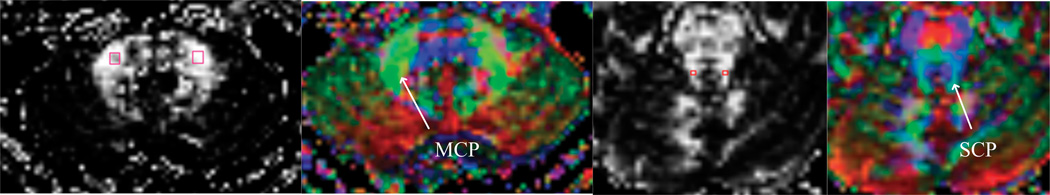
A neuroradiologist who was blinded to subject grouping was responsible for placing ROIs on each of the MCPs and SCPs (Fig. 2) according to predetermined anatomical locations. Interoperator reliability for the investigation of FA by manual tracings on the left and right MCPs and SCPs was established by two experienced operators on 10 T2-weighted images including the MCPs and SCPs, by using intraclass correlations (21) (Table 1). For the MCPs, the ROIs lay within a manually traced 4 mm×4 mm square placed over the slice where the MCP was greatest in volume. For the SCPs, the ROIs lay within a manually traced 2 mm×2 mm square placed posterior to the medial longitudinal fasciculus on the slice just superior to the slice on which the ROIs of the MCP were placed. The assumption was based on the concept that the fiber orientation is in line with the eigenvector of maximum eigenvalue. Pixels were characterized as either anterior–posterior (green), left–right (red), or vertical (blue), depending on the direction of the eigenvector of the diffusion tensor. With the color-encoded tensor image, fibers in different directions could then be easily identified, which helped localize the ROIs more accurately (9).
Fig. 2.
The first image demonstrates the placement of ROIs on the MCP; the second image demonstrates a color-coded tensor image that applies green to the MCP because of its anterior-posterior orientation. The third and fourth images display the location of ROIs on the SCP and the corresponding fiber orientation map.
Table 1.
Interoperator reliabilities measured by intraclass correlation for FA measurement from 10 scans
| FA | |
|---|---|
| Right superior cerebellar peduncles | 0.91 |
| Left superior cerebellar peduncles | 0.90 |
| Right middle cerebellar peduncles | 0.92 |
| Left middle cerebellar peduncles | 0.92 |
Statistical analysis
Independent t-tests were used to compare group differences in the FA separately for the right and left sides of the MCPs and SCPs. All t-tests were considered significant when P values were less than 0.05 (two-tailed).
Correlation analysis
To evaluate the relationship between functional connectivity and anatomical connectivity, we investigated the correlations between the strength of the altered functional connectivities and the FA value of the left and right MCPs and SCPs (22). Pearson’s correlation analysis was used, and statistical significance was set at 0.05 (two-tailed).
Results
Cerebellum functional connectivity analysis within the normal control group and within the patient group
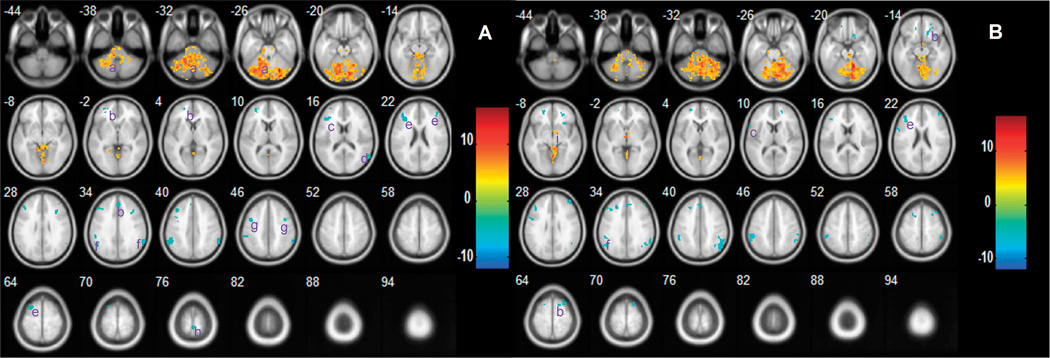
In the normal control group, the left (Fig. 3A) and right (Fig. 3B) cerebellum showed significant negative functional connectivities with a number of regions, including superior frontal gyrus, inferior frontal gyrus, superior temporal gyrus, middle frontal gyrus, supramarginal, precentral gyrus, paracentral lobule and several subcortical structures (striatum and midbrain). In addition, bilateral cerebellar regions and right thalamus showed positive connectivities with the bilateral cerebellum. In the patients with schizophrenia, the left (Fig. 4A) and right (Fig. 4B) cerebellum showed some similar regions with significant functional connectivity.
Fig. 3.
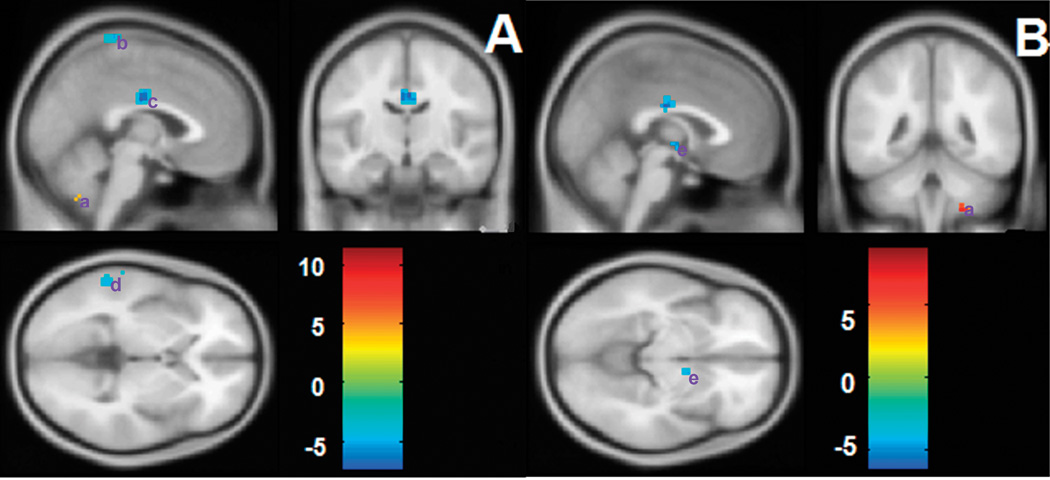
Regions showing functional connectivities to the left (A) and right (B) cerebellum in normal subjects. The left side of the image represents the left side of brain. The color bar indicates the T-score. Warm colors illustrate the regions showing positive connectivity in normal subjects, and cool colors illustrate the regions showing negative connectivity in normal subjects. Abbreviations: (a) cerebellum; (b) superior frontal gyrus; (c) inferior frontal gyrus; (d) superior temporal gyrus; (e) middle frontal gyrus; (f) supramarginal; (g) precentral gyrus; (h) paracentral lobule; (i) thalamus.
Fig. 4.
Regions showing functional connectivities to the left (A) and right (B) cerebellum in patients with schizophrenia. Details are in Fig. 3.
Altered functional connectivity of bilateral cerebellum in the patient group
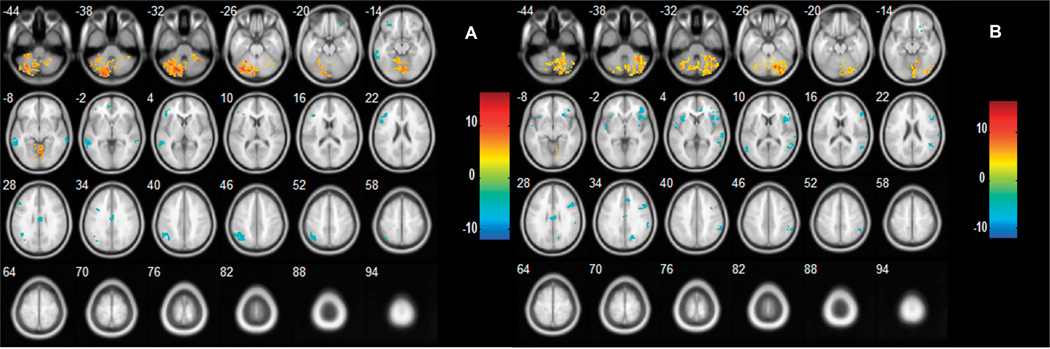
When comparing the pattern of functional connectivity of the left cerebellum between the control and patient groups, a set of regions, including the left middle temporal gyrus (MTG), right middle cingulate cortex (MCC) and right paracentral lobule, showed significantly reduced negative connectivity (i.e., the absolute value of the correlation coefficient of the control group was larger than that of the patient group) to the left cerebellum in patients with schizophrenia. In addition, one region in the left cerebellum showed reduced positive connectivity (i.e., the correlation coefficient of the control group was larger than that of the patient group) to the left cerebellum in patients with schizophrenia (Table 2, Fig. 5A). No enhanced positive or negative connectivities were observed in the patient group compared to the normal group. The right cerebellum, right thalamus and left MCC showed reduced negative connectivities to the right cerebellum in patients with schizophrenia, and one region in the right cerebellum showed reduced positive connectivity. No enhanced positive or negative connectivities were observed in patients with schizophrenia (Table 2, Fig. 5B).
Table 2.
Brain regions showing significant changes in bilateral cerebellum functional connectivity in schizophrenia
| Brain regions | Brodmann's area | No. of voxels | (x,y,z) | T score |
|---|---|---|---|---|
| Decreased positive correlation with the left cerebellum | ||||
| Left cerebellum | 266 | (−25,−60,−55) | 11.45 | |
| Decreased negative correlation with the left cerebellum | ||||
| Left middle temporal gyrus | 21 | 31 | (−63,−33,−3) | 5.78 |
| Right middle cingulate cortex | 23 | 52 | (2,−15,34) | 7.23 |
| Right paracentral lobule | 4 | 21 | (6,−33,78) | 6.57 |
| Decreased positive correlation with the right cerebellum | ||||
| Right cerebellum | 186 | (21,−57,−57) | 8.85 | |
| Decreased negative correlation with the right cerebellum | ||||
| Right thalamus | 18 | (8,−9,2) | 5.41 | |
| Left middle cingulate cortex | 23 | 33 | (−6,−18,35) | 6.05 |
(x,y,z) coordinates of primary peak locations in the Montreal Neurological Institute space.
Fig. 5.
Differences in functional connectivities of the left (A) and right (B) cerebellum between control and patient groups. The left side of the image represents the left side of the brain. The color bar indicates the T-score. Warm colors illustrate regions showing reduced positive connectivity in the patient group and cool colors illustrate regions showing reduced negative connectivity in the patient group. No regions showing increased positive or negative connectivities were found. Abbreviations: (a) cerebellum; (b) paracentral lobule; (c) middle cingulate cortex; (d) middle temporal gyrus; (e) thalamus.
FA in the superior and middle cerebellar peduncles
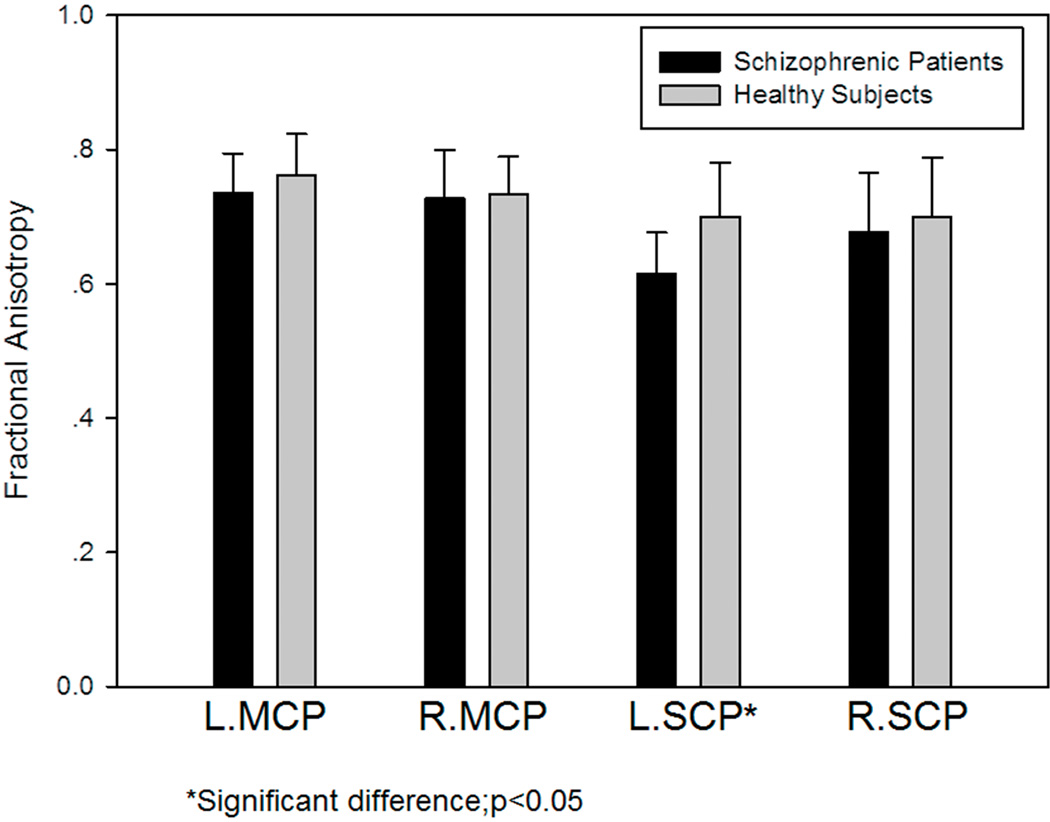
Fig. 6 presents the FA data for both patients and controls. The FA value of the left SCP in patients with schizophrenia (0.615 ± 0.061) was significantly lower (t=2.64, p=0.016) than in normal controls (0.700 ± 0.081). No statistically significant differences were detected in the three other ROIs.
Fig. 6.
FA of the left and right MCPs and SCPs in 10 patients with schizophrenia and 10 healthy subjects. There was a significant difference in the FA of the left SCP between patients with schizophrenia and healthy subjects.
Correlation between FA and the strength of functional connectivity
Significant correlations were observed between FA values of the MCP and the strength of both the left cerebellum-right paracentral lobule connectivities (Pearson's r=−0.733, p=0.015) and right cerebellum-right thalamus connectivities (Pearson's r=−0.872, p=0.001) within the control group (Table 3). There was no significant correlation between the FA of the MCP or SCP and the strength of functional connectivity within the patient group.
Table 3.
The correlation between the strength of these functional connectivities and the FA value of the MCPs and SCPs within each group is shown,
| Brain regions | FA within patients | FA within controls | ||
|---|---|---|---|---|
| MCP | SCP | MCP | SCP | |
| Decreased positive correlation with the left cerebellum | ||||
| Left cerebellum | 0.307 | −0.071 | 0.338 | 0.406 |
| Decreased negative correlation with the left cerebellum | ||||
| Left middle temporal gyrus | 0.031 | 0.154 | −0.549 | −0.037 |
| Right middle cingulate cortex | −0.322 | −0.047 | −0.416 | 0.164 |
| Right paracentral lobule | 0.012 | 0.002 | −0.733* | 0.042 |
| Decreased positive correlation with the right cerebellum | ||||
| Right cerebellum | −0.173 | −0.021 | 0.053 | 0.003 |
| Decreased negative correlation with the right cerebellum | ||||
| Right thalamus | −0.131 | −0.357 | −0.872** | −0.200 |
| Left middle cingulate cortex | −0.012 | −0.078 | −0.375 | −0.355 |
The numbers in the table are Pearson's correlation coefficients.
p<0.05;
p<0.01.
Discussion
The current study combined resting-state functional connectivity and DTI analyses. The multimodal imaging approaches can provide a unique perspective for understanding connectivity in psychiatric diseases (23). In this study, we found both reduced resting-state functional connectivities of the bilateral cerebellum and impaired MCP/SCP integrity in patients with schizophrenia. These reductions in the functional interaction and the anatomical connectivity between the cerebellum and other regions provide further evidence for implication of the cerebellum in schizophrenia.
The cerebellum has traditionally been considered to have primarily a motor role in coordination, gait and balance, but more recently, its involvement in higher cognitive and affective functions has been demonstrated. In the present study, we noticed that some regions, such as the thalamus, MTG, paracentral lobule, MCC and cerebellum itself, showed reduced functional connectivities to the left or right cerebellum in the patient group. Reduced positive connectivity with the left or right cerebellum was mainly located in the cerebellum in this study, which means the cerebellum itself is involved and is damaged in schizophrenia. There are many morphological studies that have found that the volume of the entire cerebellum is observably reduced in schizophrenic patients compared with normal controls (24–26). These results also support the opinion that the cerebellum might play a key role in schizophrenia. The cerebellum connects and regulates the cerebrum primarily through the cortico-cerebellar-thalamo-cortical circuit (CCTCC). Both the cerebellum and thalamus along the CCTCC have been implicated as contributing to cognitive dysmetria in individuals with schizophrenia (27,28). Additionally, we found that the right thalamus shows a significantly decreased negative correlation with the right cerebellum. DTI analysis has shown that the fiber tracts located between the cerebellum and the thalamus exhibit a reduced FA in patients with schizophrenia in comparison to normal controls (13). However, the functional connectivity between the cerebellum and the thalamus has not been reported in previous studies. Because anatomical connectivity constructs the basis of functional connectivity and because there is a close relationship between the two, the damaged fiber tract might induce the abnormal functional connectivity. The DTI study and our result both indicate that the connectivity between the thalamus and the cerebellum is impaired, and the CCTCC warrants more attention regarding its contribution to schizophrenia. The MTG is located between the superior temporal gyrus and the inferior temporal gyrus, and the MTG demonstrated abnormal hemodynamic activity during processing of low-probability, task-irrelevant novel stimuli in schizophrenic patients (29). In addition, schizophrenic patients with auditory hallucinations have demonstrated reduced temporal gray matter volumes compared to patients without auditory hallucinations (30). The left middle temporal gyrus in this study showed a decreased negative correlation with the left cerebellum. This might imply that the activity of the MTG is abnormal and that the connectivity between the MTG and the cerebellum is aberrant in schizophrenic patients in a resting state, although the temporal-cerebellum connectivity has not been clearly examined in previous studies. As is well known, the paracentral lobule is primarily involved in motor function, and Bilder et al. (31,32) found that patients with schizophrenia have deficits in motor control. We found that the right paracentral lobule-left cerebellum connection was reduced in patients compared with normal controls. Moreover, we found that the right MCC-left cerebellum and left MCC-right cerebellum connections were reduced in this study. The MCC area is the middle part of the cingulate gyrus in its anterior-posterior axis. Vogt et al. (33) found that the MCC contains cingulate motor areas. Furthermore, the cerebellum has traditionally been considered to have primarily a motor role and to regulate motor function through its connectivity with the cerebral motor areas. Combining the above two results, we tentatively put forward that patients with schizophrenia might possess aberrant motor function control compared to normal controls. However, due to the lack of ataxic performance in this study, this speculation needs to be validated in future studies.
DTI, a relatively new MRI technique, is capable of investigating water diffusivity and providing non-invasive information about white matter tissue structure. In the current study, FAs were utilized to evaluate tissue diffusion characteristics. The degree of diffusion anisotropy might be influenced by many factors, such as the dense packing of axons, relative membrane permeability to water, internal axonal structure, tissue water content, and the degree of myelination (8). Although the proper interpretation of water diffusion in a complex biological tissue, such as white matter, has not been achieved, changes in FA in white matter might demonstrate that the connections of related brain regions are impaired. The FA in the left SCP in patients with schizophrenia was lower than that in healthy subjects in this study, while the FA in the right SCP was not significantly decreased in the patient group. The SCP has been shown to have a decreased FA in schizophrenic patients (12), although a previous study has reported that there was no significant difference between the FA in the SCPs of 29 male schizophrenic patients and 20 healthy men (9). In addition, Park et al. (34) found asymmetry of the FA in the SCP in 32 healthy subjects, but not in 23 patients with schizophrenia, using a voxel-based approach. Their findings combined with our findings suggest that the FA is abnormal in the SCP of patients with schizophrenia and that these changes may represent a pathological process resulting in the development of schizophrenia. Statistical analysis did not demonstrate a significant difference in left and right MCPs between patients with schizophrenia and normal controls in the present study, although the MCP was shown to have a decreased FA in schizophrenic patients (10,11). One might conclude from our study that the neural fibers of the MCP remain intact in schizophrenia. However, there are some methodological limitations to our study. First, the neural fibers we measured for only a portion of the MCP. Second, the square shape of the ROI was held constant in size in the sampled regions of different subjects. We could not exclude the influence of fiber tracts with different sizes and thicknesses. Therefore, our preliminary results should be interpreted with caution.
In the present study, some regions, while showing reduced functional connectivities to the left or right cerebellum in schizophrenic patients, had direct or indirect anatomical connections with the cerebellum via the MCP or SCP, such as the thalamus, MCC and contralateral cerebellum. Changes in the MCP or SCP integrity are thought to indicate an abnormal connectivity between the cerebellum and other regions (35). In the present study, there was no significant correlation between the FA of MCP or SCP and the strength of functional connectivity within the patient group, although significant correlations were observed between FA values of MCP and the strength of both the left cerebellum-right paracentral lobule connectivity and right cerebellum-right thalamus connectivity in the control group, while not seen in the patient group. The region of the superior cerebellar peduncle or brachium conjuctivum serves as the decusation of the cerebellar fibers projecting from the cerebellum to the red nucleus and onto the thalamus. Recent evidence has shown that disruptions in the CCTCC may represent a key abnormality that underlies the clinical manifestations of the illness. The disruption in the interplay between these brain regions has been referred to as “cognitive dysmetria”, a potentially fundamental deficit in the pathology of schizophrenia (27, 36). Both the cerebellum and thalamus along the CCTCC have been implicated as contributing to cognitive dysmetria in persons with schizophrenia (27, 28). It is consistent with our current findings that anatomical-functional relationship between cerebellum and thalamus was disrupted. These changes may represent a pathologic process in schizophrenia.
Some of the methods used in our current study will require improvement, and some of the current findings will need to be clarified in future studies. First, we used a low sampling rate (TR = 2 s) and were not able to simultaneously record cardiac rate due to technical limitations. Cardiac fluctuation effects could reduce the specificity of the connectivity effect (37). However, Birn et al. (38) has confirmed that the contributions of aliased cardiac and respiratory signals to resting-state functional connectivities are relatively minor. Thus, we believe that the between-group differences observed in this study are physiologically meaningful. Second, there is an important limitation for using such a big seed region in this study. It remains a debate how to define cerebellum’s subregions according to the different anatomy or functions (39–41). Recently, O'Reilly et al identified that the cerebellum may have at least two gross functionally distinct regions (41). This may provide a useful guideline for our further study. Finally, the sample size is modest. This is a major limitation. However, it is interesting that in this modest sample, we found the altered functional connectivity between cerebral cortices and cerebellum and decreased FA values in cerebellar white matter in schizophrenia; furthermore, an association between functional and structural connectivity was identified in healthy group. It is our hope that our study could provide a model to further investigate white-grey matter structure-function interactions in the future studies.
In conclusion, by combining functional connectivity and DTI analyses, we found a reduction in the resting-state functional connectivities to the bilateral cerebellum and damage in the white matter integrity of the SCP in the same group of schizophrenic patients. The disassociation of the functional connectivity and anatomical connectivity between the cerebellum and other regions in schizophrenia suggest that the functional–anatomical relationship in schizophrenia may be impaired. The multimodal imaging approaches presently used provide a new avenue for understanding the role of the cerebellum in the pathophysiology of schizophrenia.
Acknowledgment
The authors thank Huanhuan Li, Ph.D. for her critical review, Wenge Sun, M.S. for his technical expertise, Yongfeng Wang, B.S. for his assistance with the study and the research subjects for their participation.
Grant support: Funding was sponsored by National Institution of Health (K01MH086621, Fei Wang), the National Alliance for Research on Schizophrenia and Depression (Fei Wang) and the Klingenstein Foundation (Fei Wang).
Abbreviations
- DTI
diffusion tensor imaging
- fMRI
functional magnetic resonance imaging
- FA
Fractional anisotropy
- BOLD
blood oxygen level-dependent
- MCP
middle cerebellar peduncle
- SCP
superior cerebellar peduncle
- MTG
middle temporal gyrus
- MCC
middle cingulate cortex
References
- 1.Katsetos CD, Hyde TM, Herman MM. Neuropathology of the cerebellum in schizophrenia--an update: 1996 and future directions. Biol Psychiatry. 1997;42:213–224. doi: 10.1016/S0006-3223(96)00313-7. [DOI] [PubMed] [Google Scholar]
- 2.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Liang M, Zhou Y, Jiang T, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 4.Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 5.Boksman K, Théberge J, Williamson P, et al. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophr Res. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Honey GD, Pomarol-Clotet E, Corlett PR, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon JH, Minzenberg MJ, Ursu S, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papad akis NG, Xing D, Houston GC et al. A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magn Reson Imaging. 1999;17:881–892. doi: 10.1016/s0730-725x(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Sun Z, Du X, et al. A diffusion tensor imaging study of middle and superior cerebellar peduncle in male patients with schizophrenia. Neurosci Lett. 2003;348:135–138. doi: 10.1016/s0304-3940(03)00589-5. [DOI] [PubMed] [Google Scholar]
- 10.Okugawa G, Nobuhara K, Minami T, et al. Subtle disruption of the middle cerebellar peduncles in patients with schizophrenia. Neuropsychobiology. 2004;50:119–123. doi: 10.1159/000079101. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A Diffusion Tensor Imaging Study of White Matter in Early-Onset Schizophrenia. Biol Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Okugawa G, Nobuhara K, Minami T, et al. Neural disorganization in the superior cerebellar peduncle and cognitive abnormality in patients with schizophrenia: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1408–1412. doi: 10.1016/j.pnpbp.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Magnotta VA, Adix ML, Caprahan A, Lim K, Gollub R, Andreasen NC. Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: A pilot study. Psychiatry Res. 2008;163:193–200. doi: 10.1016/j.pscychresns.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakes TR, Johnstone T, Ores Walsh KS, et al. Comparison of fMRI motion correction software tools. Neuroimage. 2005;28:529–543. doi: 10.1016/j.neuroimage.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 15.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 17.Tian L, Jiang T, Wang Y, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Jiang T, Liang M, et al. Discriminative analysis of early Alzheimer's disease based on two intrinsically anti-correlated networks with resting-state fMRI. Med Image Comput Comput Assist Interv. 2006;9:340–347. doi: 10.1007/11866763_42. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 20.Lu ML, Yeh IJ. Onset of psychosis after cerebellum pathology: a case report. Gen Hosp Psychiatry. 2001;23:41–42. doi: 10.1016/s0163-8343(00)00103-1. [DOI] [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Shu N, Liu Y, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Ramnani N, Behrens TE, Penny W, Matthews PM. New approaches for exploring anatomical and functional connectivity in the human brain. Biol Psychiatry. 2004;56:613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schröder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173:83–87. doi: 10.1016/j.pscychresns.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Keller A, Castellanos FX, Vaituzis AC, Jeffries NO, Giedd JN, Rapoport JL. Progressive loss of cerebellar volume in childhood-onset schizophrenia. Am J Psychiatry. 2003;160:128–133. doi: 10.1176/appi.ajp.160.1.128. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen NC, Paradiso S, O'Leary DS. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen NC, O'Leary DS, Flaum M, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 29.Kiehl KA, Stevens MC, Celone K, Kurtz M, Krystal JH. Abnormal Hemodynamics in Schizophrenia During an Auditory Oddball Task. Biol Psychiatry. 2005;57:1029–1040. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin SE, Lee JS, Kang MH, Kim CE, Bae JN, Jung G. Segmented volumes of cerebrum and cerebellum in first episode schizophrenia with auditory hallucinations. Psychiatry Res. 2005;138:33–42. doi: 10.1016/j.pscychresns.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia:Initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 32.Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- 33.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dicholomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HJ, Westin CF, Kubicki M, et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 37.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 38.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 39.Bloedel JR. Functional heterogeneity with structural homogeneity: how does the cerebellum operate? Behav Brain Sci. 1992;15:666–678. [Google Scholar]
- 40.Habas C, Kamdar N, Nquyen D, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]