Abstract
Neurologists and aphasiologists have debated for over a century whether right hemisphere recruitment facilitates or impedes recovery from aphasia. Here we present a well-characterized patient with sequential left and right hemisphere strokes whose case substantially informs this debate. A 72-year-old woman with chronic nonfluent aphasia was enrolled in a trial of transcranial magnetic stimulation (TMS). She underwent 10 daily sessions of inhibitory TMS to the right pars triangularis. Brain activity was measured during picture naming using fMRI prior to TMS exposure and before and after TMS on the first day of treatment. Language and cognition were tested behaviorally three times prior to treatment, and at 2 and 6 months afterwards. Inhibitory TMS to the right pars triangularis induced immediate improvement in naming, which was sustained 2 months later. FMRI confirmed a local reduction in activity at the TMS target, without expected increased activity in corresponding left hemisphere areas. Three months after TMS, the patient suffered a right hemisphere ischemic stroke, resulting in worsening of aphasia without other clinical deficits. Behavioral testing 3 months later confirmed that language function was impacted more than other cognitive domains. The paradoxical effects of inhibitory TMS and the stroke to the right hemisphere demonstrate that even within a single patient, involvement of some right hemisphere areas may support recovery, while others interfere. The behavioral evidence confirms that compensatory reorganization occurred within the right hemisphere after the original stroke. No support is found for interhemispheric inhibition, the theoretical framework on which most therapeutic brain stimulation protocols for aphasia are based.
Keywords: aphasia, transcranial magnetic stimulation, plasticity, neuromodulation, interhemispheric inhibition
1. Introduction
In 1877, Barlow described a boy who developed aphasia after a stroke involving Broca's area, recovered significantly, but then worsened after a symmetric stroke in the right hemisphere (RH) (Barlow, 1877). This case was taken as evidence that the RH can reorganize in order to assume functions of the left hemisphere (LH) in aphasia, and launched a debate regarding the role of the RH in aphasia recovery that continues today. Only a few similar cases have followed (Basso et al., 1989), and the validity of the original case has recently been subject to debate (Hellal and Lorch, 2007). Behavioral studies demonstrating a left visual field or left ear advantage for verbal stimuli, Wada studies demonstrating worsening with right carotid injection, and cases of language recovery after left hemispherectomy supported RH involvement in aphasia recovery (See Basso (1989) for review). However, as research tools have become more precise, the role of the RH has become less clear. Some functional imaging studies have supported compensatory RH recruitment (Blasi et al., 2002; Leff et al., 2002; Musso et al., 1999; Ohyama et al., 1996; Saur et al., 2006), although the RH is thought to be computationally less efficient in its language processing than native LH areas (Heiss et al., 1999; Heiss et al., 2003; Winhuisen et al., 2005). Others have concluded that RH activity is "ineffective" (Postman-Caucheteux et al., 2010; Richter et al., 2008) or is associated with nonlinguistic processes like executive control that are called upon nonspecifically when processing load is high (van Oers et al., 2010).
Another hypothesis is that the RH is aberrantly recruited after a LH stroke due to a release of left-to-right transcallosal inhibition. Over-activity in RH areas then putatively inhibits recovery of LH perilesional cortex, limiting recovery from aphasia. This “theory of interhemispheric inhibition” has motivated several studies attempting to use inhibitory RH transcranial magnetic stimulation (TMS) as a treatment to improve aphasia (Barwood et al., 2011; Naeser et al., 2005). Responses to local inhibition of the RH using TMS have varied between patients (Winhuisen et al., 2007), and between stimulation targets (Hamilton et al., 2010), but the most consistent effect of RH TMS has been sustained improvement in speech production after inhibition of the right pars triangularis (Barwood et al., 2011; Hamilton et al., 2010; Martin et al., 2009b; Naeser et al., 2005). This beneficial effect of inhibitory RH TMS on aphasia recovery has been taken as evidence that RH recruitment is detrimental to recovery.
Variation between patients in the role of the RH in aphasia recovery may explain some of the inconsistencies in the literature, but it is equally plausible that different areas within the RH play competing roles in aphasia recovery even within a single patient. Reorganization after LH injury may result in compensatory recruitment of some RH areas, while others interfere with recovery. Here we present a patient whose aphasia improved after TMS-induced inhibition of the right pars triangularis, but then worsened after a distant RH stroke. Specific patterns in performance demonstrate unequivocally that the initial LH stroke induced compensatory reorganization within RH networks supporting language. The dissociation between the effects of TMS and the RH stroke provides clear evidence that different areas of the RH can have opposing effects on aphasia recovery in a single patient, and raises questions regarding the proposed mechanism of therapeutic TMS.
2. Methods
2.1 Clinical Trial and Neuropsychology Methods
The patient was enrolled in a randomized subject-blinded sham-controlled partial crossover trial of TMS for chronic nonfluent aphasia (clinicaltrials.gov ID: NCT00608582). Procedures were approved by the University of Pennsylvania IRB. Written informed consent was obtained from the subject. The trial protocol has been described in detail previously (Martin et al., 2009b). The subject underwent three sessions of neuropsychological testing to establish a stable baseline measurement of language and cognitive function prior to TMS exposure. This battery included the first 30 items of the Boston Naming Test (BNT), selected subtests of the Boston Diagnostic Aphasia Examination (BDAE), and the Cognitive Linguistic Quick Test (CLQT). CLQT composite scores were calculated excluding the symbol cancelation subtest due to a difference in the method of administration between sessions. The same neuropsychological battery was repeated two and six months after TMS treatment to assess the long-term effects of TMS. To facilitate comparison across tests, raw neuropsychological scores at follow-up sessions were Z-transformed based on the mean and SD of the three baseline assessments (i.e. (Follow-up score – Mean of baseline scores) / SD of baseline scores)). Significance of Z-scores was tested at a Bonferroni corrected 2-tailed alpha of .05.
2.2 TMS Methods
To identify a TMS target for treatment, 1Hz repetitive TMS (rTMS) was applied at 90% resting motor threshold (rMT) for 10 minutes to each of 6 candidate RH targets in separate sessions. The rMT was determined as the intensity that induced visible contractions of the left first dorsal interosseous muscle on 5 out of 10 pulses delivered to the right motor hand area. Candidate sites were selected based on gyral anatomy on the patient’s T1-weighted MRI scan to include the three major divisions of the inferior frontal gyrus (pars opercularis, pars triangularis, and pars orbitalis) and the motor cortex mouth area, maintaining relatively equal distances along the brain surface between targets. A picture-naming task was given before and after stimulation at each session to assess for TMS-induced improvement. Items were presented on a computer monitor for 3 seconds with an additional 7-second response period. Item lists were matched for frequency and consisted of 20 repeated pictures tested at every session and 20 items only presented once across all sessions (40 items total per list, 400 seconds total task duration per administration). After all candidate sites were tested, the treatment target was selected as the site with the largest percent increase in naming accuracy from pre- to post-TMS. Ten daily 20-minute treatment sessions of 1 Hz TMS were then administered at 90% rMT to this therapeutic target over two weeks.
2.3 fMRI Methods
Block-designed fMRI was performed comparing overt picture naming to pattern viewing (3 runs using the paradigm described by Martin and colleagues (2005)). Scans were acquired on a 3T Siemens Trio scanner; T1-weighted (160 slice, TR = 1620, TE = 3.87 msec, FOV = 192 × 256, 1 × 1 × 1 mm voxels) and echoplanar images (31 slices, TR = 3000, TE = 35 msec, FOV = 64 × 64 mm, 3.75 × 3.75 × 4 mm voxels) were acquired. In the task condition, pictures from the Snodgrass and Vanderwart database (Snodgrass and Vanderwart, 1980) were presented in black on a white background (30 items per run). Patterns consisted of six different black and white checkerboard patterns presented in random order. During each stimulus presentation, a fixation cross was presented for 1 second along with an audible beep, followed by the item for 5 seconds. The subject was instructed to name the pictures aloud or simply view the patterns. Naming blocks consisted of three consecutive trials (18 seconds, 6 EPI volumes) and pattern blocks consisted of two trials (12 seconds, 4 EPI volumes). Data analysis was performed using VoxBo (http://www.voxbo.org). Preprocessing steps included sinc interpolation, rigid-intermodality realignment, and spatial smoothing (7.5mm FWHM 3D Gaussian kernel). Statistical analyses were performed as described by Martin and colleagues (2005) as follows: All volumes collected during naming blocks were discarded to avoid contamination due to jaw motion during speech. The first two acquisitions acquired during the pattern blocks (6 seconds) were labeled as naming-related volumes, and the last two (6 seconds) were labeled as pattern-related volumes, thus taking advantage of the hemodynamic delay to isolate naming-related activity after verbal responses were complete. Comparisons between naming-related volumes and pattern-related volumes were then evaluated using the general linear model, with scan effects and global signals modeled as nuisance covariates. The significance threshold was p < .05 corrected for multiple comparisons using the permutation method (Nichols and Holmes, 2002). The cluster threshold was 20 voxels.
3. Results
3.1 Case Description
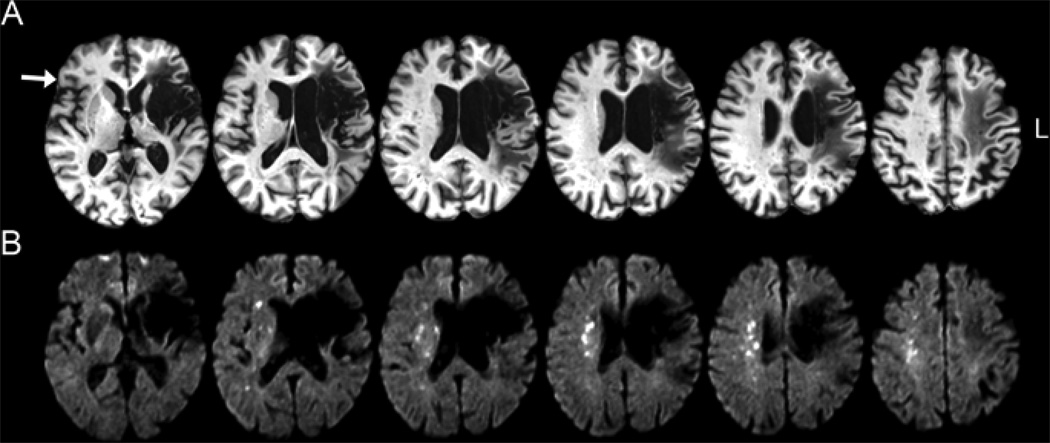
A 67-year-old female retired school counselor was found down at home with global aphasia and right hemiplegia. She was diagnosed with a left middle cerebral artery (MCA) ischemic stroke (Fig. 1A); no thrombolytic agents were administered. She was discharged to an inpatient rehabilitation hospital 3 weeks later, where she remained for 1 month. She received speech therapy 3 days per week for approximately 2 years. Four years after the stroke, she enrolled in a clinical trial of TMS as detailed above. At that time, her spontaneous speech was slow, effortful, and hesitant with short agrammatic utterances (typically 2–4 words, although occasionally longer), frequent word finding pauses, semantic and phonological errors, and moderate speech apraxia. Repetition was qualitatively better than spontaneous speech, and comprehension was relatively spared. An illustrative example of narrative speech at this time is “um ud uh uh mother drying …… uh water fall …‥ and gee (‘she’) rye (‘dry’) id de deh (‘the’) dishes” (initial phrases from a description of the cookie theft picture- each dot represents a .25 sec pause- examples below are excerpted in the same manner). She was randomized to receive a 2-week course of TMS (rather than sham stimulation). She remained blinded to this assignment until after her two-month follow up assessment. The family reported improved spontaneous speech after TMS, and evaluators noted slight improvements in word finding and hesitancy, but not articulation; an improved overall ability to convey intended meaning was noted. An example of narrative speech at this time is “now there’s uh uh uh a lady and two children and the boy is ag- uh backing up in the chair.” Three months after completing TMS, she developed sudden worsening of speech production, without a change in prior motor deficits or any new loss of vision or sensation. She was found to have an acute RH ischemic stroke affecting multiple areas of subcortical white matter, roughly in a watershed distribution at the boundary between the territories of the middle and anterior cerebral arteries (Fig. 1B). Magnetic resonance angiography revealed severe stenosis or occlusion bilaterally in the cavernous segments of the internal carotid arteries with reconstitution of flow in the left M1 segment and the right parasylvian branches. The aphasia did not improve markedly after this second stroke. At the follow-up session 6-month after TMS (3 months after the RH stroke), speech was extremely slow and effortful with only 1 or 2 word utterances. An example of narrative speech at this time is “um …‥ well …. it were wering ……………… gu- du- dult …………. running.” She died approximately 6 months later.
Fig. 1. Locations of Strokes.
(A) T1 weighted MRI showing LH stroke causing initial aphasia (acquired 4 years after the stroke). (B) Diffusion weighted image showing the acute RH stroke. The white arrow shows the right pars triangularis therapeutic TMS target.
3.2 fMRI and Acute TMS Results
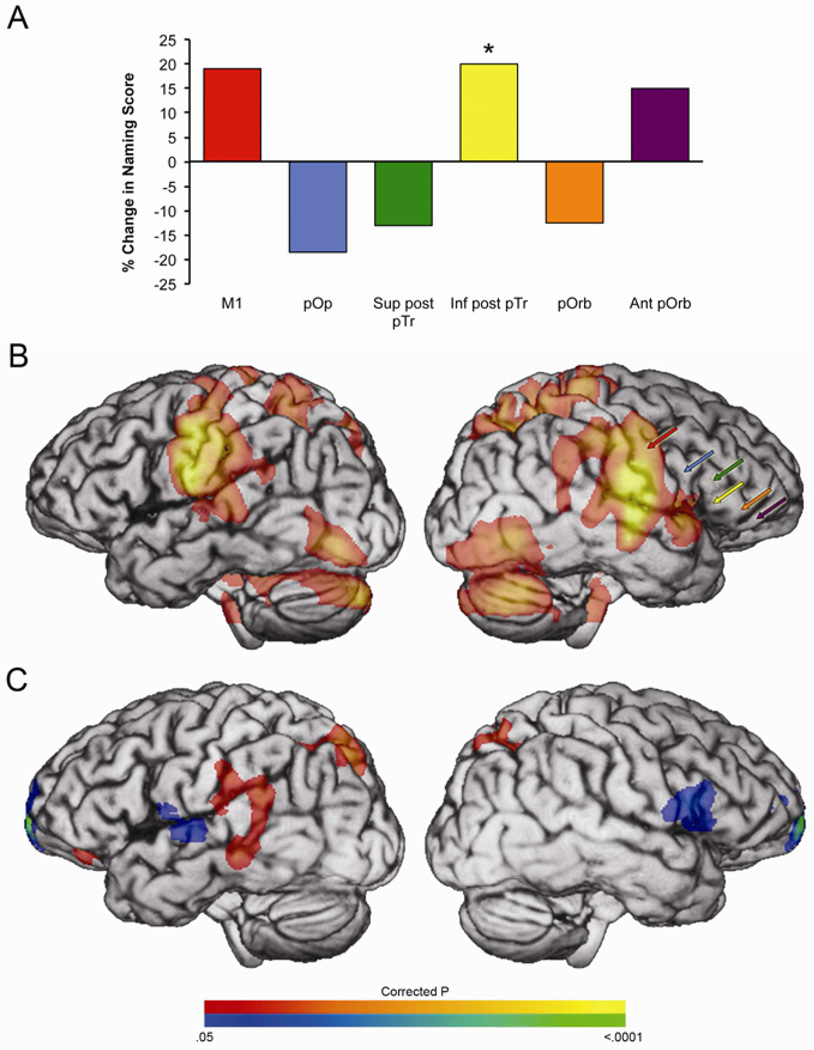
Different immediate effects of TMS were observed across the RH candidate targets (Fig. 2A). Naming improved most after inhibition of the inferior posterior pars triangularis, so this site was selected as the therapeutic target. Baseline fMRI (prior to any TMS exposure) demonstrated a bilateral pattern of motor, auditory, lateral temporal, parietal, and ventral occipitotemporal activity (Fig. 2B). The presence or absence of fMRI activity did not predict the response to TMS (note that candidate TMS sites were selected based on gyral anatomy, not fMRI activity- see Methods). Notably, the right inferior posterior pars triangularis site that responded best to TMS was not active during naming (Mean signal in 1cm spherical ROI: T(112) = −.12, p1-tailed, uncorrected = .55; Max signal T(112) = 1.25, p1-tailed, uncorrected = .107).
Fig. 2. fMRI and Acute TMS Results.
(A) Change in naming performance induced by inhibitory TMS to each of the candidate therapeutic TMS targets. (B) Baseline fMRI data (naming > pattern viewing) shown with locations of candidate TMS targets. (C) Change in fMRI activity after inhibitory TMS to the inferior posterior pars triangularis (yellow) target. The significance threshold is p < .05 corrected for multiple comparisons using the permutation method (Nichols and Holmes, 2002). The cluster threshold is 20 voxels. Red-yellow indicates increased activity. Blue-green indicates reduced activity after TMS. Activity decreased after TMS in the medial prefrontal cortex, the right caudate, right posterior insula, and the left superior temporal gyrus; activity increased in the bilateral superior parietal cortices, left supramarginal gyrus and left posterior temporal cortex.
FMRI data were also acquired on the first day of treatment, prior to TMS and immediately afterwards. Naming accuracy in the scanner improved slightly after TMS (Pre-TMS 88%, Post-TMS 93%). Only blocks without errors were analyzed to ensure that differences in activity induced by TMS were related to productive naming. Direct comparison of pre- and post-TMS scans confirmed that TMS reduced activity at the right pars triangularis stimulation site as expected (Fig. 2C). Despite predictions based on the theory of interhemispheric inhibition, no effect of TMS was observed at the LH homotope of the TMS target (p = .25), any left IFG subdivision (pars triangularis p = .44; pars opercularis p = .81; pars orbitalis p = .27), or the LH cortex as a whole (p = .12), even using anatomically defined ROIs without multiple comparisons correction.
3.3 Behavioral Results after TMS treatment and RH Stroke
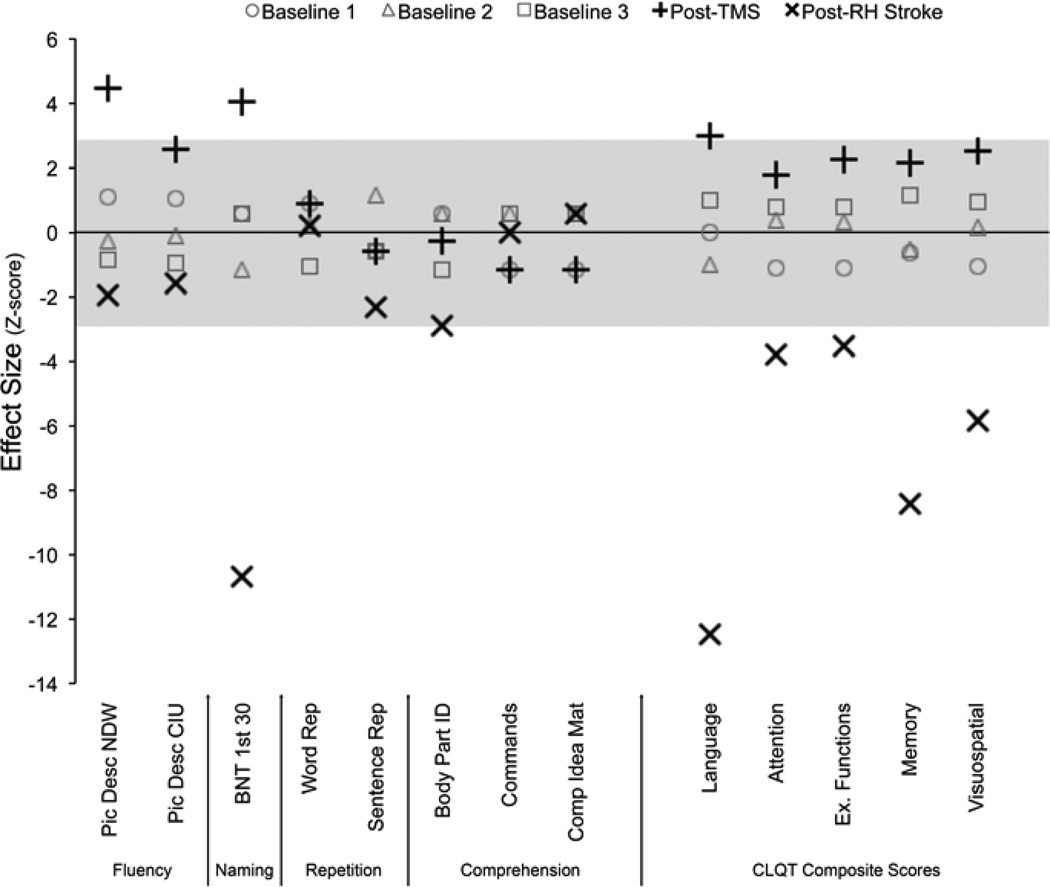
Two months after TMS treatment, the patient’s performance had improved significantly over baseline on measures of fluency and naming, but not repetition or comprehension (Fig. 3, Tab. 1). The CLQT language composite score improved significantly, while other cognitive domains demonstrated non-significant trends towards improvement.
Fig. 3. Behavioral Effects of TMS and RH Stroke.
Raw scores were Z-transformed based on mean and SD of baseline measurements to allow comparison across measures. The gray area indicates the significance threshold for a p < .05 Bonferroni corrected for multiple comparisons. CLQT composite scores were calculated excluding the symbol cancelation subtest due to a difference in the method of administration between sessions. NDW = number of different words. CIU = correct information units. BNT 1st 30 = accuracy on the first 30 items of the Boston Naming Test. Repetition and Comprehension measures are subtests of the BDAE
Tab. 1.
Scores of Neuropsychological Tests.
| Baseline Assessments | 2 month Follow-up (after TMS) |
6 Month Follow- Up (after RH stroke) |
||||||
|---|---|---|---|---|---|---|---|---|
| Test | Session 1 | Session 2 | Session 3 | Mean (SD) | Raw | Z-Score | Raw | Z-Score |
| Picture Description (Number of Different Words) | 18 | 13 | 11 | 14.0 (3.6) | 30 | 4.44* | 7 | −1.94 |
| Picture Description (Correct Information Units) | 39 | 25 | 15 | 26.3 (12.1) | 57 | 2.54 | 7 | −1.60 |
| BNT 1st 30 Items (% correct) | 66.7 | 60.0 | 66.7 | 64.4 (3.8) | 80.0 | 4.04* | 23.3 | −10.68* |
| BDAE Word Repetition (% correct) | 60.0 | 50.0 | 30.0 | 46.7 (15.3) | 60.0 | 0.87 | 50.0 | 0.22 |
| BDAE Sentence Repetition (% correct) | 40.0 | 60.0 | 40.0 | 46.7 (11.5) | 40.0 | −0.58 | 20.0 | −2.31 |
| BDAE Body Part Identification (% correct) | 95.0 | 95.0 | 85.0 | 91.7 (5.8) | 90.0 | −0.29 | 75.0 | −2.89 |
| BDAE Oral Commands (% correct) | 73.3 | 93.3 | 93.3 | 86.7 (11.5) | 73.3 | −1.15 | 86.7 | 0.00 |
| BDAE Complex Ideational Materials (% correct) | 58.3 | 66.7 | 66.7 | 63.9 (4.8) | 58.3 | −1.15 | 66.7 | 0.58 |
| CLQT Language Composite (% of max score) | 62.2 | 59.5 | 64.9 | 62.2 (2.7) | 70.3 | 3.00* | 28.4 | −12.50* |
| CLQT Attention Composite (% of max score) | 41.1 | 55.1 | 58.9 | 51.7 (9.4) | 68.2 | 1.76 | 15.9 | −3.83* |
| CLQT Executive Composite (% of max score) | 37.5 | 45.0 | 47.5 | 43.3 (5.2) | 55.0 | 2.24 | 25.0 | −3.52* |
| CLQT Memory Composite (% of max score) | 65.4 | 65.9 | 75.1 | 68.8 (5.5) | 80.5 | 2.14 | 22.7 | −8.44* |
| CLQT Visuospatial Composite (% of max score) | 53.1 | 60.5 | 65.4 | 59.7 (6.2) | 75.3 | 2.52 | 23.5 | −5.83* |
p < .05, corrected for multiple comparisons.
Three months after the RH stroke (six months after TMS treatment), the improvement on fluency and naming after TMS had reversed, with picture description scores marginally worse than baseline, and the BNT score 10.8 SD below baseline (Fig. 3, Tab. 1). There were trends toward worsening on sentence repetition and body part identification, but other repetition and comprehension tests were unchanged. CLQT composite scores decreased after the RH stroke across all cognitive domains, but the effect size was greatest for the language score. Based on Crawford and colleagues' criteria (Crawford et al., 1998), there was a strong dissociation between the language composite and both executive and visuospatial function after the RH stroke (Executive: T(3) = 3.7, p = .035; Visuospatial: T(3) = 3.2, p = .049) and trend toward a dissociation with the attention composite (T(3) = 3.1, p = .055).
4. Discussion
This case demonstrates seemingly paradoxical effects from two modes of RH disruption in the same aphasic patient: first, inhibiting the right pars triangularis using TMS improved aspects of aphasia both in the short and long-term, and second, a RH stroke worsened aphasia, even in comparison to its impact on other cognitive domains. Together, these findings demonstrate that the RH is not unitary in its role in aphasia recovery. Even within a single patient, involvement of some RH areas may support recovery, while other RH areas interfere.
It is not the case that the RH played a functional role in one language process, but a dysfunctional role in another. Both TMS and the RH stroke primarily impacted naming and word retrieval during picture description, with comparatively less effect on comprehension and repetition. This likely indicates that the RH as a whole in this case was more involved in word retrieval than other linguistic operations, but this finding may not generalize to other individual patients. For example, both production and comprehension were impacted by RH strokes in two previous cases of aphasia (Basso et al., 1989). RH recruitment, at least in terms of fMRI activity, varies depending on the distribution of the lesion such that RH regions are typically recruited if their LH counterparts have been damaged (Blank et al., 2003; Turkeltaub et al., 2011). This patient’s primary deficits from the original LH stroke were in speech production, and correspondingly her lesion was largely restricted to anterior language cortex. Thus, hemispheric shifts in posterior temporal lobe language processes may not have occurred because this territory was largely spared by the original stroke.
This case provides unambiguous clinical evidence that a LH lesion can induce compensatory reorganization in the RH, as no other competing explanation can explain the pattern of results seen here. General motor impairment after the RH stroke cannot explain the patient's language production deficits, as no left hemiparesis or other motor deficits were observed with the RH stroke, and repetition was spared relative to naming. Nor can generally decreased cognitive capacity due to an increased lesion burden explain the effects, as measures of attention and executive function were relatively spared compared to language. Reorganization after TMS cannot entirely account for the data either, as the RH stroke did not simply reverse the beneficial effects of TMS, but worsened speech production even relative to the pre-TMS baseline assessments. Rather, the relatively specific worsening of language functions after a RH stroke in a person with native left-lateralization (as demonstrated by the effects of the original LH stroke), strongly suggests that language networks reorganized after the LH stroke, resulting in an increased reliance on RH structures for some language functions. As the RH stroke involved multiple areas of subcortical white matter, this case does not allow us to ascribe a compensatory role to specific RH cortical sites, but rather suggests a compensatory role of RH networks in general.
Further, this case provides evidence against the putative mechanism by which TMS improves aphasia. Improvements in aphasia after inhibitory TMS to the RH have been attributed to a reduction in right-to-left interhemispheric inhibition, which theoretically allows the LH to recover function (Barwood et al., 2011; Martin et al., 2009a). According to this theory, the RH is normally inhibited by transcallosal fibers from the LH, but becomes over-active in aphasia because of a loss of these inhibitory pathways. This over-activity then theoretically inhibits the surviving homologous tissue in LH language areas, preventing them from regaining function. Inhibition of select RH targets using TMS is thus expected to reduce right-to-left inhibition, aiding recovery in the LH. The existence of interhemispheric connections that can affect cortical activity is well established in the motor system (Ferbert et al., 1992; Ilmoniemi et al., 1997), and inhibitory inputs originating from the intact contralesional hemisphere have been shown to contribute to motor deficits after lateralized strokes (Duque et al., 2005; Murase et al., 2004). Inhibitory rTMS over the contralesional motor cortex has been shown to decrease interhemispheric inhibition of motor evoked potentials (Pal et al., 2005), supporting the notion that the therapeutic benefit of inhibitory rTMS to the contralesional hemisphere may derive from a release of inhibition on perilesional cortex. However, the existence and importance of these interhemispheric interactions in language systems is largely theoretical, and is based on extrapolation from findings in the motor system.
This case provides a unique opportunity to test specific predictions based on an interhemispheric interaction account of RH recruitment and its suppression using TMS. Several predictions based on this theory can be tested in this case based on the immediate behavioral effects of TMS, the fMRI response to TMS, and behavioral effects of the RH stroke: (1) RH areas contralateral to the LH lesion should be over-active during language tasks, (2) the beneficial effect of TMS should be predicted by the presence of this over-activity, (3) inhibiting a RH target using TMS should decrease activity at the stimulation site and increase activity in homologous LH structures, (4) other modes of RH disruption should disinhibit the LH in a similar fashion to TMS, improving language functions further. None of these predictions were supported by this case. The TMS target that produced the greatest benefit to naming was not " over-active" prior to TMS as would be predicted if it were disinhibited by a loss of transcallosal projections from the LH. In fact, across candidate RH target sites, presence or absence of activity did not predict a beneficial effect to TMS. Although TMS produced the expected reduction in fMRI activity at the target site, no release of inhibition was seen in expected LH areas. Some increased activity was observed in other LH sites, notably in the lateral temporal lobe, but accounting for this finding on the basis of a release of interhemispheric inhibition would require substantial revision of the theory. It should be noted that although portions of the left pars triangularis and pars opercularis were involved in the original stroke, apparently viable tissue remained in both of these areas. Activation of such “peri-lesional” tissue is common in fMRI studies of aphasia (e.g. Rosen et al., 2000), but it remains possible that the lack of increased activity in these areas is due to perturbations in the hemodynamic response (Bonakdarpour et al., 2007), or functional deafferentation due to loss of subcortical white matter. The severe internal carotid stenoses might also have reduced the sensitivity to detect BOLD effects, but it is difficult to account for the lack of fMRI support for interhemispheric inhibition on this basis, as naming-related activity and changes in this activity from pre- to post-TMS were observed within the MCA distributions of each hemisphere in patterns that did not support interhemispheric inhibition. Finally, the detrimental effect of the RH stroke is incompatible with inter-hemispheric inhibition as a general mechanism for RH recruitment in aphasia. Although only a single case, the failure to support any of the predictions of inter-hemispheric inhibition strongly suggest that other mechanisms may account for RH recruitment in aphasia and the beneficial effects of right pars triangularis suppression on aphasia recovery.
What mechanism then accounts for the paradoxical effects of TMS inhibition and the RH stroke in this case? The negative impact of the stroke, which affected multiple areas of subcortical white matter, indicates the RH as a whole was involved in word retrieval. The paradoxical improvement seen after specific disruption of the pars triangularis then suggests that certain nodes within the RH may have limited the effectiveness of this adaptation. Consistent with these results, a recent imaging meta-analysis suggested that right frontal areas served similar functional roles to their normal LH counterparts in all cases except the pars triangularis (Turkeltaub et al., 2011). Likewise, immediate effects of inhibitory TMS in previous cases have indicated that improvements in naming are reliably seen after inhibition of the pars triangularis, whereas inhibiting other areas typically produces no effect or worsens naming (Hamilton et al., 2010; Martin et al., 2009b). This heterogeneity in RH neuroplastic mechanisms may account for seemingly contradictory results in prior imaging studies and the paradoxical effects of TMS and the RH stroke observed here. It is likely that latent RH networks capable of compensating for LH disruption are recruited when critical LH nodes are lost, and that this reorganization facilitates recovery (Heiss et al., 1999). However, the right pars triangularis, which is recruited as part of the latent RH network, limits the efficacy of this compensation either due to dysfunctional processing (Postman-Caucheteux et al., 2010), or an exaggerated role in a competing function, like response inhibition (Simmonds et al., 2008). The potential for these simultaneous opposing effects on recovery from different parts of the RH must be taken into consideration when designing interventions to treat aphasia.
In particular, a relatively new neuromodulatory technique called transcranial direct current stimulation (tDCS) has shown promise in improving language functions in aphasia (Baker et al., 2010; Fiori et al., 2010 EPub Ahead of Print; Monti et al., 2008). TDCS applies a low level of electrical current to the scalp to slightly increase or decrease cortical excitability. Its effects are less focal than those of TMS, so the potential for opposing roles of neighboring RH areas on language recovery must be taken into account when designing electrode configurations. Based on the evidence for interhemispheric inhibition in the motor system, a successful trial of tDCS for hemiparesis after stroke recently employed an electrode configuration designed to facilitate the lesioned hemisphere and simultaneously inhibit the intact side (Lindenberg et al., 2010). Our findings here suggest that similar electrode configurations may be less successful for treatment of aphasia since widespread inhibition of the RH may be counterproductive. In contrast, new tDCS devices that allow relatively focal inhibition with mild excitation of surrounding cortex (Datta et al., 2009) may be ideally suited to treatment of aphasia when applied to the right pars triangularis.
We report a patient with aphasia who received TMS, then had a right hemisphere stroke
Inhibition of right pars triangularis using TMS improved word retrieval
FMRI data did not support a release of transcallosal inhibition as a mechanism of TMS
A subsequent right hemisphere stroke specifically worsened language function
The right hemisphere supports aphasia recovery, but the pars triangularis interferes
Acknowledgments
This work was supported by the American Academy of Neurology Foundation (Clinical Research Training Fellowship to P.E.T), and the NIH (K01NS060995 to R.H.H and RO1 DC05672). We thank the patient and her family for their participation in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow T. On a case of double cerebral hemiplegia, with cerebral symmetrical lesions. British Medical Journal. 1877;2:103–104. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, JD OS, Coulthard A, Wong A. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. European Journal of Neurology. 2011;18(7):935–943. doi: 10.1111/j.1468-1331.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia. Two case studies. Cortex. 1989;25(4):555–566. doi: 10.1016/s0010-9452(89)80017-6. [DOI] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJ. Speech production after stroke: the role of the right pars opercularis. Annals of Neurology. 2003;54(3):310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36(1):159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B, Parrish TB, Thompson CK. Hemodynamic response function in patients with stroke-induced aphasia: implications for fMRI data analysis. Neuroimage. 2007;36(2):322–331. doi: 10.1016/j.neuroimage.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Howell DC, Garthwaite PH. Payne and Jones Revisited: Estimating the Abnormality of Test ScoreDifferences Using a Modified Paired Samples t Test. Journal of Clinical & Experimental Neuropsychology. 1998;20(6):898. doi: 10.1076/jcen.20.6.898.1112. [DOI] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation. 2009;2(4):201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28(4):940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. The Journal of Physiology. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, Provinciali L, Tomaiuolo F, Marangolo P. Transcranial Direct Current Stimulation Improves Word Retrieval in Healthy and Nonfluent Aphasic Subjects. Journal of Cognitive Neuroscience. 2010 doi: 10.1162/jocn.2010.21579. EPub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, Martin P, Coslett HB. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain and Language. 2010;113(1):45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annals of Neurology. 1999;45(4):430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Kessler J, Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage. 2003;20(Suppl 1):S42–S49. doi: 10.1016/j.neuroimage.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hellal P, Lorch MP. The validity of Barlow's 1877 case of acquired childhood aphasia: case notes versus published reports. Journal of the History of the Neurosciences. 2007;16(4):378–394. doi: 10.1080/09647040600653931. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8(16):3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, Turkheimer F, Howard D, Wise R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Annals of Neurology. 2002;51(5):553–558. doi: 10.1002/ana.10181. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Doron KW, Bogdan A, Baker EH, Kurland J, Renshaw P, Yurgelun-Todd D. Overt naming in aphasia studied with a functional MRI hemodynamic delay design. Neuroimage. 2005;28(1):194–204. doi: 10.1016/j.neuroimage.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker EH, Alonso M, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain and Language. 2009a;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Treglia E, Kaplan E, Baker EH, Pascual-Leone A. Research with transcranial magnetic stimulation in the treatment of aphasia. Current Neurology and Neuroscience Reports. 2009b;9(6):451–458. doi: 10.1007/s11910-009-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. Journal of neurology, neurosurgery, and psychiatry. 2008;79(4):451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122(Pt 9):1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain and Language. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Senda M, Kitamura S, Ishii K, Mishina M, Terashi A. Role of the nondominant hemisphere and undamaged area during word repetition in poststroke aphasics. A PET activation study. Stroke. 1996;27(5):897–903. doi: 10.1161/01.str.27.5.897. [DOI] [PubMed] [Google Scholar]
- Pal PK, Hanajima R, Gunraj CA, Li JY, Wagle-Shukla A, Morgante F, Chen R. Effect of low-frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. Journal of Neurophysiology. 2005;94(3):1668–1675. doi: 10.1152/jn.01306.2004. [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, McArdle J, Braun AR. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience. 2010;22(6):1299–1318. doi: 10.1162/jocn.2009.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain : a journal of neurology. 2008;131(Pt 5):1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, Hamilton RH. Are networks for residual language function and recovery consistent across aphasic patients? Neurology. 2011;76(20):1726–1734. doi: 10.1212/WNL.0b013e31821a44c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers CA, Vink M, van Zandvoort MJ, van der Worp HB, de Haan EH, Kappelle LJ, Ramsey NF, Dijkhuizen RM. Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010;49(1):885–893. doi: 10.1016/j.neuroimage.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36(8):1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke. 2007;38(4):1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]